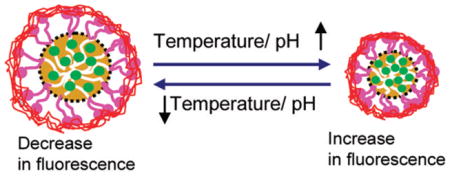
Abstract
Polymeric near-infrared (NIR) fluorescent nanocapsules were developed, of which the fluorescence exhibited reversible response to local thermal/pH modulation. Our strategy was to use polymeric micelles made of temperature-sensitive Pluronic F-127 to encapsulate an amphiphilic NIR fluorescent dye—indocyanine green (ICG)—within the core and then cross-link the micelle corona by pH-sensitive poly(ethylenimine) (PEI). The size swelling/shrinking property of the micelles induced by temperature decrease/increase was used as a switch to control the fluorescence yield of the nanocapsules. It was found that the fluorescence yield significantly increased with the increase in temperature. The PEI cross-link made the fluorescence yield also sensitive to local pH change and enhanced intracellular delivery of the nanocapsules as well. Preliminary results suggest the NIR fluorescent probes could be potentially used as a contrast agent sensitive to local environment for translational optical imaging/sensing.
Graphical abstract
INTRODUCTION
Optical molecular imaging technology has emerged as a powerful tool for early disease detection, cell tracking, drug screening, and monitoring of various physiological activities in a noninvasive fashion, and fluorescent dyes as contrast agents are the integral part of this emerging imaging technology.1 Polymer-conjugated fluorescent dyes, of which the fluorescence properties are sensitive to local stimuli such as temperature and pH changes, have received significant attention.2–5 Most of those environmentally sensitive probes are in the visible spectral range, and one associated challenge is the strong interference from the background tissue autofluorescence when used for tissue imaging/sensing.6,7 It is well-known that near-infrared (NIR) cyanine dyes with excitation/emission spectra within the “optical window” of biological tissues (i.e., 700–1000 nm) have much reduced interference from tissue autofluorescence. In addition, NIR fluorescent dyes can also provide deep imaging/sensing penetration depth due to the reduced optical attenuation of biological tissues in the NIR wavelength range.8–10 In principle, NIR dyes can be made sensitive to local temperature or pH, e.g., by incorporating temperature or pH-sensitive compounds into the cyanine molecular framework.11 However, this approach is often rather complex since it requires a precise choice of functional groups and a feasible synthetic methodology.12 Here we report a novel and general yet simple method to develop an NIR fluorescent probe that exhibits reversible sensitivity to both thermal and pH modulation. The probe is based on temperature-sensitive Pluronic nanocapsules with an NIR dye (indocyanine green, ICG) loaded in the hydrophobic core and poly(ethylenimine) polymers cross-linked in the hydrophilic corona. It was found that the fluorescence yield of ICG could be tuned by changing the hydrophobicity of the nanocapsules following local temperature or pH modulation.
ICG is a water-soluble, amphiphilic tricarbocyanine dye with the adsorption and emission maxima around 780 and 810 nm, respectively.13,14 Up to date, ICG is the only NIR fluorescent dye approved by the United States Food and Drug Administration (FDA) for routine clinical use,15,16 for example, for visualizing blood and clearance, studying liver function, and recently for guiding biopsies.17–19 However, the use of ICG for imaging applications is limited by several drawbacks of ICG such as aqueous, photo, and thermal instability and nonspecific binding with serum proteins.20,21 Recently, we have demonstrated that some of the drawbacks, particularly the aqueous and thermal instability of ICG, can be overcome by encapsulating ICG molecules with polymeric micelles.22,23 It has also been shown that the amphiphilic property of ICG induces aggregation in an aqueous solution, resulting in a reduced quantum yield due to self-quenching.16,24 Considering aggregation and fluorescence yield of an amphiphilic molecule (such as ICG) is dependent on solvent polarity,25,26 it is anticipated that the change of the hydrophobicity/hydrophilicity of the micelle interior could potentially induce a change in ICG fluorescence yield.
In this report we chose Pluronic F-127 micelle to encapsulate ICG. Pluronic F-127 is an amphiphilic triblock copolymer composed of poly(ethylene oxide) [PEO]–poly(propylene oxide) [PPO]–poly(ethylene oxide) [PEO] which can self-assemble into micelle in aqueous solution with a hydrophobic core and hydrophilic corona.27 The micelle and gel formation of Pluronic copolymers in aqueous solution has been extensively investigated. Because of the block architecture and lower critical solution temperature (LCST) of PEO and PPO, aqueous solution of Pluronic micelle exhibits temperature sensitivity. As temperature rises, the hydrophobicity of the PPO and PEO segments on the Pluronic polymer increases, which leads to the packing of more PPO segments in the core of the micelle, expulsion of more water from the core, and shrinkage of the PEO segment in the corona of the micelle; therefore, the overall size of the micelle decreases.28–30 As a consequence, the fluorescence yield of ICG encapsulated within the micelle becomes sensitive to temperature. To make the nanocapsules sensitive to local pH, poly(ethylenimine) (PEI), which contains abundant amino groups and exhibits pH-dependent property (such as deprotonation at higher pH),31,32 is used to cross-link the corona of the ICG-loaded micelles. The cross-link can also improve micelle stability, which will be particularly useful for future in vivo optical imaging/sensing applications. In addition, the PEI cross-link can also significantly enhance intracellular delivery of the micellar nanocapsules considering PEI is the most commonly used cationic polymer for gene delivery.33,34 In the present study, we investigated the fluorescence characteristics of the micellar ICG nanocapsules and demonstrated their reversible responsiveness to local thermal and pH modulation. To the best of our knowledge, this is the first report of a stable, reversible NIR fluorescent probe that is responsive to both local temperature and pH modulation.
EXPERIMENTAL METHODS
Materials
Pluronic F-127 (PEO100–PPO65–PEO100, average MW ~12 600) was obtained from BASF Corp. (Mount Olive, NJ). ICG, poly(ethylenimine) (MW ~ 2000, 50% solution in water), and p-nitrophenyl chloroformate (p-NPC) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM), streptomycin/penicillin, trypsin, fetal bovine serum (FBS), and all other cell culture supplements were purchased from Invitrogen (Carlsbad, CA). All chemical reagents used in this study were analytical grade.
Activation of Pluronic F-127 for Cross-Linking Micelles with PEI
The hydroxyl groups of Pluronic F-127 were activated by p-NPC to prepare amine-reactive Pluronic F-127.35,36 Pluronic F-127 was first completely dried in vacuum overnight. 2 g of Pluronic F-127 was then dissolved in 6 mL of anhydrous benzene and slowly added to a stirred 6 mL solution of anhydrous benzene containing p-NPC (192 mg) in a dropwise manner. The reaction proceeded at room temperature with gentle stirring under nitrogen purge for 3 h, and the product was precipitated three times in ice-cold diethyl ether and then dried under vacuum.
Preparation of ICG Encapsulation with Activated Pluronic Micelles
A solvent evaporation method was employed to encapsulate ICG into the activated Pluronic micelles. Briefly, a 1 mM solution of ICG was prepared in chloroform, and then the ICG solution was added dropwise to a 2% activated Pluronic solution (pH ~ 6.0) under stirring to achieve a final ICG concentration of 50 μM. The chloroform was removed by evaporation with ICG partitioning into the core of the micelles. Free ICG molecules were removed by using cellulose centrifuge filters (MWCO 10 kDa, Millipore, Billerica, MA), and the remaining ICG-loaded micelle solution was rinsed two times with deionized water (pH ~ 6.0). Rinsed micelles were concentrated in 200 μL of DI water (pH ~ 6.0).
PEI Cross-Linking of ICG-Loaded Micelles
200 μL of aqueous solution of ICG-loaded micelles was added dropwise to 2 mL aqueous solution of PEI (0.5% w/v at pH ~ 9.0). The reaction proceeded at room temperature with gentle stirring for 3 h. The byproduct was removed using cellulose centrifuge filters (MWCO 10 kDa, Millipore, Billerica, MA), and the remaining PEI cross-linked ICG/micelle solution was rinsed three times using deionized water and the final volume of the ICG–micelle solution was kept at 1 mL.
ICG Concentration Assay
50 μL of the above ICG–micelle solution was added to 950 μL of dimethyl sulfoxide (DMSO), resulting in complete disassembling of the micelles and release of the encapsulated ICG molecules. A solution of 50 μL of DI water plus 950 μL of DMSO was used as blank. The equivalent ICG concentration of the ICG–micelle solution was determined by comparing the absorbance of the (dissolved) ICG–micelle DSMO solution at 794 nm to a standard absorption curve of free ICG in the same solution. All measurements were performed in triplicate.
Particle Size and Zeta Potential Measurements
The effective (or hydrodynamic) diameter and zeta potential of the ICG-Pluronic/PEI micelles were measured using a dynamic light scattering (DLS) instrument (ZetaPlus, Brookhaven Instrument Co., Holtsville, NY) at a wavelength of 632 nm and a 90° detection angle. The concentration of the micelles was 0.5% in water (pH ~ 7.2), and the temperature was controlled at 22–40 °C. The measurements were carried out in triplicate.
Transmission Electron Microscopy (TEM) Imaging of ICG Micellar Nanocapsules
For TEM imaging, 0.5% (w/v) ICG nanocapsule solutions were pre-equilibrated at 22, 28, 34, and 40 °C. One drop of each solution was deposited onto a 300 mesh Formvar/carbon coated copper grid and dried at 22, 28, 34, and 40 °C, and then the samples were stained for 1 min with 1 drop of 2% (w/v) uranyl acetate solution. Negatively stained samples were imaged with a Philips F20 transmission electron microscope.
ICG Fluorescence Measurements under Temperature and pH Modulation
The temperature dependence of the fluorescence yield of ICG-Pluronic/PEI micelles was determined by measuring the variation of the fluorescence intensity of a given ICG micellar solution using Fluorolog-3 spectrofluorometer (Jobin Yvon) which was equipped with a temperature controller. The monochromator-based spectrofluorometer offered a spectral resolution better than 0.5 nm and thus enabled effective separation of the ICG emission spectrum (with a peak around 810 nm) from the excitation light (780 nm). The ICG micellar solutions were pre-equilibrated at different temperatures (i.e., 22–40 °C) for 30 min before the spectrofluorometry measurements. During fluorescence measurements, the sample temperature was maintained constant by putting the cuvette in a thermostated fluid with a preset digitally controlled temperature. Fluorescence intensities of the ICG micelle solutions at various pH values (4, 7, and 10) were acquired at a fixed wavelength (i.e., 810 nm which was around the ICG peak emission wavelength). During the measurements, HCl and NaOH were used to adjust the solution pH to a desired value, and the fluorescence intensity of the sample was measured at different digitally controlled temperature ranging from 22 to 40 °C. The pH of the samples was measured by a digital pH meter.
Test of Intracellular Uptake of ICG–Pluronic/PEI Micelles
Cancer cells (A431) were cultured in DMEM medium with 10% FBS and 1% penicillin/streptomycin. Cells were seeded at a density of 5 × 105 cells/well over cover glass in a 6-well plate and cultivated at 37 °C and 5% CO2 in a humidified incubator. After 24 h, the cell culture medium in each well was replaced with 2 mL of serum-free DMEM medium plus 200 μL of ICG–Pluronic/PEI micelle solution (with an ICG dose of ~10 μg) in each well. The cells were then incubated for 30 min. As a control, the cells were incubated with a solution of ICG–Pluronic micelles (which were not cross-linked with PEI but had the same ICG dose). After incubation, the cells were washed three times with PBS prewarmed to 37 °C. ICG fluorescence images of the cells were then acquired by a microscope (Zeiss Axiovert 200) with an ICG filter set (49030 ET, Chroma Technology Corp., excitation 775/50 nm, emission 845/55 nm) and a 20× objective (Zeiss, Ecplan-NEOFLUAR). Images were recorded using an EMCCD camera (Andor) with a gain of 200 and an exposure time of 0.2 s.
In Vitro Cytotoxicity Assay
The in vitro cytotoxicity of PEI cross-linked ICG nanocapsules was investigated by performing methyl thialzolyl tetrazolium (MTT) assays on cancer cells (A431). Cells were first seeded at a density 5 × 104 cells per well in a 96-well plate. After 24 h of incubation at 37 °C with 5% CO2 under 100% humidity, the cells were divided into four groups with each group incubated with a 200 μL DMEM medium containing PEI cross-linked ICG nano-capsules at a different concentration (0.01, 0.1, 1, and 10 mg/mL), and each concentration was tested in triplicate. After 3 h incubation, MTT aqueous solution (20 μL, 5 mg/mL) was added to each well, and the plate was incubated for an additional 2 h. The medium in each well was then removed, and 200 μL of DMSO was added to dissolve any purple formazan crystals. The absorbance at 570 nm of each well was measured by a microplate reader (Tecan Safire2), and the cell viability in each well was directly proportional to the measured absorbance. For comparison, the same experiment was repeated, except that cells in this case were incubated with ICG nanocapsules instead of PEI cross-linked ICG nanocapsules. Cells incubated with standard culture medium were served as control. The cell viability is expressed as the percentage of viable cells relative to the survival of the control group.
RESULTS AND DISCUSSION
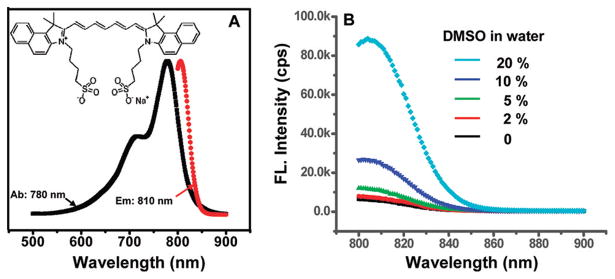
ICG is a water-soluble and amphiphilic tricarbocyanine NIR dye. It has two hydrophobic polycyclic parts and a hydrophilic sulfate group bound to each polycyclic part as shown in the inset of Figure 1A. In general, the fluorescence quantum yield of an amphiphilic dye (such as ICG) depends on the solvent polarity. Figure 1B shows the fluorescence intensity of ICG at a concentration ~1 μg/mL in a solvent (water mixed with DMSO with different volume ratios). The ICG fluorescence intensity in 20% DMSO is much higher than in pure water. This is mainly because the latter is more hydrophilic and ICG molecules are more subjective to aggregation.25,26 Supported by the above observation, we hypothesize that the fluorescence yield of ICG encapsulated within the micelles can be tuned by modulating the hydrophobicity/hydrophilicity of the micelle core, which in turn can be modulated by local temperature and pH. This forms the basis for developing the thermo/pH-sensitive NIR fluorescent probes.
Figure 1.
Absorption and emission spectra and molecular structure of ICG (A) and ICG fluorescence intensity versus solvent polarity (B), where the ICG concentration was 1 μg/mL.
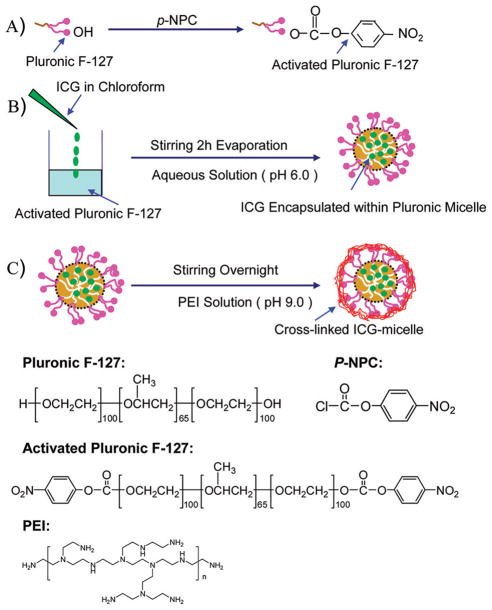
In this study we chose temperature-sensitive Pluronic micelles to encapsulate ICG and then cross-linked the corona of the micelles by pH-sensitive polymer–PEI, aiming to develop a dual thermo/pH-sensitive fluorescent probe in the NIR wavelength range. Figure 2 illustrates the detailed steps to synthesize the fluorescent nanoprobes and structures of the chemicals involved. In essence, the terminal hydroxyl groups of Pluronic F-127 were preactivated by p-nitrophenyl chloroformate (p-NPC) (Figure 2A), and ICG molecules were then encapsulated into the micelles made of activated Pluronic F-127 by using a solvent evaporation method (Figure 2B). After ICG encapsulation, the poly(ethylenimine) (PEI) polymer which has a positively charged amino group was used to cross-link the corona of the micelles and achieve sensitivity to local pH (Figure 2C), and the ICG concentration within cross-linked micelles was determined by DMSO extraction as described in the Experimental Methods section. The absorption curve of the ICG micelles dissolved in the DSMO solution was compared with the standard absorption curve of free ICG dissolved in the same solution (DSMO) in the concentration range of 0–3.0 μg/mL, and an R2 correlation coefficient of 0.999 was achieved. The hydrophobicity/hydrophilicity of the micelle interior and the ICG fluorescence yield can be then modulated by local temperature/pH variation. Furthermore, considering PEI is the most common cationic polymer for gene delivery, the PEI cross-linkage within the micelle corona can also significantly enhance intracellular delivery of the micellar nanocapsules.
Figure 2.
Steps for engineering ICG-loaded Pluronic micellar nanocapsules that are cross-linked by PEI polymers. Molecular structures are also shown in this figure for the chemicals involved in engineering the polymeric ICG nanocapsules.
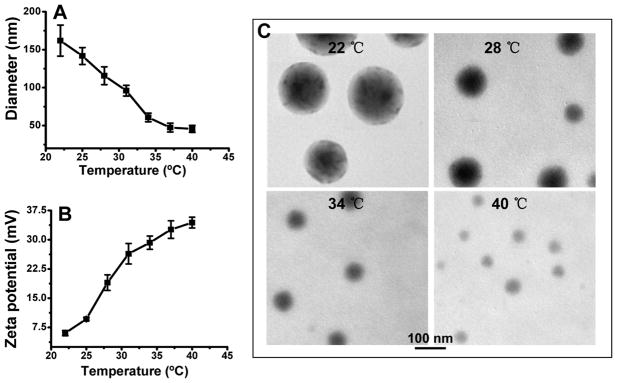
The size and the temperature/pH-sensitive swelling/shrinking behavior of the ICG nanocapsules were characterized by using dynamic light scattering (DLS) and TEM imaging (Figure 3). Figure 3A shows the thermosensitive swelling/shrinking hydrodynamic size of the nanoprobes at various temperatures measured by DLS. The size is very sensitive to temperature change (22–40 °C), and the hydrodynamic diameter reduces dramatically from 162 ± 20.5 nm at 22 °C down to 45.6 ± 4.6 nm at 40 °C. The cross-linkage by the positively charged PEI on the surface of the nanocapsules makes the surface zeta potential sensitive to nanocapsule size and thus to temperature as shown in Figure 3B. The zeta potential greatly increases from 6 ± 0.6 mV at 22 °C up to 34 ± 1.4 mV at 40 °C. The reduced size at an elevated temperature increases the charge density on the nanocapsule surface, resulting in an increase in zeta potential. Temperature-induced size change of the ICG nanocapsules was also directly revealed by TEM imaging. Figure 3C shows representative TEM images of ICG–Pluronic/PEI nanocapsules pre-equilibrated at different temperature on a 300 mesh Formvar/carbon support grid. To improve the image quality, the nanocapsules were stained with 1 drop of 2% (w/v) uranyl acetate solution for 1 min. The average sizes of ICG nanocapsules at 22, 28, 34, and 40 °C were 155 ± 23, 95 ± 17.4, 69 ± 10.5, and 37 ± 9.8 nm, respectively. Increasing the solution temperature from 22 to 40 °C caused the nanocapsule to shrink by almost 5-fold. Temperature-dependent micellization and gel formation are two most characteristic properties of aqueous Pluronic copolymers solutions. Above the critical micellar temperature (CMT), the Pluronic polymer molecules become more lipophilic and self-assemble to micelles with hydrophobic PPO groups at the core of the micelle.30 A unique property of Pluronics micelles is that its core becomes increasingly hydrophobic with a shrinking size by increasing the temperature, which results in the enhancement of fluorescence yield/intensity of the encapsulated ICG.
Figure 3.
Size and zeta potential of ICG–Pluronic/PEI nanocapsules measured by DLS (A) and a zeta potential analyzer (B) over a temperature range of 22–40 °C and TEM images of the nanocapsules at different temperatures (C).
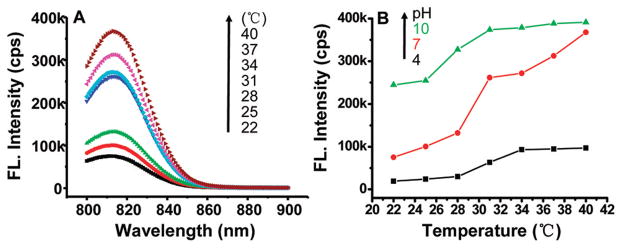
The changes in fluorescence intensity/yield of the ICG nanocapsules versus temperature and pH are shown in Figure 4. In Figure 4A, it is noted that the fluorescence intensity (over the emission spectrum of ICG) greatly increased with the increase in temperature at a given pH (i.e., 7.0). From 22 to 40 °C, the fluorescence intensity of the ICG nanocapsules at the same concentration (~3 μM) was enhanced by about 6 times. As discussed above, ICG is amphiphilic molecules, and its fluorescence yield is dependent on solvent polarity. As the temperature increases, the size of the ICG micelles decreases and the increased hydrophobicity within the micellar core resulted in the enhancement of ICG fluorescence. It should be noted that the fluorescence intensity of ICG-Pluronic/PEI micelles exhibited pH-dependent behavior as well owing to the positively charged amine groups of PEI within the micelle corona. The influence of local pH on the ICG nanocapsule fluorescence at the peak wavelength around 814 nm is shown in Figure 4B. The detailed fluorescence spectra of the nano-capsules versus temperature (22–40 °C) at three different pH values (4.0, 7.0, and 10.0) are illustrated in Figure S-1 (Supporting Information). The positively charged amine groups in the cross-linked corona of the micelles can be neutralized by anions in solution, which will influence the micelle core hydrophobicity and thus induces the sensitivity of the encapsulated ICG fluorescence yield to local pH. At a high pH (i.e., 10.0), the nanocapsules would be more hydrophobic due to deprotonation of the amine groups of PEI in the micelle shell, resulting in an increased hydrophobicity in the nano-capsule interior and thus an increase in the ICG fluorescence intensity/yield. In comparison, at a low pH (i.e., 4.0), the nanocapsules would be more hydrophilic due to protonation of the amine groups in the shell, and thus the nanocapsule interior hydrophobicity would decrease, resulting in a decrease in the ICG fluorescence intensity/yield.
Figure 4.
ICG nanocapsule fluorescence intensity versus temperature at a given pH (i.e., 7.0) (A) and fluorescence intensity versus pH at a given temperature (i.e., 22 °C) (B) in aqueous solution. cps: counts per second.
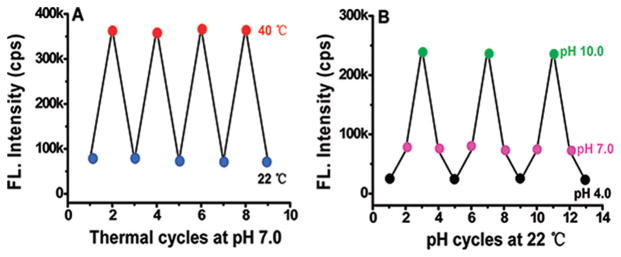
Our study has also shown that the above response of the nanocapsule fluorescence to local temperature and pH modulation is reversible (see Figure 5). These results are consistent with the thermoreversible sol–gel transition of the Pluronic F-127 micelles.37 It was found that the resulted reversible change of the micelle hydrophobicity accompanies with a reversible modulation of the encapsulated ICG fluorescence intensity/yield. During each cycle of temperature or pH modulation, the fluorescence could almost come back its previous value, and the time to reach an equilibrium was ~30 (~10) min for thermo (pH) modulation.
Figure 5.
Reversible response of the ICG nanocapsule fluorescence to temperature (A) and pH (B) modulation in aqueous solution. For temperature modulation in (A), the pH was kept at 7.0. For pH modulation in (B), the temperature was kept at 22 °C. It took about 30 min to reach equilibrium during thermal modulation and about 10 min during pH modulation.
It has been shown that salt can influence the critical micellization temperature (CMT);38 thus, it is naturally interesting to assess the performance of the micellar ICG nanocapsules in electrolytes at different concentrations. Figure 6 illustrates the change of the ICG nanocapsule fluorescence (at a given ICG concentration of ~1 μM) in response to the varying medium temperature at different ionic strengths (i.e., different NaCl concentrations). At a given temperature, it was found that the fluorescence intensity increased with sodium chloride concentration (as shown in Figure 6). The detailed fluorescence spectra of the nanocapsules versus temperature (22–40 °C) at different concentrations of sodium chloride are illustrated in Figure S-2 of the Supporting Information. The underlying mechanism is that salt can change the hydrogen-bonding interactions in water, which decreases the interaction of polymer molecules with water. Both the EO and PO blocks of Puronic-127 are dehydrated by the addition of salt in a similar way, which results in a decrease in the critical micellization temperature (CMT).38 It is found that the addition of salt favors a more compact micellar core, where the water content is decreased and the PO–PO block interaction is increased, thus leading to an increase in the hydrophobicity of the nanocapsule and consequently an increase in the fluorescence yield of the encapsulated ICG.39
Figure 6.
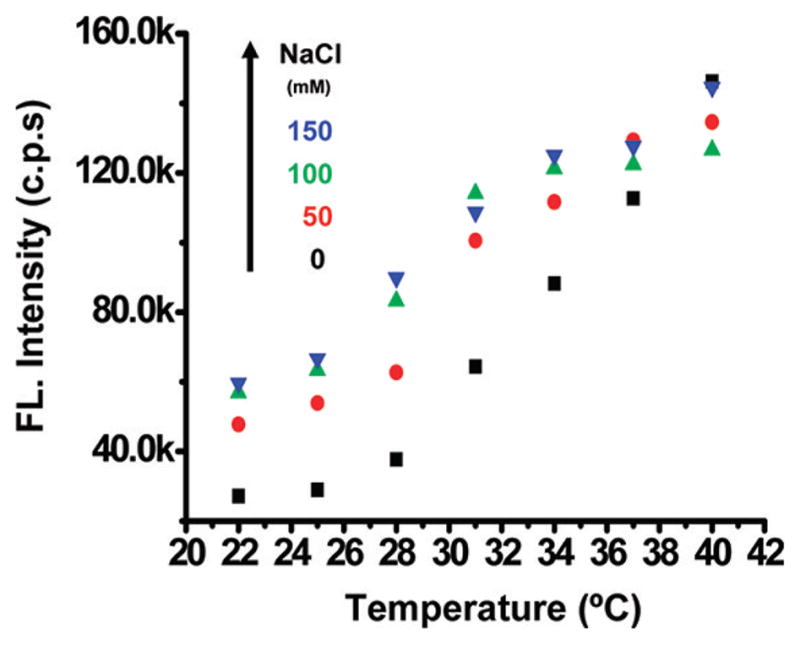
Fluorescence response of the ICG nanocapsules to the change in medium temperature at different ionic strengths.
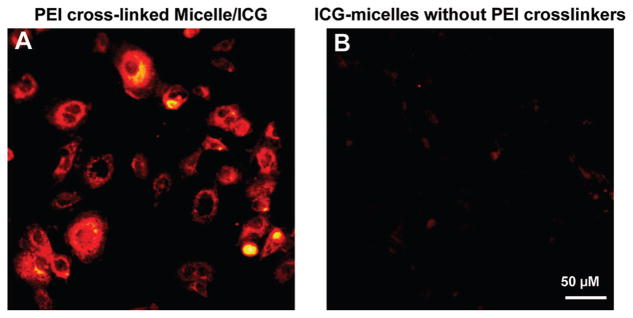
As mentioned previously, PEI is the most commonly used cationic polymer for gene delivery. The PEI cross-link in the micelle corona can thus significantly enhance intracellular delivery of the micellar nanocapsules. To demonstrate the feasibility of enhanced intracellular delivery, A431 cancer cells were incubated with the nanocapsules for about 30 min, followed by PBS wash and fluorescence microscopy imaging. Significantly enhanced ICG fluorescence was observed from the cells incubated with the PEI cross-linked ICG nanocapsules (as shown in Figure 7A) compared with the cells incubated with the ICG nanocapsules that did not have PEI cross-linkers (as shown in Figure 7B) under the same imaging condition. We noticed in Figure 7A that strong fluorescence signals were detected throughout the cytoplasm in some cells, while in other cells strong fluorescence signals were only observed around the cell membrane. We suspected in some cells some ICG nanocapsules had traversed the cell membrane at the time the fluorescence microscopy image was taken, and the cytoplasm thus exhibited strong fluorescence. In other cells, the majority ICG nanocapsules might be in the process of going through the membrane when the microscopy image was taken, and thus only the cell membranes exhibited strong fluorescence. The effective intracellular transfer was facilitated by the highly basic and positively charged aliphatic polymers–PEI. The results also suggest that PEI-cross-linked ICG–Pluronic nanocapsules can potentially serve as NIR fluorescent contrast agents for intracellular imaging and for monitoring intracellular gene/drug delivery (once gene/drug is coencapsulated with ICG within the micelles).
Figure 7.
ICG fluorescence images of A431 cells after incubation with PEI-cross-linked ICG nanocapsules (A) and ICG nanocapsules without PEI cross-linkers (B) for 30 min with an ICG concentration of 5 μg/mL. After washing with PBS three times, fluorescence microscopy images of the cells were acquired by a microscope (Zeiss Axiovert 200) with an ICG filter set (49030 ET, Chroma Technology Corp., excitation 775/50 nm, emission 845/55 nm) and a 20× objective (Zeiss, Ecplan-NEOFLUAR). Images were recorded using an EMCCD camera (Andor) with a gain of 200 and an exposure time of 0.2 s.
Considering the potential cytotoxicity associated with PEI.40,41 In vitro cell viability experiments based on MTT assay were performed on A431 cancer cells, and the results are shown in Figure S-3. Drastic cell death (e.g., about 50%) was observed after cells were incubated with PEI cross-linked ICG nanocapsules at a concentration of 10 mg/mL for 3 h, which corresponded to a LD50 value of about 10 mg/mL for the PEI cross-linked ICG nanocapsules. In comparison, cells incubated with ICG nanocapsules (10 mg/mL) that did not have the PEI cross-linkers exhibited much less cytotoxicity, and the cell viability loss was less than 10%. It is also noted that the LD50 of the PEI cross-linked ICG nanocapsules is still much higher (i.e., by about 300-fold) than the critical micelle concentration (CMC) of the ICG Pluronic F-127 micelles (i.e., 0.035 mg/mL),23 leaving a fairly large concentration range for using the PEI cross-linked ICG nanocapsules in vitro and potentially in vivo. Nonetheless, for future in vivo applications, it will be desirable to pursue more biocompatible polymers (as opposed to PEI) for developing pH/thermo NIR fluorescent probes.
CONCLUSIONS
In summary, NIR fluorescent molecular probes based on PEI-cross-linked ICG-loaded Pluronic micelles were successfully developed. It has been shown that the ICG nanocapsules are responsive to local thermal/pH modulation in a reversible fashion. The nanocapsules also exhibit significant enhancement for intracellular delivery which may be very valuable for intracellular optical molecular imaging.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (NIH) (R01CA120480 and R01CA153023) and the National Science Foundation (NSF) Career Award (XDL). The authors thank Dr. Yong Ren for his assistance with DLS and zeta potential measurements. We also thank Mr. Jiefeng Xi and Dr. Toufic Jabbour for their critical reading of the manuscript.
Footnotes
Detailed fluorescence spectra of the nanocapsules versus temperature (22–40 °C) at three different pH values (4.0, 7.0, and 10.0) and at different concentrations of sodium chloride; cell viability study results based on MTT assay of the ICG nanocapsules with and without PEI cross-linkers. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ke S, Wen XX, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, Li C. Cancer Res. 2003;63(22):7870–7875. [PubMed] [Google Scholar]
- 2.Tang L, Jin JK, Qin AJ, Yuan WZ, Mao Y, Mei J, Sun JZ, Tang BZ. Chem Commun. 2009;33:4974–4976. doi: 10.1039/b907382e. [DOI] [PubMed] [Google Scholar]
- 3.Gota C, Okabe K, Funatsu T, Harada Y, Uchiyama S. J Am Chem Soc. 2009;131(8):2766–2767. doi: 10.1021/ja807714j. [DOI] [PubMed] [Google Scholar]
- 4.Chen DY, Xu QF, Xia XW, Ge JF, Lu JM, Li NJ. Mater Chem Phys. 2010;120(2–3):614–618. [Google Scholar]
- 5.Maruyama S, Kikuchi K, Hirano T, Urano Y, Nagano T. J Am Chem Soc. 2002;124(36):10650–10651. doi: 10.1021/ja026442n. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Berezin MY, Guo K, Kao J, Achilefu S. Org Lett. 2009;11(1):29–32. doi: 10.1021/ol802363x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miltsov S, Encinas C, Alonso JN. Tetrahedron Lett. 1998;39(50):9253–9254. [Google Scholar]
- 8.Mata JP, Majhi PR, Guo C, Liu HZ, Bahadur P. J Colloid Interface Sci. 2005;292(2):548–556. doi: 10.1016/j.jcis.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Licha K, Olbrich C. Adv Drug Delivery Rev. 2005;57(8):1087–1108. doi: 10.1016/j.addr.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Klohs J, Wunder A, Licha K. Basic Res Cardiol. 2008;103(2):144–151. doi: 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- 11.Cooper ME, Gregory S, Adie E, Kalinka S. J Fluoresc. 2002;12(3–4):425–429. [Google Scholar]
- 12.Briggs MS, Burns DD, Cooper ME, Gregory SJ. Chem Commun. 2000;23:2323–2324. [Google Scholar]
- 13.Saxena V, Sadoqi M, Shao J. Int J Pharm. 2004;278(2):293–301. doi: 10.1016/j.ijpharm.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Altinoglu EI, Russin TJ, Kaiser JM, Barth BM, Eklund PC, Kester M, Adair JH. ACS Nano. 2008;2(10):2075–2084. doi: 10.1021/nn800448r. [DOI] [PubMed] [Google Scholar]
- 15.Rao JH, Dragulescu-Andrasi A, Yao HQ, Yao HQ. Curr Opin Biotechnol. 2007;18(1):17–25. doi: 10.1016/j.copbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Saxena V, Sadoqi M, Shao J. J Pharm Sci. 2003;92(10):2090–2097. doi: 10.1002/jps.10470. [DOI] [PubMed] [Google Scholar]
- 17.Paumgart G, Probst P, Kraines R, Leevy CM. Ann NY Acad Sci. 1970;170(1):134. [Google Scholar]
- 18.Lauterburg BH, Sautter V, Preisig R, Bircher J. Gastroenterology. 1976;71(2):221–227. [PubMed] [Google Scholar]
- 19.Murawa D, Hirche C, Dresel S, Hunerbein M. Br J Surg. 2009;96(11):1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 20.Saxena V, Sadoqi M, Shao J. J Photochem Photobiol, B. 2004;74(1):29–38. doi: 10.1016/j.jphotobiol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Yaseen MA, Yu J, Wong MS, Anvari B. J Biomed Opt. 2007;12(6) doi: 10.1117/1.2821423. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez VB, Henry SM, Hoffman AS, Stayton PS, Li XD, Pun SH. J Biomed Opt. 2008;13(1) doi: 10.1117/1.2834296. [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Chen YP, Mount CW, Gombotz WR, Li XD, Pun SH. Pharm Res. 2010;27(9):1900–1913. doi: 10.1007/s11095-010-0190-y. [DOI] [PubMed] [Google Scholar]
- 24.Cardillo JA, Jorge R, Costa RA, Nunes SMT, Lavinsky D, Kuppermann BD, Tedesco AC, Farah ME. Br J Ophthalmol. 2008;92(2):276–280. doi: 10.1136/bjo.2007.129395. [DOI] [PubMed] [Google Scholar]
- 25.Benson RC, Kues HA. Phys Med Biol. 1978;23(1):159–163. doi: 10.1088/0031-9155/23/1/017. [DOI] [PubMed] [Google Scholar]
- 26.Desmettre T, Devoisselle JM, Mordon S. Surv Ophthalmol. 2000;45(1):15–27. doi: 10.1016/s0039-6257(00)00123-5. [DOI] [PubMed] [Google Scholar]
- 27.Allen C, Maysinger D, Eisenberg A. Colloids Surf, B. 1999;16(1–4):3–27. [Google Scholar]
- 28.Bohorquez M, Koch C, Trygstad T, Pandit N. J Colloid Interface Sci. 1999;216(1):34–40. doi: 10.1006/jcis.1999.6273. [DOI] [PubMed] [Google Scholar]
- 29.Alexandridis P, Nivaggioli T, Hatton TA. Langmuir. 1995;11(7):2847–2847. [Google Scholar]
- 30.Chandaroy P, Sen A, Alexandridis P, Hui SW. Biochim Biophys Acta, Biomembr. 2002;1559(1):32–42. doi: 10.1016/s0005-2736(01)00431-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee SC, Choi HS, Ooya T, Yui N. Macromolecules. 2004;37(20):7464–7468. [Google Scholar]
- 32.Tian HY, Chen XS, Lin H, Deng C, Zhang PB, Wei Y, Jing XB. Chem— Eur J. 2006;12(16):4305–4312. doi: 10.1002/chem.200501322. [DOI] [PubMed] [Google Scholar]
- 33.Godbey WT, Wu KK, Mikos AG. J Controlled Release. 1999;60(2–3):149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 34.Godbey WT, Wu KK, Mikos AG. Proc Natl Acad Sci U S A. 1999;96(9):5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi SH, Lee JH, Choi SM, Park TG. Langmuir. 2006;22(4):1758–1762. doi: 10.1021/la052549n. [DOI] [PubMed] [Google Scholar]
- 36.Lee JI, Yoo HS. Eur J Pharm Biopharm. 2008;70(2):506–513. doi: 10.1016/j.ejpb.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Jeong B, Kim SW, Bae YH. Adv Drug Delivery Rev. 2002;54(1):37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 38.Carale TR, Pham QT, Blankschtein D. Langmuir. 1994;10(1):109–121. [Google Scholar]
- 39.Su YL, Liu HZ, Wang J, Chen JY. Langmuir. 2002;18(3):865–871. [Google Scholar]
- 40.Zintchenko A, Philipp A, Dehshahri A, Wagner E. Bioconjugate Chem. 2008;19(7):1448–1455. doi: 10.1021/bc800065f. [DOI] [PubMed] [Google Scholar]
- 41.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. Mol Ther. 2005;11(6):990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.