Abstract
Polysulfates and polysulfonates possess exceptional mechanical properties making them potentially valuable engineering polymers. However, they have been little explored due to a lack of reliable synthetic access. Here we report bifluoride salts (Q+[FHF]−, where Q+ represents a wide range of cations) as powerful catalysts for the sulfur(VI) fluoride exchange (SuFEx) reaction between aryl silyl ethers and aryl fluorosulfates (or alkyl sulfonyl fluorides). The bifluoride salts are significantly more active in catalysing the SuFEx reaction compared to organosuperbases, therefore enabling much lower catalyst-loading (down to 0.05 mol%). Using this chemistry, we are able to prepare polysulfates and polysulfonates with high molecular weight, narrow polydispersity and excellent functional group tolerance. The process is practical with regard to the reduced cost of catalyst, polymer purification and by-product recycling. We have also observed that the process is not sensitive to scale-up, which is essential for its future translation from laboratory research to industrial applications.
Click chemistry features the formation of carbon–heteroatom bonds to generate molecules with desired functionality using highly efficient, selective, modular and benign reaction processes1. From a synthetic perspective, polymer chemistry depends on many of the same basic principles as click chemistry —modularity and efficiency are inherent in practical polymer syntheses2, which explains the growing impact of click chemistry on materials science over the past decade3, especially in the field of polymer preparation and modification4,5.
Recently, we introduced sulfur(VI) fluoride exchange (SuFEx) as another embodiment of click chemistry6. The SuFEx click chemistry allows the activation of SVI–F bond with a ‘Si+’ species in the presence of organosuperbase catalysts, such as 1,8-diazabi-cyclo[5.4.0]undec-7-ene (DBU) and 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP, a phosphazene base—full name cited from Reaxys). With these catalysts, the reaction between aryl fluorosulfates (or aryl sulfonyl fluorides) and aryl silyl ethers exclusively affords diaryl sulfates (or diaryl sulfonates). The SuFEx reaction has been successfully applied to the synthesis of aromatic polysulfates and polysulfonates by our group7,8, and to post-polymerization modifications by others9–11.
Polysulfates and polysulfonates with their sulfur(VI) linkages (–SO2–) possess excellent mechanical properties and high chemical stability, as needed for engineering polymers12–15. For instance, the bisphenol A (BPA) polysulfate has slightly higher tensile modulus, similar yield stress and significantly lower oxygen permeability compared to its polycarbonate counterpart7. Nevertheless, they have been rarely used for industrial applications due to a lack of reliable and scalable synthetic access. Our SuFEx process represents the first efficient protocol for the preparation of these polymers. Considering the availability of bisphenols (BPA has an annual production of millions of tonnes, most of which are used for polycarbonates and epoxy resins industry) and sulfuryl fluoride gas (SO2F2, widely used as fumigant) as commercial feed-stocks, it is attractive to further explore this process for potential industrial applications.
However, several disadvantages of the base-catalysed SuFEx process have hampered its scale-up. First, the catalyst loading is quite high. For a typical polymerization reaction, no less than 10 mol% of DBU has to be used. One limitation of high catalyst-loading is that a tedious purification procedure is required to remove catalyst residue prior to polymer processing. Second, BEMP requires only 1.0 mol% of catalyst-loading, but is quite expensive and lacks bulk accessibility. Third, the strong basic nature of the catalysts restricts the substrate scope of the process. For instance, alkyl sulfonyl fluoride monomers are not well compatible with this process due to the facile deprotonation of their α-H when subjected to strong bases16,17.
Addressing these issues, we report a new set of catalysts for SuFEx-based polysulfate and polysulfonate synthesis, namely the bifluoride salts (Q+[FHF]−, where Q+ refers to a wide range of organic and inorganic cations)18,19. The bifluoride species has been little appreciated by the synthetic community. Its presence is nearly certain wherever a fluoride ion is exposed to any potential proton sources20. The 4-electron-3-centered anion ([FHF]−) has one proton trapped between two fluoride anions via the strongest hydrogen bond ever recorded21. Such a formation gives the bifluoride ion modest acidity (pH ≈ 3.0 for the saturated aqueous solution of potassium bifluoride at room temperature)22, moderate nucleophilicity23 and excellent stability21. While the strongly basic fluoride sources, such as cesium fluoride, only exhibited moderate activity in our SuFEx reaction7, we hypothesized that the acidic bifluoride species could facilitate SuFEx reactivity.
Results and discussion
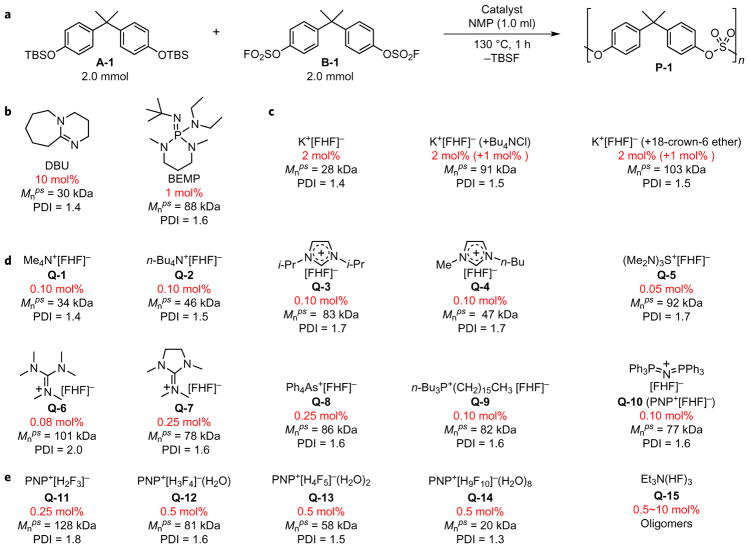
We first investigated potassium bifluoride (K+[FHF]−) as catalyst for the polycondensation of A-1 and B-1 in N-methyl-2-pyrrolidone (NMP) at 130 °C (Fig. 1a). The polymerization reaction catalysed by DBU (10 mol%) and BEMP (1 mol%) served as controls (Fig. 1b; catalyst loadings are given in molar ratio to the total amount of both monomers). The number-average molecular weight (Mnps) (ps, polystyrene) and polydispersity (PDI) were determined by gel permeation chromatography (GPC), which was calibrated with commercial polystyrenes. Our results indicated that 2.0 mol% of potassium bifluoride was indeed able to promote the polysulfate formation. But it was much slower compared to the reaction catalysed with DBU or BEMP (17 h versus 1 h), affording the polysulfate P-1 with a low molecular weight (Mnps = 28 kDa). However, the molecular weight of P-1 could be dramatically improved to as high as 100 kDa by combining 2.0 mol% of potassium bifluoride with 1.0 mol% of tetrabutylammonium chloride or 18-crown-6 ether as catalyst. In all entries, the PDI remained narrow (1.4~1.6, Fig. 1c).
Figure 1. Catalyst screening for the SuFEx-based synthesis of polysulfates.
a, The synthesis of P-1 from monomers A-1 and B-1 is employed as a model reaction for catalyst screening. b, Control experiments with DBU or BEMP as catalysts. c, Evaluation of K+[FHF]− (with/without phase transfer reagents) as the catalyst with a reaction time of 17 h. d, Evaluation of onium bifluoride (Q+[FHF]−) salts as catalysts. e, Evaluation of poly(hydrogen fluoride) salts as catalysts. NMP, N-methyl-2-pyrrolidone; TBS, tert-butyldimethylsilyl; Mnps, number-average molecular weight with polystyrene as standard; PDI, polydispersity index.
Subsequently, we investigated the impact of cations on the activities of the bifluoride catalysts (Q+[FHF]−, Fig. 1d). Various onium bifluoride salts were prepared through ion-exchange of their chloride/bromide precursors (Q+X−, X = Cl, Br) with silver bifluoride (Ag+[FHF]−), and were used as 0.1 M stock solution in acetonitrile (Supplementary Section 2-3-1). We found that a wide variety of onium bifluorides were highly active at promoting the reaction, requiring only 0.1 mol% loading in most cases (the minimum amount required to produce P-1 with Mnps > 20 kDa). Upon the addition of the bifluoride catalyst, the reaction was triggered instantly with the release of tert-butyldimethylsilyl fluoride (TBSF, b.p. 89 °C)24, and yet not much heat was released. Within one hour P-1 was obtained with Mnps ranging from 30 kDa to 100 kDa (PDI = 1.4~1.7). We found that tris(dimethylamino)sulfonium bifluoride (Q-5)25 was the most effective catalyst—at a 0.05 mol% catalyst loading it was able to generate P-1 with a molecular weight up to 100 kDa. Hexamethyl guanidium bifluoride (Q-6) also exhibited remarkable activity at 0.08 mol% catalyst loading (PDI = 2.0). However, a confined, five-membered cyclic version of the cation (Q-7) had decreased activity (0.25 mol% loading).
Inspired by the extraordinary activity of bifluoride salts in catalysing the SuFEx polymerization reaction, we investigated whether poly(hydrogen fluoride) salts (Q+[HnFn+1]−, n > 1, Fig. 1e) could promote the transformation as well18. The dihydrogentrifluoride salt Q-11 was prepared via ion-exchange of its chloride precursor with excess K+[FHF]− (ref. 26). Thereafter, equilibrations of Q-11 with hydrofluoric acid (48 wt.% in H2O) in acetonitrile gave catalysts Q-12–Q-14 in situ, which were used directly without the removal of water (Supplementary Section 2–3). We found Q-11–Q-13 were excellent catalysts for P-1 formation, although slightly higher catalyst-loadings were required (0.5 mol% in most cases). Intriguingly, we confirmed the formation of P-1 with a moderate molecular weight (Mnps = 20 kDa) even when the HF:F− ratio was 9:1 (Q-14), suggesting that the polymerization reaction has an excellent tolerance for the presence of a few per cent of water and HF in the system. In contrast, we only obtained a mixture of oligomers and partly hydrolysed monomers when triethylamine trihydrofluoride, pyridinium poly(hydrogen fluoride)27, or hydrofluoric acid were employed as catalysts (see Supplementary Section 2–4 for more information on catalyst evaluation).
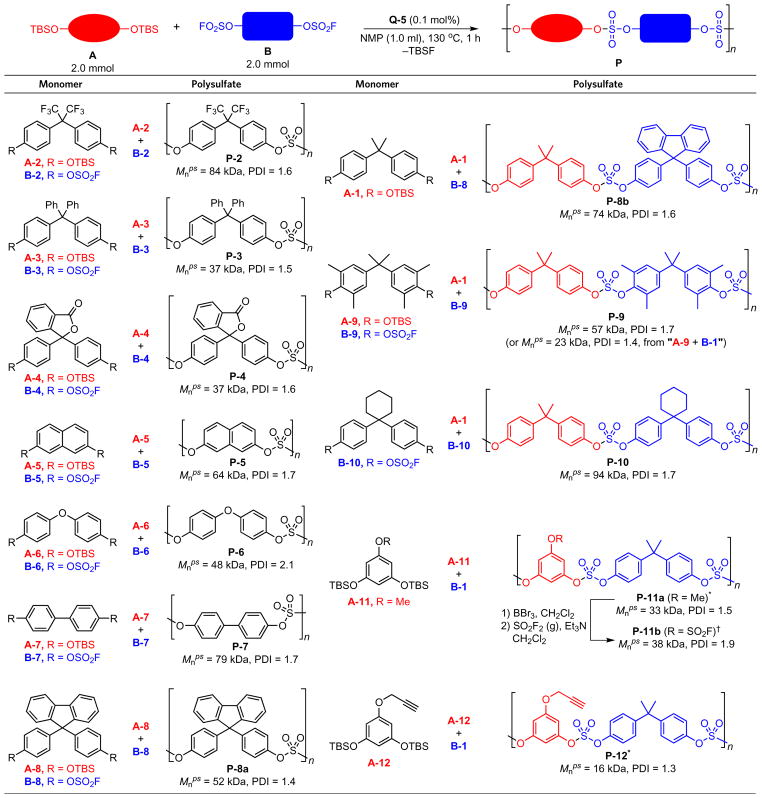
To evaluate the fidelity of the bifluoride catalytic system, we examined monomers with diverse functional groups (Table 1). The polysulfates P-2, P-5 and P-6 had previously been prepared under the DBU-catalysed polycondensation reaction7. When the bifluoride catalyst Q-5 was used, we obtained these polysulfates with comparable molecular weights and polydispersities. For instance, the Mnps/PDI of P-2 was 46 kDa/1.5 with the DBU catalyst versus 84 kDa/1.6 with the Q-5 catalyst. Under the same reaction conditions, monomers A-3/B-3, A-4/B-4 and A-8/B-8 were converted to polysulfates with molecular weights ranging from 36 to 52 kDa. Even the sterically hindered monomer B-9 underwent cross-condensation with A-1 giving polymer P-9 with high molecular weight and good polydispersity (Mnps = 58 kDa, PDI = 1.7), alternatively A-9 reacted with B-1 giving P-9 with a slightly lower molecular weight (Mnps = 23 kDa, PDI = 1.4). The orthogonal nature of the SuFEx click reaction to many other standard chemical reactions enables us to incorporate latent functional groups on the emergent polymer backbones for post-polymerization modification. For example, propargyl groups in P-12 can serve as handles for the CuAAC-mediated functionalization5. Alternatively, the methoxy groups in P-11a were converted into fluorosulfates (P-11b) to undergo further SuFEx modification11.
Table 1.
Synthesis of polysulfates from diverse building blocks.
Typical reaction conditions: A (2.0 mmol) and B (2.0 mmol) in NMP (1.0 ml) at 130 °C for 1 h with Q-5 (0.004 mmol) as the catalyst.
5.0 mol% of Q-5 was used.
P-11a was treated with BBr3 (10.0 equiv.) in dichloromethane first, then with SO2F2 (balloon)/Et3N in dichloromethane.
NMP, N-methyl-2-pyrrolidone; TBS, tert-butyldimethylsilyl; Mnps, number-average molecular weight with polystyrene as standard; PDI, polydispersity index.
We have also examined Q-5 (0.1 mol%) catalysed polycondensation of AB-1, in which the two functional groups, –OSO2F and –OTBS, were residents in a single molecule (AB-1 is bench-stable for at least one year at room temperature). Under the same reaction conditions, polysulfate P-1 was obtained with a Mnps of 65 kDa (PDI = 1.5), which is comparable to polymers obtained from the A-1/B-1 polycondensation reaction (Fig. 2a). Intriguingly, we were also able to realize the direct polycondensation of A-1 with SO2F2 gas catalysed by Q-5, affording P-1 with moderate molecular weight (Mnps = 22 kDa, PDI = 1.4, Fig. 2b). This strategy is generally applicable to other monomers like A-7, with which polysulfate P-7 was prepared in near quantitative conversion (Mnps = 28 kDa, PDI = 1.5, Fig. 2c). In these cases, aryl fluorosulfate intermediates (–C6H4OSO2F) were generated in situ via the reaction between aryl silyl ether (–C6H4OTBS) and sulfuryl fluoride gas catalysed by Q-5, which underwent further condensation with another molecule of aryl silyl ether to extend the backbone and finally resulted in the polysulfate.
Figure 2. Two alternative strategies for the synthesis of polysulfates via the bifluoride-catalysed SuFEx reaction.
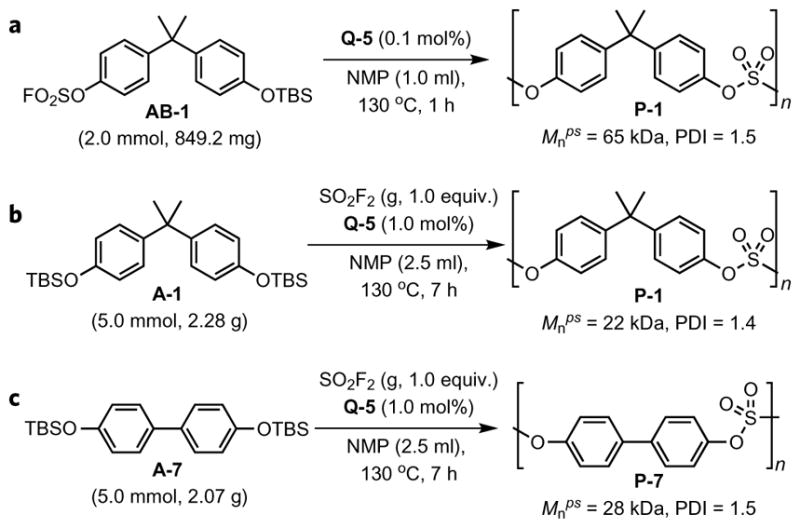
a, The synthesis of P-1 from the bifunctional monomer AB-1. b, The synthesis of P-1 from the polycondensation of A-1 with sulfuryl fluoride gas (SO2F2). c, The synthesis of P-7 from the polycondensation of A-7 with sulfuryl fluoride gas (SO2F2). NMP, N-methyl-2-pyrrolidone; TBS, tert-butyldimethylsilyl; Mnps, number-average molecular weight with polystyrene as standard; PDI, polydispersity index.
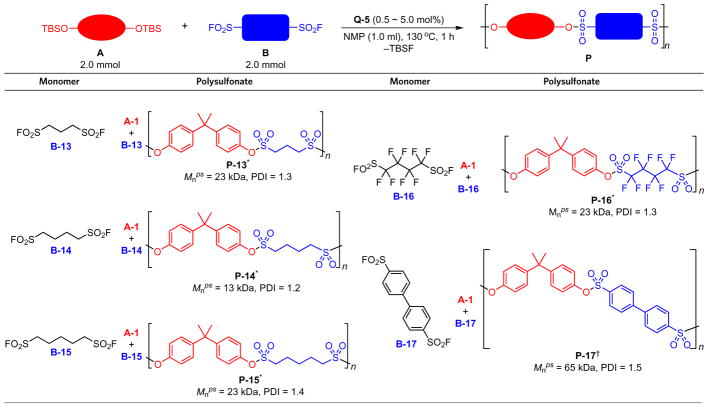
When DBU or BEMP was employed as the catalyst, the reactions of aryl silyl ether with alkyl sulfonyl fluoride afforded a mixture of oligomers and decomposition adducts of both monomers and oligomers, which might be due to the deprotonation of the α-H of the sulfonyl fluoride group17. Hydrogen fluoride catalysts, however, provide an acidic reaction environment that is compatible with those alkyl sulfonyl fluorides22. To demonstrate this, we combined the aliphatic bis(sulfonyl fluorides) B-13, B-14 and B-15 respectively with A-1 in NMP in 1:1 ratio and in the presence of 5 mol% catalyst Q-5 at 130 °C. The resulting polysulfonates P-13, P-14 and P-15 were afforded in quantitative yields and moderate molecular weights (Table 2). On the other hand, the polycondensation of the aromatic bis(sulfonyl fluoride) B-17 required lower catalyst-loading (0.5 mol%) and gave polysulfonate with a higher molecular weight. The bifluoride-catalysed SuFEx polycondensation reaction is also applicable to the synthesis of the fluoropolymer P-16 from the perfluorobutane-1,4-disulfonyl fluoride B-16 and the silyl ether A-1 (Mnps = 23 kDa, PDI = 1.3). Fluoropolymers often possess excellent physical and chemical properties, such as low dielectric constants and coefficients of friction28.
Table 2.
Synthesis of polysulfonates from aliphatic and aromatic bis(sulfonyl fluoride).
Typical reaction conditions: A (2.0 mmol) and B (2.0 mmol) were polymerized in NMP (1.0 ml) at 130 °C for 1 h with Q-5 as catalyst.
5.0 mol% of Q-5 was used.
0.5 mol% of Q-5 was used.
NMP, N-methyl-2-pyrrolidone; TBS, tert-butyldimethylsilyl; Mnps, number-average molecular weight with polystyrene as standard PDI, polydispersity index.
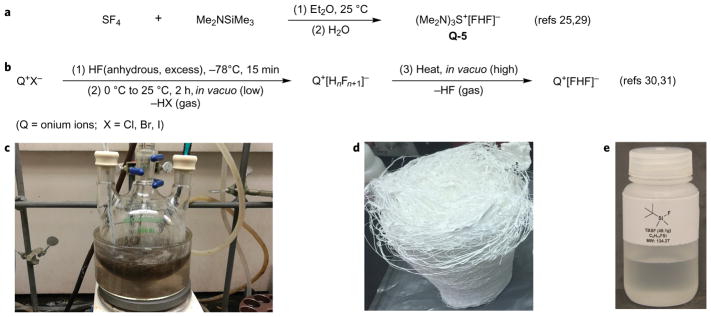
For laboratory use of the bifluoride-catalysed SuFEx polymerization or ligation, there are several options for catalyst preparation summarized in Supplementary Section 2–3. For industrial interests, however, bulk supplies of high-quality catalysts at low cost is required. Fortunately, a reliable protocol for the large-scale preparation of Q-5 from commercially available SF4 has already been reported (Fig. 3a)25,29. In addition, there is a practical access to other bifluoride salts which involves the ion-exchange of onium halides (Q+X−, X = Cl, Br, I) with anhydrous hydrogen fluoride (Fig. 3b). Anhydrous HF and many onium halides are bulk chemicals, therefore practical industrial scale syntheses of bifluoride catalysts is expected. Demonstrating this, we carried out a 5-gram scale preparation of 1-ethyl-3-methylimidazolium bifluoride (EMI+[FHF]−) (modified procedure based on refs 30,31). (Caution! Anhydrous HF is dangerous for its strong corrosiveness and toxicity, and must be manipulated in specific apparatus under safety guidance. See Supplementary Section 2-3-6).
Figure 3. Catalysts preparation and bulk synthesis of P-1.
a, Reported procedure for the preparation of catalyst Q-5. b, A schematic protocol for the preparation of bifluoride catalysts via the ion-exchange reaction of onium halides (Q+X−, X = Cl, Br, I) with anhydrous hydrogen fluoride (see Supplementary Section 2-3-6 for details). c, The 100-gram scale preparation of P-1 from the polycondensation of A-1 (0.2 mol) and B-1 (0.2 mol) catalysed by 0.05 mol% of Q-5 in NMP (50 ml). d, 112.7 g P-1 was obtained from bulk polymerization (Mnps = 110 kDa, PDI = 1.7). e, 49.1 g TBSF was recycled from the bulk polymerization process.
Having established the optimal conditions for the bifluoride-catalysed SuFEx polymerization reaction, as well as the protocols for catalysts preparation, we were further interested in enlarging the synthesis of polysulfate P-1 to 100-gram scale in our laboratory. A bulk polymerization with 0.2 mol of A-1 and 0.2 mol of B-1 was carried out at ~120 °C (internal temperature) in NMP (50 ml), using only 0.05 mol% of catalyst Q-5 (Fig. 3c). Polysulfate P-1 was obtained as white fibrous materials in near quantitative yield (112.7 g, Fig. 3d). GPC analysis gave a Mnps of 110 kDa (PDI = 1.7, Supplementary Fig. 8), which was even higher than the P-1 product obtained at the 2.0 mmol scale (Mnps = 92 kDa, PDI = 1.7, Fig. 1d). Differential scanning calorimetry and thermal gravimetric analysis results (Supplementary Section 2–8) were comparable with the data obtained from the BEMP-catalysed bulk polycondensation7, indicating these two batches of polymers had the same quality. We also examined the end-capping effect, which is a standard strategy for molecular weight-control (Supplementary Section 2–6)32. The only by-product of the SuFEx-polymerization process is the TBSF (Fig. 3e). Nevertheless, we found it can be used in place of tert-butyl-dimethylsilyl chloride for the production of the silyl ether monomer A and hence recycled (Supplementary Section 2–9).
Conclusion
In summary, we have developed bifluoride salts as a new class of catalysts for the SuFEx click reaction, and have applied this chemistry to the preparation of polysulfates and polysulfonates. Compared to organosuperbase catalysts (for example, DBU and BEMP), the bifluoride ion [FHF]− is acidic. It allows the SuFEx polymerization reaction to be extended to a broader substrates scope, such as aliphatic sulfonyl fluorides. Most importantly, the bifluoride catalyst is significantly more active, therefore the polymerization reaction requires much lower catalyst-loading, which is essential for cost-effective industrial scale production. Our large-scale experiment further confirms the high fidelity of the bifluoride catalytic process for scale-up. Moreover, we have also provided practical solutions for catalyst preparation and for by-product recycling (TBSF). This work sets a foundation for future translation of polysulfate synthesis from laboratory research to industrial applications.
Methods
Process for the bulk preparation of polysulfate P-1
Monomers A-1 (91.36 g, 0.2 mol) and B-1 (78.48 g, 0.2 mol) were combined in a dry 1 l three-neck round-bottom flask equipped with a Teflon-coated magnetic stir bar, a thermometer and a reflux condenser (Fig. 3c). The flask was purged with nitrogen gas, into which dry NMP (50 ml) was then added. Next, it was placed in a pre-heated oil bath (130 °C, oil temperature). With stirring, monomers gradually dissolved in NMP, and the internal temperature stabilized at 123 °C. Under vigorous stirring, catalyst Q-5 (0.1 M in dry CH3CN, 2.0 ml, 0.0002 mol) was added. The reaction initiated instantaneously. The internal temperature rose to 135 °C, then quickly dropped to 100–110 °C due to the refluxing of TBSF. The reaction was allowed to run for 1 h, during which the reaction mixture turned highly viscous and the internal temperature stabilized at around 120 °C. The flask was subsequently cooled to room temperature. TBSF was then distilled out from the reaction mixture as colourless liquid at 80 °C (diaphragm pump; 49.1 g product, Fig. 3e). After the distillation, 250 ml DMF was added to the reaction flask to dissolve the polymer at 120 °C. The warm DMF solution was then slowly poured into 2 l methanol with vigorous mechanical stirring at room temperature. White fibres of P-1 crashed out and aggregated like a bird’s nest in methanol, which was collected and dried at 70 °C for 12 h to give 112.7 g dry polymer (Mnps = 110 kDa, PDI = 1.7, Fig. 3d). Full experimental details and characterization of compounds are given in Supplementary Information.
Supplementary Material
Acknowledgments
Financial support was provided by the National Science Foundation (CHE-1610987 to K.B.S.) and the National Institutes of Health (GM093282 to P.W.). Part of the work was carried out as a user project at the Molecular Foundry, which was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. B.G. is grateful for the postdoctoral fellowship support from the Shanghai Institute of Organic Chemistry (SIOC), Chinese Academy of Science (CAS), Pharmaron and Zhejiang Medicine. J.D. is grateful for the One Hundred Talents Program supported by SIOC, CAS. J.D. is financially supported by the Strategic Priority Research Program of the CAS (XDB200203) and the National Natural Science Foundation of China (21672240). We thank P. Dawson at The Scripps Research Institute (TSRI) for the anhydrous HF experiment. We also thank S. Li and C. J. Hawker at University of California, Santa Barbara, Q. Xu and B. Wu at Soochow University, S. Li and H. Wang at TSRI for helpful discussions on this project.
Footnotes
Author contributions
K.B.S. and P.W. led the project. J.D. and B.G. designed the experiments. B.G., J.D. and L.Z. carried out the experiments. F.Z., L.M.K., J.L. and Y.L. collected and analysed the TGA and DSC data of all polymers and provided helpful suggestion on the project. B.G. wrote the manuscript. P.W., Q.Z., J.D., Y.L. and K.B.S. edited the manuscript.
Competing financial interests
A patent application covering this work has been filed by TSRI (US patent application no. 62/182755, International patent application no. PCT/US2016/038701).
Supplementary information and chemical compound information are available in the online version of the paper.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Kricheldorf HR, Nuyken O, Swift G. Handbook of Polymer Synthesis. Marcel Dekker; 2004. [Google Scholar]
- 3.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Hawker CJ, Wooley KL. The convergence of synthetic organic and polymer chemistries. Science. 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- 5.Golas PL, Matyjaszewski K. Marrying click chemistry with polymerization: expanding the scope of polymeric materials. Chem Soc Rev. 2010;39:1338–1354. doi: 10.1039/b901978m. [DOI] [PubMed] [Google Scholar]
- 6.Dong JJ, Krasnova L, Finn MG, Sharpless KB. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew Chem Int Ed. 2014;53:9430–9448. doi: 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]
- 7.Dong JJ, Sharpless KB, Kwisnek L, Oakdale JS, Fokin VV. SuFEx-based synthesis of polysulfates. Angew Chem Int Ed. 2014;53:9466–9470. doi: 10.1002/anie.201403758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J, et al. Condensation polymerization of fluoro-substituted compounds with silyl-substituted compounds. WO2014089078A1. World patent. 2014
- 9.Li S, Beringer LT, Chen S, Averick S. Combination of AGET ATRP and SuFEx for post-polymerization chain-end modifications. Polymer. 2015;78:37–41. [Google Scholar]
- 10.Yatvin J, Brooks K, Locklin J. SuFEx on the surface: a flexible platform for postpolymerization modification of polymer brushes. Angew Chem Int Ed. 2015;54:13370–13373. doi: 10.1002/anie.201506253. [DOI] [PubMed] [Google Scholar]
- 11.Oakdale JS, Kwisnek L, Fokin VV. Selective and orthogonal post-polymerization modification using sulfur(VI) fluoride exchange (SuFEx) and copper-catalyzed azide-alkyne cycloaddition (CuAAC) reactions. Macromolecules. 2016;49:4473–4479. [Google Scholar]
- 12.Thomson DW, Ehlers GFL. Aromatic polysulfonates: preparation and properties. J Polym Sci Part A. 1964;2:1051–1056. [Google Scholar]
- 13.Schlott RJ, Goldberg EP, Scardigl F, Hoeg DF. Preparation and properties of aromatic polysulfonates. Adv Chem. 1969;91:703–716. [Google Scholar]
- 14.Firth WC. Preparation of aromatic polysulfates and copoly(sulfate carbonates) J Polym Sci Part B. 1972;10:637–641. [Google Scholar]
- 15.Firth WC. Aryl sulfate polymers and methods for their production. 3733304. US patent. 1973
- 16.Ishikawa T. Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts. Wiley; 2009. [Google Scholar]
- 17.Shirota Y, Nagai T, Tokura N. Reactions of alkanesulphonic acid derivatives with organoalkali metal compounds. Tetrahedron. 1969;25:3193–3204. [Google Scholar]
- 18.Cady GH. Freezing points and vapor pressures of the system potassium fluoride-hydrogen fluoride. J Am Chem Soc. 1934;56:1431–1434. [Google Scholar]
- 19.Mootz D, Boenigk D. Poly(hydrogen fluorides) with the tetramethylammonium cation: preparation, stability ranges, crystal structures, [HnFn+1]− anion homology, hydrogen bonding F–H–F. Z Anorg Allg Chem. 1987;544:159–166. [Google Scholar]
- 20.Sharma RK, Fry JL. Instability of anhydrous tetra-n-alkylammonium fluorides. J Org Chem. 1983;48:2112–2114. [Google Scholar]
- 21.Emsley J. Very strong hydrogen bonding. Chem Soc Rev. 1980;9:91–124. [Google Scholar]
- 22.Subbarao SN, Yun YH, Kershaw R, Dwight K, Wold A. Electrical and optical properties of the system TiO2–xFx. Inorg Chem. 1979;18:488–492. [Google Scholar]
- 23.Landini D, Maia A, Rampoldi A. Dramatic effect of the specific solvation on the reactivity of quaternary ammonium fluorides and poly(hydrogen fluorides), (HF)n·F−, in media of low polarity. J Org Chem. 1989;54:328–332. [Google Scholar]
- 24.Kumada M, Ishikawa M, Maeda S, Ikura K. The preparation and some reactions of (chloromethyl)-tert-butyldimethylsilane. J Organomet Chem. 1964;2:146–153. [Google Scholar]
- 25.Farnham WB, Middleton WJ, Sogah DY. Tris(dialkylamino)sulfonium bifluoride catalysts. 4598161 A. US patent. 1986
- 26.Landini D, Molinari H, Penso M, Rampoldi A. Convenient procedures for the preparation of lipophilic quaternary onium fluorides, hydrogendifluorides and dihydrogentrifluorides via ion-exchange in 2-phase systems. Synthesis. 1988;1988:953–955. [Google Scholar]
- 27.Olah GA, et al. Synthetic methods and reactions. 63 Pyridinium poly (hydrogen fluoride) (30 PYRIDINE-70 hydrogen fluoride): a convenient reagent for organic fluorination reactions. J Org Chem. 1979;44:3872–3881. [Google Scholar]
- 28.Teng HX. Overview of the development of the fluoropolymer industry. Appl Sci. 2012;2:496–512. [Google Scholar]
- 29.Middleton WJ. Tris(substituted amino) sulfonium salts. 3940402 A. US patent. 1976
- 30.Matsumoto K, Tsuda T, Hagiwara R, Ito Y, Tamada O. Structural characteristics of 1-ethyl-3-methylimidazolium bifluoride: HF-deficient form of a highly conductive room temperature molten salt. Solid State Sci. 2002;4:23–26. [Google Scholar]
- 31.Muttenthaler M, Albericio F, Dawson PE. Methods, setup and safe handling for anhydrous hydrogen fluoride cleavage in Boc solid-phase peptide synthesis. Nat Protoc. 2015;10:1067–1083. doi: 10.1038/nprot.2015.061. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto M. Relationship between the end-cap structure of polycarbonates and their impact resistance. Polymer. 2001;42:8355–8359. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.