Abstract
Growing evidence points to a disruption of cortico-thalamo-cortical circuits in schizophrenia (SZ) and bipolar disorder (BD). Clues for a specific involvement of the thalamic reticular nucleus (TRN) come from its unique neuronal characteristics and neural connectivity, allowing it to shape thalamo-cortical information flow. A direct involvement of the TRN in SZ and BD has not been tested thus far. We used a combination of human postmortem and rodent studies to test the hypothesis that neurons expressing parvalbumin (PV neurons), a main TRN neuronal population, and associated Wisteria floribunda agglutinin-labeled perineuronal nets (WFA/PNNs) are altered in SZ and BD, and that these changes may occur early in the course of the disease as a consequence of oxidative stress. In both disease groups, marked decreases of PV neurons (immunoreactive for PV) and WFA/PNNs were observed in the TRN, with no effects of duration of illness or age at onset. Similarly, in transgenic mice with redox dysregulation, numbers of PV neurons and WFA/PNN+PV neurons were decreased in transgenic compared with wild type mice; these changes were present at postnatal day (P) 20 for PV neurons and P40 for WFA/PNN+PV neurons, accompanied by alterations of their firing properties. These results show profound abnormalities of PV neurons in the TRN of subjects with SZ and BD, and offer support for the hypothesis that oxidative stress may play a key role in impacting TRN PV neurons at early stages of these disorders. We put forth that these TRN abnormalities may contribute to disruptions of sleep spindles, focused attention and emotion processing in these disorders.
INTRODUCTION
Growing evidence points to a disruption of neural networks involved in emotion, cognitive, and sensory processing as a key component of the pathophysiology of schizophrenia (SZ). In particular, imaging studies point to altered functional thalamo-cortical connectivity in SZ and bipolar disorder (BD) patients and high risk subjects who later convert to psychosis (1–14). In this context, the Thalamic Reticular Nucleus (TRN) is of particular interest, as it shapes the information flow between the thalamus and the cortex (15). The TRN receives collaterals from cortico-thalamic and thalamo-cortical neurons, and in turn exerts powerful inhibition on these latter neurons, thus gating thalamo-cortical information flow. This thalamic circuitry, and the intrinsic activity of TRN neurons, is thought to underlie several key functions of high relevance to the pathogenesis of psychiatric disorders, including sensory gating, regulation of arousal state, focused attention, emotional salience, cognitive flexibility, and the generation of cortical sleep spindles and modulation of cortical γ oscillations (16–26). Therefore, abnormalities affecting the TRN may affect sleep, emotion processing, and cognitive performance relying on sensory processing and attention, and have been postulated to contribute to the genesis of hallucinations (4, 8, 13, 25, 27–29). Importantly, sleep spindles have been shown to be robustly disrupted in SZ, further supporting the hypothesis that the TRN is affected in this disorder; sleep spindle disruption was reported in a group of people with mood disorder, including a small number affected by bipolar disorder (22, 23, 30–33). However, direct evidence for TRN abnormalities in SZ and BD is lacking. To our knowledge, only two postmortem studies have been published thus far, showing a modest decrease of nicotinergic receptor binding and altered expression of excitatory amino acid transporters in TRN of SZ patients (34, 35).
A key question toward testing the hypothesis that the TRN may be involved in the pathophysiology of SZ and BD is whether its main neuronal populations are affected. The TRN is composed entirely by GABAergic neurons, a large proportion of which expresses the calcium-binding protein, parvalbumin (PV). Deficits of GABAergic neurons, and particularly molecular abnormalities of neurons immunoreactive for PV (PV neurons) in prefrontal cortex and limbic regions, represent a key pathological feature of SZ and BD (36–43)}. In addition, decreases of perineuronal nets (PNNs), including those labeled with the lectin Wisteria Floribunda agglutinin (WFA/PNNs) and predominantly ensheating PV interneurons, have been reported in the amygdala, entorhinal cortex, and prefrontal cortex of SZ and BD patients (44–47). PNNs are organized extracellular matrix structures known to regulate neuronal maturation, synaptic connectivity and plasticity, and to provide protection against oxidative stress (48–52). PNN disruption in these disorders is of note, as it may contribute to altered synaptic connectivity and increased neuronal vulnerability to oxidative stress (50, 53, 54). Data from several animal models relevant to SZ indicate that oxidative stress is a convergent mechanism inducing PV interneurons and WFA/PNNs impairment in the prefrontal cortex (55). By analogy, PV neurons in the TRN are here postulated to be vulnerable to oxidative stress.
On the basis of these considerations, we hypothesized that PV neurons and WFA/PNNs may be altered in the TRN of subjects with SZ and BD, and that redox dysregulation may contribute to TRN abnormalities. Indeed, accumulating evidence supports the idea that abnormal redox homeostasis and oxidative stress play a role in the etiology of SZ and BD (56–61). We used a combination of human postmortem and animal model studies to examine whether PV neurons and PNNs in the TRN are altered in SZ and BD and whether they are susceptible to oxidative stress. To address the question of susceptibility to oxidative stress, we examined the TRN in mice with a knockout of the modulatory subunit of the glutamate cysteine ligase (GCLM KO, (62, 63)), an animal model of redox dysregulation caused by a weakened synthesis of the main cellular antioxidant and redox regulator glutathione (GSH).
METHODS AND MATERIAL
Human Postmortem Study
Human Subjects
Tissue blocks containing the whole thalamus from a cohort of healthy subjects (n=20), SZ (n=15), and BD (n=15) subjects were obtained from the Harvard Brain Tissue Resource Center (HBTRC), McLean Hospital, Belmont, MA, USA, and used for all histochemical and immunocytochemical investigations (Supplementary Tables S1, S2). Retrospective diagnoses and inclusion criteria were conducted as described in previous studies (47, 64) (see also Supplementary Information).
Tissue Processing and Immunocytochemistry
Tissue blocks for immunohistochemistry were dissected from fresh brains and post-fixed in 0.1M phosphate buffer (PB) containing 4% paraformaldehyde and 0.1M Na azide at 4 °C for 3 weeks, then cryoprotected at 4 °C for 3 weeks (30% glycerol, 30% ethylene glycol and 0.1% Na azide in 0.1M PB). Tissue blocks were then sectioned for stereological analysis as described in previous studies (43, 47, 64). Immunohistochemistry and histological labeling for PV neurons, WFA/PNNs, and multiplex immunofluorescence (used to phenotype PV and WFA/PNNs) were carried out as described in our previous study (47) (See Supplementary Information for methods and antibodies and lectin labeling specificity).
Data Collection
Total numbers (Tn) and numerical densities (Nd) of WFA/PNNs and PV neurons were assessed in the TRN using standard stereology-based methods (43, 45, 47). Tn was calculated as Tn= i • Σn, where i is the section interval and Σn = sum of neurons (or WFA/PNNs) counted; Nd was calculated as Nd = Tn / V, where V is the volume. The volume of the TRN (V) was calculated according to the Cavalieri principle (65) as V= z • i • Σ a, where z is the thickness of the section (40 μm) and i is the section interval (26; i.e. number of serial sections between each section and the following one within a compartment). The borders of the TRN were identified according to specific landmarks, such as the internal capsule laterally and the subthalamic nucleus ventromedially. The medial border was identified at high magnification (40x) according to cytoarchitectonic criteria, i.e., the edge created by thick myelinated fiber bundles entering the dense gray matter of the lateral thalamus. Adjacent Nissl sections were used as reference (see Supplementary Information, Fig. S1).
Statistical Analysis
Differences between groups relative to the main outcome measures were assessed for statistical significance using an ANCOVA stepwise linear regression process as described in our previous studies (43, 45, 47). Effect sizes were calculated according to Hedges’ g. A logarithmic transformation was uniformly applied to all original values because the data were not normally distributed. Covariates including pharmacological exposure and demographic variables were obtained from medical records available for each donor (see Sullivan et al (66) and listed in Supplementary Tables S1, S2). Covariates were tested systematically for their effects on the main outcome measures in all group comparisons, and included in the model if they significantly improved the model goodness-of-fit. Values relative to the t ratio and p value for main outcome measure differences found to be statistically significant are reported in Supplementary Table S3. Any and all covariates found to affect an outcome measure significantly are also reported (see also Supplementary Information, Table S3).
Animal study
Experiments were performed on males GCLM KO (62) and WT mice and were approved by the Local Veterinary Office. Further details on breeding is given in supplementary information.
Immunohistology
Tissue preparation, immunostaining, and analyses were similar to previous studies (67). Detailed descriptions can be found in supplementary information.
Electrophysiology
The firing mode (bursting versus tonic) of spontaneously active TRN neurons recorded in slices was analysed. Separate intracellular recordings were performed using sharp glass electrodes to preserve the intracellular redox state. Depolarization step currents were injected into the cell while its membrane was maintained at various potentials (from ~−60 to ~−90 mV). The responses to these depolarization currents were quantified. Details about slice preparation, recordings, and analyses are provided in supplementary information.
RESULTS
Postmortem Human Studies
PV neuron colocalization with WFA/PNNs in healthy human subjects
PV neurons were found to be densely represented in the TRN. Our estimates show that the average Tn of PV neurons in the human TRN is 52,901.8 (± 30,695.1 SD), making it a predominant neuronal population in this nucleus (58.8% of the total TRN neuron numbers 89,903.7 ± 22,457.8 – Nissl staining, unpublished observations). WFA/PNNs are less numerous, with an estimated average Tn of 25,078.6 (± 26,768.6 SD). Dual fluorescent immunolabeling was carried out to assess percentages of PV neurons associated with WFA/PNNs. Our results show that 63.4% of WFA/PNNs are associated with PV neurons (Supplementary Fig. 2). Conversely, only 20.5% of PV neurons are enwrapped by WFA/PNNs.
PV neurons and WFA/PNNs decreases in SZ
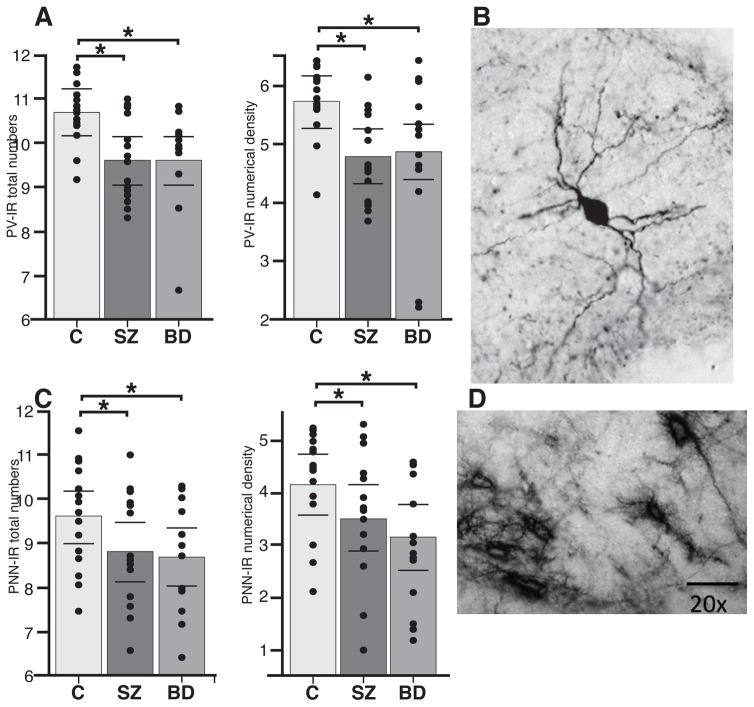
In subjects with SZ, Tn and Nd of PV neurons were markedly decreased as compared to healthy controls (PV neurons - Tn: p<0.0001, Hedges’ g = −2.08, 71.1% decrease; Nd: p<0.0001, Hedges’ g= −2.09, 66.4% decrease; Figure 1, Table 1 and Supplementary Table S3). WFA/PNNs were also significantly decreased in SZ as compared to healthy controls (WFA/PNNs - Tn: p<0.006, Hedges’ g = −1.40, 81.3% decrease; Nd: p<0.003, Hedges’ g= −1.52, 67.2% decrease; Figure 1, Table 1 and Supplementary Table S3). Statistical models for Tn of PV neurons included the effects of exposure to antipsychotics during the last 6 months’ of life (CPZ 6m, p=0.002, t ratio=2.31); similarly, Tn and Nd of WFA/PNNs were adjusted for antipsychotics exposure (CPZ 6M, Tn, p=0.007, t ratio= 2.93; Nd, p=0.0019, t ratio=3.52) and for cause of death (Tn, p=0.012, t ratio=2.71; Nd, p=0.002, t ratio= 3.39). Note that effects of antipsychotic exposure were only significant for the last 6 months’ life (CPZ 6m). Importantly, the effects are positive, suggesting that these drugs tend to bring these values toward normality. PV neuron and WFA/PNN decreases did not significantly vary along the rostrocaudal axis of the TRN.
Figure 1.
PV neurons and WFA/PNNs are decreased in the TRN of subjects with SZ and BD. (a) Total number and Nd of PV neurons in the TRN of healthy subjects, SZ and BD subjects. Marked decreases were observed in each disorder with respect to healthy controls. SZ: PV neurons - Tn: p < 0.0001, Hedges’ g = −2.08, 71.1% decrease; Nd: p < 0.0001, Hedges’ g= −2.09, 66.4% decrease; BD: PV neurons - Tn: p < 0.0007, Hedges’ g = −1.88, 72.1% decrease; Nd: p < 0.003, Hedges’ g= −1.55, 55.9% decrease. (b) Example of a PV neuron in the TRN of a healthy human subject. (c) Total number and Nd of WFA/PNNs in the TRN of healthy subjects, SZ and BD subjects. Significant decreases were observed in each disorder with respect to healthy controls. SZ: WFA/PNNs - Tn: p < 0.006, Hedges’ g = −1.40, 81.3% decrease; Nd: p < 0.003, Hedges’ g= −1.52, 67.2% decrease; BD: (WFA/PNNs - Tn: p < 0.04, Hedges’ g = −0.77, 57.1% decrease; Nd: p < 0.001, Hedges’ g= −0.92, 51.9% decrease). (d) Example of WFA/PNNs in the healthy human TRN. Note that all bar graphs show logarithmically transformed values and do not reflect the effects of confounding variables included in ANCOVA models.
Table 1. Summary of results.
Percent differences for Tn and volume in disease groups with respect to the controls. Tn of PV neurons and WFA/PNNs were markedly decreased in the TRN of both SZ and BD groups, with large effect sizes. Modest volume decreases, measured on Nissl-stained sections, were not statistically significant.
| Diagnosis | Total Number PV neurons | Total Number of PNNs | TRN Volumes | |||
|---|---|---|---|---|---|---|
| Percent Diff. | g value | Percent Diff. | g value | Percent Diff. | g value | |
| SZ | ⇓ −71.1 %* | ⇓ −2.08 %* | ⇓ −81.3 %* | ⇓ −1.40 %* | ⇓ −18.1 % | ⇓ −0.75 |
| BD | ⇓ −72.1 %^ | ⇓ −1.88 %^ | ⇓ −57.1 % | ⇓ −0.77 % | ⇓ −21.7 % | ⇓ −0.83 |
Bold values and arrows indicate statistically significant changes (ANCOVA analysis on log-transformed values). Percent changes are calculated on raw values, adjusted for the effects of the covariates with significant impact in the model; g values are calculated on log transformed values, adjusted for the effects of the covariates with significant impact in the model.
adjusted for exposure to antipsychotics during last 6 months;
adjusted for lifetime exposure to lithium.
Abbreviations: BD, bipolar disorder; SZ, schizophrenia.
PV neurons and WFA/PNNs decreases in BD
In subjects with BD, Tn and Nd of PV-IR neurons are significantly decreased in the TRN with respect to controls (PV neurons - Tn: p<0.0007, Hedges’ g = −1.88, 72.1% decrease; Nd: p<0.003, Hedges’ g= −1.55, 55.9% decrease; Figure 1, Table 1 and Supplementary Table S3). WFA/PNNs are also significantly decreased in BD as compared to healthy controls (WFA/PNNs - Tn: p<0.04, Hedges’ g = −0.77, 57.1% decrease; Nd: p<0.01, Hedges’ g= −0.92, 51.9% decrease; Figure 1, Table 1 and Supplementary Table S3). Statistical models for PV neurons included lifetime exposure to lithium (Tn, p = 0.01, t ratio = 2.53; Nd, p = 0.04, t ratio = 2.05). PV neuron and WFA/PNN decreases did not significantly vary along the rostrocaudal axis of the TRN.
TRN volume in SZ and BD
To help interpret cell count results, the volume of the TRN was measured in Nissl-stained sections in each case from the same cohort. Subjects with SZ showed a modest, not statistically significant, volume decrease (p=0.07, t ratio= −1.86; Hedges’ g = − 0.75; Table 1 and Table 3S). In subjects with BD, TRN volume decreases were also relatively modest, but significant (p=0.02; t ratio= −2.35; Hedges’ g = − 0.83; Table 1 and Table 3S).
Experimental Animal Studies
Increased oxidative stress and reduced PV and WFA/PNNs in TRN of adult GCLM KO mice
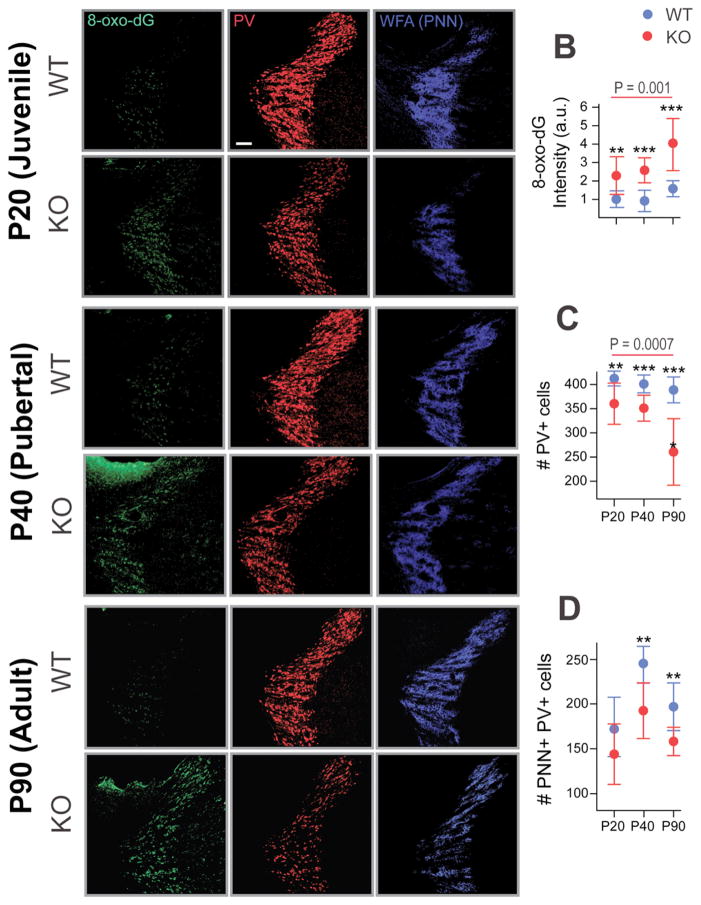
We first investigated whether a redox dysregulation, as in GCLM KO mice, would render PV neurons and WFA/PNNs vulnerable to oxidative stress in the TRN. The degree of oxidative stress was demonstrated with an antibody against 8-oxo-dG, which reveals mitochondrial DNA damage. In the TRN, we observed a significantly higher 8-oxo-dG labeling, by about 150%, in adult GCLM KO compared to WT mice (Figure 2a, b; p < 0.001). We found that the numbers of PV neurons and WFA/PNN+PV neurons were significantly decreased by about 33% (Figure 2a, c; p < 0.001) and 17% (Figure 2a, d; p < 0.01) respectively in the KO compared with WT. The immunolabeling intensities (a.u.) of PV neurons and WFA/PNNs in the TRN were also both significantly decreased in KO compared to WT (PV: p = 0.04; WFA/PNNs: p < 0.001: not shown in the figure). These results suggest that oxidative stress due to redox dysregulation could lead to impaired PV neurons and WFA/PNN circuitry of TRN in adult GCLM KO mice.
Figure 2.
Early increased in oxidative stress and PV neurons and WFA/PNN deficit in the TRN of adult GCLM KO mice. (a) Micrographs show immunofluorescent labeling for 8-oxo-dG (green), WFA/PNN (blue) and PV neurons (red) in the TRN of P20 (Juvenile), P40 (Pubertal), and P90 (Adult) WT and GCLM KO mice. (b) The increased in 8-oxo-dG immunolabeling (in arbitrary unit, a.u.) in KO (red) was already present at P20, increased further in P40 and even higher at P90. (c) As the animal aged, the number of PV neurons decreased in TRN of KO compared to WT mice. (d) The number of WFA/PNN+PV neurons in the TRN of KO mice were also reduced in P40 and P90 when compared to WT mice. For each group, n = 4–5. Scale: 100 μm. Bars in all graphs represent SD. **p < 0.01; ***p < 0.001 (pair-wise Dunnett tests).
Early oxidative stress and PV/PNN impairment in the TRN of GCLM KO mice
The second objective was to test whether the observed elevated oxidative stress and the altered PV/PNN circuitry in the TRN of GCLM KO mice in adulthood was already present in the postnatal developmental period, i.e. postnatal day (P) 20 (juvenile) and 40 (pubertal). In the TRN of both P20 and P40 KO mice, 8-oxo-dG immunolabeling was significantly increased (126% and 146%, respectively), when compared to WT mice (Figure 2a). At P20, numbers of PV neurons in KO were significantly reduced (approximately 13%) (Figure 2a, c; p = 0.002) compared to WT mice. Pubertal (P40) KO mice showed significantly reduced numbers of both PV neurons (p<0.001; 13% decrease) and WFA/PNN+PV neurons (p<0.001; 20% decrease) (Figure 2a, c, d). Intensity of 8-oxo-dG immunolabeling significantly increased with age in KO mice (ANOVA, F(1,28) = 8.9, p = 0.0001), while PV neurons decreased with age in the KO (ANOVA, F(1,28) = 9.6, p = 0.0007), compared to WT mice (ANOVA, F(1,28) = 3.2, p = 0.06) (Figure 2b). 8-oxo-dG intensity was significantly higher in Gclm KO at P90 when compared to both P20 (Tukey-Kramer, p = 0.005) and P40 (p =0.016). No significant increase of 8-oxo-dG intensity was found between P20 and P40. Collectively, oxidative stress appears in the TRN of KO mice early during development and worsens from peripubertal to adulthood. This is accompanied by PV and WFA/PNN deficits which also persist until adulthood.
Alterations of spike/bursting properties of TRN neurons in GCLM KO mice
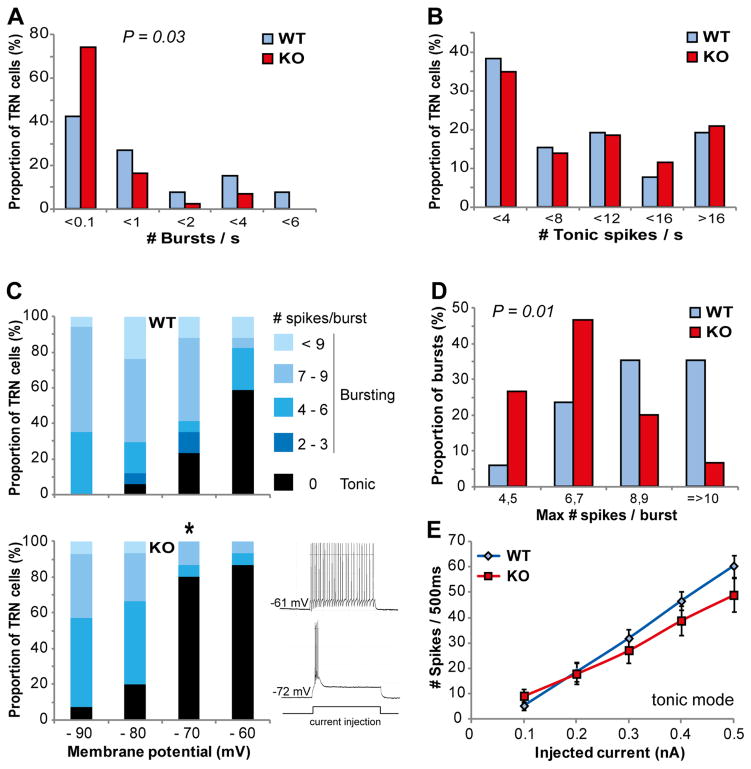
We then assessed whether PV and WFA/PNN abnormalities, and increased oxidative stress in GCLM KO mice were accompanied by functional alterations of TRN neurons in adulthood. We first recorded the spontaneous activity of TRN neurons (Supplementary Figures S2, S3). The frequency of spike bursts was significantly altered in GCLM KO mice, with more neurons generating no or very few bursts, as compared to WT mice (Figure 3a; p = 0.03). In contrast, the frequency of tonic spikes were similar in WT and KO mice (Figure 3b). We then examined the firing properties of TRN neurons using intracellular sharp electrodes. The proportion of neurons displaying bursting behavior at resting membrane potential was similar in both genotypes: 74% (17/23) in WT and 75% (15/20) in KO mice. Likewise, the resting membrane potential of these bursting TRN neurons was not significantly different (mean ± sem: −68.5 ± 2.4 mV for WT and −67.4 ± 2.4 mV for KO) as well as the input resistance. As expected from the inactivation curve of the low-threshold T-calcium current responsible for burst firing in TRN neurons, the proportion of neurons that exhibited a burst of action potentials upon depolarizing step currents increased as the membrane potentials became more negative (Figure 3c). However at a membrane potential of ~−70 mV, a vast majority of TRN neurons still fired in a tonic mode in KO mice while most neurons in WT mice already displayed a bursting behavior (Figure 3c; p = 0.015). Thus at their resting membrane potential, most TRN neurons were excited in bursting mode in WT but in tonic mode in KO mice. Moreover, single bursts contained significantly less action potentials in KO as compared to WT mice (Figure 3d; p = 0.009). By contrast the tonic firing mode observed in most neurons upon depolarization from a membrane potential kept at ~−60 mV did not differ between genotypes (Figure 3e). Altogether, these data indicate that, at least in-vitro, TRN neurons burst less in KO compared to WT mice, while the tonic mode is unaffected.
Figure 3.
The burst-firing mode of TRN neurons was impaired in GCLM KO mice in vitro. (a) and (b) Spiking pattern of spontaneously active TRN neurons was altered in GCLM KO mice. (a) Frequency of bursts. More TRN neurons did not burst in GCLM KO as compared to WT mice (p = 0.03; Mann-Whitney U test). (b) Frequency of tonic spikes. No significant difference in the tonic mode between both genotypes. Data are based on 26 and 43 recorded neurons in 4 WT and 4 KO mice, respectively. (c) Proportion of TRN neurons exhibiting a burst versus a tonic firing when kept at 4 different membrane potential levels. Note the significantly smaller number of neurons bursting at ~–70 mV in GCLM KO as compared to WT mice (total number of recorded neurons: 17 and 15 in WT and KO, respectively). * significantly different between genotypes (p = 0.015; Fisher exact test, p value corrected for multiple comparisons). The traces in the inset show a TRN neuron exhibiting a tonic (upper trace) and a bursting (lower trace) response upon a depolarization current while its membrane potential was at −61 and −71 mV, respectively. (d) The maximum number of action potentials generated within a single burst was significantly smaller in TRN neurons of KO as compared to WT mice (p = 0.009; ANOVA). (e) When the membrane potential was kept at ~−60mV, the tonic response to depolarization currents was not different between genotypes (number of neurons: 10 and 12 in WT and KO, respectively).
DISCUSSION
Our results provide direct evidence of structural and cellular anomalies in the TRN of SZ and BD patients and compelling data pointing to potential mechanisms and functional consequences of such anomalies. Postmortem findings add to previous neurochemical support for TRN involvement in SZ (34, 35) and represent the first evidence for its role in BD. Specifically, a predominant TRN neuronal population, i.e. neurons expressing PV, in part ensheathed by WFA/PNNs, was found to be decreased in these disorders. Results from an animal model of redox dysregulation due to low GSH synthesis capacity (GCLM KO mice) show that the TRN is particularly prone to oxidative stress. In the TRN of these mice, both PV neurons and WFA/PNNs are decreased from early postnatal age onward and TRN neurons are less inclined to generate bursts of action potentials. We put forth that oxidative stress may represent a mechanism contributing to TRN neuronal abnormalities in SZ and BD, and that these changes may be present at early stages of these disorders and may be associated with electrophysiological abnormalities. Indeed, oxidative stress and altered antioxidant systems are consistently reported in individuals suffering from these disorders, including reduced GSH levels in some patients (56, 58–60, 68–70) and altered in vivo redox NAD+/NADH ratio (71).
Tn and Nd of PV neurons and WFA/PNNs were markedly decreased in the TRN of subjects with SZ and BD. In patients with SZ, positive correlations with exposure to antipsychotics during the last 6 months’ of life suggest that these drugs may protect from, and/or counteract, decreases of PV neurons and WFA/PNNs in the TRN. Together with the strikingly similar results in the mouse model studies, lack of effects of all other covariates tested, including duration of illness, age of disease onset, exposure to other pharmacological agents tested, substance abuse, cause of death, is consistent with the possibility that these changes are inherent to each disorder rather than representing secondary factors, and may occur early on, perhaps before these illnesses become clinically manifest. Further studies will be needed to corroborate this possibility. Large effect sizes for these decreases were detected in both SZ and BD, suggesting that these disorders are similarly impacted. Finally, we note that despite the overwhelming similarities, there are small discrepancies relative to percentages of PV neurons and WFA/PNN decreases between the mouse model and human postmortem studies. We suggest that these differences may plausibly reflect species-specific differences in PV neurons association with WFA/PNNs (see for instance 95, 96) and/or the far more complex multigenic pathology in patients as compared to the mouse model.
In SZ and BD subjects, parallel decreases of TRN volume, PV neurons and WFA/PNNs, of which 63% is associated with PV neurons, raise the possibility of PV cell loss in the TRN. An alternative, non-reciprocally exclusive, interpretation of these findings is that decreased numbers of PV neurons and WFA/PNNs reflect a decrease of PV expression below detectable levels associated with altered WFA/PNNs molecular composition. Together, these two anomalies could be interpreted as reflecting neuronal immaturity (72, 73). This interpretation may also be consistent with a previous report of increased glutamate transporter expression in the TRN of subjects with schizophrenia, and suggestions that such changes may reflect a ‘diseased neuron’ signature in psychiatric disorders (35, 74, 75). On going studies are designed to distinguish between the ‘neuronal loss’ versus ‘diseased neuron’ pathological scenarios in the TRN as well as other thalamic nuclei. However, in either case, the present results do show robust deficits of PV-IR neurons and WFA/PNNs.
TRN neurons, among which PV cells represent the predominant neuronal population, exert a powerful inhibitory control over thalamo-cortical neurons. They are implicated in selected attention (26, 76) and in the generation of sleep spindles. Moreover, PNNs modulate synaptic plasticity and neuronal firing patterns (77, 78). Thus, PV neurons and WFA/PNNs deficits in the TRN of SZ and BD patients may profoundly impact TRN functions, contributing to disruption of sleep patterns, hallucinations, emotional and cognitive processing, and attention. Of note, a deficit of sleep spindles is already present in first episode, antipsychotic-naïve SZ patients and first degree relatives of SZ patients (79, 80), further supporting the possibility that TRN abnormalities in this disorder may precede its clinical manifestations. Although disruption of these functions has been most extensively described in SZ, sleep disturbances, hallucinations and emotion processing disturbances are also observed in BD, consistent with parallel PV neurons and WFA/PNNs decreases in the TRN of both disorders (81–86). Speculatively, differential sleep spindles disruption in SZ versus BD (30, 32, 80, 87) may reflect different pathophysiological mechanisms affecting PV neurons and/or WFA/PNN in the TRN, e.g. cell loss versus ‘diseased neuron’ pathology. While similar findings in the TRN of SZ and BD subjects reported here are consistent with increasing evidence for overlapping neurogenetic, clinical, pharmacological and pathological features (including oxidative stress) in these and other psychiatric disorders (88–91), the specific pathophysiological mechanisms underlying PV and WFA/PNN decreases may align with distinct clinical domains.
Electrophysiological studies reported here point to a mechanism potentially linking findings in GCLM KO mice and human to sleep spindles deficits. We found that TRN neurons of GCLM KO mice are less inclined to burst, a firing mode often observed in free behaving mice particularly during slow wave sleep (92). Speculatively, such a deficit in burst firing might diminish the inhibitory modulation of the thalamocortical neurons and affect sleep spindles. The mechanisms underlying the alteration in the firing mode of TRN neurons in GCLM KO mice remain to be elucidated. Interestingly, Cav3.3 T-type calcium channels, which contribute largely to the calcium T-currents responsible for TRN neuron burst firing and sleep spindles (93), are encoded by a candidate risk gene for SZ, CACNA1i (94).
Our results in rodents offer important clues on one potent pathophysiological mechanism underlying PV neurons and WFA/PNNs deficits in the TRN. Oxidative stress is a common pathological endpoint leading to PV neurons and WFA/PNNs anomalies in the medial prefrontal cortex of many animal models carrying genetic and/or environmental risks relevant to SZ (55). The present results suggest that this could hold true for the TRN, as this region is particularly prone to oxidative stress. In the TRN of GCLM KO mice, oxidative stress is accompanied by a reduction of the number of PV neurons and WFA/PNN+PV neurons. Likewise, in an other model relevant to psychosis, acute ketamine administration induces oxidative stress and decreases PV immunoreactivity in mouse TRN (97). In TRN of GCLM KO mice, oxidative stress became more severe from postnatal development into adulthood. A decrease of PV neuron numbers was already present at P20, while a significant reduction of WFA/PNNs was observed from P40 onward. In this animal model, the number of PV neurons in the TRN was affected earlier than those of the anterior cingulate cortex (67) and the hippocampus (63), suggesting that the TRN is particularly susceptible to redox dysregulation and can be affected early on during postnatal development. Such high susceptibility may be due to the predominance of highly active PV cells within the TRN. Indeed, fast-spiking activities of PV neurons implies enhanced oxidative metabolism, making them particularly vulnerable to redox imbalance (98). Thus, oxidative stress may represent an important contributor to PV and WFA/PNN decreases in the TRN, potentially adding to other factors such as altered excitatory/glutamatergic transmission (26, 35), altered function of T-type calcium channels (99) and reduced cholinergic modulation (34).
CONCLUSIONS
This study provides a direct and compelling evidence for anomalies in the TRN of SZ and BD. Data from mice also show that TRN neurons are susceptible to redox dysregulation, a potent mechanism by which this thalamic nucleus could be affected in both diseases, already in early stages. Given the key role that the TRN plays in gating thalamo-cortical information, altered neuronal firing patterns and neuron decrease, and/or neurochemical abnormalities in this nucleus, may profoundly affect thalamo-cortical connectivity, and therefore contribute to sensory, attentional, cognitive, emotional, and sleep deficits observed in these disorders.
Supplementary Material
Acknowledgments
(Pascal Steullet, Jan-Harry Cabungcal, Thomas E. Salt, Michel Cuenod, Kim Q. Do) The authors would like to thank Adeline Cottier and Rudolf Kraftsik for their helpful technical support. We thank our financial supports: National Center of Competence in Research (NCCR) “SYNAPSY - The Synaptic Bases of Mental Diseases” from the Swiss National Science Foundation (n° 51NF40-158776), the Banque Lombard Odier &CieSA, Damm-Etienne Foundation and Alamaya Foundation.
(Syed A. Bukhari, Magdalena I. Ardelt, Harry Pantazopoulos, Fadi Hamati, Sabina Berretta) The authors thank NIH (NIMH R01 MH105608) for funding this work and the Harvard Brain Tissue Resource Center, funded through NIH-NeuroBiobank HHSN-271-2013-00030C (The National Institute of Mental Health (NIMH), National Institute of Neurological Diseases and Stroke (NINDS) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and brain donors and their families for the tissue samples used in these studies.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, et al. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull. 2014;40(6):1227–43. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA psychiatry. 2015;72(9):882–91. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, et al. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37(2):499–507. doi: 10.1038/npp.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013 doi: 10.1007/s00406-013-0417-0. [DOI] [PubMed] [Google Scholar]
- 6.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness. Cereb Cortex. 2013 doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anticevic A, Repovs G, Krystal JH, Barch DM. A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res. 2012;141(1):8–14. doi: 10.1016/j.schres.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinn AK, Baker JT, Cohen BM, Ongur D. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr Res. 2013;143(2–3):260–8. doi: 10.1016/j.schres.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64(2):81–8. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774–81. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–22. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–9. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Chu KW, Teague EB, Newmark RE, Buchsbaum MS. fMRI assessment of thalamocortical connectivity during attentional performance. Magnetic resonance imaging. 2013;31(7):1112–8. doi: 10.1016/j.mri.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46(1):1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Luthi A. Sleep Spindles: Where They Come From, What They Do. Neuroscientist. 2014;20(3):243–56. doi: 10.1177/1073858413500854. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald KD, Fifkova E, Jones MS, Barth DS. Focal stimulation of the thalamic reticular nucleus induces focal gamma waves in cortex. J Neurophysiol. 1998;79(1):474–7. doi: 10.1152/jn.1998.79.1.474. [DOI] [PubMed] [Google Scholar]
- 18.Lewis LD, Voigts J, Flores FJ, Schmitt LI, Wilson MA, Halassa MM, et al. Thalamic reticular nucleus induces fast and local modulation of arousal state. eLife. 2015;4:e08760. doi: 10.7554/eLife.08760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells MF, Wimmer RD, Schmitt LI, Feng G, Halassa MM. Thalamic reticular impairment underlies attention deficit in Ptchd1(Y/−) mice. Nature. 2016;532(7597):58–63. doi: 10.1038/nature17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26(28):7348–61. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist. 2007;13(5):532–45. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. Journal of psychopharmacology (Oxford, England) 2015;29(2):127–37. doi: 10.1177/0269881114565805. [DOI] [PubMed] [Google Scholar]
- 23.Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37(2):306–15. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young A, Wimmer RD. Implications for the thalamic reticular nucleus in impaired attention and sleep in schizophrenia. Schizophr Res. 2017;180:44–7. doi: 10.1016/j.schres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Behrendt RP. Dysregulation of thalamic sensory “transmission” in schizophrenia: neurochemical vulnerability to hallucinations. Journal of psychopharmacology (Oxford, England) 2006;20(3):356–72. doi: 10.1177/0269881105057696. [DOI] [PubMed] [Google Scholar]
- 26.Ahrens S, Jaramillo S, Yu K, Ghosh S, Hwang GR, Paik R, et al. ErbB4 regulation of a thalamic reticular nucleus circuit for sensory selection. Nat Neurosci. 2015;18(1):104–11. doi: 10.1038/nn.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreasen NC, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O’Leary D, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266(5183):294–8. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–18. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 29.Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66(2):77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- 30.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 31.Ferrarelli F, Tononi G. Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr Res. 2016 doi: 10.1016/j.schres.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Maertelaer V, Hoffman G, Lemaire M, Mendlewicz J. Sleep spindle activity changes in patients with affective disorders. Sleep. 1987;10(5):443–51. doi: 10.1093/sleep/10.5.443. [DOI] [PubMed] [Google Scholar]
- 33.Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry. 2016;80(8):599–608. doi: 10.1016/j.biopsych.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73(4):1590–7. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158(9):1393–9. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- 36.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12(4):335–44. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169(10):1082–91. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in Cortical Network Oscillations and Parvalbumin Neurons in Schizophrenia. Biol Psychiatry. 2015;77(12):1031–40. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24(3):349–55. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 41.Beasley C, Zhang Z, Patten I, Reynolds G. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52(7):708. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55(1–2):1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 43.Pantazopoulos H, Lange N, Baldessarini RJ, Berretta S. Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;61(5):640–52. doi: 10.1016/j.biopsych.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA. Reduced Labeling of Parvalbumin Neurons and Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia. Neuropsychopharmacology. 2016;41(9):2206–14. doi: 10.1038/npp.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantazopoulos H, Markota M, Jaquet F, Ghosh D, Wallin A, Santos A, et al. Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala. Translational psychiatry. 2015;5:e496, 1–11. doi: 10.1038/tp.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74(6):427–35. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67(2):155–66. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernard C, Prochiantz A. Otx2-PNN Interaction to Regulate Cortical Plasticity. Neural plasticity. 2016;2016:7931693. doi: 10.1155/2016/7931693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Developmental neurobiology. 2011;71(11):1073–89. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 50.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110(22):9130–5. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–51. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 52.Frischknecht R, Gundelfinger ED. The brain’s extracellular matrix and its role in synaptic plasticity. Adv Exp Med Biol. 2012;970:153–71. doi: 10.1007/978-3-7091-0932-8_7. [DOI] [PubMed] [Google Scholar]
- 53.Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: Potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015 doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pantazopoulos H, Berretta S. In Sickness and in Health: Perineuronal Nets and Synaptic Plasticity in Psychiatric Disorders. Neural plasticity. 2016;2016:9847696. doi: 10.1155/2016/9847696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry. 2017;22(7):936–43. doi: 10.1038/mp.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1–2):61–8. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19(2):220–30. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Koga M, Serritella AV, Sawa A, Sedlak TW. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr Res. 2016;176(1):52–71. doi: 10.1016/j.schres.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 59.Munkholm K, Vinberg M, Berk M, Kessing LV. State-related alterations of gene expression in bipolar disorder: a systematic review. Bipolar Disord. 2012;14(7):684–96. doi: 10.1111/bdi.12005. [DOI] [PubMed] [Google Scholar]
- 60.Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxidants & redox signaling. 2011;15(7):2011–35. doi: 10.1089/ars.2010.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17(2):125–34. doi: 10.1038/nrn.2015.19. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/ −) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277(51):49446–52. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- 63.Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30(7):2547–58. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62(8):884–93. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 65.Cavalieri B. Geometria degli Indivisibili. Torino: Unione Tipografico-Editrice Torinese; 1966. [Google Scholar]
- 66.Sullivan KM, Pantazopoulos H, Liebson E, Woo TUW, Baldessarini RJ, Hedreen J, et al. What can we learn about brain donors? Use of clinical information in human postmortem brain research. In: Huitinga I, Webster M, editors. Brain Banking in Neurologic and Psychiatric Disorders. Elsevier; 2017. p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013;73(6):574–82. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 68.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12(10):3721–8. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 69.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400–9. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xin L, Mekle R, Fournier M, Baumann PS, Ferrari C, Alameda L, et al. Genetic Polymorphism Associated Prefrontal Glutathione and Its Coupling With Brain Glutamate and Peripheral Redox Status in Early Psychosis. Schizophr Bull. 2016;42(5):1185–96. doi: 10.1093/schbul/sbw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim SY, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, et al. Redox Dysregulation in Schizophrenia Revealed by in vivo NAD+/NADH Measurement. Schizophr Bull. 2017;43(1):197–204. doi: 10.1093/schbul/sbw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30(45):14964–71. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 74.Huerta I, McCullumsmith RE, Haroutunian V, Gimenez-Amaya JM, Meador-Woodruff JH. Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse. 2006;59(7):394–402. doi: 10.1002/syn.20250. [DOI] [PubMed] [Google Scholar]
- 75.McCullumsmith RE, Sanacora G. Regulation of extrasynaptic glutamate levels as a pathophysiological mechanism in disorders of motivation and addiction. Neuropsychopharmacology. 2015;40(1):254–5. doi: 10.1038/npp.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature. 2015;526(7575):705–9. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balmer TS. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro. 2016;3(4) doi: 10.1523/ENEURO.0112-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience. 2001;104(2):359–69. doi: 10.1016/s0306-4522(01)00082-3. [DOI] [PubMed] [Google Scholar]
- 79.Schilling C, Schlipf M, Spietzack S, Rausch F, Eisenacher S, Englisch S, et al. Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. Eur Arch Psychiatry Clin Neurosci. 2017;267(3):213–24. doi: 10.1007/s00406-016-0725-2. [DOI] [PubMed] [Google Scholar]
- 80.Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, et al. Sleep spindle deficits in antipsychotic-naive early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014;8:762. doi: 10.3389/fnhum.2014.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cardenas SA, Kassem L, Brotman MA, Leibenluft E, McMahon FJ. Neurocognitive functioning in euthymic patients with bipolar disorder and unaffected relatives: A review of the literature. Neurosci Biobehav Rev. 2016;69:193–215. doi: 10.1016/j.neubiorev.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duarte W, Becerra R, Cruise K. The Relationship Between Neurocognitive Functioning and Occupational Functioning in Bipolar Disorder: A Literature Review. Europe’s journal of psychology. 2016;12(4):659–78. doi: 10.5964/ejop.v12i4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Rheenen TE, Rossell SL. Auditory-prosodic processing in bipolar disorder; from sensory perception to emotion. J Affect Disord. 2013;151(3):1102–7. doi: 10.1016/j.jad.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 84.Toh WL, Thomas N, Rossell SL. Auditory verbal hallucinations in bipolar disorder (BD) and major depressive disorder (MDD): A systematic review. J Affect Disord. 2015;184:18–28. doi: 10.1016/j.jad.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 85.Mercer L, Becerra R. A unique emotional processing profile of euthymic bipolar disorder? A critical review. J Affect Disord. 2013;146(3):295–309. doi: 10.1016/j.jad.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 86.Cullen B, Ward J, Graham NA, Deary IJ, Pell JP, Smith DJ, et al. Prevalence and correlates of cognitive impairment in euthymic adults with bipolar disorder: A systematic review. J Affect Disord. 2016;205:165–81. doi: 10.1016/j.jad.2016.06.063. [DOI] [PubMed] [Google Scholar]
- 87.Altena E, Micoulaud-Franchi JA, Geoffroy PA, Sanz-Arigita E, Bioulac S, Philip P. The bidirectional relation between emotional reactivity and sleep: From disruption to recovery. Behav Neurosci. 2016;130(3):336–50. doi: 10.1037/bne0000128. [DOI] [PubMed] [Google Scholar]
- 88.Lee PH, Baker JT, Holmes AJ, Jahanshad N, Ge T, Jung JY, et al. Partitioning heritability analysis reveals a shared genetic basis of brain anatomy and schizophrenia. Mol Psychiatry. 2016;21(12):1680–9. doi: 10.1038/mp.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Owen MJ, Craddock N, Jablensky A. The genetic deconstruction of psychosis. Schizophr Bull. 2007;33(4):905–11. doi: 10.1093/schbul/sbm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, et al. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158(4):808–21. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108(33):13823–8. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pantazopoulos H, Lange N, Hassinger L, Berretta S. Subpopulations of neurons expressing parvalbumin in the human amygdala. The Journal of comparative neurology. 2006;496(5):706–22. doi: 10.1002/cne.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pantazopoulos H, Murray EA, Berretta S. Total number, distribution, and phenotype of cells expressing chondroitin sulfate proteoglycans in the normal human amygdala. Brain Res. 2008;1207:84–95. doi: 10.1016/j.brainres.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–7. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 98.Kann O. The interneuron energy hypothesis: Implications for brain disease. Neurobiol Dis. 2016;90:75–85. doi: 10.1016/j.nbd.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Andrade A, Hope J, Allen A, Yorgan V, Lipscombe D, Pan JQ. A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity. Scientific reports. 2016;6:34233. doi: 10.1038/srep34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.