Summary
Endometrial endometrioid carcinoma is related to estrogen excess and expression of estrogen and progesterone receptors. Epidemiological evidence suggests that exposure to elevated androgens, as in polycystic ovarian syndrome, increases the risk of endometrial cancer. Factors impacting androgen receptor expression are not well studied. Mismatch repair deficiency due to MLH1 gene methylation is one of the most common molecular alterations in endometrial cancer, occurring in 15–20% of cases. MLH1 methylation can be associated with decreased expression of other genes, so we examined the effect of mismatch repair status on androgen receptor expression. As NF-κB is known to induce AR, this transcription factor was also examined. 344 unselected endometrial carcinomas were evaluated for DNA mismatch repair. Loss of expression of MLH1 with MLH1 methylation was defined as mismatch repair deficient, and positive expression of mismatch repair proteins was defined as mismatch repair intact. A case-control cohort of 96 grade 2 endometrioid carcinomas was studied from this set (47 mismatch repair deficient, 49 mismatch repair intact). Cases were matched for histotype, grade, and age. Androgen receptor and NF-κB immunohistochemical expression were evaluated by two different scoring systems (CAP/ASCO and Allred) used for estrogen receptor. Despite higher levels of NF-κB, mismatch repair deficiency was associated with a significantly lower mean percentage of androgen receptor expression. The mismatch repair deficient group had more variable androgen receptor expression, with more cases scoring on the lower end of the spectrum. These findings have implications for clinical trials of androgen receptor antagonists in gynecological cancers.
Keywords: Endometrial endometrioid carcinoma, Androgen receptor, NF-κB, Mismatch repair genes
Introduction
Steroid hormones including estrogen and progesterone play an important role in the development of endometrial endometrioid carcinoma (EEC) 1. Androgen and androgen receptor (AR) are well-known to be critical in the development of prostate cancer, but they have gradually been recognized to be important in EEC as well 2–4. For example, endometrial cancer risk is tightly linked to obesity which is associated with a hyperandrogenic state. Polycystic ovarian syndrome is associated with androgen excess and an increased risk of EEC 5. Data from the European Prospective Investigation into Cancer and Nutrition (EPIC) have shown that elevated circulating levels of free testosterone positively correlated with increasing body mass index and endometrial carcinoma risk 1. Both testosterone and dihydrotestosterone are elevated in EEC compared to normal endometrium 6. AR expression was previously studied in 85 endometrial cancers (37 endometrioid grades 1 and 2, and 48 endometrioid grade 3, serous, and clear cell carcinomas and carcinosarcomas). Similar to estrogen and progesterone receptors, AR expression declined as endometrial cancer grade increased 2. Another study of 86 EEC demonstrated that AR expression was inversely correlated with tumor grade, presence of lymphatic/vascular space invasion, and Ki67 and positively correlated with ER expression. AR expression was not correlated to clinical stage 6. Recently, Mahdi et al. detected AR expression (defined as expression in 1% or more tumor cell nuclei) in 51% of the 261 endometrial cancers examined. AR expression was detected in both endometrioid and non-endometrioid carcinomas, with lower frequencies of positive cases in the serous and carcinosarcoma groups 7.
Other than tumor differentiation, molecular factors impacting AR expression in endometrial cancer remain poorly understood. High levels of microsatellite instability/DNA mismatch repair deficiency is one of the most common molecular defects in endometrial cancer, occurring in 15–20% of cases, especially in the endometrioid histotype 8,9. In sporadic endometrial cancer, defective DNA mismatch repair is due to MLH1 gene methylation with subsequent loss of MLH1 protein. A methylator phenotype involving methylation of numerous other genes in addition to MLH1 has been demonstrated for endometrial cancer 10,11. Hypermethylation of a CpG region spanning the transcription start site of the AR gene is associated with AR gene inactivation in EEC 12. We therefore hypothesized that DNA mismatch repair (MMR) deficiency due to MLH1 gene methylation and subsequent MLH1 protein loss would be associated with decreased AR expression in EEC. As NF-κB has been previously shown to activate the AR promoter in prostate cancer cells, inducing AR expression and cell proliferation 13, we also examined expression of this transcription factor in the same tumors.
Materials and methods
Patient population
Unselected patients who underwent hysterectomy for endometrial cancer at M.D. Anderson Cancer Center from 01/01/2013 to 10/30/2015 were identified. Relevant clinical data were extracted from the electronic medical record, and all hysterectomies were pathologically reviewed by a gynecologic pathologist (R.R.B.). The carcinomas were graded according to the International Federation of Gynecology and Obstetrics (FIGO) grading system. A total of 335 endometrial cancer patients were identified, and their tumors were subjected to immunohistochemistry testing for MLH1, MSH2, MSH6, and PMS2 (Figure 1). MLH1 methylation was performed in patients with immunohistochemical loss of MLH1. A total of 91 endometrial cancers with MMR defects were identified, and the majority of these tumors were endometrioid FIGO grade 2 with loss of MLH1 (Figure 1). To eliminate any possible variation in biomarker expression that could be attributed to the histotype or grade of tumor, we selected for further examination grade 2 endometrioid tumors. Specific MMR-deficient cases were then selected based on the presence of adequate tumor and age of patient for matching to a MMR-intact control. 47 MMR-deficient (all with MLH1 loss due to MLH1 gene methylation) and 49 MMR-intact controls made up the final cohort.
Figure 1.
Schematic overview of methodology. A total of 335 endometrial cancers were tested for mismatch repair deficiency using immunohistochemistry and MLH1 gene methylation analysis when a tumor had loss of MLH1 protein expression. To control for possible impact of tumor histology and grade on AR expression, this study was restricted to grade 2 endometrioid carcinomas.
Assessment of DNA mismatch repair
All tissue-based analyses were performed using formalin-fixed, paraffin-embedded sections. All of the cases were fixed in the same pathology laboratory, for approximately the same length of time, and under the same protocols. After review of all the hematoxylin and eosin-stained slides for each case, one representative block that had an adequate amount of viable tumor for immunohistochemistry studies and was best representative of Grade 2 endometrioid histology was selected for study. Immunohistochemistry of MMR proteins was performed using standard techniques for MLH1 (G168–15 1:25; BD Biosciences Pharmingen), MSH2 (FE11, 1:100; Calbiochem), MSH6 (44, 1:300; BD Biosciences Phar-Demographic datamingen), and PMS2 (Alb-4, 1:125; BD Biosciences Pharmingen). MLH1, MSH2, MSH6, and PMS2 immunohistochemistry was scored as protein intact or deficient using light microscopic examination. Complete absence of MMR protein expression was required in order for a case to be designated as MMR deficient. Stromal cells served as an internal positive control.
For tumors with loss of MLH1 protein expression, polymerase chain reaction (PCR)-based MLH1 promoter methylation analysis was performed. DNA was isolated from mapped formalin fixed, paraffin-embedded tissue sections from the same block in which immunohistochemistry was performed. Tissue sections were dissected with a scalpel blade to provide relatively pure tumor samples for analysis. Isolated DNA was treated with bisulfite to convert unmethylated cytosine nucleotides to uracil using the Zymo EZ DNA Methylation-Gold Kit according to the manufacturer’s instructions (Zymo Research, Orange, CA). Methylation of MLH1 was assessed using a modified version of methylation-specific PCR followed by capillary electrophoresis using FAM-labeled reverse primer and unalabeled forward primers (Intergrated DNA Technology). The primer sequences were the following: methylated forward, 5′-GAT AGC GAT TTT TAA CGC-3′, unmethylated forward, 5′-AGA GTG GAT AGT GAT TTT TAA TGT-3′ and labeled reverse primer, 5′-FAM-TCT ATA AAT TAC TAA ATC TCT TC-3′ and labeled reverse primer, 5′ FAM-TCT- ATA AAT TAC TAA ATC TCT TC-3′. The forward primers were designed to distinguish the methylated amplicon from the unmethylated by difference in size. The bisulfite-treated DNA was then amplified by PCR using primers specific for methylated and unmethylated DNA. The methylated PCR product of 85 bp was separated from unmethylated PCR product of 91 bp by capillary electrophoresis using an ABI Prism 3130 Genetic Analyzer. Chromatograms for tumor were compared with those generated for the RKO colon carcinoma cell line (positive control known to have loss of MLH1 protein due to MLH1 promoter methylation).
Assessment of AR and NF-κB
Immunohistochemical analysis for AR was performed using the AR441 clone (Dako) at 1:30 dilution. For NFκB, anti-p65 antibody (Abcam 47423) was used at 1:300 dilution. AR and NFκB expression were evaluated by light microscopic examination, blinded as to tumor mismatch repair status, using both a clinically validated cut-off established by ASCO/CAP for ER in breast cancer and an Allred scoring system because no clinically validated system has been established for hormonal receptor evaluation in EEC 14–16. In the Allred scoring system, the proportion of positive staining is scored on a scale of 0–5 and staining intensity is on a scale of 0–3. The final score adds both together to yield a total score. A score of 0–2 was regarded as negative and 3–8 as positive. Representative photomicrographs of the different staining intensities and proportions positive for AR are shown in Figure 2. For both scoring systems, only staining in tumor epithelial cell nuclei was considered. Allred and CAP scores for AR and NF-κB were assigned at the same time for each case. The data were analyzed using the Fisher exact test and Chi-Square analysis to compare AR expression by MSI status.
Figure 2.
Representative examples of the range of Allred expression of AR in grade 2 endometrial endometrioid adenocarcinomas. A–C, range of expression intensity (low, intermediate, and high); D–I, range of proportion of tumor staining (0–5).
Results
Overall, the mean age for the 96 patients included in the study was 66.3 years. The mean age for the MMR deficient group was 67.5 years, and the mean age for the MMR intact group 65.1 years (p=0.25). The FIGO stage distribution included 80 stage I, 3 stage II, 12 stage III, and 1 stage IV. Stage distribution between the MMR deficient and MMR intact groups was comparable. The MMR deficient group had 87.2% early stage (stages I and II combined) and 12.8% late stage (stages III and IV combined), while the MMR intact group had 85.7% early stage and 14.3% late stage cases.
Despite controlling for endometrial cancer histotype (endometrioid), grade (FIGO 2), and matching for patient age, a broad spectrum of nuclear AR expression was encountered (Figure 2). No cytoplasmic expression was identified. Among all cases, AR was positive in 73.9% by using CAP criteria and 59.4% by Allred criteria (Figure 3A). Overall, 39/96 (40.6%) tumors had an Allred AR score of 0–2, so these were considered negative (Figure 4B). Stage had no effect on AR expression (p=0.178). In both scoring systems, the MMR-intact group had a significantly greater percentage of positive cases (Figures 3B and 3C).
Figure 3.
Summary of CAP and Allred AR immunohistochemical expression in grade 2 endometrial endometrioid adenocarcinoma. Using the CAP scoring system, a significantly higher number of cases were considered AR positive (A). In both the CAP (B) and Allred (C) systems, mismatch repair deficiency was associated with a significantly lower percentage of tumors considered AR positive.
Figure 4.
AR immunohistochemical expression in Allred-positive endometrial carcinomas. For the tumors considered AR-positive by the Allred system, the mean score was not significantly different between the MMR-deficient and –intact groups (A). The mismatch repair deficient group was more likely to have lower Allred scores of 0–2 (B).
When considering only the positive cases (n=34 MMR intact; n=23 MMR deficient), the mean Allred scores for AR expression of the MMR deficient and MMR intact groups were not significantly different (Figure 4A). Inspection of the distribution of Allred scores demonstrated that the MMR intact group had more cases with an AR score ranging from 3–5 compared to the MMR deficient group. The MMR deficient group was much more likely to have an AR score of zero. The two groups had comparable numbers in the higher scoring range of 6-7-8.
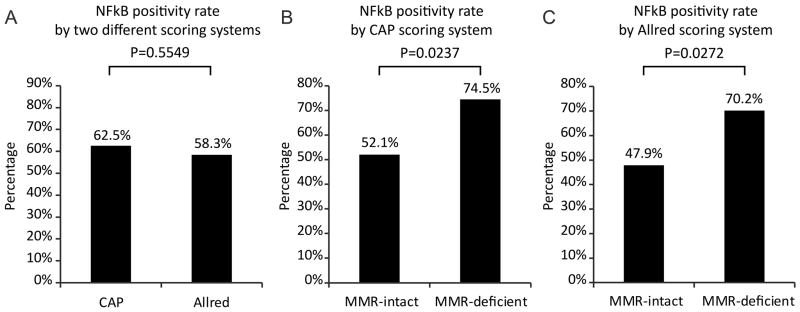
The transcription factor NF-κB is known to induce expression of AR in prostate cancer cells 12. Thus, we also examined its nuclear expression in the mismatch repair intact and deficient groups. As with AR, there was a spectrum of different NF-κB expression patterns despite the fact that tumor histotype, tumor grade, and patient age were controlled for (Figure 5). NF-κB positivity rate did not differ between the two different scoring systems (Figure 6A). There were significantly more MMR deficient cases that were positive for NF-κB (Figure 6B and 6C). Tumors positive for NF-κB did not have distinctive histopathological features compared to NFκB negative cases.
Figure 5.
NFκB immunohistochemical expression in endometrial carcinoma. Representative examples of Allred intensities of low (A), intermediate (B), and strong (C) are shown.
Figure 6.
Summary of CAP and Allred NFκB immunohistochemical expression in grade 2 endometrial endometrioid adenocarcinoma. The percentage of NFκB-positive cases was comparable between the 2 scoring systems (A). Using both the CAP (B) and Allred (C) scoring systems, the percentage of NFκB-positive tumors was significantly higher in the MMR-deficient group.
Discussion
To our knowledge, this is the first published report examining the effect of MMR status on AR expression. We chose to examine MMR status, as loss of mismatch repair is one of the most common molecular alterations in endometrial cancer, especially the endometrioid subtype. Applying two different scoring systems to a large set of grade 2 endometrioid carcinomas, we found that the incidence of AR positivity is significantly lower in the MMR-deficient group. Interestingly, a transcription factor known to up-regulate AR in prostate cancer, NF-κB, was significantly increased in the MMR-deficient group. These results imply that possible NF-κB regulation of AR expression in endometrial cancer is uncoupled in the setting of MMR deficiency.
Our finding of decreased AR expression in MMR-deficient EECs has important clinical implications. Recently, the AR antagonist Enzalutamide has been introduced in on-going clinical trials for advanced gynecological cancers including endometrial cancer (NCT02684227 Enzalutamide in Combination with Carboplatin and Paclitaxel in Endometrial Cancer; NCT01974765 Enzalutamide in Treating Patients with Advanced or Recurrent Androgen Receptor-Positive Ovarian, Primary Peritoneal, or Fallopian Tube Cancer; both trials summarized at https://clinicaltrials.gov.). For the endometrial cancer clinical trial, tumor AR positivity is not an entrance criterion, but it will be assessed during the study and correlated to treatment response. For the ovarian/tubal/peritoneal cancer trial, AR expression in 5% or more of tumor cells is required for trial enrollment. In surveying the breast cancer clinical trials at this website, there is variable AR immunohistochemistry requirements. Some trials do not require a tumor to be tested, while others require more than 1% or at least 10% AR-positive tumor cells. Nevertheless, results of our study imply that mismatch repair status of the endometrial cancer should be taken into account when designing clinical trials of AR antagonists.
It has been reported by several groups, including ours, that expression of clinically relevant biomarkers is different in MMR-deficient compared to MMR-intact colon and endometrial cancers. Immunohistochemistry for cytokeratins 7 and 20 can be useful in helping to distinguish carcinomas of lower GI tract origin (typically CK20 positive, CK7 negative) from gynecological carcinomas (typically CK7 positive, CK20 negative). Cytokeratin 20 expression is decreased in MMR deficient colorectal adenocarcinoma 17,18. MMR deficient endometrial carcinomas have a lower percentage of cytokeratin 7 positive cells 19. In addition, the mismatch repair deficient group had greater variability in ER expression, with more cases having less than 55% ER-positive tumor cells 19.
Mismatch repair status has been previously shown in endometrial carcinoma to be associated with distinctive histopathological features. For example, localization of endometrial tumor in the lower uterine segment is significantly associated with defective DNA MMR 20. MMR deficiency is associated with increased tumor infiltrating lymphocytes and increased mutation-generated neoantigens 21,22. Recent publications suggest that the specific type of mismatch repair deficiency may also influence endometrial carcinoma histologic features. For example, EEC with MLH1 loss due to MLH1 methylation have intermediate PD-L1 expression, with higher levels seen in EEC with loss of MSH6, MSH2 and MSH6, and PMS2 23. Compared to endometrial cancers with MSH2/MSH6/PMS2 loss, endometrial cancers with loss of MLH1 due to MLH1 gene methylation have significantly greater incidence of mucinous differentiation and more tumor infiltrating lymphocytes 24.
Given its role in regulating lymphocytes and their associated inflammatory responses, it was not surprising to us that NF-κB was significantly increased in the MMR deficient group, which is known to have increased tumor infiltrating lymphocytes 21,22. In a previous study of 95 endometrial cancers, NF-κB expression was not related to MMR deficiency 25. Our study differs in a number of important ways that may account for the discrepant results. The previous study used a tissue microarray rather than whole tissue sections from each tumor and a different NF-κB p65 antibody that was detecting much more cytoplasmic signal than we observed. Also, the previous study included 17 non-endometrioid endometrial carcinomas, which are very unlikely to be mismatch repair deficient. The relationship between mismatch repair and inflammation is likely complex. Most studies in endometrial cancer and colorectal cancer focus on mismatch repair deficiency leading to increased tumor inflammatory infiltrates 21–24. However, there is evidence that inflammation inhibits DNA mismatch repair. For example, liver cancer cell lines treated with the pro-inflammatory cytokine TNF-α have significantly decreased expression of MSH2. This inhibition is NF-κB–dependent 26. The loss of MSH2 is important, as human hepatocellular carcinomas also have loss of MSH2 and MSH2-deficient mice develop liver cancers 26. NF-κB likely plays an important role in endometrial cancer, as it has been previously linked to endometrial carcinogenesis induced by KRAS mutation 27 and activation of the PI3K/AKT signaling pathway 28. Endometrial cancer cell lines treated with estrogen show activation of NF-κB, leading to increased expression of MMP-9, which helps to promote invasion 29. The mechanistic interplay between inflammation and mismatch repair in endometrial cancer development and progression has not yet been resolved.
In our series, elevated NF-κB in the MMR deficient group was not associated with increased AR expression. The exact molecular mechanisms regulating AR expression in the endometrium are unknown. Given that AR expression was lower in the MMR deficient group, which was defined as MLH1 loss due to MLH1 gene methylation, it is possible that methylation and subsequent silencing of the AR gene is also occurring in at least a subset of these tumors. This scenario would be consistent with the existence of a methylator phenotype for endometrial cancer 10,11 and the previously reported hypermethylation of the AR gene being associated with decreased immunohistochemical expression of AR protein 12. Such gene methylation would be expected to interfere with the binding of transcription factors such as NF-κB, which activate AR gene transcription 13, even when expression of such transcription factors is elevated.
Acknowledgments
Financial support: NIH SPORE in Uterine Cancer 3P50 CA098258 (R.R.B.)
Footnotes
Presented in part at the 106th USCAP Annual Meeting, March 4–10, 2017, San Antonio, Texas
The authors declare no conflict of interest.
References
- 1.Dossus L, Lukanova A, Rinaldi S, et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort--a factor analysis. Am J Epidemiol. 2013;177(8):787–799. doi: 10.1093/aje/kws309. [DOI] [PubMed] [Google Scholar]
- 2.Schweizer MT, Yu EY. AR-signaling in human malignancies: prostate cancer and beyond. Cancers (Basel) 2017;9(1) doi: 10.3390/cancers9010007. pii E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamal AM, Bulmer JN, DeCruze SB, et al. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br J Cancer. 2016;114(6):688–696. doi: 10.1038/bjc.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tangen IL, Onyango TB, Kopperud R, et al. Androgen receptor as potential therapeutic target in metastatic endometrial cancer. Oncotarget. 2016;7(31):49289–49298. doi: 10.18632/oncotarget.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piltonen TT. Polycystic ovary syndrome: endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2016;37:66–79. doi: 10.1016/j.bpobgyn.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka S, Miki Y, Hashimoto C, et al. The role of 5alpha-reductase type 1 associated with intratumoral dihydrotestosterone concentrations in human endometrial carcinoma. Mol Cell Endocrinol. 2015;401:56–64. doi: 10.1016/j.mce.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Mahdi Z, Abdulfatah E, Pardeshi V, et al. The impact of androgen receptor expression on endometrial carcinoma recurrence and survival. Int J Gynecol Pathol. 2017;36(5):405–411. doi: 10.1097/PGP.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 8.Djordjevic B, Barkoh BA, Luthra R, et al. Relationship between PTEN, DNA mismatch repair, and tumor histotype in endometrial carcinoma: retained positive expression of PTEN preferentially identifies sporadic non-endometrioid carcinomas. Mod Pathol. 2013;26(10):1401–1412. doi: 10.1038/modpathol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banno K, Kisu I, Yanokura M, et al. Endometrial Cancer and Hypermethylation: Regulation of DNA and MicroRNA by Epigenetics. Biochem Res Int. 2012;2012:738274. doi: 10.1155/2012/738274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitcomb BP, Mutch DG, Herzog TJ, et al. Frequent HOXA11 and THBS2 promoter methylation and a methylator phenotype in endometrial adenocarcinoma. Clin Cancer Res. 2003;9(6):2277–2287. [PubMed] [Google Scholar]
- 11.Trimarchi MP, Yan P, Groden J, et al. Identification of endometrial cancer methylation features using combined methylation analysis methods. PLoS One. 2017;12(3):e0173242. doi: 10.1371/journal.pone.0173242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki M, Oh BR, Dharia A, Fujimoto S, et al. Inactivation of the human androgen receptor gene is associated with CpG hypermethylation in uterine endometrial cancer. Mol Carinog. 2000;29(2):59–66. doi: 10.1002/1098-2744(200010)29:2<59::aid-mc2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Altuwaijri S, Deng F, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175(2):489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgibbons PL, Dillon DA, Alsabeh R, et al. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch Pathol Lab Med. 2014;138(5):595–601. doi: 10.5858/arpa.2013-0566-CP. [DOI] [PubMed] [Google Scholar]
- 15.Longacre TA, Broaddus R, Chuang LT, et al. Template for Reporting Results of Biomarker Testing of Specimens From Patients With Carcinoma of the Endometrium. Arch Pathol Lab Med. 2017 Mar 16; doi: 10.5858/arpa. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Qureshi A, Pervez S. Allred scoring for ER reporting and it’s impact in clearly distinguishing ER negative from ER positive breast cancers. J Pak Med Assoc. 2010;60(5):350–353. [PubMed] [Google Scholar]
- 17.Lugli A, Tzankov A, Zlobec I, et al. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod Pathol. 2008;21(11):1403–1412. doi: 10.1038/modpathol.2008.117. [DOI] [PubMed] [Google Scholar]
- 18.McGregor DK, Wu TT, Rashid A, et al. Reduced expression of cytokeratin 20 in colorectal carcinomas with high levels of microsatellite instability. Am J Surg Pathol. 2004;28(6):712–718. doi: 10.1097/01.pas.0000126757.58474.12. [DOI] [PubMed] [Google Scholar]
- 19.Okoye EI, Bruegl AS, Fellman B, et al. Defective DNA Mismatch Repair Influences Expression of Endometrial Carcinoma Biomarkers. Int J Gynecol Pathol. 2016;35(1):8–15. doi: 10.1097/PGP.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westin SN, Lacour RA, Urbauer DL, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol. 2008;26(36):5965–5971. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howitt BE, Shukla SA, Sholl LM, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1(9):1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 22.Pakish JB, Zhang Q, Chen Z, et al. Immune microenvironment in microsatellite-instable endometrial cancers: hereditary or sporadic origin matters. Clin Cancer Res. 2017 Mar 6; doi: 10.1158/1078-0432. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloan EA, Ring KL, Willis BC, et al. PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including Lynch syndrome-associated and MLH1 promoter hypermethylated tumors. Am J Surg Pathol. 2017;41(3):326–333. doi: 10.1097/PAS.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 24.Sloan EA, Moskaluk CA, Mills AM. Mucinous differentiation with tumor infiltrating lymphocytes is a feature of sporadically methylated endometrial carcinomas. Int J Gynecol Pathol. 2017;36(3):205–216. doi: 10.1097/PGP.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 25.Pallares J, Martinez-Guitarte JL, Dolcet X, et al. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J Pathol. 2004;204(5):569–577. doi: 10.1002/path.1666. [DOI] [PubMed] [Google Scholar]
- 26.Eso Y, Takai A, Matsumoto T, et al. MSH2 dysregulation is triggered by proinflammatory cytokine stimulation and is associated with liver cancer development. Cancer Res. 2016;76(15):4383–4393. doi: 10.1158/0008-5472.CAN-15-2926. [DOI] [PubMed] [Google Scholar]
- 27.Mizumoto Y, Kyo S, Kiyono T, et al. Activation of NF-kappaB is a novel target of KRAS-induced endometrial carcinogenesis. Clin Cancer Res. 2011;17(6):1341–1350. doi: 10.1158/1078-0432.CCR-10-2291. [DOI] [PubMed] [Google Scholar]
- 28.St-Germain ME, Gagnon V, Parent S, et al. Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-kappaB/IkappaB pathway. Mol Cancer. 2004;3:7. doi: 10.1186/1476-4598-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh JH, Kim JH, Ahn HJ, et al. Syndecan-1 enhances the endometrial cancer invasion by modulating matrix metalloproteinase-9 expression through nuclear factor kappaB. Gynecol Oncol. 2009;114(3):509–515. doi: 10.1016/j.ygyno.2009.05.027. [DOI] [PubMed] [Google Scholar]