Abstract
Objective
To determine if walking speed collected at 6 and 12 months following anterior cruciate ligament reconstruction (ACLR) associates with inter-limb differences in proteoglycan density, measured via T1ρ magnetic resonance imaging (MRI), in tibiofemoral articular cartilage 12 months following ACLR.
Methods
Twenty-one individuals with a unilateral patellar-tendon autograft ACLR (10 females, 11 males, 23.9±2.7 years, 23.9±2.7 BMI) were recruited for participation in this study. Walking speed was collected using 3-dimensional motion capture at 6 and 12 months following ACLR. The articular cartilage of the medial and lateral condyles of the femur (MFC and LFC) and tibia (MTC and LTC) was manually segmented and sub-sectioned into three regions of interest (Anterior, Central and Posterior) based upon the location of the meniscus in the sagittal plane. Inter-limb mean T1ρ relaxation time ratios (T1ρ-ACLR limb/T1ρ-contralateral limb) were calculated and used for analysis. Pearson product-moment correlations were used to determine associations between walking speed and inter-limb differences in T1ρ relaxation time ratios.
Results
Slower walking speed 6 months post-ACLR significantly associated with higher T1ρ relaxation time ratios in the medial femoral condyle of the ACLR limb 12 months following ACLR (Posterior-MFC, r=−0.51, P=0.02, Central-MFC, r=−0.47, P=0.04). Similarly, slower walking speed at 12 months post-ACLR significantly associated with higher T1ρ relaxation time ratios in the Posterior-MFC ACLR limb (r=−0.47, P=0.04) 12 months following ACLR.
Conclusion
Slower walking speed at 6 and 12 months following ACLR may be associated with early proteoglycan density changes in medial femoral compartment cartilage health in the first 12 months following ACLR.
Keywords: Posttraumatic Osteoarthritis, Gait, Proteoglycan Density, ACL Injury
Approximately 250,000 anterior cruciate ligament (ACL) injuries are sustained each year in the United States.(1, 2) Individuals who sustain an ACL injury often elect to undergo surgical reconstruction (ACLR) followed by 6-9 months of rehabilitation, with the goal of acutely stabilizing the knee and returning to physical activity.(3) However, these patients are at greater risk for developing post-traumatic knee osteoarthritis (PTOA).(4–6) Between 30-50% of individuals with ACLR develop PTOA within 10-15 years following injury.(5–7) Signs of altered cartilage composition, specifically diminished proteoglycan density, have been observed in these individuals as early as 1-2 years following ACL injury.(8–11) Radiographic imaging is typically used for detecting structural joint changes that are consistent with late stage osteoarthritis (OA).(12) However, radiographs are not sensitive to pre-morphologic changes in the articular cartilage.(12) T1ρ magnetic resonance imaging (MRI) relaxation times have been used to measure proteoglycan density of the articular cartilage.(13–15) Decreased proteoglycan density in tibiofemoral articular cartilage has been identified as an early compositional change consistent with the early development of knee OA.(15, 16) Lesser proteoglycan density has been detected using T1ρ MRI in tibiofemoral cartilage of the injured limb as early as one year following ACLR.(9–12) A comparison of the T1ρ MRI relaxation times between the ACLR and contralateral limbs may contribute to our understanding of the early signs of OA development in these individuals.
Walking speed is a simple performance outcome that clinicians can use to measure the functional status of individuals with knee OA(17) and predict likelihood for OA development in those without knee OA.(18, 19) Slower walking speed has been found to predict fall risk,(20) diminished performance in activities of daily living,(18, 21) and mortality in elderly individuals.(22, 23) Purser et al.(20) demonstrated that older healthy adults (60.07±9.86 years old) without knee OA who walked more slowly were more likely to develop knee OA over the following 6 years. In younger individuals with an ACLR (22.00±3.62 years old) slower walking speeds associated with serum markers of type-II collagen breakdown, which may be indicative of early changes in cartilage metabolism.(21) Aberrant joint loading following ACLR may contribute to accelerated cartilage breakdown and hasten the development of knee OA.(22) It is hypothesized that individuals who are at risk for developing knee OA slow their habitual walking speed in an effort to reduce the energy distributed through knee tissues.(23) Previous research(24) found that faster walking speed associated with higher peak vertical ground reaction force (vGRF) magnitudes and loading rates shortly following heel-strike during the first half of the stance phase of walking gait.(24) Together, these findings suggest that walking speed may associate with changes in articular cartilage homeostasis in individuals following ACLR. Therefore, the purpose of this study was to determine if walking speed at 6 and 12 months following ACLR associates with inter-limb differences in proteoglycan density in the tibiofemoral articular cartilage measured 12 months following ACLR via T1ρ MRI. We hypothesized that those with a slower walking speed at 6 and 12 months following ACLR would demonstrate lesser proteoglycan density in the injured limb relative to the contralateral limb 12 months following ACLR.
Materials and Methods
Participants
All participants underwent arthroscopic ACLR using a graft from the middle third of the patellar tendon as previously described.(21) Participants were excluded if they were pregnant or planned to become pregnant within 12 months of study enrollment. We also excluded participants who had any form of arthritis or needed a multi-ligament reconstruction. All individuals with a history of cochlear implant, clinical hypertension, claustrophobia, hepatic disease, diabetes, seizures, or cardiac pacemaker were excluded. Individuals with a history of a previous traumatic knee injury were also excluded. Biomechanical analyses were conducted during the 6 and 12 month follow-up exams after ACLR. Electronic mail and telephone communication were used to retain study participants and schedule 6 and 12 month follow-up visits. All participants provided informed consent to participate in the study, and the university’s Institutional Review Board approved all aspects of the study. Participants completed the KOOS Knee Survey during the 6 and 12 month follow-up following ACLR (Table 1) in order to determine the self-perceived function of our cohort. Each of the KOOS subscales (KOOS Pain, KOOS Symptoms, KOOS ADL, KOOS Sport, and KOOS QOL) have demonstrated acceptable construct validity and reliability (ICCs 0.75-0.96) compared to the Short Form-36 questionnaire in individuals with an ACLR.(27) We estimated needing 19 participants in order to detect a significant moderate association between walking speed and T1ρ MRI relaxation times (r=0.59) with an alpha level of 0.05 and a 1-β of 0.8, which is similar to the magnitude of the association that we found between biomechanical and biochemical measures in a previous study that examined a separate cohort of individuals with an ACLR.(25) We elected to recruit and evaluate outcomes in 21 participants in case multiple participants were found to be outliers during analysis.
Table 1.
Patient Demographics
| Participants | 10 males, 11 females |
|---|---|
| Age | 23.89 ± 2.70 years old |
| Height | 178.37 ± 11.49 cm |
| Weight | 76.34 ± 13.17 kg |
| BMI | 23.88 ± 2.70 kg/m2 |
| Days between ACL injury and ACLR | 32.35 ± 14.17 days |
| 6 month after ACLR Walking Speed | 1.28 ± 0.13 m/s |
| 12 month after ACLR Walking Speed | 1.24 ± 0.13 m/s |
| Months between surgery and 6 month follow-up | 6.83 ± 0.95 months |
| Months between surgery and 12 month follow-up | 12.33 ± 0.61 months |
| 6 month KOOS Symptoms | 76.2 ± 16.82 |
| 6 month KOOS Pain | 83 ± 13.79 |
| 6 month KOOS Activities of Daily Living | 92.8 ± 13.54 |
| 6 month KOOS Sports | 62.5 ± 20.74 |
| 6 month KOOS Quality of Life | 53.15 ± 18.24 |
| 12 month KOOS Symptoms | 82.42 ± 10.3 |
| 12 month KOOS Pain | 91.32 ± 8.37 |
| 12 month KOOS Activities of Daily Living | 96.74 ± 4.27 |
| 12 month KOOS Sports | 79.21 ± 16.85 |
| 12 month KOOS Quality of Life | 71.21 ± 19.97 |
ACL: Anterior Cruciate Ligament, ACLR: Anterior Cruciate Ligament Reconstruction
Data Collection Procedures
Walking Speed Analysis
All participants were outfitted with 25 retroreflective markers, along with a cluster of 3 additional markers that was secured over the sacrum.(21) Prior to collecting walking gait trials, a static trial was performed to create the segment-linkage model. Three-dimensional marker coordinates were sampled at 120 Hz using a 10-camera motion capture system (Vicon, Nexus), and all data were processed with the Vicon Nexus motion capture software. Participants walked over a 6-meter distance that included 2 embedded force-plates (40 × 60 cm, FP406010, Bertec Corporation), sampled at 1200 Hz, positioned in a staggered formation, which allowed for the entire stance phase to be collected from both limbs during a single trial. All participants struck the first force-plate with their right foot and the second force-plate with their left foot in order to standardize order of limb contact across all participants. To eliminate any bias from multiple types of footwear, all participants walked barefoot and were instructed to walk at a “normal comfortable walking speed as if they were normally walking down the sidewalk” for all trials. Participants completed an acclimatization period during which they practiced the walking protocol in the motion capture area while being outfitted with the retroreflective markers and barefoot. Once the participants vocalized that they felt comfortable performing the protocol, all participants performed 5 practice walking trials in which walking speed was assessed via infrared timing gates (TF100, TracTronix) and averaged in order to maximize consistency in subsequent testing trials. During data collection, participants performed 5 gait trials for which they were required to: 1) individually strike an individual force plate with each foot; 2) maintain walking speed within ±5% of the average speed during practice trials; and 3) not undergo any visible alterations to gait during the trial (e.g., trip or stutter step).(20, 24) Trajectories form the anterior superior iliac spine and sacral cluster markers were low-pass filtered at 10 Hz using a 4th order recurrent Butterworth filter and used to estimate the pelvic center of mass.(26) To more accurately determine habitual walking speed for data analysis, we located the point of initial ground contact for the injured limb striking the force plate during each gait trial and measured the velocity of the pelvis center of mass during a 1-meter distance that began 0.5 meter prior to initial ground contact and ended 0.5 meter after initial ground contact.(20)
Magnetic Resonance Image Acquisition
T1 MRI outcomes were acquired with a Siemens Magnetom TIM Trio 3T scanner using a 4-channel Siemens large flex coil (516 mm × 224 mm, Siemens, Munich, Germany) for 18 of the 21 participants. Due to a MRI systems upgrade in our Biomedical Research Imaging Center, the 12 month T1ρ MRI outcomes for 3 of the 21 participants were acquired using a Siemens Magnetom Prisma 3T PowerPack scanner with a XR 80/200 gradient coil (60 cm × 213 cm, Siemens, Munich, Germany). Inter-scanner reliability was assessed in 6 knees using intra-class correlation coefficients (ICC), which were found to be within an acceptable range (ICC≥0.75) for all regions of interest. Additionally, all T1ρ relaxation times in the ACLR limb were normalized to the regions of interest in the uninjured contralateral limb for each participant thereby minimizing any effect from utilizing multiple MRI scanners. Prior to acquiring MR images, participants remained seated for 30 minutes to unload the knee cartilage.(27) We used a T1ρ prepared three-dimensional Fast Low Angle Shot (FLASH) with a spin-lock power at 500Hz, five different spin-lock durations (40, 30, 20,10, 0 ms) and a voxel size of 0.8mm × 0.4mm × 3mm (field of view= 288mm, slice thickness=3.0mm, TR= 9.2ms, 160 × 320 matrix, gap= 0mm, flip angle=10°, echo-train duration time= 443ms, phase encode direction of anterior/posterior).
Segmentation of the Articular Cartilage
The articular cartilage in the medial and lateral condyles of the femur and tibia was manually segmented using ITK-SNAP software(28) with a T1ρ MRI image that was acquired during the 0 ms spin-lock duration, which has been found to be reliable in our laboratory for all regions of interest (intra-segmentor reliability, N=8, ICC range 0.80 - 0.97; inter-segmentor reliability, N=10 ICC range 0.75 - 0.98). A fellowship-trained musculoskeletal radiologist (DN) confirmed the anatomical accuracy of the segmentations. The medial and lateral femoral condyles (MFC and LFC) and tibial condyles (MTC and LTC) were further sub-sectioned into three regions of interest, based upon the location of the meniscus in the sagittal plane.(9) The three regions of interest that were sub-sectioned represent load-bearing regions and included: 1) the cartilage that corresponds with the anterior horn of the meniscus (Anterior-MFC/LFC & Anterior-MTC/LTC); 2) the central portion of the cartilage that lies between the anterior and posterior meniscus (Central-MFC/LFC & Central-MTC/LTC; and 3) the cartilage corresponding with the posterior horn of the meniscus (Posterior-MFC/LFC & Central-MTC/LTC, Figure 1). Volumes for each region of interest (ROI) were determined for both limbs using the ITK-Snap software(28) and were used to account for potential cartilage volume differences between limbs.
Figure 1.
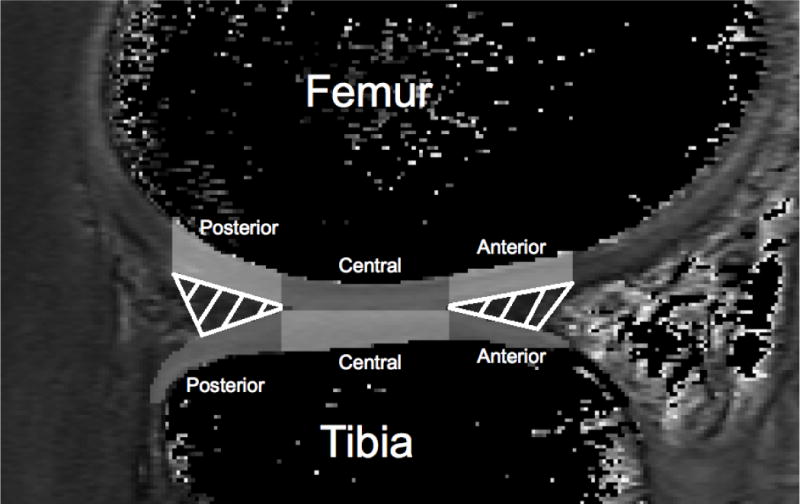
The weight-bearing articular cartilage of the femur and tibia were segmented and sub-sectioned into three different sections (Posterior, Central, and Anterior) that corresponded to the area articulating with the meniscus in the sagittal plane.
T1ρ Relaxation Time Quantification
Voxel by voxel T1ρ relaxation times were calculated using a five image sequence created with a MatLab program (MatLab R2014b [8.4.0] MathWorks, Natick, MA, USA) with the following equation: S(TSL) = S0 exp(-TSL/T1ρ).(9) In this equation TSL is the duration of the spin-lock time, S0 is signal intensity when TSL equals zero, S corresponds to signal intensity, and T1ρ is the T1 relaxation time in the rotating frame. The segmented T1-weighted MRI image was transposed over the T1ρ image to establish T1ρ relaxation times for each ROI. Mean T1ρ values for each ROI were extracted using the ITK-SNAP software. Higher T1ρ relaxation times are indicative of lower proteoglycan density.(9) As others have described,(29) we calculated similar inter-limb mean T1ρ relaxation time ratios that were normalized to the T1ρ relaxation time for the ROI of the ACLR limb to the same ROI in the contralateral limb (Inter-limb mean T1ρ relaxation time ratio = ACLR limb/contralateral limb). This provides a value for each participant that describes the percent difference in proteoglycan density of specific regions of interest in the ACLR limb compared to the same ROI in the contralateral limb (i.e. ratio of 1.10 equates to 10% greater mean relaxation time of the ROI in the ACLR limb compared to the contralateral limb). Inter-limb mean T1ρ relaxation time ratios with a value greater than 1.00 indicated lower proteoglycan density in the ROI for the ACLR limb compared to the contralateral limb.
Statistical Analyses
Descriptive data were calculated for participant demographics (Table 1) as well as inter-limb T1ρ relaxation time ratios (Table 2). Dependent t-tests were used to determine if differences existed for walking speed between the 6 and 12 month assessments, and to compare cartilage volume and T1ρ relaxation times in each ROI between the ACLR and contralateral limbs (Table 2). Data distributions for walking speed and inter-limb T1ρ relaxation time ratios were assessed using Shapiro-Wilk’s test for normality and stem and leaf plots. Data points that were greater than 2.5 SD from the mean were identified as outliers, which we planned to remove prior to the final analysis. Pearson product-moment correlations were used to determine associations between 6 month and 12 month walking speed and inter-limb T1ρ relaxation time ratios for each ROI in the medial and lateral compartments of the femur and tibia. Next, we conducted partial correlations between walking speed and inter-limb T1ρ relaxation time ratios while accounting for differences in the cartilage volumes between limbs for all regions of interest (ACLR limb ROI volume/contralateral limb ROI volume). Associations were classified as negligible (0.0-0.3), low (.31-0.5), moderate (0.51- 0.7), high (0.71- 0.9), and very high (0.9-1.0).(30) Due to the preliminary nature of the analysis, we chose to interpret significant associations at an alpha level of 0.05 for all correlations but have provided the 95% confidence intervals for each correlation coefficient (Table 3) in order to demonstrate the variability in addition to the magnitude for each bivariate or partial correlation. Owing to the preliminary nature of the study, the two-tailed alpha level was set a priori as α≤0.05 for all correlations. All statistics were performed using the Statistical Package for the Social Sciences software (SPSS, Version 21.0, IBM Corp., Somers, NY).
Table 2.
12 month mean T1ρ relaxation times and volumes.
| T1ρ Volume (mm3) | Mean T1ρ Relaxation Time (ms) | Mean Inter-limb T1ρ Relaxation Time Ratio | |||||
|---|---|---|---|---|---|---|---|
| Region of Interest | ACLR Limb | Uninjured Limb | p-value | ACLR Limb | Uninjured Limb | p-value | (T1ρ-ACLR Limb/T1ρ-Uninjured Limb) |
| Medial Posterior Femoral | 1364± 626 | 1413 ± 639 | 0.57 | 53 ± 5 | 51 ± 3 | 0.02* | 1.04 ± 0.05 |
| Medial Central Femoral | 1581 ± 733 | 1534 ± 590 | 0.67 | 54 ± 5 | 48 ± 3 | 0.01* | 1.11 ± 0.06 |
| Medial Anterior Femoral | 762 ± 390 | 766 ± 284 | 0.94 | 51 ± 6 | 50 ± 3 | 0.01* | 1.13 ± 0.12 |
| Lateral Posterior Femoral | 988 ± 508 | 1021 ± 374 | 0.75 | 59 ± 7 | 53 ± 7 | 0.01* | 1.13 ± 0.15 |
| Lateral Central Femoral | 1240 ± 501 | 1212 ± 437 | 0.79 | 53 ± 5 | 47 ± 5 | 0.01* | 1.14 ± 0.13 |
| Lateral Anterior Femoral | 832 ± 388 | 821 ± 342 | 0.88 | 50 ± 6 | 43 ± 4 | 0.01* | 1.13 ± 0.14 |
| Medial Posterior Tibial | 1219 ± 549 | 1424 ± 518 | 0.03* | 47 ± 4 | 46 ± 4 | 0.41 | 1.01 ± 0.08 |
| Medial Central Tibial | 1713 ± 613 | 1689 ± 525 | 0.83 | 48 ± 4 | 45 ± 4 | 0.01* | 1.05 ± 0.07 |
| Medial Anterior Tibial | 458 ± 272 | 484 ± 241 | 0.50 | 54 ± 9 | 50 ± 4 | 0.03* | 1.09 ± 0.19 |
| Lateral Posterior Tibial | 1246 ± 573 | 1461 ± 509 | 0.02* | 52 ± 4 | 48 ± 5 | 0.01* | 1.09 ± 0.11 |
| Lateral Central Tibial | 1852 ± 624 | 1783 ± 545 | 0.56 | 44 ± 4 | 43 ± 4 | 0.25 | 1.03 ± 0.11 |
| Lateral Anterior Tibial | 765 ± 579 | 803 ± 385 | 0.74 | 53 ± 14 | 54 ± 7 | 0.71 | 0.98 ± 0.26 |
ACLR=Anterior Cruciate Ligament Reconstructed,
indicates significance (P<0.05)
Table 3.
Bivariate and partial associations between inter-limb T1ρ relaxation time ratios and 6 and 12 month walking speed 12 months following anterior cruciate ligament reconstruction.
| Inter-limb T1ρ Relaxation Time ROI Ratio | 6 Month Walking Speed r (95% CI’s for correlation) |
12 Month Walking Speed r (95% CI’s for correlation) |
6 Month Walking Speed (partial correlation controlling for ROI volume differences) r (95% CI’s for correlation) |
12 Month Walking speed (partial correlation controlling for ROI volume differences) r (95% CI’s for correlation) |
|---|---|---|---|---|
| Medial Posterior Femoral | −0.45** (−0.93, −0.03) |
−0.36 (−0.83, 0.07) |
−0.49** (−1.0, −0.08) |
−0.47** (−0.97, −0.05) |
| Medial Central Femoral | −0.45** (−0.93, −0.03) |
−0.34 (−0.80, 0.10) |
−0.47** (−0.97, −0.05) |
−0.37 (−0.85, 0.07) |
| Medial Anterior Femoral | 0.15 (−0.30, 0.60) |
−0.02 (−0.47, 0.43) |
0.20 (−0.26, 0.66) |
0.01 (−0.45, 0.47) |
| Lateral Posterior Femoral | 0.02 (−0.43, 0.50) |
0.14 (−0.31, 0.59) |
−0.05 (−0.51, 0.51) |
0.07 (−0.39, 0.53) |
| Lateral Central Femoral | −0.03 (−0.48, 0.46) |
−0.13, (−0.58, 0.32) |
−0.16 (−0.62, 0.30) |
−0.18 (−0.64, 0.28) |
| Lateral Anterior Femoral | 0.21 (−0.24, 0.66) |
−0.29 (−0.75, 0.15) |
0.13 (−0.33, 0.59) |
−0.26 (−0.73, 0.19) |
| Medial Posterior Tibial | −0.11 (−0.56, 0.34) |
−0.20 (−0.65, 0.25) |
−0.07 (−0.53, 0.39) |
−0.17 (−0.63, 0.29) |
| Medial Central Tibial | −0.08 (−0.53, 0.37) |
0.11 (−0.34, 0.56) |
−0.06 (−0.52, 0.40) |
0.12 (−0.34, 0.58) |
| Medial Anterior Tibial | 0.01 (−0.48, 0.46) |
0.07 (−0.38, 0.52) |
0.06 (−0.40, 0.52) |
0.10 (−0.36, 0.56) |
| Lateral Posterior Tibial | −0.03 (−0.48, 0.46) |
0.39 (−0.04, 0.86) |
−0.03 (−0.49, 0.43) |
0.42 (−0.01, 0.91) |
| Lateral Central Tibial | −0.28 (−0.74, 0.16) |
0.12 (−0.33, 0.57) |
−0.29 (−0.76, 0.16) |
0.12 (−0.34, 0.58) |
| Lateral Anterior Tibial | −0.29 (−0.15, 0.15) |
−0.27 (−0.73, 0.17) |
−0.20 (−0.66, 0.26) |
−0.28 (−0.75, 0.17) |
indicates significance (P<0.05), ROI: Region of Interest, CI:Confidence Interval
Post Hoc Analyses
In order to determine whether pain during the walking speed acquisition had a confounding effect on the associations between walking speed and inter-limb mean relaxation time ratios, the KOOS Pain subscale scores were used in a post hoc analysis. We performed additional partial correlations between walking speed and inter-limb mean relaxation time ratios while controlling for the KOOS Pain subscale scores.
Results
Demographics
Only 7 of the 21 (33%) participants sustained an isolated ACL injury while the majority (n=14, 67%) presented with a concomitant knee injury that was sustained at the time of the ACL injury (medial meniscus injury [n=2, 9.5%], lateral meniscus injury [n=14, 67%], chondral injury [n=4, 19%]). ROI T1ρ volumes were lesser in the ACLR limb of the Posterior-LTC (P=0.022) and Posterior-MTC (P=0.031, Table 2) regions of interest. Mean T1ρ relaxation times were greater in the ACLR limb compared to the contralateral knee for all regions of interest except the Central-LTC (P=0.249), Anterior-LTC (P=0.708), and Posterior-MTC (P=0.404; Table 2). Walking speed was not significantly different between the 6 month (1.28±0.13 m/s) and 12 month time points (1.24±0.13 m/s; P=0.10). No outliers were found during the evaluation for normality.
Association between Walking Speed and Proteoglycan Density
Slower walking speed at 6 months following ACLR significantly associated with higher 12 month inter-limb mean T1ρ relaxation time ratios of regions of interest on the medial femoral condyle (Posterior-MFC r=−0.448, P=0.027, Central-MFC r=−0.448, P=0.042, Table 3, Figure 2). After accounting for inter-limb differences in volume we found similar significant associations in the same regions of interest (Posterior-MFC r=−0.494, P=0.041; Central-MFC r=−0.467, P=0.038). The remainder of the bivariate associations between 6 month walking speed and all other regions of interest for the femoral and tibial condyles were negligible and were not statistically significant (Table 3). Slower walking speed at 12 months following ACLR did not significantly associate with higher 12 month inter-limb mean T1ρ relaxation time ratios of any of the regions of interest. However, after accounting for inter-limb differences in volume we found that those who walked more slowly at 12 months following ACLR significantly associated with 12 month inter-limb mean T1ρ relaxation time ratio for Posterior-MFC (r=−0.471, P=0.036, Figure 3). No other associations between 12 month walking speed and the inter-limb T1ρ relaxation time ratio were significant (Table 3).
Figure 2.
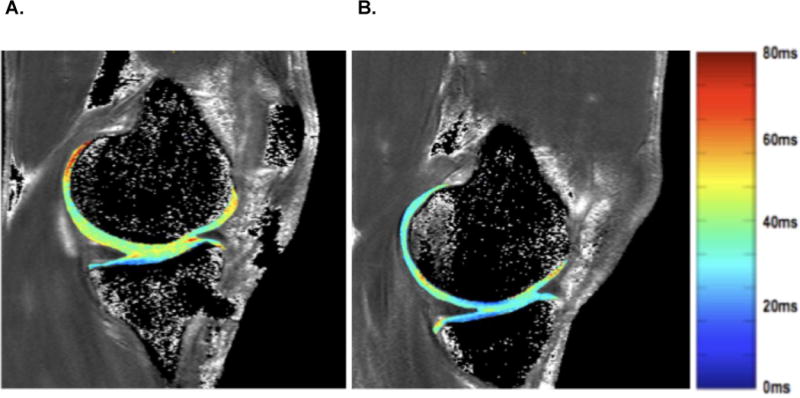
Represents a sample map of T1ρ relaxation times of an anterior cruciate ligament reconstructed (ACLR) knee (1A) and contralateral knee (1B). Figure 1A depicts the articular cartilage from an ACLR knee with greater T1ρ relaxation times compared to the contralateral uninjured knee (1B). The color of the cartilage corresponds with a specific T1ρ relaxation time value (ms) that is outlined in the color scale.
Figure 3.
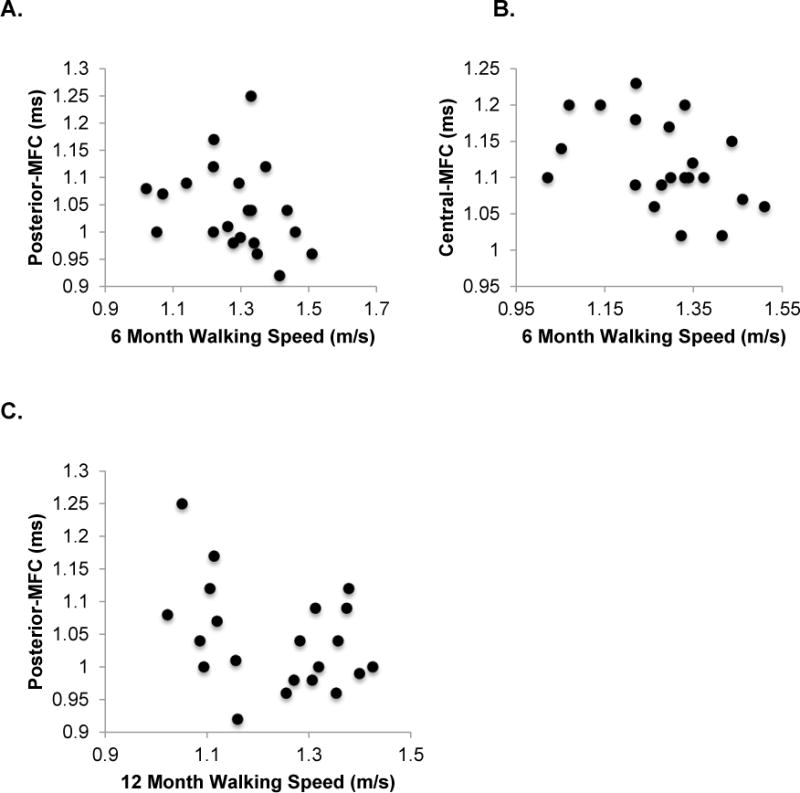
Scatter-plots depicting significant associations between 6 (2A & 2B) and 12 (2C) month walking speed (x-axis) and 12 month inter-limb T1ρ relaxation time ratios (y-axis). Slower walking speed at 6 months following ACLR significantly associated with higher 12 month inter-limb mean T1ρ relaxation time ratios of regions of interest in the articular cartilage of the posterior medial femoral condyle (MFC; r=−0.448, P=0.027; 2A) and central MFC (r=−0.448, P=0.042; 2B). Those who walked slower at 12 months following ACLR significantly associated with 12 month inter-limb mean T1ρ relaxation time ratio for posterior MFC (r=−0.471, P=0.036, 2C).
Post Hoc Analysis
Scores for each KOOS subscale from our cohort were within a previously established range of KOOS scores at 6 months following ACLR in other cohorts.(31, 32) After controlling for 6 month post-ACLR KOOS Pain subscale scores, the significant associations observed between 6 month walking speed and 12 month inter-limb mean T1ρ relaxation time ratio for Posterior-MFC (r=−.481, P=0.031) and Central-MFC (r=−.454, P=0.05) remained significant. Similarly, the significant association observed between 12 month walking speed and 12 month inter-limb T1ρ relaxation time ratio in the Posterior-MFC (r=−.438, P=0.042) remained after controlling for 12-month post-ACLR KOOS Pain subscale scores.
Discussion
Individuals with slower walking speeds at 6 months following ACLR demonstrated greater 12 month inter-limb mean T1ρ relaxation time ratios, interpreted as lesser proteoglycan density, in the articular cartilage of the medial femoral condyle of the ACLR knee relative to the uninjured knee. Similarly, slower 12 month walking speed associated with greater inter-limb mean T1ρ relaxation time ratios in the posterior-MFC ROI of the medial compartment in the ACLR limb compared to the uninjured knee, after accounting for inter-limb differences in cartilage volume, at the 12 month follow-up. While significant associations were found between walking speed and measures of cartilage composition, the strength of the associations in this study would be classified as “low.” Therefore, the majority of variance in altered proteoglycan density is explained by factors other than walking speed which may include altered tissue metabolism(33) and water content(13) in the cartilage. We are cautious about concluding that these associations are robust enough to allow for walking speed to take the place of more sophisticated means of assessing changes in cartilage composition. Walking speed may be best used as part of a future comprehensive evaluation strategy that uses functional and self-reported outcomes, biochemical measures and imaging techniques to assess the change in cartilage composition. This is the first study to investigate the association between walking speed and compositional measures of cartilage health at early time points following ACLR. While our study may be preliminary, the knowledge of the association between slower walking speeds and lesser proteoglycan density may assist in detecting individuals with deleterious changes in cartilage composition following ACLR.
The mechanisms influencing walking speed and OA onset are not fully understood. Walking speed has previously been found to detect functional limitations in individuals with diagnosed radiographic knee OA(17) and is a useful clinical predictor of idiopathic knee OA onset in older individuals.(20) Previous studies(24, 34) have hypothesized that individuals with knee OA slow their habitual walking speed in order to reduce impulsive loading during gait. Higher loading rates have been observed in the ACLR limb compared to the uninjured limb and uninjured control subjects.(35, 36) An alternate explanation for the association between slower walking and decreased cartilage health is that walking slower following injury insufficiently loads joint tissues and negatively influences cartilage health. Insufficient loading has been observed more commonly in individuals with an ACLR who developed PTOA at a 5-year follow up examination.(37) Slower walking speed may influence walking kinematics and lead to insufficient loading of the articular cartilage, thus accelerating the development of PTOA.(38, 39) Future research is needed to determine the causal nature of the relationship between walking speed and markers of cartilage composition following ACLR.
Individuals who sustain an ACL injury and undergo ACLR are at a greater risk for developing knee PTOA compared to individuals without a history of a lower extremity injury.(5, 6) Unfortunately, there are limited clinical methods for predicting early PTOA onset following ACLR. A recent study found that individuals with ACLR (43±36 months post-ACLR) who demonstrated slower walking speeds had greater serum concentrations of type-II collagen breakdown.(21) Greater collagen breakdown may be linked to more deterioration of the cartilage matrix and may be an early indicator of PTOA. Similarly, the findings of the current study demonstrate that slower walking speed within the first 6 months following ACLR associates with lesser proteoglycan density on the posterior and central regions of the medial femoral condyle at the 12 month follow-up. We are cautious about concluding the presence of a robust association between 12 month walking speed and medial compartment articular cartilage proteoglycan density at 12 months, as there was only a single ROI (posterior-MFC) demonstrating a significant association after accounting for inter-limb differences in cartilage volume.
Significant associations were found between slower walking speed and lower proteoglycan density in medial weight bearing regions of the femur. The development of knee OA in the medial tibiofemoral compartment is common and has been linked to aberrant medial compartment loading in those with idiopathic phenotypes of knee OA.(40, 41) Individuals with knee OA who walk more slowly demonstrate greater peak knee adduction moments (KAM) compared to healthy controls.(42, 43) It remains unknown if the slower walking speeds influence the development of greater KAM or if slower walking speeds develop due to greater KAM. Greater KAM also leads to greater mechanical loading to the articular cartilage of the medial compartment of the knee joint following ACLR.(43, 44) In a cohort of healthy individuals who walked under different speed conditions (i.e. slow, self-selected, fast), slower walking speeds led to higher KAM when compared to self-selected and faster walking speeds.(45) This exposure to repetitive compressive force with greater magnitudes is theorized to be one factor driving the high prevalence of OA observed in the medial tibiofemoral compartment, compared to the lateral.(43, 45) A recent study(44) identified KAM as a significant predictor of medial compartment contact forces at an early time point (<7 months) in individuals after an ACLR. Similarly, previous research has reported that following an ACLR, some patients have an increased knee flexion angle at heel strike during walking.(46) An increased knee flexion angle at heel strike may lead to a posterior shift of contact force in the tibiofemoral joint, thereby distributing load through portions of cartilage in the posterior compartment that is not conditioned to handle these loads. Slower walking speed, combined with increased knee flexion angle and KAM, may lead to portions of the cartilage on the medial femoral condyle being subject to increased loads over a greater period of time, thereby accelerating the degenerative process and PTOA development.
While the findings of this study provide valuable information that may further the development of walking speed as a potential clinical indicator of early changes in cartilage health following injury and PTOA risk, there were limitations to this study that may inform future analyses. Our study was preliminary in nature with a relatively small sample size (n=21), limiting our ability to evaluate patient sub-groups. Additionally, the significant associations found between walking speed and cartilage composition were only found in two ROI’s in our analysis of both femoral and tibial condyles and the magnitudes of these associations were classified as “low.” Long-term follow-up exams are needed to determine if proteoglycan density changes in these ROI lead to PTOA onset. Future studies may seek to increase the sample size to determine mechanisms that may further explain differences between 6 and 12 month walking speeds and the relationship to 12 month proteoglycan density. Evaluation of a larger sample size will build on our study by determining the effects of specific covariates, which could also increase PTOA risk (i.e. sex, concomitant meniscal injury, and chondral injury), on the associations between walking speed and cartilage health following ACLR. All participants had a unilateral ACLR, which improved the control in this initial study. Further research should determine what associations exist between walking speed and proteoglycan density in those with multiple injuries. This preliminary analysis was part of a larger study that was designed to capture specific outcomes related to heelstrike biomechanics. Therefore, the participants in this current study performed all walking trials barefoot in order to eliminate any bias of footwear; however, different shoed conditions may affect habitual walking speed, which should be accounted for in future studies. Additionally, pain was not assessed at the time of the walking trials which is an assessment that could be added to future studies. Our collection distance for walking speed was relatively short (1 meter). Future studies should also examine whether collecting walking speed over greater distances (20 or 40 meters) would influence associations with cartilage health. Additionally, pain was not assessed at the time of the walking trials. This current study also focused primarily on habitual walking speed and its relationship to articular cartilage composition. A further analysis determining the relationship between specific kinetic and kinematic variables and cartilage composition in these individuals may allow for more effective diagnosis and treatment of PTOA in individuals with an ACLR.
In conclusion, individuals who walked slower at 6 months following ACLR demonstrated greater inter-limb mean T1ρ relaxation time ratios, which can be interpreted as lesser proteoglycan density in the posterior and central regions of the medial femoral articular cartilage in the ACLR limb relative to the contralateral limb, at 12 months following ACLR. These alterations may be indicative of early changes in cartilage composition that may be associated with early changes in joint homeostasis that influence the development of future PTOA. The current study was preliminary in nature and further research is needed to determine if walking speed may be used as part of a more comprehensive evaluation strategy to clinically assess deleterious changes in cartilage health in a relatively younger cohort of patients at risk of developing PTOA.
Significance and Innovations.
Individuals with an anterior cruciate ligament reconstruction (ACLR), identified as being at a greater risk of developing post-traumatic knee osteoarthritis, who demonstrate slower habitual walking speed at 6 and 12 months following ACLR were found to have greater T1ρ relaxation times in the medial femoral condyle of the ACLR limb compared to their uninjured limb at 12 months following ACLR.
This is the first study to identify an association between walking speed and T1ρ relaxation times, which is a marker of proteoglycan density in articular cartilage, following ACLR.
Walking speed has the potential to serve as a simple, feasible clinical measure to assist in the assessment of risk for post-traumatic osteoarthritis development following ACLR.
Acknowledgments
ROLE OF FUNDING SOURCE: Research reported in this original manuscript was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (1R03AR066840-01A1), North Carolina Translational and Clinical Sciences (TRaCS) Institute, and National Athletic Trainers Association Research and Education Foundation (#14NewInv001).
References
- 1.Flynn RK, Pedersen CL, Birmingham TB, Kirkley A, Jackowski D, Fowler PJ. The familial predisposition toward tearing the anterior cruciate ligament: a case control study. The American journal of sports medicine. 2005;33(1):23–8. doi: 10.1177/0363546504265678. [DOI] [PubMed] [Google Scholar]
- 2.Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. The Journal of bone and joint surgery American volume. 1997;79(10):1556–76. doi: 10.2106/00004623-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Beynnon BD, Johnson RJ, Naud S, Fleming BC, Abate JA, Brattbakk B, et al. Accelerated versus nonaccelerated rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind investigation evaluating knee joint laxity using roentgen stereophotogrammetric analysis. The American journal of sports medicine. 2011;39(12):2536–48. doi: 10.1177/0363546511422349. [DOI] [PubMed] [Google Scholar]
- 4.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. British journal of sports medicine. 2011;45(7):596–606. doi: 10.1136/bjsm.2010.076364. [DOI] [PubMed] [Google Scholar]
- 5.Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. Journal of athletic training. 2014;49(6):806–19. doi: 10.4085/1062-6050-49.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, Engebretsen L, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. The American journal of sports medicine. 2010;38(11):2201–10. doi: 10.1177/0363546510373876. [DOI] [PubMed] [Google Scholar]
- 7.Roemer FW, Jarraya M, Niu J, Silva JR, Frobell R, Guermazi A. Increased risk for radiographic osteoarthritis features in young active athletes: a cross-sectional matched case-control study. Osteoarthritis Cartilage. 2015;23(2):239–43. doi: 10.1016/j.joca.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis and rheumatism. 2004;50(10):3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 9.Theologis AA, Haughom B, Liang F, Zhang Y, Majumdar S, Link TM, et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2014;22(2):298–307. doi: 10.1007/s00167-013-2397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theologis AA, Kuo D, Cheng J, Bolbos RI, Carballido-Gamio J, Ma CB, et al. Evaluation of bone bruises and associated cartilage in anterior cruciate ligament-injured and -reconstructed knees using quantitative t(1rho) magnetic resonance imaging: 1-year cohort study. Arthroscopy. 2011;27(1):65–76. doi: 10.1016/j.arthro.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2–initial experience with 1-year follow-up. Radiology. 2011;258(2):505–14. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage. 2013;21(8):1058–67. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Academic radiology. 2002;9(12):1388–94. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 14.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. Journal of magnetic resonance imaging: JMRI. 2006;23(4):547–53. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 15.Hatcher CC, Collins AT, Kim SY, Michel LC, Mostertz WC, 3rd, Ziemian SN, et al. Relationship between T1rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. Journal of biomechanics. 2017;55:18–26. doi: 10.1016/j.jbiomech.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43(11):782–8. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(8):1042–52. doi: 10.1016/j.joca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.White DK, Zhang Y, Niu J, Keysor JJ, Nevitt MC, Lewis CE, et al. Do worsening knee radiographs mean greater chances of severe functional limitation? Arthritis Care Res (Hoboken) 2010;62(10):1433–9. doi: 10.1002/acr.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzog MM, Driban JB, Cattano NM, Cameron KL, Tourville TW, Marshall SW, et al. Risk of Knee Osteoarthritis Over 24 Months in Individuals Who Decrease Walking Speed During a 12-Month Period: Data from the Osteoarthritis Initiative. J Rheumatol. 2017;44(8):1265–70. doi: 10.3899/jrheum.170093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purser JL, Golightly YM, Feng Q, Helmick CG, Renner JB, Jordan JM. Association of slower walking speed with incident knee osteoarthritis-related outcomes. Arthritis Care Res (Hoboken) 2012;64(7):1028–35. doi: 10.1002/acr.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrosimone B, Troy Blackburn J, Harkey MS, Luc BA, Hackney AC, Padua DA, et al. Walking Speed As a Potential Indicator of Cartilage Breakdown Following Anterior Cruciate Ligament Reconstruction. Arthritis Care Res (Hoboken) 2016;68(6):793–800. doi: 10.1002/acr.22773. [DOI] [PubMed] [Google Scholar]
- 22.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis and rheumatism. 1991;34(12):1560–70. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 23.Zeni JA, Jr, Higginson JS. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: a result of altered walking speed? Clinical biomechanics. 2009;24(4):372–8. doi: 10.1016/j.clinbiomech.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung MJ, Wang MJ. The change of gait parameters during walking at different percentage of preferred walking speed for healthy adults aged 20-60 years. Gait & posture. 2010;31(1):131–5. doi: 10.1016/j.gaitpost.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Pietrosimone B, Blackburn JT, Harkey MS, Luc BA, Hackney AC, Padua DA, et al. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. The American journal of sports medicine. 2016;44(2):425–32. doi: 10.1177/0363546515618380. [DOI] [PubMed] [Google Scholar]
- 26.Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics six-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017 doi: 10.1002/jor.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souza RB, Stehling C, Wyman BT, Hellio Le Graverand MP, Li X, Link TM, et al. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010;18(12):1557–63. doi: 10.1016/j.joca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Su F, Pedoia V, Teng HL, Kretzschmar M, Lau BC, McCulloch CE, et al. The association between MR T1rho and T2 of cartilage and patient-reported outcomes after ACL injury and reconstruction. Osteoarthritis Cartilage. 2016;24(7):1180–9. doi: 10.1016/j.joca.2016.01.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 31.Ingelsrud LH, Granan LP, Terwee CB, Engebretsen L, Roos EM. Proportion of Patients Reporting Acceptable Symptoms or Treatment Failure and Their Associated KOOS Values at 6 to 24 Months After Anterior Cruciate Ligament Reconstruction: A Study From the Norwegian Knee Ligament Registry. The American journal of sports medicine. 2015;43(8):1902–7. doi: 10.1177/0363546515584041. [DOI] [PubMed] [Google Scholar]
- 32.Lefevre N, Klouche S, Mirouse G, Herman S, Gerometta A, Bohu Y. Return to Sport After Primary and Revision Anterior Cruciate Ligament Reconstruction: A Prospective Comparative Study of 552 Patients From the FAST Cohort. The American journal of sports medicine. 2017;45(1):34–41. doi: 10.1177/0363546516660075. [DOI] [PubMed] [Google Scholar]
- 33.Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyere O, Chapurlat R, et al. Republished: Value of biomarkers in osteoarthritis: current status and perspectives. Postgrad Med J. 2014;90(1061):171–8. doi: 10.1136/postgradmedj-2013-203726rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu MC, Wang MJ. The effect of gait speed and gender on perceived exertion, muscle activity, joint motion of lower extremity, ground reaction force and heart rate during normal walking. Gait & posture. 2007;25(3):385–92. doi: 10.1016/j.gaitpost.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn JT, Pietrosimone B, Harkey MS, Luc BA, Pamukoff DN. Inter-limb differences in impulsive loading following anterior cruciate ligament reconstruction in females. Journal of biomechanics. 2016 doi: 10.1016/j.jbiomech.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Noehren B, Wilson H, Miller C, Lattermann C. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Medicine and science in sports and exercise. 2013;45(7):1340–7. doi: 10.1249/MSS.0b013e318285c6b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–57. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 38.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. The American journal of sports medicine. 2016;44(1):143–51. doi: 10.1177/0363546515608475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxby DJ, Bryant AL, Modenese L, Gerus P, Killen BA, Konrath J, et al. Tibiofemoral Contact Forces in the Anterior Cruciate Ligament-Reconstructed Knee. Medicine and science in sports and exercise. 2016;48(11):2195–206. doi: 10.1249/MSS.0000000000001021. [DOI] [PubMed] [Google Scholar]
- 40.Leong DJ, Li YH, Gu XI, Sun L, Zhou Z, Nasser P, et al. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011;25(1):182–91. doi: 10.1096/fj.10-164277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. The American journal of sports medicine. 2014;42(5):1049–57. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 42.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. British journal of sports medicine. 2009;43(5):366–70. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 43.Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(7):573–9. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 44.Webster KE, McClelland JA, Palazzolo SE, Santamaria LJ, Feller JA. Gender differences in the knee adduction moment after anterior cruciate ligament reconstruction surgery. British journal of sports medicine. 2012;46(5):355–9. doi: 10.1136/bjsm.2010.080770. [DOI] [PubMed] [Google Scholar]
- 45.Wellsandt E, Khandha A, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Predictors of knee joint loading after anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(3):651–6. doi: 10.1002/jor.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robbins SM, Maly MR. The effect of gait speed on the knee adduction moment depends on waveform summary measures. Gait & posture. 2009;30(4):543–6. doi: 10.1016/j.gaitpost.2009.08.236. [DOI] [PubMed] [Google Scholar]


