Abstract
Background and Purpose
Estradiol is a sex steroid hormone known to protect the brain against damage related to transient and global cerebral ischemia. In the present study, we leverage an experimental murine model of bilateral carotid artery stenosis (BCAS) to examine the putative effects of estradiol therapy on chronic cerebral hypoperfusion. We hypothesize that long-term estradiol therapy protects against white matter injury and declarative memory deficits associated with chronic cerebral hypoperfusion.
Methods
Adult male C57BL/6J mice underwent either surgical bilateral carotid artery stenosis (BCAS) or sham procedures. Two days after surgery the mice were given oral estradiol (Sham−E; BCAS−E) or placebo (Sham−P; BCAS−P) treatments daily for 31–34 days. All mice underwent Novel Object Recognition (NOR) testing 31–34 days after the start of oral treatments. Following sacrifice, blood was collected and brains fixed, sliced, and prepared for histological examination of white matter injury and ERK expression.
Results
Animals receiving long-term oral estradiol therapy (BCAS−E2 and Sham−E2) had higher plasma estradiol levels than those receiving placebo treatment (BCAS−P and Sham−P). BCAS−E2 mice demonstrated less white matter injury (Kluver-Barrera staining) and performed better on the NOR task compared to BCAS−P mice. ERK expression in the brain was increased in the BCAS compared to shams cohorts. Among the BCAS mice, the BCAS−E2 cohort had a greater number of ERK+ cells.
Conclusion
This study demonstrates a potentially protective role for oral estradiol therapy in the setting of white matter injury and declarative memory deficits secondary to murine chronic cerebral hypoperfusion.
Keywords: Estrogen, Neuroprotection, White Matter Disease, Chronic Hypoperfusion, Ischemia
INTRODUCTION
Vascular cognitive impairment is a leading cause of dementia in older adults (1). Chronic cerebral hypoperfusion (CCH) is implicated in the pathogenesis of subcortical white matter injury and resultant loss of neurons that contributes to neurocognitive decline (2). Studies support a critical role for cerebral vascular dysfunction in the onset and progression of both sporadic and familial forms of Alzheimer’s disease (3, 4). To date, therapeutic efforts to target vascular cognitive impairment have been unsuccessful.
Mechanisms regulating cerebral protection are not well understood (5–7). Clinical studies demonstrate that the sex steroid estradiol (17β-estradiol; E2) can protect the brain by activating pro-survival pathways in neurons and regulating cerebral blood flow (8, 9). Non-human, experimental models show that activation of estrogen receptors (ER) is a critical step in the hormone’s ability to protect neurons (2, 10, 11). It is known that activation of the ERα isoform in neurons enhances the genomic expression of anti-apoptotic genes. However, research suggests that activation of this genomic mechanism alone cannot fully account for the steroid hormone’s neuroprotective actions (12).
Understanding mechanisms involved in cerebral protection is increasingly important as the prevalence of age-related brain disease rises. In this study, we leverage the murine bilateral carotid artery stenosis (BCAS) model to study protective effects of long-term oral estradiol administration on white matter injury in the setting of chronic cerebral hypoperfusion. We evaluate white matter ischemic injury in the corpus callosum and neurocognitive outcome. Further, we explore activation of the extracellular-signal regulated kinase (ERK) pathway
METHODS AND MATERIALS
Animals
All procedures utilized in this study were approved by the Institutional Animal Care and Use Committee (IACUC; protocol # 20036) of the University of Southern California and carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NIH). All mice were male C57BL/6J (12 weeks of age) and housed in a barrier facility with free access to food and water on a 12-hour light dark cycle. 42 mice (25–27 g; The Jackson Laboratory) were chosen and assigned random numbers for use in this study. Control mice (n = 14) did not undergo any surgical procedure. 14 mice underwent the BCAS surgery and 14 mice underwent sham surgery (see below). Within each group (control, sham, BCAS) mice were either treated with oral estradiol therapy or placebo. This yielded six groups: [Placebo (P); Estradiol (E2); Sham + P; Sham + E2; BCAS + P; BCAS + E2]. Each cohort of mice underwent behavioral testing (NOR paradigm). Following sacrifice, brains were harvested for quantification of white matter injury and immunohistochemical analysis. At the time of sacrifice, blood was drawn for measurement of estradiol levels.
Bilateral Carotid Artery Stenosis Procedure
14 male mice were subjected to the bilateral carotid artery stenosis procedure using external micro-coils (Sawane Spring Co., Ltd.) as previously described (13, 14). Briefly, after a seven-day quarantine period, mice were anesthetized (4% isoflurane and maintained with 2% isoflurane in 30–50% oxygen and 70–50% nitrogen) and placed in the prone position. A Laser Doppler Flowmetry microtip fiber probe was fixed to the skull (Bregma point: posterior +1mm/right +5mm). The mouse was then placed in the supine position. Through a midline cervical incision, both common carotid arteries were exposed and an external micro-coil (0.18 mm diameter, 2.5 mm length) was applied to each. Sham operated animals (n = 14) underwent the same procedure, except the microcoils were not placed. Cerebral blood flow (CBF) values were recorded in the supine position just prior to surgery, following application of the first microcoil, and following application of the second microcoil using a Probe 418-1 master probe/PF 5010 laser Doppler Perfusion Monitoring Unit (Perimed AB, Sweden). Unless otherwise stated, mice were humanely euthanized 35–38 days after the BCAS/sham procedure (including 4 days of behavioral testing). One BCAS-operated animal (BCAS+Placebo) was euthanized due to surgical procedure complications.
Oral Estradiol Preparation and Treatments
Estradiol dosage and method of preparation was performed according to Strom et al. (15). To prepare experimental oral steroid hormone treatments a 1 mg 17β-estradiol tablet (Watson Labs) was pulverized using a mortar and pestle. Then 1.12 μg of crushed 17β-estradiol tablet was added to 0.312 μL of sesame oil and allowed to dissolve for 2 days while rocking at 4°C. The total volume of treated oil was then added to 60 mg Nutella (hazelnut cocoa spread). For each mouse 17β-estradiol- or placebo- treated Nutella (2 mg) was given per 1 g animal weight (15). Placebo and estradiol tablets contain the following inactive ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, and polacrilin potassium. Mice were handled and fed with treated Nutella daily between 8 am and 10 am as follows: each mouse was removed from its home cage, weighed, and then individually placed into a separate clean cage containing the Nutella treatment. Treated Nutella was placed onto the centers of white glass tiles (4 cm2) and placed into the center of the cage. Mice were given no more than 5 min to consume treatments. Animals were given oral estradiol or placebo treatments for 31–34 days; oral treatments started 2 days following BCAS or sham surgical procedures to allow for recovery. During task phases mice were given oral treatments post behavior training throughout (see below). Plasma estradiol was measured (see below).
Novel Object Recognition (NOR) Task
Declarative memory was tested in experimental and control mice (n = 41) using a novel object recognition task (16). Testing commenced 31–34 days after the start of oral estradiol and placebo treatments and continued for 4 days. The object recognition task was conducted using a black plexiglass open field box with a white colored bottom. Three objects were used for the task: 2 red wood cubes (familiar objects) and 1 yellow wood triangle (novel object). Immediately before testing, mice were taken from the home cage and individually placed into a separate clean cage. For each phase, mice were placed into the southern portion of the open field box. During the sample and test phases of the task, two objects were placed equal distance (5 cm) from the northwest and northeast sides of the box walls. A video camera was mounted above the box to digitally record the behavior of each mouse in order to determine the amount of time (seconds) spent with each object. Prior to the behavior task, mice were habituated to the test arena for 15 min on testing days 1 and 2. On testing day 3, mice were exposed to two familiar objects (O1 and O2) located in the arena and allowed to explore for a 5 min test period. On testing day 4 mice were exposed to one novel object (N1) and one familiar that were placed in the same locations as day 3 for a 5 min test period. Exploration was defined as touching the object with the snout. To be counted the mouse had to be on all four feet; time when the mouse sat on or chewed the object was not included. All phases of the task were conducted between 8 am and 10 am. The open field box and objects were washed and cleaned with 70% ethanol between each use. Analysis of NOR behavior video was performed blind to experimental treatment conditions. Cognitive outcomes were assessed by computation of a novel exploration index score and the total time spent with objects. The novel object exploration index is a discriminatory measure that is calculated as follows: (time exploring novel object-time exploring familiar)/(time exploring novel + familiar) *100.
Klüver-Barerra Staining/Plasma ELISA
Mice were transcardially perfused with PBS + heparin saline and fixed (4% paraformaldehyde and 0.2% picric acid in PBS). Blood samples were collected at the very beginning of the cardiac puncture to determine 17β-estradiol levels in blood plasma with an ELISA kit (Calbiotech Inc, Spring Valley, CA) following the manufactures instructions. After perfusion, the brains were removed from the cranium and dehydrated using a sucrose gradient (10%–30%), and then the brains were paraffin embedded; one brain (Sham + Placebo) was damaged during histological processing. To visualize changes to white matter tracts, the paraffinized brain located approximately 1 mm posterior to the Bregma (adjusted according to mouse atlas) was then sliced into serial 20 μm-thick coronal sections and Klüver-Barrera staining was performed (n = 40) (14). White matter integrity was evaluated at 400x magnification (Keyence BZ-X1000) in the area (ROI) of the corpus callosum and cingulate bundle according to the 4 point scale developed by Wakita et al. used for assessment of injury in the BCAS model by our group and others.(17–19) It is a derivative of the clinical Fazekas sacle (1987).(20) The scale is as follows: normal (grade 0), disarrangement of the nerve fibers (grade 1), formation of marked vacuoles (grade 2), and disappearance of myelinated fibers (grade 3) (21). Scores were assigned to the left and right ROIs and the average scores from three independent blind observers were used for data analysis.
ERK Identification
To examine whether chronic cerebral hypoperfusion was capable of enhancing ERK pathway activation we examined the retrosplenial cortex in fixed brain slices. The retrosplenial cortex is a brain area involved in head movement and temporal discrimination learning task in rodents and networks with the prefrontal cortex via the corpus callosum and cingulate bundle as well as the hippocampus (22–25). To visualize changes in ERK expression in experimental mice a subset (n = 16) of paraffinized brains were sliced into serial 20 μm-thick coronal sections (located approximately 1.25 mm posterior to the Bregma; adjusted according to mouse atlas) and mounted onto glass slides. Slices were then permeabilized (1% Tween-20) and incubated with antisera consisting of anti-active phospho-p44/42 ERK1/2 (Thr202/Tyr204; 1:500; Cell Signaling Technology, #9101) antibodies overnight at 4°C. Primary antibodies were detected using anti-mouse-Alexa488 secondary antibodies for 1 h at room temperature (Jackson Immuno Research). ProLong Gold anti-fade mounting medium (Thermo Fisher Scientific) was applied to slices and then cover slipped. The number of active phospho-p44/42 ERK1/2 positive cells was collected from one field of view (4x magnification; Keyence BZ-X1000) that included the left and right side of the retrosplenial cortices. Cells from each side of the cortex were counted using ImageJ (1.47v) software and the numbers were averaged. Cells were counted by an independent observer that was blind to the experimental conditions.
Statistics Analysis
GraphPad Prism 5 software was used to analyze data results. One-way ANOVA with Bonferroni’s Multiple Comparisons Test was used to test for significance differences between groups when investigating weight, blood plasma, and behavior testing. A ranked One-way ANOVA (Kruskal-Wallis) with Dunn’s multiple comparisons post hoc test was used for ordinal data to investigate white matter pathology. Student’s unpaired two-tailed t-test with Welch’s correction was used to assess differences between two similar groups. For NOR total time with familiar and novel objects, a two-tailed paired t test was used. Box graphs represent the median value and interquartile range (IQR) whereas the plus sign (+) within boxes represents the mean value. Data in the text are presented as mean ± SEM for continuous data. Specific n numbers are stated for each experiment in the results section.
RESULTS
Cerebral Blood Flow (CBF)
CBF values were recorded after BCAS (placement of bilateral microcoils) or sham surgery. Values were calculated as percentage of baseline CBF levels recorded prior to midline cervical incision. Mean CBF changes in the entire Sham and. BCAS cohorts (including all sub-groups) and in each of the 4 experimental subgroups (Sham+P, BCAS+P, Sham+E, BCAS+E) are shown in Table 1. There were significant difference in CBF changes between the entire cohorts of Sham and BCAS mice (p < 0.0001). There were no significant differences in CBF changes between the two Sham (Sham+P vs. Sham+E; p=0.56) or the two BCAS (BCAS+P vs BCAS+E; p = 0.29) groups (see Table 1).
Table 1. Cerebral blood flow (CBF) measurements using Laser Doppler Flowmetry following placement of microcoils around the bilateral carotid arteries.
Values shown represent the mean percent (%) change in CBF from baseline after the placement of the 2nd coil. There were no significant differences in CBF changes among the E2 and Placebo treated groups (Sham+P vs Sham+E2 or BCAS+P vs BCAS+E2). As expected there were significant differences in CBF changes between the Sham and BCAS cohorts.
| Sham | BCAS | |
| 5.07% ± 10.11 | −27.46% ± 14.72 | p < 0.0001 |
|
| ||
| Sham + P | Sham + E2 | |
| 6.71% ± 9.59 | 3.43% ± 11.10 | p = 0.56 |
|
| ||
| BCAS + P | BCAS + E2 | |
| −23.29% ± 10.7 | −32.33% ± 18.16 | p = 0.29 |
Long-term oral estradiol treatment
Mice consumed oral estradiol (approximately 970 ng of 17β-estradiol per animal per day; based on average starting weight of 26 g per animal) for approximately 35–38 days. During this period no significant weight changes (One-way ANOVA p = 0.62) were observed from the start (26.4 ±1.04 g) to the end (28.0 ±1.06 g, p=ns) of testing. On average, placebo treated cohorts gained 1.7 ±0.12 g (n = 20) while the estradiol treated cohorts gained 1.4 ±0.38 g (n = 21). The BCAS + E2 (~1.1 g, n = 7) and Sham + E2 (~1.2 g, n = 7) groups had the lowest mean weight gain values when compared to the remaining groups (~1.6–1.8 g).
Blood plasma estradiol levels
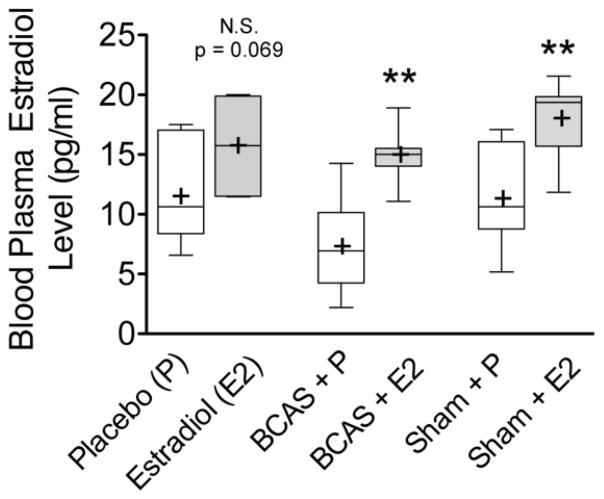
Plasma ELISA results for (Figure 1) demonstrate that mice treated with oral estradiol had significantly higher plasma estradiol levels than those treated with placebo (One-way ANOVA p = 0.0002, F(5,35) = 6.775). BCAS + E2 (p = 0.005, median 15.0 pg/ml, IQR 14–15.5 pg/ml, n = 7) and Sham + E2 (p = 0.014, median 19.4 pg/ml, IQR 15.7–19.9 pg/ml n = 7) groups had a higher levels of plasma estradiol than BCAS + P (median 6.95 pg/ml, IQR 4.25–10.2 pg/ml, n = 6) and Sham + P (median 10.6 pg/ml, IQR 8.79–16.1 pg/ml, n= 7) groups, respectively. There was a biologic difference between plasma estradiol levels between control mice that received oral P (median 10.6 pg/ml, IQR 8.38–17.1 pg/ml, n = 7) and E2 (median 15.7 pg/ml, IQR 11.5–19.9 pg/ml, n = 7) treatments that approached significance (two-tailed unpaired t test with Welch’s correction, p = 0.069).
Figure 1. Elevated blood plasma estradiol levels in mice receiving oral estradiol.
Blood was collected from male mice after 32–35 days of oral treatments and plasma estradiol levels determined using an ELISA assay. Analysis of the optical density means (+) reveals a significant difference between the placebo (P) and estradiol (E2) treated groups for both BCAS and Sham animals. There difference in plasma estradiol levels between control animals in each treatment arm approaches significance (p = 0.069). **p < 0.01 (unpaired two tailed t-test comparisons are between P and E2 groups within each treatment cohort)
Oral estradiol reduces deficits in Novel Object Recognition (NOR) behavior induced by BCAS
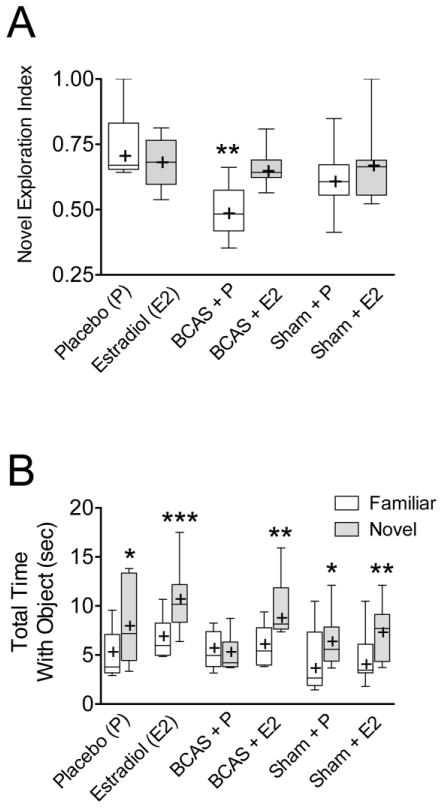
Novel object exploration index (Figure 2A) and total time with familiar and novel objects (Figure 2B) were determined for each group. Sample-testing phase demonstrated that mice spent the same amount of time with the two familiar objects (O1 and O2), suggesting a lack of lateral preference (i.e. left vs. right side object). The BCAS + P mice had lower novel object exploration indexes (Fig 2A; 0.48, IQR 0.42–0.57, n = 6) and spent less relative time with the novel object (Fig 2B; 4.2 sec, IQR 3.8–6.3 sec, n = 6) than did the Sham + P mice (0.60, IQR 0.56–0.67; 5.6 sec, IQR 4.4–7.9 sec, n = 7). The BCAS + E2 mice exhibited significantly better (higher) novel object exploration indices (0.64, IQR 0.62–0.69, n = 7) than did the BCAS + P mice (0.48, IQR 0.42–0.57, n = 6; Fig 2A; unpaired two tailed t-test with Welch’s correction p = 0.012, t = 3.21, df = 8). They also spent more relative time with the novel object (8.2 sec, IQR 7.6–11.9 sec, n = 7) than did the BCAS + P mice (4.2 sec, IQR 3.8–6.3 sec, n = 6); In fact, the BCAS + E2 mice had novel object indices (0.64, IQR 0.62–0.69, n = 7; One-way ANOVA p = 0.022, F(5,35) = 3.05) and exploration times (8.2 sec, IQR 7.6–11.9, n = 7; One-way ANOVA p = 0.013, F(5,35) = 3.41; Bonferroni’s Multiple Comparisons Test p < 0.05) similar to those of the sham operated mice (both Placebo, 5.6 sec, IQR 4.4–6.4 sec, n = 7, and E2 treated, 7.7 sec, IQR 4.4–9.2 sec, n = 7) suggesting a protective effect of the estradiol.
Figure 2. Estradiol reduces deficits secondary to BCAS on Novel Object Recognition task.
After long-term estradiol treatment, male mice underwent novel object recognition testing. (A) BCAS+E2 had a significantly higher novel object exploration (discriminatory) index than BCAS−P (p=0.012). (B) BCAS+P group spends an equal amount of time exploring both objects and illustrates why this group has a low discrimination index shown in Figure 2A. *p < 0.05, **p < 0.01, ***p < 0.001
Oral estradiol treatment decreases white matter injury secondary to BCAS in the corpus callosum
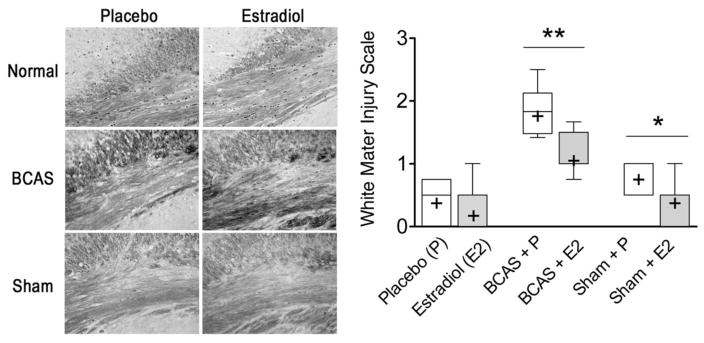
A Kruskal-Wallis test shows a significant global difference in the median white matter injury scores between groups (p < 0.0001). Post hoc assessment of the groups using a Dunn’s multiple comparisons test showed (Figure 3) significant differences in median white matter injury score between BCAS + P (1.83, IQR 1.48–2.12, n = 6) and Sham + P (1.00, IQR 0.50–1.00, n = 6) mice. Further, BCAS + E2 mice (1.00, IQR 1.00–1.50, p < 0.01, n = 7) demonstrated less white matter injury than BCAS + P mice (1.83, IQR 1.48–2.12, n = 6). No significant differences in white matter injury existed between Sham + E2 (0.50, IQR 0–0.50, p < 0.05, n = 7) and BCAS + E2 mice (1.00, IQR 1.00–1.50, n = 7).
Figure 3. There is decreased white matter injury in the corpus callosum in BCAS and sham mice who receive oral estradiol.
One day after behavior testing the brains from male mice were fixed, sliced, and prepared for Klüver-Barerra staining (left side, representative sections) to assess the changes to white matter tracks of the corpus callosum. Analysis using a white matter injury scale (right side; see methods for injury scale ranking) shows that the oral estradiol treated BCAS group had decreased white matter injury compared to the placebo counterpart. Estradiol-treated sham animals also demonstrated less white matter injury than their placebo treated counterparts. *p < 0.05, **p < 0.01 (unpaired two tailed t-test comparisons are between P and E2 groups within each treatment cohort)
Oral estradiol treatment enhanced the expression of active ERK following BCAS
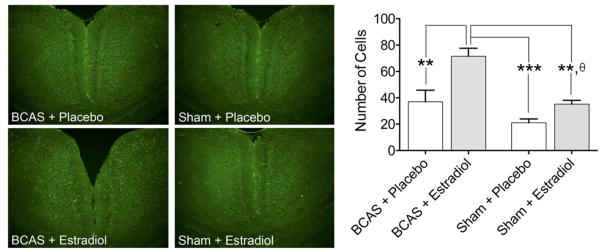
Immunohistochemistry and cell counting demonstrated that active ERK expression (Figure 4) was enhanced in the retrosplenial cortices in the BCAS mice compared to Sham-operated mice (One-way ANOVA p = 0.0003, F(3,12) = 13.88). Further, there was an increase in ERK positive cells in the BCAS + E2 mice (71.5 ±12.23, n = 4) when compared to the BCAS + P (37 ±17.66, n = 4; Bonferroni’s Multiple Comparison Test, p < 0.01), the Sham + E2 (32.75 ±3.59, n = 4, p < 0.01), and the Sham + P (21 ±5.94, n = 4, p < 0.001) cohorts. There was also a significant increase in ERK phosphorylation when the Sham + E2 group was compared to the Sham + P treated mice (p < 0.05, two-tailed unpaired t test with Welch’s correction, p = 0.018, t = 3.21, df = 6).
Figure 4. Oral estradiol treatment increases ERK phosphorylation in the brain of BCAS and Sham animals.
Analysis of brain slices using immunohistochemistry (left side, representative sections of BCAS and Sham) revealed that oral estradiol treatment increased the level of ERK phosphorylation in BCAS mice (right side, bar graph). The increase in ERK phosphorylation was also evident in estradiol treated sham animals when compared to placebo treated-sham animals (right side, bar graph). (Bonferroni’s Multiple Comparisons Test, **p < 0.01, ***p < 0.001; unpaired two-tailed t-test with Welch’s correction, θp < 0.01, Sham + Placebo vs. Sham + Estradiol)
DISCUSSION
Our data demonstrates that long-term estradiol treatment results in decreased white matter injury and cognitive deficits in the setting of experimental chronic cerebral hypoperfusion secondary to bilateral carotid artery stenosis. The finding of increased active ERK expression with oral estradiol administration suggests a possible mechanism for future study. The BCAS model used in these experiments generates reproducible white matter injury and behavioral deficits. This model system is an attractive paradigm for testing estradiol administration as prior studies suggest that estrogen both exhibits neuroprotective effects in the setting of ischemic stroke and influences neurocognition in normal aging and a range of disease processes.
Changes in endogenous estrogen levels have been shown to affect cognitive function. Human studies demonstrate that formal memory testing scores decline following menopause (26, 27) and that pre-menopausal women who undergo surgical oophorectomy exhibit similar neurocognitive impairments to post-menopausal women (28, 29). Analogous findings have been described in rodents and non-human primates. Spatial memory deficits appear after 12 months of age in female mice (30). This timeframe corresponds to late middle age, menopause, and estrogen decline (31). Likewise, young female mice exhibit cognitive impairment following surgical ovariectomy (32). In aged rhesus monkeys, ovariectomy causes impairment in a delayed response task. The addition of estradiol to these aged female animals reverses the impairment to a level of function similar to young animals (11). Taken together, these data suggest a potential role for estrogen therapy in the prevention and/or treatment of cognitive decline.
Estrogen-mediated protection may be relevant to both normal aging processes and diseases involving cognitive decline. (33–35). Studies have clearly demonstrated an association between chronic cerebral hypoperfusion, cognitive decline, and Alzheimer’s disease (3). It is well known that β-amyloid and tau proteins play a role in Alzheimer’s disease pathology. Data suggests that estradiol protects against Aβ/tau protein toxicity and neuronal cell death (36–39). Changes in ERα expression level may be involved in the development of Alzheimer’s disease neuropathology (40). ERα co-localizes with neurofibrillary tangles and interacts with tau protein in hippocampal and cortical tissue from individuals (female and male) with Alzheimer’s disease.
Chronic cerebral hypoperfusion and low sex steroid hormone levels are both known risk factors for cognitive impairment and dementia (11, 29, 41–47). However, potential therapeutic avenues resulting from interactions of these two exposures remain understudied. (5, 6). Gonadal hormones are known to influence working memory tests affected by injury to the medial and orbital prefrontal cortices (48). Prior studies have demonstrated white matter ischemic changes and novel object recognition deficits in the setting of chronic cerebral hypoperfusion secondary to BCAS. In these experiments, we sought to determine whether estradiol therapy could reverse these injuries.
One potential mechanism of estradiol-mediated protection is thought to be related to rapid activation of pro-survival signal transduction pathways, regulated by a sub-population of ERα localized to the plasma membrane (10). Although several pathways may be involved, estradiol-dependent activation of ERK appears to be a key early step (10, 49, 50). The mechanisms by which cell surface estradiol receptors activate ERK and other pro-survival pathways are not completely clear. However, evidence strongly suggests G protein-coupled signaling cascades are involved (51). In addition to neuroprotection, activation of the ERK pathway by membrane localized ERα modulates memory consolidation in rats and mice (52–54). On novel object recognition testing, memory consolidation is evident with either pre- or post-treatment with estradiol (55, 56). Inhibition of estradiol-induced ERK pathway activation, using pathway blockers, prevents enhancement on novel object memory testing (52–54, 57). Our data shows increased activated ERK expression in the setting of BCAS as well as with oral estradiol administration, with the greatest ERK levels evident in the BCAS−E2 cohort. It is possible that the decreased white matter injury and improved NOR performance in BCAS−E2 mice (compared to BCAS−P mice) is related to ERK activation. Future studies aimed at probing this mechanism through the use of ERK-inhibitors could be informative.
As expected, our BCAS mice exhibited increased white matter injury and deficits in NOR testing when compared to their sham-operated counterparts (13, 76). Oral estradiol administration resulted in decreased white matter damage and fewer neurobehavioral deficits in the BCAS cohort, supporting a protective effect of estradiol. Interestingly, estradiol treated sham-operated mice (that did not undergo BCAS) demonstrated improved white matter injury scores when compared to their placebo treated counterparts as well. However, novel object recognition testing did not indicate behavioral differences among these groups. Differences may be explained by the activation of the ERK pathway in sham-operated animals that received oral estradiol treatment.
The estrogen-mediated effects on cognition may derive, in part, from protection against subtle ischemic injury resulting from cerebral hypoperfusion. Robust evidence exists for the protective effects of estrogen in the setting of stroke/cerebral ischemia. Studies have suggested that female rodents sustain less injury following ischemic stroke than their male counterparts. However the protection is eliminated when females are ovariectomized (58). In vivo studies show an accumulation of estradiol binding in the cortex of rats following experimental stroke (59). Animal studies suggest that estradiol-mediated neuroprotection requires the activation of ERα and ERK (10, 11, 60, 61). Cortical up-regulation of ERα mRNA in neurons and glial cells (59, 61–63) has been demonstrated in rodent, ovine, and non-human primate stroke models. This effect is consistent across sexes.
Rodent studies have demonstrated neuroprotective capabilities of estradiol therapy across a spectrum of disease models (64). However, these findings have not translated to clinical application (29, 41–43, 65). This discordance may result from species-specific differences in brain structure/function, route of treatment administration, estradiol composition, and prescribed dosing parameters (58). The current study leverages a noninvasive oral treatment protocol and pharmaceutical grade estradiol to recapitulate a practical clinical treatment regimen. Serum ELISA analysis demonstrated that plasma estradiol levels were significantly elevated in the estradiol treated mice (when compared to placebo treated) at the time of behavioral/histological analysis (Figure 1). While many studies demonstrate neuroprotective effects of estradiol, some report toxic sequellae (66–68). These differences may be due to prescribed dosages and route of estradiol administration (69). Ingberg et al. (70) utilized a low dose administration protocol in ovariectomized female mice to achieve steady physiological serum hormone levels that ranged from 5–50 pg/ml. This dose was protective against transient focal ischemia. A low dose, peroral method was also protective in our current BCAS studies.
The potential model paradigms for testing the effects of exogenous estrogen therapy include male, or ovariectomized female, mice. We chose to use male mice in the current study to eliminate the influence of endogenous gonadal estradiol and limit the number of surgical procedures (ovariectomy) prior to behavioral testing and histological assessment (71–74). Further, ovariectomy has been found to cause dysregulation of glucose homeostasis in female mice (75).
CONCLUSIONS
This study leverages a BCAS model of CCH to examine the neuroprotective effects of long-term oral estradiol therapy on white matter integrity and neurobehavior. The results demonstrate that estradiol reduces CCH-induced white matter injury in the corpus callosum and ameliorates resultant deficits on NOR testing. Our data show an increase in activated ERK expression in estradiol treated animals, suggesting a possible mechanism through which estradiol could exert its protective role. Details regarding estradiol dosing parameters, timing, and effects on other neurocognitive domains remain to be studied.
Acknowledgments
Sources of Funding: This research was supported by National Institute of Health grant ES024936 to W.J.M. and from a USC Provost Postdoctoral Research Award to R.D.
This research was supported by National Institute of Health grant ES024936 to WJM and from a USC Provost Postdoctoral Research Award to RD. All authors have discussed the results and commented on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. The Lancet Neurology. 2002;1(7):426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 2.Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49(2):246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Torre JC. Pathophysiology of neuronal energy crisis in Alzheimer’s disease. Neurodegener Dis. 2008;5(3–4):126–132. doi: 10.1159/000113681. [DOI] [PubMed] [Google Scholar]
- 4.Miners JS, Palmer JC, Love S. Pathophysiology of hypoperfusion of the precuneus in early Alzheimer’s disease. Brain Pathology. 2015 doi: 10.1111/bpa.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, et al. Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab. 2011;31(2):413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep. 2010;12(1):6–13. doi: 10.1007/s11886-009-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spychala MS, Honarpisheh P, McCullough LD. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. Journal of Neuroscience Research. 2017;95(1–2):462–471. doi: 10.1002/jnr.23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34(2):171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 9.Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacological Research. 2005;52(2):119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74(7):555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey ME, et al. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience. 2011;191:148–158. doi: 10.1016/j.neuroscience.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jover T, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. Journal of Neuroscience. 2002;22(6):2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35(11):2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, et al. White matter injury due to experimental chronic cerebral hypoperfusion is associated with C5 deposition. PLoS One. 2013;8(12):e84802. doi: 10.1371/journal.pone.0084802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strom JO, Theodorsson A, Ingberg E, Isaksson IM, Theodorsson E. Ovariectomy and 17beta-estradiol replacement in rats and mice: a visual demonstration. J Vis Exp. 2012;(64):e4013. doi: 10.3791/4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1(3):1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- 17.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta neuropathologica. 1994;87(5):484–492. doi: 10.1007/BF00294175. [DOI] [PubMed] [Google Scholar]
- 18.Shibata M, et al. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38(10):2826–2832. doi: 10.1161/STROKEAHA.107.490151. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, et al. White matter injury due to experimental chronic cerebral hypoperfusion is associated with C5 deposition. PloS one. 2013;8(12):e84802. doi: 10.1371/journal.pone.0084802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. American journal of roentgenology. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 21.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Protective effect of cyclosporin A on white matter changes in the rat brain after chronic cerebral hypoperfusion. Stroke. 1995;26(8):1415–1422. doi: 10.1161/01.str.26.8.1415. [DOI] [PubMed] [Google Scholar]
- 22.Sugar J, Witter MP, van Strien NM, Cappaert NL. The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinform. 2011;5:7. doi: 10.3389/fninf.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata H, Kondo S, Naito J. Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neurosci Res. 2004;49(1):1–11. doi: 10.1016/j.neures.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Todd TP, Bucci DJ. Retrosplenial Cortex and Long-Term Memory: Molecules to Behavior. Neural Plast. 2015;2015:414173. doi: 10.1155/2015/414173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell AL, et al. The retrosplenial cortex and object recency memory in the rat. Eur J Neurosci. 2017;45(11):1451–1464. doi: 10.1111/ejn.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrag AK, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord. 2002;13(3):193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- 27.Greendale GA, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010;171(11):1214–1224. doi: 10.1093/aje/kwq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nappi RE, et al. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47(1):29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- 29.Rocca WA, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 30.Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19(18):8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21(1):193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 32.Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically-used hormone therapies in the female rat: models, mazes, and mechanisms. Brain Res. 2013;1514:18–39. doi: 10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collij LE, et al. Application of Machine Learning to Arterial Spin Labeling in Mild Cognitive Impairment and Alzheimer Disease. Radiology. 2016:152703. doi: 10.1148/radiol.2016152703. [DOI] [PubMed] [Google Scholar]
- 34.Merlini M, Wanner D, Nitsch RM. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 2016;131(5):737–752. doi: 10.1007/s00401-016-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iturria-Medina Y, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng H, et al. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80(1):191–196. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- 37.Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. J Alzheimers Dis. 2002;4(6):449–457. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- 38.Shi J, Yang SH, Stubley L, Day AL, Simpkins JW. Hypoperfusion induces overexpression of beta-amyloid precursor protein mRNA in a focal ischemic rodent model. Brain Res. 2000;853(1):1–4. doi: 10.1016/s0006-8993(99)02113-7. [DOI] [PubMed] [Google Scholar]
- 39.Shi J, et al. Estrogen attenuates over-expression of beta-amyloid precursor protein messager RNA in an animal model of focal ischemia. Brain Res. 1998;810(1–2):87–92. doi: 10.1016/s0006-8993(98)00888-9. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, et al. Estrogen receptor-alpha is localized to neurofibrillary tangles in Alzheimer’s disease. Sci Rep. 2016;6:20352. doi: 10.1038/srep20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker WH. Bilateral oophorectomy versus ovarian conservation: effects on long-term women’s health. J Minim Invasive Gynecol. 2010;17(2):161–166. doi: 10.1016/j.jmig.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Wroolie TE, et al. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17beta-estradiol versus conjugated equine estrogens. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2011;19(9):792–802. doi: 10.1097/JGP.0b013e3181ff678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tierney MC, et al. Lifelong Estrogen Exposure and Memory in Older Postmenopausal Women. Journal of Alzheimer’s disease: JAD. 2012 doi: 10.3233/JAD-122062. [DOI] [PubMed] [Google Scholar]
- 44.Iemolo F, Martiniuk A, Steinman DA, Spence JD. Sex differences in carotid plaque and stenosis. Stroke. 2004;35(2):477–481. doi: 10.1161/01.STR.0000110981.96204.64. [DOI] [PubMed] [Google Scholar]
- 45.Schierbeck LL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 46.Sacco S, Totaro R, Baldassarre M, Carolei A. Morphological variations of the internal carotid artery: Prevalence, characteristics and association with cerebrovascular disease. Int J Angiol. 2007;16(2):59–61. doi: 10.1055/s-0031-1278249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarti C, Pantoni L, Bartolini L, Inzitari D. Cognitive impairment and chronic cerebral hypoperfusion: what can be learned from experimental models. Journal of the neurological sciences. 2002;203:263–266. doi: 10.1016/s0022-510x(02)00302-7. [DOI] [PubMed] [Google Scholar]
- 48.Aubele T, Kaufman R, Montalmant F, Kritzer M. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54(2):244–252. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135(1):59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 50.Yang LC, et al. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5(5):e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29(13):4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan L, et al. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30(12):4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez SM, et al. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28(35):8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160(1):6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84(1):112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 56.Gervais NJ, Jacob S, Brake WG, Mumby DG. Systemic and intra-rhinal-cortical 17-beta estradiol administration modulate object-recognition memory in ovariectomized female rats. Horm Behav. 2013;64(4):642–652. doi: 10.1016/j.yhbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49(2):246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19(15):6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wappler EA, et al. Neuroprotective effects of estrogen treatment on ischemia-induced behavioural deficits in ovariectomized gerbils at different ages. Behav Brain Res. 2010;209(1):42–48. doi: 10.1016/j.bbr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Ovejero D, Veiga S, Garcia-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450(3):256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 62.Wood CE. Cerebral hypoperfusion increases estrogen receptor abundance in the ovine fetal brain and pituitary. Neuroendocrinology. 2008;87(4):216–222. doi: 10.1159/000112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blurton-Jones M, Tuszynski MH. Reactive astrocytes express estrogen receptors in the injured primate brain. J Comp Neurol. 2001;433(1):115–123. doi: 10.1002/cne.1129. [DOI] [PubMed] [Google Scholar]
- 64.Pabon M, et al. Estrogen Replacement Therapy for Stroke. Cell Med. 2014;6(3):111–122. doi: 10.3727/215517913X672263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neuro-degenerative diseases. 2012;10(1–4):175–178. doi: 10.1159/000334764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macrae IM, Carswell HV. Oestrogen and stroke: the potential for harm as well as benefit. Biochem Soc Trans. 2006;34(Pt 6):1362–1365. doi: 10.1042/BST0341362. [DOI] [PubMed] [Google Scholar]
- 67.Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26(9):1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 68.Maki PM, Sundermann E. Hormone therapy and cognitive function. Hum Reprod Update. 2009;15(6):667–681. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strom JO, Ingberg E. Impact of methodology on estrogens’ effects on cerebral ischemia in rats: an updated meta-analysis. BMC Neurosci. 2014;15:22. doi: 10.1186/1471-2202-15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ingberg E, Theodorsson E, Theodorsson A, Strom JO. Effects of high and low 17beta-estradiol doses on focal cerebral ischemia in rats. Sci Rep. 2016;6:20228. doi: 10.1038/srep20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11(2):292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 72.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29(8):1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 73.Sudo S, et al. Beta-estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neurosci Res. 1997;29(4):345–354. doi: 10.1016/s0168-0102(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 74.Saleh TM, Cribb AE, Connell BJ. Estrogen-induced recovery of autonomic function after middle cerebral artery occlusion in male rats. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1531–1539. doi: 10.1152/ajpregu.2001.281.5.R1531. [DOI] [PubMed] [Google Scholar]
- 75.Santos RS, et al. Lacking of estradiol reduces insulin exocytosis from pancreatic beta-cells and increases hepatic insulin degradation. Steroids. 2016;114:16–24. doi: 10.1016/j.steroids.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 76.Patel A, et al. Chronic cerebral hypoperfusion induced by bilateral carotid artery stenosis causes selective recognition impairment in adult mice. Neurological Research. 2017;39:910–917. doi: 10.1080/01616412.2017.1355423. [DOI] [PMC free article] [PubMed] [Google Scholar]