Abstract
BACKGROUND CONTEXT
Advances in the development of biomaterials and stem cell therapy provide a promising approach to regenerating degenerated discs. The normal nucleus pulposus (NP) cells exhibit the similar phenotype as chondrocytes. Because dental pulp stem cells (DPSCs) can be differentiated into chondrogenic cells, the DPSCs and DPSCs-derived chondrogenic cells encapsulated in type I and type II collagen hydrogels can potentially be transplanted into degenerated nucleus pulposus (NP) to repair damaged tissue. The motility of transplanted cells is critical because the cells need to migrate away from the hydrogels containing the cells of high density and disperse into the NP tissue after implantation.
PURPOSE
The purpose of this study was to determine the motility of DPSC and DPSC-derived chondrogenic cells in type I and type II collagen hydrogels.
STUDY DESIGN/SETTING
The time lapse imaging that recorded cell migration was analyzed to quantify the cell migration velocity and distance.
METHODS
The cell viability of DPSCs in native or 4S-StarPEG – crosslinked type I and type II collagen hydrogels was determined using LIVE/DEAD® cell viability assay and AlamarBlue® assay. DPSCs were differentiated into chondrogenic cells. The migration of DPSCs and DPSC-derived chondrogenic cells in these hydrogels was recorded using a time lapse imaging system. This study was funded by Regional Institute on Aging and Wichita Medical Research and Education Foundation and the authors declare no competing interest.
RESULT
DPSCs showed high cell viability in non-crosslinked and crosslinked collagen hydrogels. DPSCs migrated in collagen hydrogels, and the cell migration speed was not significantly different in either type I collagen or type II collagen hydrogels. The migration speed of DPSC-derived chondrogenic cells was higher in type I collagen hydrogel than in type II collagen hydrogel. Crosslinking of type I collagen with 4S-StarPEG significantly reduced the cell migration speed of DPSC-derived chondrogenic cells.
Conclusions
After implantation of collagen hydrogels encapsulating DPSCs or DPSC-derived chondrogenic cells, the cells can potentially migrate from the hydrogels and migrate into the NP tissue. This study also explored the differential cell motility of DPSCs and DPSC-derived chondrogenic cells in these collagen hydrogels.
Keywords: dental pulp stem cells, collagen, migration, chondrogenic cell, nucleus pulposus
Introduction
The nucleus pulposus (NP) maintains intervertebral disc function by absorbing shock and relieving stress between adjacent vertebrae. The pathological change in a degenerative disc causes the overall alteration of the biomechanics of the spinal column, thereby resulting in low back pain [1]. Although surgical procedures such as discectomy, spinal fusion, or nucleotomy are generally the clinical therapeutic treatment for degenerative disc disease, these methods result in restricted flexibility of the spine. The biological approach targets therapy that preserves the intervertebral disc (IVD) function by regenerating the degenerated and damaged NP[2–4]. Advances in the development of biomaterials and stem cell therapy provide a promising approach to regenerating degenerative discs. Biomaterial hydrogels that mimic the NP extracellular matrix (ECM) can serve as stem cell carriers for cell transplantation and a matrix for replacement of the degenerative NP[5–8].
The ECM of NP mainly consists of type II collagen (20% of dry weight), and proteoglycans (50% of dry weight) as well as a small amount of other collagen types (VI, IX, and XI) [9]. The characterized alterations of a degenerative disc include increased cell death, swelling of the disc, immune privilege unbalance, and aberrant gene expression. The ECM of the degenerative NP is condensed, and the jelly-like material becomes replaced by fibrous tissue. In the process of aging and degeneration, NP cells lose their ability to proliferate because of tissue damage and stress.
In clinical trials, patients diagnosed with degenerative disc disease were treated with intra-discal injection of bone marrow-derived mesenchymal stem cells (MSCs) [10, 11]. The clinical studies showed the safety and feasibility of the clinical use of MSCs for the treatment of degenerative disc and some patients reported the improvement in mobility [11]. Dental pulp stem cells (DPSCs) are primarily derived from the dental pulp tissues of primary incisors and permanent third molar teeth. Stem cells derived from dental pulp have received growing attention because they have a similar differentiation capability as other MSCs and they are relatively easy to obtain. It has been shown that normal NP expresses a chondrocytic phenotype because these cells express chondrocyte markers such as sox 9, collagen type II, and aggrecan [12]. Studies have shown that DPSCs can be differentiated into chondrogenic cells [13,14]. DPSCs have become an attractive cell source that may replace chondrocytes and NP cells for cartilage and NP regeneration.
Type II collagen, as a major component of NP, has been fabricated as a hydrogel and studied for the growth of NP cells and MSCs [15–17]. Type II collagen hydrogel can be remodeled by local NP cells and grafted cells after implantation, and they degrade via a natural process without producing toxic byproducts. In previous studies, we fabricated and characterized type I collagen and type II collagen hydrogels and studied rat astrocyte growth and human NP cells in these gels, respectively [15,18]. We found that crosslinking collagen hydrogel with 1-ethyl-3 (3-dimethyl aminopropyl) carbodiimide (EDC) or poly(ethylene glycol) ether tetrasuccinimidyl glutarate (4S-StarPEG) increased the gel stability against collagenase digestion without reducing the cell viability. Both types of collagen hydrogels can potentially serve as carriers for the transplantation of DPSCs into degenerated NP to repair the damaged tissue. In the cell implantation process, a large number of cells encapsulated in collagen hydrogels are injected into the degenerative NP. The migration of transplanted cells is critical because the cells of high density need to move away from the hydrogels and disperse into the NP tissue. However, the motility of DPSC cells and DPSCs-derived chondrogenic cells in types I and II collagen hydrogels for regeneration of NP has not yet been reported.
In this study, we grew DPSCs and DPSC-derived chondrogenic cells in type I and type II collagen hydrogels, with the aim of determining the cell motility in these materials by means of time-lapse imaging. We explored the differential cell motility of DPSCs and DPSC-derived chondrogenic cells in these collagen hydrogels.
Materials and Methods
DSPC Culture in Type I And Type II Collagen Hydrogels
Type I collagen and type II collagen were extracted from bovine achilles tendon and bovine cartilage, respectively in our lab. The pH of the collagen solution was adjusted to 7 by adding a NaOH aqueous solution (1M) and phosphate-buffered saline (PBS) solution (10X) as we have reported before [15,18]. The hydrogel was crosslinked with different concentrations of 4S-StarPEG. The final concentrations of 4S-StarPEG in the collagen solutions was 0.1 mM, and 0.5 mM. The collagen hydrogel without any crosslinker was used as the control. Human DPSCs (Lonza, Walkersville, MD) were mixed with type I and type II collagen hydrogels containing 4S-StarPEG in a cell culture dish. Then the cell culture medium was added to the cell culture dish, and the cells were cultured in an incubator (37°C, 5% CO2). Cell culture was maintained with DPSC culture medium (DMEM/F12 medium supplemented with 10% FBS and 1% antibiotics (100 IU/ml penicillin and streptomycin) and DPSC medium (Lonza, Walkersville, MD)).
Chondrogenic Differentiation of DPSCs
To induce chondrogenic differentiation, DSPC cells (250,000) were centrifuged to form a cell pellet. The cell pellet was cultured with chondrogenic medium (high-glucose DMEM supplemented with 10 ng/mL TGF-β1, 50 g/mL ascorbate-2-phosphate, 0.1 M dexamethasone, 100 g/mL sodium pyruvate, 40 g/mL proline, 50 mg/mL ITS premix, and 1% penicillin–streptomycin) for 3 weeks. Control cell pellets were cultured with DPSC culture medium. The medium was changed twice a week.
LIVE/DEAD® Cell Viability Assay
DPSCs (40,000) were seeded in 0.4 ml of type I or type II collagen hydrogels containing 4S-StarPEG (0 mM, 0.1 mM, and 0.5 mM). The LIVE/DEAD® cell vitality assay (Lifetechnology, Grand Island, NY) was performed after the DPSCs were cultured in the hydrogels for 6 days. An ethidium homodimer-1 (EthD-1) stock solution (2 μl, 2 mM) and calcein AM stock solution (0.5 μl, 4 mM) were added to a sterile PBS solution (1 ml). The solution (300 μl) was added directly to each cell culture well and incubated for 30 minutes at room temperature. The cells were then viewed under a fluorescent microscope. At least three independent experiments were performed in this study. Four images of the cells within the hydrogel in each experiment were recorded. The live and dead cells in the images were counted, and the ratio of live cells to total cells was quantified.
AlamarBlue® Assay
The viability and proliferation of DPSCs in the hydrogel was studied by monitoring their metabolic activity using the AlamarBlue® assay (Pierce Biotechnology, Rockford, IL). DPSCs (40,000 cells) were seeded in 0.4 ml collagen hydrogels or crosslinked collagen hydrogel (0.5 mM 4S-StarPEG). The AlamarBlue® assay was performed after the cells were cultured for 3 days and 6 days. Then the cells were incubated in a cell culture medium containing 10% (v/v) AlamarBlue® reagent for 4 hours. Absorbance was measured at wavelengths of 570 nm and 600 nm in a microplate reader (Synergy Mx Monochromator-Based Multi-Mode Microplate Reader, Winooski, VT).
Migration of DPSCs in Collagen Hydrogel
To study the migration of DPSC cells in the hydrogel, 40,000 cells were seeded in 400 μl type I or type II collagen hydrogels or collagen hydrogel crosslinked with 4S-StarPEG (0.5 mM). After the cells were cultured for 24 hours, the migration of DPSC cells was recorded with a time-lapse microscope (Zeiss Axio Observer microscope) placed in a plastic incubator with 5% CO2 at 37ºC. Time-lapse image recording was performed to record the cell migration using ZEN 2011 imaging microscope software. The migration of cells was recorded by capturing images every 5 minutes for 3 hours. The time-lapse images were analyzed using NIH ImageJ software, and the cell migration distance and speed were quantified.16
Immunocytochemistry
To study the phenotype of DPSC cell-derived chondrogenic cells, the undifferentiated DPSC cells, the pellets of DPSC cells-derived chondrogenic cells, and the control pellets were cultured in collagen type I-coated cell culture dishes for 6 days. Then the cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% triton-100. Cells were labeled with polyclonal anti-aggrecan antibody (Santa Cruz Biotechnology, Dallas, TX), monoclonal antibody anti-type II collagen antibody (Santa Cruz Biotechnology, Dallas, TX), and polyclonal anti-sox 9 antibody (EMD Millipore, Billerica, MA).
DMMB Assay
To measure the amount of glycosaminoglycans (GAGs) generated by the cells, the culture medium for control pellet growth and culture medium for pellets of chondrogenic differentiation were collected every week for 3 weeks. The content in the cell culture medium was concentrated by freeze-drying the medium and reconstituting the content in 100 μl water. The GAGs were measured by dimethylmethylene blue (DMMB) assay. A DMMB (Sigma-Aldrich, St. Louis, MO) solution was prepared by dissolving 16 mg DMMB in 1 L water containing 3.04 g glycine, 1.6 g NaCl, and 95 ml of 0.1 M acetic acid. The pH of the solution was adjusted to 3.0. The solution was then filtered using Whattman® filter paper. The standard curve of chondroitin 4 sulfate solution was prepared. The mixtures of the sample solution (50 μl) and DMMB solution (200 μl) were measured using a plate reader (Synergy Mx Monochromator-Based Multi-Mode Microplate Reader, Winooski, VT) at 525 nm.
Migration of DPSC-Derived Chondrogenic Cells in Collagen Hydrogels
DPSC-derived chondrogenic cell pellets and control pellets were cut into four pieces and seeded on type I collagen-coated dishes, in type I and type II collagen hydrogels or type I and type II collagen hydrogels crosslinked with 4S-StarPEG (0.5 mM). After culturing for 3 days and 6 days, images for the cultured pellets on cell culture dishes or in the collagen hydrogels were recorded. The distance of cell migration from the edge of cultured cell pellets was measured and quantified.
To study the motility of DPSC-derived chondrogenic cells in the hydrogels, the dissociated chondrogenic cells were grown in collagen hydrogels, and the cell migration was recorded with a time-lapse imaging system. DPSC-derived chondrogenic cell pellets were dissociated using a digestion solution prepared with cell culture medium containing 10% FBS, 0.15% collagenase type I (Thermo Fisher Scientific), and 2 mM CaCl2. The dissociated cells were cultured in cell culture dishes for 6 days. Then the cultured cells were harvested, and about 20,000 cells were seeded in 200 μl type I or type II collagen hydrogels or collagen hydrogel crosslinked with 4S-StarPEG (0.5 mM). After the cells were cultured for 24 hours, the migration of the cells was recorded with a time-lapse microscope placed in a plastic incubator with 5% CO2 at 37ºC. The migration of cells was recorded by capturing images every 5 minutes for 3 hours.
Statistical Analysis
Statistical analysis was conducted using a two-tailed Student’s t-test. A p-value of 0.05 was considered to be statistically significant. Data were expressed as means ± standard deviation.
Results
Cell Viability and Proliferation in Collagen Hydrogels
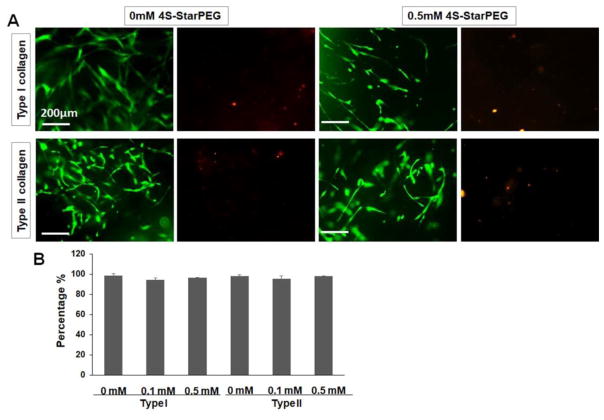
Cell viability of DPSCs in the type I and type II collagen hydrogels was determined by a LIVE/DEAD® cell vitality assay kit. After the cells were cultured for 6 days, most cells were live cells in the hydrogels (Figure 1A). The cells showed elongated morphology with multiple-processes in the hydrogels. Quantification of live and dead cells in the hydrogels showed that the ratio of live DPSCs was above 95% in all groups (Figure 1B). There was no significant difference between groups.
Figure 1.
LIVE/DEAD® cell viability assay for DPSCs grown in collagen hydrogels: (A) Most cells grown in non-crosslinked and 4S-StarPEG crosslinked collagen hydrogels exhibiting high cell viability. Scale bar: 200 μm. The images of same field were taken to show the green or red fluorescence – labeled cells. Live cells labeled with calcein AM (green). Dead cells labeled with ethidium homodimer-1 (red). (B) Percentage of live cells in collagen hydrogels as determined by LIVE/DEAD® cell assay.
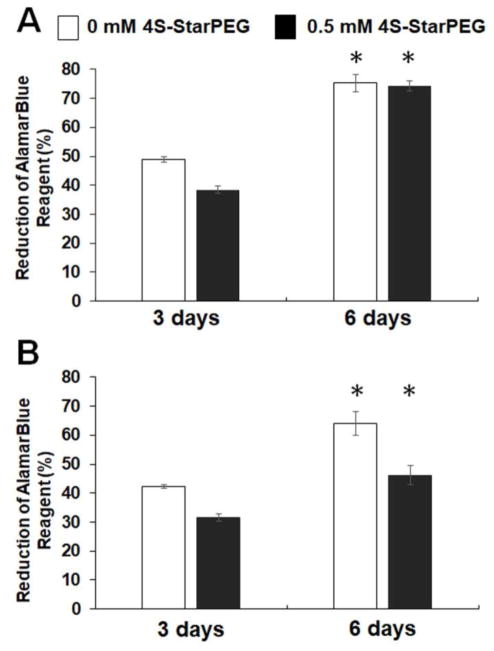
The AlamarBlue® assay showed the metabolic activity and proliferation of DPSCs in collagen hydrogels (Figure 2). For type I collagen hydrogels, after culturing for 3 days, the reduction of AlamarBlue® reagent for DPSC cells in the non-crosslinked and crosslinked hydrogels was 48.9 ± 0.9% and 38.4 ± 1.3%, respectively. The values increased to 75.2 ± 2.9% and 74.2 ± 1.6% after culturing for 6 days. For type II collagen hydrogels, the values in non-crosslinked and crosslinked hydrogels were 42.3 ± 0.7% and 31.7 ± 1.2%, respectively, after culturing for 3 days, and these values increased to 64.0 ± 4.0% and 46.1 ± 3.2%, respectively, after culturing for 6 days. The increase in AlamarBlue® reagent reduction indicated cell proliferation in the hydrogels.
Figure 2.
AlamarBlue® assay for DPSCs grown in hydrogels for 6 days demonstrating cell proliferation in these hydrogels. *, p < 0.05, compared with cell culturing for 3 days.
Migration of DPSCs in Collagen Hydrogels
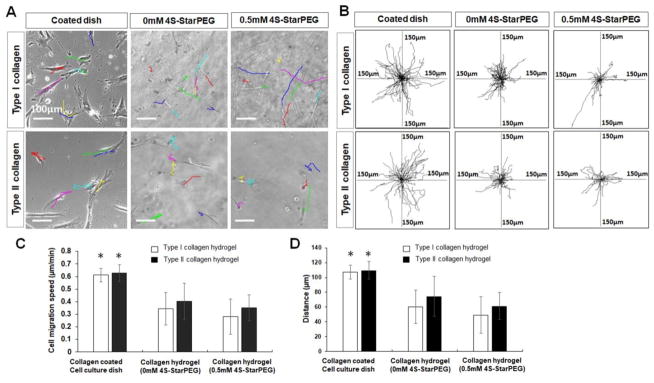
Migration of the DPSC cell culture in collagen hydrogels was studied. As shown in Figure 3A, the cell migration pathways are labeled with lines of different colors. The cell migration distance on the cell culture dish was higher than that in the non-crosslinked and crosslinked collagen hydrogels. In Figure 3B, each frame shows all superimposed migration tracks of DPSCs in at least four independent experiments. The position of all cells at t = 0 minute is represented by the origin (0, 0). Each line represents the migration track of one single cell over a 3-hour period.
Figure 3.
Migration of DPSCs in hydrogels. (A) DPSC cells migrated in cell culture dish or in hydrogels (each line indicates one migration track of a cell). Scale bar: 100 μm. (B) Cell migration paths determined by video monitor tracings (position of all cells at t = 0 min represented by origin position (center of frame), with migratory track of each cell at 3 hours plotted as single line on graph; each axis arm represents 150 μm of translocation distance). (C) Quantification of DPSC migration speed in hydrogels. (D) Quantification of cell migration distance in hydrogels. *, p < 0.05, compared with DPSC cell migration in corresponding type I or type II collagen hydrogel.
The migration speed and distance of DPSCs were quantified. The DPSC migration speed in type I collagen hydrogel (see supplemental video 1) and type I collagen hydrogel crosslinked with 4S-StarPEG (0.5 mM) was 0.34 ± 0.12 μm/min and 0.28 ± 0.14 μm/min, respectively, which were significantly lower than that on the type I collagen-coated cell culture dish of 0.6 ± 0.05 μm/min (P<0.05). The DPSC migration speed in type II collagen hydrogel (see supplemental video 2) and type II collagen hydrogel crosslinked with 4S-StarPEG (0.5 mM) was 0.40 ± 0.14 μm/min and 0.35 ± 0.10 μm/min, respectively, which were significantly lower than that on the type II collagen-coated cell culture dish of 0.62 ± 0.06 μm/min (P<0.05). The migration speed of DPSC cells in type I and type II collagen hydrogels was not significantly different.
The DPSC 3-hour migration distance on the type I collagen-coated cell culture dish, in type I collagen hydrogel, and crosslinked type I collagen hydrogel was 107.1 ± 9.2 μm, 60.0 ± 22.5 μm, and 49.1 ± 24.6 μm, respectively. The DPSC 3-hour migration distance of the type II collagen-coated cell culture dish, in type II collagen hydrogel and crosslinked type II collagen hydrogel was 109.6 ± 11.7 μm, 74.4 ± 26.8 μm, and 61.4 ± 18.1 μm, respectively.
Chondrogenic Differentiation of DPSCs
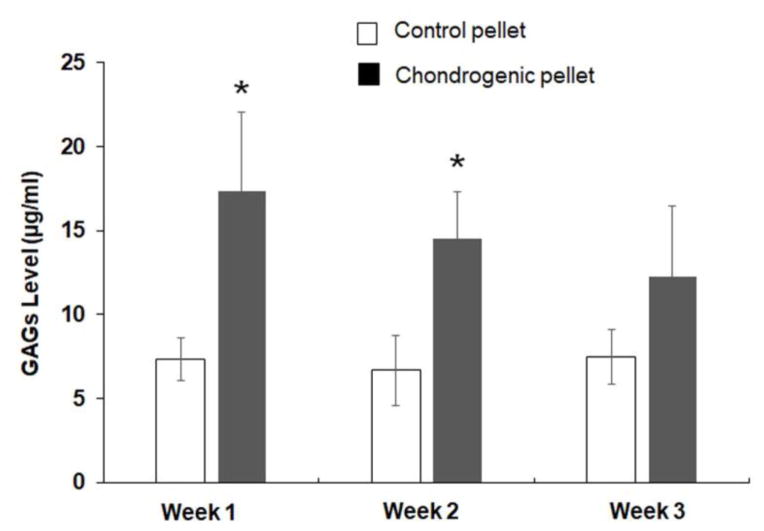
The chondrogenic differentiation of DPSCs was determined by the type II collagen antibody, aggrecan antibody, and sox 9 antibody. The undifferentiated DPSC cells and control pellets expressed aggrecan, but not type II collagen or sox 9. The chondrogenic pellets expressed type II collagen, aggrecan, and sox 9. After the chondrogenic pellets were cultured for 1 and 2 weeks, the DMMB assay showed that the levels of GAGs were 17.3 ± 4.7 μg/ml and 14.5 ± 2.7 μg/ml, respectively, which were significantly higher than that of the control pellets. However, after culturing for 3 weeks, the level of GAGs in the chondrogenic pellet medium was 12.2 ± 4.1 μg/ml, which is not significantly different from that in the control pellet medium of 7.4 ± 1.5 μg/ml.
Migration of DPSC-Derived Chondrogenic Cells in Collagen Hydrogels
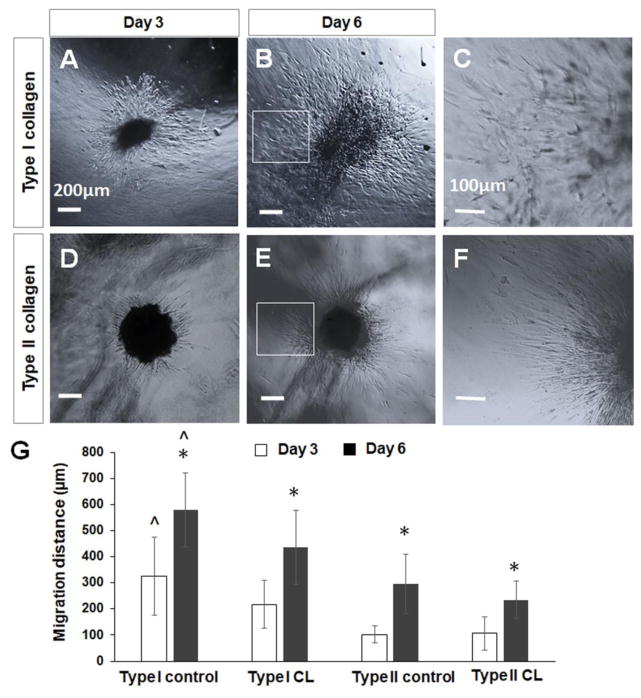
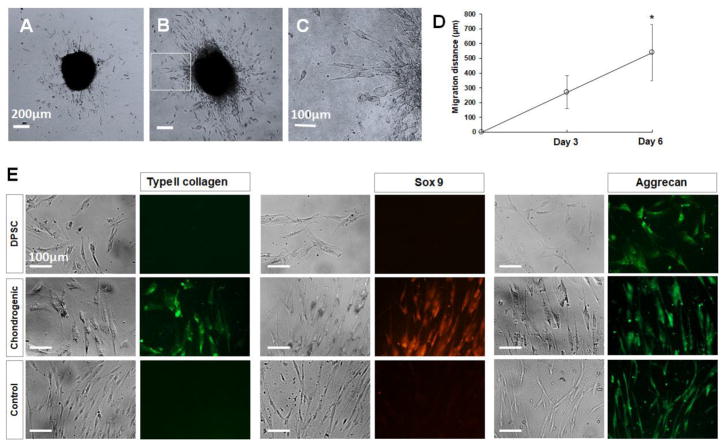
DPSC-derived chondrogenic cells migrated into the collagen hydrogels after the cell pellets were seeded in the hydrogels (Figures 6A–6F). The cell migration distance of chondrogenic cells in non-crosslinked collagen hydrogel was significantly higher (325.7 ± 150.8 μm, P<0.05) than that of cells in the type II collagen hydrogel (102.5 ± 32.8 μm) or 4S-StarPEG-crosslinked type I hydrogel (218 ± 92.5 μm) after culturing for 3 days. After culturing for 6 days, the cell migration distance in the non-crosslniked collagen hydrogel increased to 579.4 ± 142.5 μm, which is significantly higher than that in the type II collagen hydrogel (295 ± 140.4 μm, P<0.05) and crosslinked type I collagen hydrogel (436.3 ± 112.6 μm, P<0.05). The cell migration distances in the crosslinked-type II collagen hydrogels after culturing for 3 days or 6 days were 106.5 ± 54.6 μm and 234.2 ± 71.9 μm, respectively, which were significantly lower than that in the corresponding crosslinked type I collagen hydrogels.
Figure 6.
Migration of DPSC-derived chondrogenic cells of cell pellets in collagen hydrogels. (A–F) DPSC-derived chondrogenic cells migrated into collagen hydrogels from cell pellets. (A, B) Cell migration in type I collagen hydrogel. (C, D) Cell migration in type II collagen hydrogel. Scale bar: 200 μm. (C) Magnified images of inset indicated in (B). (F) Magnified images of inset indicated in (E). Scale bar: 100 μm. (G) Quantification of cell migration distance in collagen hydrogels from cell pellets. *, p < 0.05, compared with cell migration in corresponding collagen hydrogels after cell culturing for 3 days. ^, p < 0.05, compared with cell migration in corresponding type II collagen hydrogels and crosslinked type I and type II collagen hydrogels after cell culturing for 3 days and 6 days. CL, crosslinked collagen.
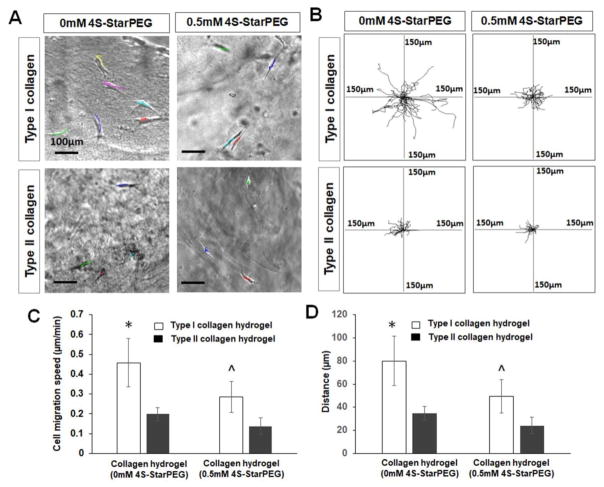
The migration of dissociated DPSC-derived chondrogenic cells was studied by a time-lapse imaging system. Figure 7A shows the cell migration pathways labeled with lines of different colors. The cell migration distance in type I collagen hydrogel (see supplemental video 3) was longer than that in type II collagen hydrogel (see supplemental video 4). Each frame of Figure 7(B) shows the superimposed cell migration tracks in at least four independent experiments. Each line represents the migration track of one single cell over a 3-hour period. The cell migration speed in type I collagen hydrogel was 0.45 ± 0.12 μm/min, which was significantly greater than that in type II collagen hydrogel (0.19 ± 0.03 μm/min, P<0.05) and in the crosslinked type I collagen hydrogel (0.27 ± 0.07 μm/min, P<0.05) (Figure 7C). The cell migration distance in type I collagen hydrogel was 80.0 ± 21.2 μm, which was significantly greater than that in type II collagen hydrogel (34.7 ± 5.9 μm, P<0.05) and in the crosslinked type I collagen hydrogel (47.1 ± 14.3 μm, P<0.05) (Figure 7D). The cell migration speed and distance in crosslinked type II collagen hydrogels were 0.13 ± 0.04 μm/min and 24.1 ± 7.3 μm, respectively, which were significantly lower than the corresponding migration speed and distance in crosslinked type I collagen hydrogel.
Figure 7.
Migration of DPSC-derived chondrogenic cells in hydrogels. (A) Cells migrated in collagen hydrogels (each line indicates one migration track of a cell). Scale bar: 100 μm. (B) Cell migration paths determined by video monitor tracings (position of all cells at t = 0 minute represented by origin position (center of frame), with migratory track of each cell at 3 hours plotted as single line on graph; each axis arm represents 150 μm of translocation distance). (C) Quantification of cell migration speed in hydrogels. (D) Quantification of cell migration distance in hydrogels. *, p < 0.05, compared with cell migration in type II collagen hydrogel and crosslinked type I and type II collagen hydrogels. ^, p < 0.05, compared with cell migration crosslinked type II collagen hydrogel.
Discussion
Stem cell therapy is a potential approach for the repair of the NP and the restoration of the function degenerated discs. MSCs isolated from bone marrow, cartilage endplate, umbilical cord blood, synovial tissue, and adipose tissue have been studied in the investigation of NP regeneration [8,19–27]. In animal studies, the transplantation of MSCs in intervertebral discs can restore the disc height, increase the extracellular matrix, and improve spine function [28–33]. In a clinical trial, the patients showed the preservation of biomechanics and the pain relief after the bone marrow-derived MSCs were injected into the nucleus pulposus of patients with degenerative disc disease [10]. DPSCs are neural crest-derived MSCs that are extracted from tooth pulp [13,14]. DPSCs have exhibited multipotency and regenerative capacity. DPSCs are an attractive postnatal type of stem cell because they are easily accessible and can be easily expanded in cell culture. DPSCs demonstrated similar differentiation capability as other MSCs. Although transplantation of bone marrow MSCs for the therapy of degenerative disc has been widely investigated, the research for the potential of DPSCs-based disc therapy has not been reported. Although no specific marker has been identified for NP cells, their trait resembles that of chondrocytes. A previous study showed that healthy adult human NP cells expressed the classical chondrocyte markers, including sox 9, collagen II, and aggrecan [12]. That study suggested that the chondrogenic differentiation of MSCs can potentially be applied to repair degenerated NP. In this study, DPSCs were differentiated as chondrogenic cells. The differentiation of the cells was confirmed by chondrogenic cell markers—type II collagen, aggrecan, and sox 9. We further investigated the motility of DPSCs and DPSCs-derived chondrogenic cell migration in type I and type II collagen hydrogels.
The function of natural biomaterials such as collagen, HA, fibrin, gelatin, alginate, and chitosan have been investigated as the matrix for NP cell growth and the carrier for cell transplantation for IVD regeneration [5,34–39]. The mechanical property of biomaterial hydrogel mimics that of NP, and it can potentially retain its original dimensions and water content after multicycle compression. Type II collagen is a major component of IVD, and the ratio of type II collagen and hyaluronic acid is 9:1 (w/w) in native NP tissue. Studies showed that type II collagen hydrogels can support bovine and human nucleus pulposus cell growth [15,17]. Crosslinking can enhance the material properties of collagen hydrogel against enzyme degradation. A previous study showed that crosslinking of collagen hydrogels with 4S-Star PEG reduced collagenase-induced type II collagen degradation [16]. We reported that when collagen microspheres were crosslinked with 4S-Star PEG, the degradation rate of these microspheres in collagenase solution was reduced [40].
Studies have shown that rat MSCs or bovine NP cells grew and proliferated in type II collagen hydrogels. The expression of type II collagen by rat MSCs in collagen hydrogel increased, compared to that of cells grown in a culture dish [16,17]. We reported that human NP cells have shown high cell viability when grown in type II collagen hydrogel or EDC-crosslinked type II collagen hydrogels [15]. After human-derived adipose tissue stromal cells (hADSCs) were cultured in hydrogel for 14 days, the expression of type II collagen and aggrecan increased [41]
Cell motility in collagen hydrogels using time-lapse imaging system has also been studied [18]. In a previous study, we showed that astrocytes dynamically migrated in type I collagen hydrogels (3.5 mg/ml) at the rate of 0.47μm/min. The crosslinking of type I collagen with 4S-StarPEG (0.05 mM) did not significantly alter the speed of astrocyte migration in type I collagen hydrogels. Here we studied the growth of DPSC growth and migration in both type I and type II collagen. DPSCs exhibited high cell viability in collagen hydrogels and 4S-Star PEG crosslinked collagen hydrogel. The DPSCs migrated in these hydrogels, and the migration speed was not significantly different in either hydrogel. We also observed that the crosslinking of collagen did not reduce the cell migration speed. This study indicates that after transplantation of DPSC cells in nucleus pulposus, the DPSC cells can dynamically migrate in the hydrogel, and potentially the cells can move toward the native nucleus pulposus and perform repair function.
Collagen has been investigated for the implantation in NP and cartilage. Cell motility is critical for the transplanted cells encapsulated within the hydrogel because the cells need to migrate into the host NP tissue to generate function. Studies have demonstrated the effect of collagen on chondrocyte and NP cell motility. The presence of type I collagen and type II collagen in a cell culture medium promoted chondrocyte migration in a modified Boyden chamber [42]. When NP cells were cultured on type I collagen matrices, they invaded the materials. That study suggested that the NP cells in local NP tissue can migrate into the collagen matrices and remodel the biomaterial scaffolds [43]. In this study, the cell pellets of DPSC-derived chondrogenic cells were seeded in collagen hydrogels, and the cells migrated into the collagen hydrogels. More cells migrated into the type I collagen hydrogels than those in type II collagen hydrogels. To investigate the motility of DPSC-derived chondrogenic cells in collagen hydrogels, the chondrogenic pellets were dissociated, and individual cell migration was recorded and analyzed by time-lapse imaging. Different from the findings of DPSC cell migration in collagen hydrogel, the DPSC-derived chondrogenic cells showed higher migration speed in type I collagen hydrogels than that in type II collagen hydrogels. The single-cell migration study confirmed that DPSC-derived chondrogenic cells are more motile in type I collagen hydrogels.
Biomaterials scaffolds fabricated by type I and type II collagen for the repair of cartilage defect have been broadly investigated [44–51]. However, few studies were performed to explore the potential of type I and type II collagen for NP repair. Studies showed that the NP can generate proteoglycan and collagens when they were seeded in type I and type II collagen scaffolds [52, 53]. In one study, the cell metabolism was analyzed after rabbit NP cells were grown in type I and type II collagen scaffolds and treated with TGF-β1 and/or BMP-2 [52]. The NP cells showed high proliferation rate in type I collagen scaffolds compared with that in type II collagen scaffolds. The proteoglycan synthesis of NP cells in type II collagen was significantly increased compared with that in type I collagen scaffolds. However, the aggrecan, collagen type I, and collagen type II gene expression of rabbit NP cells in type I and type II collagen scaffolds was significantly higher than the control group. In another study, bone marrow MSCs were grown in four types of matrixes and the chondrogenic markers were tested [54]. MSCs in type I collagen and gelatin matrixes produced more chondrogenic markers such as collagen type I, collagen type II (COL2) and aggrecan (ACAN) than the cells embedded in alginate or chitosan matrixes. In this study, we demonstrated the higher cell motility of DPSC and DPSCs-derived chondrogenic cells in type I collagen hydrogel compared with the cells in type II collagen hydrogels. These studies indicated that both type I and type II collagen can act as a carrier of DPSCs and DPSCs-derived chondrogenic cells for the repair the degenerative NP. The implanted cells can disperse into the native NP tissue. The implanted collagen hydrogels are degradable in vivo and they will be replaced by the proteoglycan and collagen produced by the implanted cells. In this study, we observed the decreased GAG production in cell culture medium by DPSCs-derived chondrogenic cells after the cell pellets were cultured for 3 weeks. However, to repair the generative disc, DPSCs-derived chondrogenic cell pellets will be digested as single cells and the dissociated cells will be encapsulated in the collagen hydrogels. Further studies will be needed to determine the generation of the GAGs and type II collagen by dissociated chondrogenic cells in collagen hydrogels.
Supplementary Material
DPSC migration in type I collagen hydrogels.
DPSC migration in type II collagen hydrogels.
DPSC-derived chondrogenic cell migration in type I collagen hydrogels.
DPSC-derived chondrogenic cell migration in type II collagen hydrogels.
Figure 4.
Migration and differentiation of DPSC-derived chondrogenic cells. (A–D) DPSC-derived chondrogenic cells migrated out of cell pellet after culturing on collagen-coated cell culture dish. Scale bar Figures A and B: 200 μm. Scale bar Figure C: 100 μm. (E) Cells that migrated out of the pellets labeled with anti-type II collagen, anti-sox 9, and anti-aggrecan antibodies. Scale bar: 100 μm.
Figure 5.
DMMB assay of level of GAGs in cell culture medium produced by chondrogenic pellets and control pellets. *, p < 0.05, compared with corresponding control pellets after pellet culturing for 1 week and 2 weeks.
Acknowledgments
We are grateful to Dr. Michael Heggeness for assistance in the preparation of this manuscript. This work was supported by Graduate Student Fellowship, Regional Institute on Aging; Wichita Medical Research and Education Foundation (WMREF); National Institute of General Medical Sciences (P20 GM103418) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 2.Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J. 2008;17(Suppl 4):452–458. doi: 10.1007/s00586-008-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008;17(Suppl 4):480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mern DS, Beierfuss A, Thome C, Hegewald AA. Enhancing human nucleus pulposus cells for biological treatment approaches of degenerative intervertebral disc diseases: a systematic review. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1583. [DOI] [PubMed] [Google Scholar]
- 5.Gan Y, Li P, Wang L, Mo X, Song L, Xu Y, Zhao C, Ouyang B, Tu B, Luo L, Zhu L, Dong S, Li F, Zhou Q. An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration. Biomaterials. 2017 Aug;136:12–28. doi: 10.1016/j.biomaterials.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Blanquer SB, Sharifi S, Grijpma DW. Development of poly(trimethylene carbonate) network implants for annulus fibrosus tissue engineering. J Appl Biomater Funct Mater. 2012;10:177–184. doi: 10.5301/JABFM.2012.10354. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Wu Y, Shao Z, Yang S, Che B, Sun C, et al. Functionalized self-assembling peptide nanofiber hydrogel as a scaffold for rabbit nucleus pulposus cells. J Biomed Mater Res A. 2012;100:646–653. doi: 10.1002/jbm.a.33300. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Zhou Y, Huang B, Liu LT, Liu MH, Wang J, et al. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A. 2014;20:908–920. doi: 10.1089/ten.TEA.2012.0703. [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1089/ten.TEA.2012.0703. [DOI] [PubMed] [Google Scholar]
- 10.Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822–828. doi: 10.1097/TP.0b013e3182298a15. [DOI] [PubMed] [Google Scholar]
- 11.Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016;14:253. doi: 10.1186/s12967-016-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaöz E, Demircan PC, Sağlam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol. 2011;136:455–473. doi: 10.1007/s00418-011-0858-3. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth CL, Janebodin K, Yuan AE, Dennis JE, Reyes M, Kim DH. Enhanced Chondrogenic Differentiation of Dental Pulp Stem Cells Using Nanopatterned PEG-GelMA-HA Hydrogels. Tissue Eng Part A. 2014;20:2817–2829. doi: 10.1089/ten.TEA.2013.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priyadarshani P, Li Y, Yang S, Yao L. Injectable hydrogel provides growth-permissive environment for human nucleus pulposus cells. J Biomed Mater Res A. 2016;104:419–26. doi: 10.1002/jbm.a.35580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collin EC, Grad S, Zeugolis DI, Vinatier CS, Clouet JR, Guicheux JJ, et al. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–70. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A. Type II collagen-hyaluronan hydrogel--a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134–48. doi: 10.22203/eCM.v020a12. [DOI] [PubMed] [Google Scholar]
- 18.Seyedhassantehrani N, Li Y, Yao L. Dynamic behaviors of astrocytes in chemically modified fibrin and collagen hydrogels. Integr Biol (Camb) 2016;8:624–34. doi: 10.1039/c6ib00003g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Zhou Y, Huang B, Liu LT, Liu MH, Wang J, et al. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A. 2014;20:908–920. doi: 10.1089/ten.TEA.2012.0703. [DOI] [PubMed] [Google Scholar]
- 20.Mercuri J, Addington C, Pascal R, 3rd, Gill S, Simionescu D. Development and initial characterization of a chemically stabilized elastin-glycosaminoglycan-collagen composite shape-memory hydrogel for nucleus pulposus regeneration. J Biomed Mater Res A. 2014;102:4380–4393. doi: 10.1002/jbm.a.35104. [DOI] [PubMed] [Google Scholar]
- 21.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 22.Feng G, Zhao X, Liu H, Zhang H, Chen X, Shi R, et al. Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: a comparison of 2 cell types as potential candidates for disc regeneration. J Neurosurg Spine. 2011;14:322–329. doi: 10.3171/2010.11.SPINE10285. [DOI] [PubMed] [Google Scholar]
- 23.Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28:1267–1275. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 24.Pei M, Shoukry M, Li J, Daffner SD, France JC, Emery SE. Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine (Phila Pa 1976) 2012;37:1538–1547. doi: 10.1097/BRS.0b013e31825150bf. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longo UG, Papapietro N, Petrillo S, Franceschetti E, Maffulli N, Denaro V. Mesenchymal stem cell for prevention and management of intervertebral disc degeneration. Stem Cells Int. 2012;2012:921053. doi: 10.1155/2012/921053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chun HJ, Kim YS, Kim BK, Kim EH, Kim JH, Do BR, et al. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg. 2012;78:364–371. doi: 10.1016/j.wneu.2011.12.084. [DOI] [PubMed] [Google Scholar]
- 28.Jin ES, Min J, Jeon SR, Choi KH, Jeong JH. Analysis of molecular expression in adipose tissue-derived mesenchymal stem cells: prospects for use in the treatment of intervertebral disc degeneration. J Korean Neurosurg Soc. 2013;53:207–212. doi: 10.3340/jkns.2013.53.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francisco AT, Mancino RJ, Bowles RD, Brunger JM, Tainter DM, Chen YT, et al. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials. 2013;34:7381–7388. doi: 10.1016/j.biomaterials.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 31.Park SH, Gil ES, Cho H, Mandal BB, Tien LW, Min BH, et al. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng Part A. 2012;18:447–458. doi: 10.1089/ten.TEA.2011.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson DG, Markova D, An HS, Chee A, Enomoto-Iwamoto M, Markov V, et al. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013;92:420–429. doi: 10.1097/PHM.0b013e31825f148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson DG, Markova D, An HS, Chee A, Enomoto-Iwamoto M, Markov V, et al. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013;92:420–429. doi: 10.1097/PHM.0b013e31825f148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J Bone Joint Surg Am. 2002;84:2022–2028. doi: 10.2106/00004623-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A. Type II collagen-hyaluronan hydrogel--a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134–148. doi: 10.22203/eCM.v020a12. [DOI] [PubMed] [Google Scholar]
- 36.Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine (Phila Pa 1976) 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 37.Abbushi A, Endres M, Cabraja M, Kroppenstedt SN, Thomale UW, Sittinger M, et al. Regeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degeneration. Spine (Phila Pa 1976) 2008;33:1527–1532. doi: 10.1097/BRS.0b013e3181788760. [DOI] [PubMed] [Google Scholar]
- 38.Mern DS, Beierfuss A, Thome C, Hegewald AA. Enhancing human nucleus pulposus cells for biological treatment approaches of degenerative intervertebral disc diseases: a systematic review. J Tissue Eng Regen Med. 2014;8:925–936. doi: 10.1002/term.1583. [DOI] [PubMed] [Google Scholar]
- 39.Chan SC, Gantenbein-Ritter B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy--feasible or fiction? Swiss Med Wkly. 2012;142:w13598. doi: 10.4414/smw.2012.13598. [DOI] [PubMed] [Google Scholar]
- 40.Berndt M, Li YC, Seyedhassantehrani N, Yao L. Fabrication and characterization of microspheres encapsulating astrocytes for neural regeneration. ACS Biomaterials Science & Engineering. 2016 Jul 5; doi: 10.1021/acsbiomaterials.6b00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirzner Y, Marcolongo M, Bhatia SK. Advances in biomaterials for the treatment of intervertebral disc degeneration. J Long Term Eff Med Implants. 2012;22:73–84. doi: 10.1615/JLongTermEffMedImplants.v22.i1.80. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu M, Minakuchi K, Kaji S, Koga J. Chondrocyte migration to fibronectin, type I collagen, and type II collagen. Cell Struct Funct. 1997;22:309–315. doi: 10.1247/csf.22.309. [DOI] [PubMed] [Google Scholar]
- 43.Bron JL, Mulder HW, Vonk LA, Doulabi BZ, Oudhoff MJ, Smit TH. Migration of intervertebral disc cells into dense collagen scaffolds intended for functional replacement. J Mater Sci Mater Med. 2012;23:813–821. doi: 10.1007/s10856-011-4545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulkkinen HJ, Tiitu V, Valonen P, Jurvelin JS, Rieppo L, Töyräs J, et al. Repair of osteochondral defects with recombinant human type II collagen gel and autologous chondrocytes in rabbit. Osteoarthritis Cartilage. 2013;21:481–490. doi: 10.1016/j.joca.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Han L, Zhang ZW, Wang BH, Wen ZK. Construction and biocompatibility of a thin type I/II collagen composite scaffold. Cell Tissue Bank. 2017 Aug 14; doi: 10.1007/s10561-017-9653-2. [DOI] [PubMed] [Google Scholar]
- 46.Lazarini M, Bordeaux-Rego P, Giardini-Rosa R, Duarte ASS, Baratti MO, Zorzi AR, et al. Natural Type II Collagen Hydrogel, Fibrin Sealant, and Adipose-Derived Stem Cells as a Promising Combination for Articular Cartilage Repair. Cartilage. 2017;8:439–443. doi: 10.1177/1947603516675914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, et al. Results 2 Years After Matrix-Associated Autologous Chondrocyte Transplantation Using the Novocart 3D Scaffold: An Analysis of Clinical and Radiological Data. Am J Sports Med. 2014;42:1618–27. doi: 10.1177/0363546514532337. [DOI] [PubMed] [Google Scholar]
- 48.Baek J, Sovani S, Glembotski NE, Du J, Jin S, Grogan SP, et al. Repair of Avascular Meniscus Tears with Electrospun Collagen Scaffolds Seeded with Human Cells. Tissue Eng Part A. 2016;22:436–448. doi: 10.1089/ten.TEA.2015.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan L, Li B, Yang J, Ni Y, Teng Y, Guo L, et al. Effects of Composition and Mechanical Property of Injectable Collagen I/II Composite Hydrogels on Chondrocyte Behaviors. Tissue Eng Part A. 2016;22:899–906. doi: 10.1089/ten.TEA.2015.0513. [DOI] [PubMed] [Google Scholar]
- 50.Bertolo A, Häfner S, Taddei AR, Baur M, Pötzel T, Steffen F, et al. Injectable microcarriers as human mesenchymal stem cell support and their application for cartilage and degenerated intervertebral disc repair. Eur Cell Mater. 2015;29:70–80. doi: 10.22203/eCM.v029a06. [DOI] [PubMed] [Google Scholar]
- 51.Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648–57. doi: 10.1177/0363546513518007. [DOI] [PubMed] [Google Scholar]
- 52.Lee KI, Moon SH, Kim H, Kwon UH, Kim HJ, Park SN, et al. Tissue engineering of the intervertebral disc with cultured nucleus pulposus cells using atelocollagen scaffold and growth factors. Spine (Phila Pa 1976) 2012;37:452–8. doi: 10.1097/BRS.0b013e31823c8603. [DOI] [PubMed] [Google Scholar]
- 53.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine (Phila Pa 1976) 2003;28:446–54. doi: 10.1097/01.BRS.0000048672.34459.31. [DOI] [PubMed] [Google Scholar]
- 54.Bertolo A, Mehr M, Aebli N, Baur M, Ferguson SJ, Stoyanov JV. Influence of different commercial scaffolds on the in vitro differentiation of human mesenchymal stem cells to nucleus pulposus-like cells. Eur Spine J. 2012;21(Suppl 6):S826–38. doi: 10.1007/s00586-011-1975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DPSC migration in type I collagen hydrogels.
DPSC migration in type II collagen hydrogels.
DPSC-derived chondrogenic cell migration in type I collagen hydrogels.
DPSC-derived chondrogenic cell migration in type II collagen hydrogels.