Abstract
Orofacial neuropathic pain caused by trigeminal nerve injury is a debilitating condition with limited therapeutic options. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels regulate neuronal excitability and are involved in the development and maintenance of chronic pain. However, the impact of HCN channel activity in the gasserian ganglion on trigeminal neuropathic pain has not been examined. We evaluated nociceptive behaviors after microinjection of the HCN channel blockers ZD7288 or ivabradine into the gasserian ganglion in rats with trigeminal nerve injury. Both blockers dose-dependently ameliorated evoked and spontaneous nociceptive behavior in rats with trigeminal neuropathic pain. Moreover, clinically available HCN channel blocker ivabradine showed a prolonged anti-nociceptive effect. In the gasserian ganglion, HCN1 and HCN2 are major HCN isoforms. After trigeminal nerve injury, the counts of both HCN1 and HCN2 immuno-positive punctae were increased in the ipsilateral gasserian ganglions. These results indicate that the increased HCN channel activity in the gasserian ganglion directly contributes to neuropathic pain resulting from trigeminal nerve injury.
Perspective
Trigeminal nerve damage-induced orofacial pain is severe and more resistant to standard pharmacological treatment than other types of neuropathic pain. Our study suggests that targeting HCN channel activities in the gasserian ganglion may provide an alternative treatment of trigeminal neuropathy including trigeminal neuralgia.
Keywords: trigeminal neuropathic pain, HCN channel, ZD7288, ivabradine, gasserian ganglion
Background
Orofacial pain is estimated to affect up to 7% of the general population36. Chronic pain caused by trigeminal nerve damage is serve and more debilitating than other types of neuropathic pain 4. Furthermore, neuropathic pain associated with the trigeminal nerve system is more resistant to treatments25, 36. Studies have shown that pain caused by facial nerve injury has different physiological and pathological characteristics compared with pain induced by spinal nerve injury 2, 20, 21, possibly due to the anatomical and molecular differences between facial and body sensory pathways 3, 31. Indeed, comprehensive RNA-Seq expression analysis reveals differences in the expression of ion channels and G-protein coupled receptors (GPCR) between the gasserian ganglion (GG) and the dorsal root ganglion (DRG) 13, 19.
Hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels (HCN1-4 isoforms) are widely expressed in peripheral sensory neurons in the DRG 15, 22 and GG 8, 9. In DRG neurons, inward current (Ih) generated by HCH channels contributes to nociceptor sensitization and pain by facilitating ectopic firing and hyper-excitability7, 15, 33, 34. Up-regulation of Ih current as well as changes in HCN protein expression has been observed in neurons in the DRG 1 and spinal cord 29 of rodents with peripheral nerve injury. Inhibition of HCN channel activity alleviates neuropathic and inflammatory pain 24, 29, 34. To date, it remains unclear whether HCN activity in the GG would impact nociceptive behavior and whether HCN protein expression in the GG would change after trigeminal nerve injury.
In this study, we utilized an improved trigeminal nerve injury model, in which the distal infraorbital nerve was subjected to chronic constriction injury (dIoN-CCI) 11, to investigate the role of HCN channels in trigeminal neuropathic pain. The results of behavioral study indicated that microinjection of HCN channel blockers into the GG ameliorated both evoked and non-evoked nociceptive behavior in dIoN-CCI rats and that clinically available HCN channel blocker ivabradine had a better anti-nociceptive effect than ZD7288. Immunohistochemical analysis showed that HCN1 and HCN2 immuno-positive puncta counts were increased in the GG of rats with dIoN-CCI injury.
Methods
Animals
Adult male Sprague–Dawley rats weighing 270-300 g were purchased from Charles River Laboratories (Wilmington, MA). The experimental protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Surgical procedures
Chronic constriction injury of infraorbital nerve (dIoN-CCI) and sham operation
The facial surface between the eye and whisker pad of a rat was gently shaved without damaging the whiskers. A 0.5 cm incision parallel to the mid-line was made starting at the caudal end of the third row of whisker lines towards the ipsilateral orbit. The superficial fascia was bluntly separated to expose the IoN trunk at its distal segment outside the orbital cavity. Two chromic catgut ligatures (4–0) were loosely tied around the distal part of the IoN (2 mm apart)11.
Rats in sham groups underwent the same surgical procedure including skin incision and the IoN nerve dissection except for the actual nerve ligation.
Implantation of a guide cannula for intra-gasserian ganglion microinjection
Drug or vehicle was microinjected into the GG of rats16, 18. Rats were anesthetized with intraperitoneal pentobarbital and placed in a Stoelting stereotaxic instrument. A guide cannula (C315G with an infusion cannula C315I, Plastics One, Roanoke, VA) was implanted next to the GG ipsilateral to the injury side. The implantation of guide cannula was performed according to the following coordinates from the rat brain atlas 26: 3.6 mm posterior to the Bregma, 2.8 mm lateral to the midline, and 11.2 mm ventral to the skull surface. The guide cannula was fixed to the skull using dental acrylic and jeweler's screws. A dummy cannula (33 gauge stainless steel wire) was inserted into the guide cannula to reduce the incidence of occlusion. Saline and drug solutions were microinjected into the GG site using a microinjection unit (33 gauge cannula) that extended 0.5 mm beyond the tip of the guide cannula. The microinjection unit was attached to a Hamilton microsyringe via polyethylene tubing (PE-10), and an infusion pump was programmed to deliver a volume of 1 μL over a period of 1 min into the GG site. The needle was held for 1 minute before retraction, and the injection site was confirmed by visual examination of GG upon the completion of each experiment. ZD7288 and ivabradine were purchased from Selleckchem and dissolved in sterile saline.
Behavioral tests
All behavioral experiments were carried out with the investigators being blinded to treatment conditions. Animals were habituated to the test environment for two consecutive days (30 minutes per day) before baseline testing.
Mechanical allodynia
Orofacial sensitivity to mechanical stimulation was tested using von Frey filaments 11, 32. The stimuli were applied within the IoN territory, near the center of the vibrissal pad. This area was stimulated on both sides of the face after surgery, i.e., ipsilateral and contralateral to the side where surgery had been performed. Stimuli were applied in an ascending order of intensity. The ipsilateral and contralateral sides were stimulated in a randomized order for each rat. The following criteria were used to determine a positive response to a filament: an immediate withdrawal reaction, attacking the filament by biting and grabbling, escaping by moving away from the filament, or asymmetric face stroke to the stimulated facial area. A threshold force of response (in grams) was defined as the first filament that evoked at least two reactions out of five applications 30.
Face grooming
The rat was placed into the Plexiglas enclosure and video recorded. Face-grooming episodes were counted over a period of 10 min after the rat was placed into the enclosure. A face-grooming episode was defined as an uninterrupted sequence of face-grooming action 11, 32.
Real time qRT-PCR
Total RNA was isolated from brain tissue using TRIzol (Life Technologies). cDNA was synthesized using ProtoScirpt II cDNA synthesis kit (New England Biolabs). qPCR was performed on QuantaStudio3 using the following Taqman probes from Applied Biosystems: HCN1 (Rn00670384_m1 and Rn01490048_m1), HCN2 (Rn01408575_gH and Rn01408575_gH), HCN3 (Rn00586666_m1), HCN4 (Rn00572232_m1), and GAPDH (Rn99999916_s1).
Immunohistochemistry and image analysis
Immunostaining was carried out as previously reported 37 using the following primary and secondary antibodies: mouse anti-HCN1 antibody (1:800; ab84816, Abcam), mouse anti-HCN2 antibody (1:1000; ab84817, Abcam), and FITC conjugated goat anti-mouse antibody (1:300; Jackson ImmunoResearch Laboratories Inc.). Primary antibody omission was used as a control. Two GG sections from each rat (4 sham rats and 4 dIoN-CCI rats) were stained and two micrographs from each GG section were captured with an Olympus fluorescence microscope. Micrographs taken with 40× objective were used for adequate resolution and saved in 16 bit format. ImageJ from NIH was used for puncta counting. To make the counting more robust to random noise, all images were smoothed first (using a 3×3 mean filter). As there were only a small number of neurons in each field, only the first four large neurons with clear morphology were selected and measured individually. To get a count, ImageJ's FindMaxima command was employed with a tolerance of 700.
Statistical analysis of behavioral data
Behavioral data were analyzed using two-way analysis of variance (ANOVA)37 as appropriate. One-way ANOVA was used to examine real time qRT-PCR and image analysis data. Post-hoc Waller-Duncan K-ratio t test was performed to determine the source(s) of differences. GraphPad Prizm5 software was used for the statistical analyses. All data were expressed as mean ± SEM and the statistically significant level was set at P<0.05.
Results
Gasserian ganglion microinjection of the HCN channel blocker ZD7288 improved nociceptive behavior in dIoN-CCI rats
Unilateral ligation of infraorbital nerve induces nociceptive behavior such as mechanical allodynia and intense unilateral facial grooming, which lasted for weeks (Fig 1A and B). To determine the impact of Ih inhibition in the gasserian ganglion (GG) on trigeminal neuropathic pain, nociceptive behavior was assessed in dIoN-CCI rats after microinjection of ZD7288 into the GG site ipsilateral to the dIoN-CCI side at fourteen days after injury. Both 0.1 μg and 1 μg of ZD7288 improved mechanical allodynia and the effect of 1 μg of ZD7288 lasted for 90 min (Fig 1C and E). Measured at 30 min after 1 μg of ZD7288 injection, the threshold of dIoN-CCI rats to mechanical stimulus nearly returned to its pre-injury (baseline) level. Asymmetry facial grooming was also reduced after 1 μg of ZD7288 infusion in the dIoN-CCI rats (Fig 1D). In contrast, the nociceptive behaviors in dIoN-CCI rats were not affected by saline (Fig 2A and B). ZD7288 (1μg) did not alter the baseline of either evoked or non-evoked nociceptive behavior in sham rats (Fig 2A and B). The injection site was confirmed by visual examination or methylene injection of the GG site upon the completion of each experiment (Fig 3A-C).
Fig 1. Gasserian ganglion microinjection of ZD7288 reduced nociceptive behavior in dIoN-CCI rats.
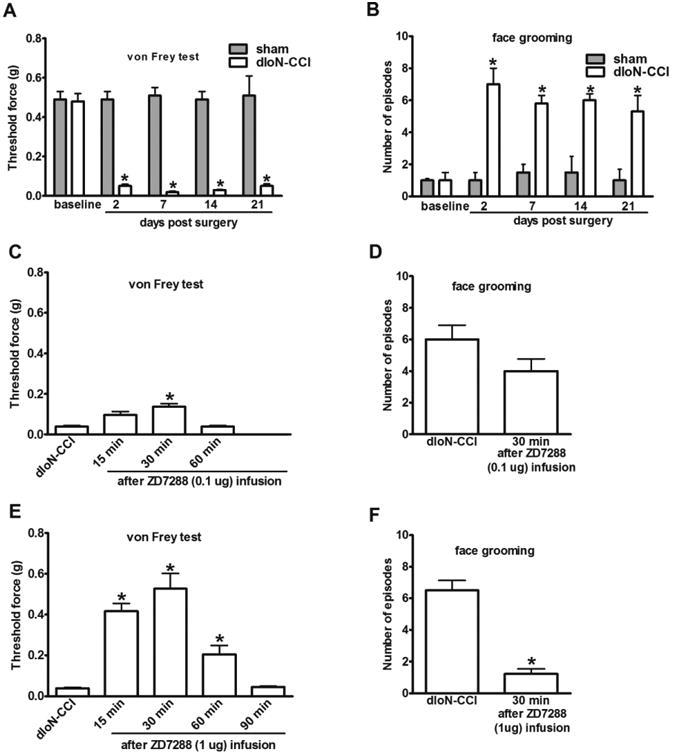
(A, B) dIoN-CCI induced mechanical allodynia and intense unilateral facial grooming, which lasted for weeks in rats (sham vs dIoN-CCI. *P<0.05, n = 6). (C, D) Infusion of 0.1 μg of ZD7288 to the GG site ipsilateral to the dIoN-CCI side transiently reduced mechanical allodynia measured at 30 min after drug administration. (E, F) Microinjection of 1μg of ZD7288 to the ipsilateral side of the GG reduced mechanical allodynia for at least 60 min. Facial grooming was also significantly reduced in dIoN-CCI rats. (before vs. after ZD7288 infusion * p<0.05, n = 6).
Fig 2. HCN blockers did not alter baseline nociception in sham rats when administered to the GG site.
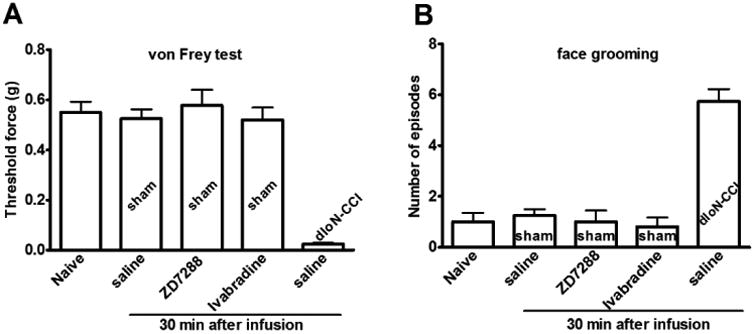
Saline, ZD7288 (1 μg) or ivabradine (1 μg) did not alter mechanical threshold force (A) or facial grooming (B) in sham rats measured at 30 min after being injected into the ipsilateral side of the GG. Naive vs. sham groups, p>0.05, n = 5. Microinjection of saline to the GG site did not change nociceptive behavior in dIoN-CCI (day 14) rats. Before vs. after saline injection, p>0.05, n=5.
Fig 3. Schematic drawing and anatomical demonstration of the GG injection site.
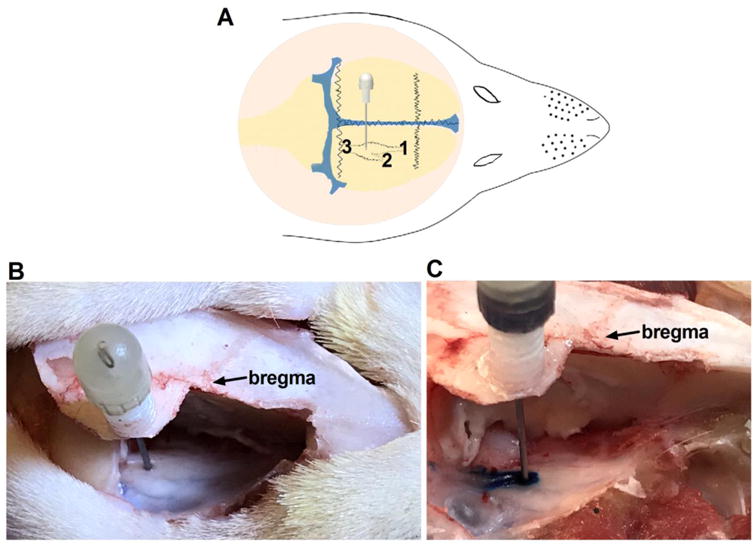
(A) Schematic drawing showing the site of the GG injection. The structure details: 1) V1/V2 (ophthalmic/maxillary), 2) V3 (mandibular), and 3) sensory root. (B) Anatomical position of the cannulation. (C) Methylene blue injection showing the drug injection site.
Clinically available HCN blocker ivabradine had a prolonged anti-nociceptive effect in IoN-CCI rats
Ivabradine is a specific and selective HCN blocker currently being used to treat heart failure 6. To further demonstrate that increased HCN channel activity in the GG contributes to trigeminal neuropathic pain, we examined the anti-nociceptive effect of ivabradine. At fourteen days after dIoN-CCI, microinjection of 0.01 μg of ivabradine into the ipsilateral GG only slightly improved mechanical allodynia after 15 min of infusion (Fig 4A). The nociceptive threshold was significantly increased at 15 min after microinjection of 0.1 μg or 1 μg of ivabradine, which lasted for 90 min or 120 min respectively (Fig 4C and E). Asymmetry facial grooming was also reduced after ivabradine (0.1 or 1 μg) infusion in the dIoN-CCI rats (Fig 4D and F). Ivabradine (1 μg) infusion into the GG did not alter the baseline of either evoked or non-evoked nociceptive behavior in sham rats (Fig 2A and B).
Fig 4. Gasserian ganglion microinjection of ivabradine produced a prolonged analgesic effect in dIoN-CCI rats.
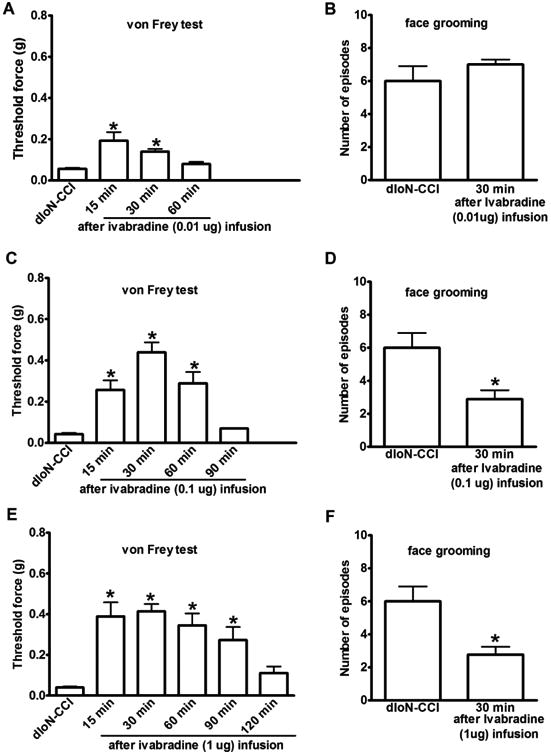
(A, B) Microinjection of 0.01 μg of ivabradine to the GG site ipsilateral to dIoN-CCI slightly reduced mechanical allodynia measured at 15 and 30 min, but facial grooming was not improved when examined at 30 min. (C-F) Microinjection of 0.1μg or 1μg of ivabradine to the ipsilateral GG site reduced mechanical allodynia and facial grooming in dIoN-CCI rats. Before vs. after ivabradine injection, *p<0.05, n=6.
HCN1 and HCN2 immuno-positive puncta counts were increased in the GG of dIoN-CCI rats
Previous studies suggest that four HCN isoforms are expressed in the DRG 17 and GG 8. HCN1 and HCN2 isoforms are considered to be relevant to pain signaling in peripheral and central nerve systems1, 10, 37. We analyzed mRNA levels of HCN isoforms in the GG using qPCR. We found that the mRNA levels of HCN1 and HCN2 were much higher than HCN3 and HCN4 in the GG (Fig 5). Immunohistochemistry also showed a high expression of both HCN1 and HCN2 protein in the GG of a sham rat (Fig 6A and D). Furthermore, at fourteen days after dIoN-CCI, the counts of HCN1 and HCN2 immuno-positive punctae were increased in the ipsilateral GG of dIoN-CCI rats compared with sham rats (Fig 6).
Fig 5. HCN1 and HCN2 isoforms were predominately expressed in the rat GG.
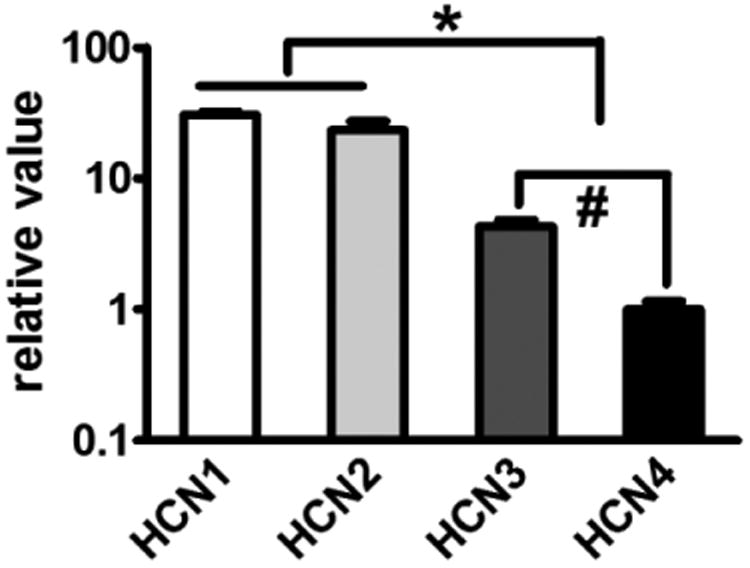
qRT-PCR analysis of HCN1-4 mRNA levels in the rat GG. HCN1-2 vs. HCN3-4, * p<0.05, n=5; HCN3 vs. HCN4, #p<0.05, n=5.
Fig 6. HCN1 and HCN2 immunostaining of the GG sections.
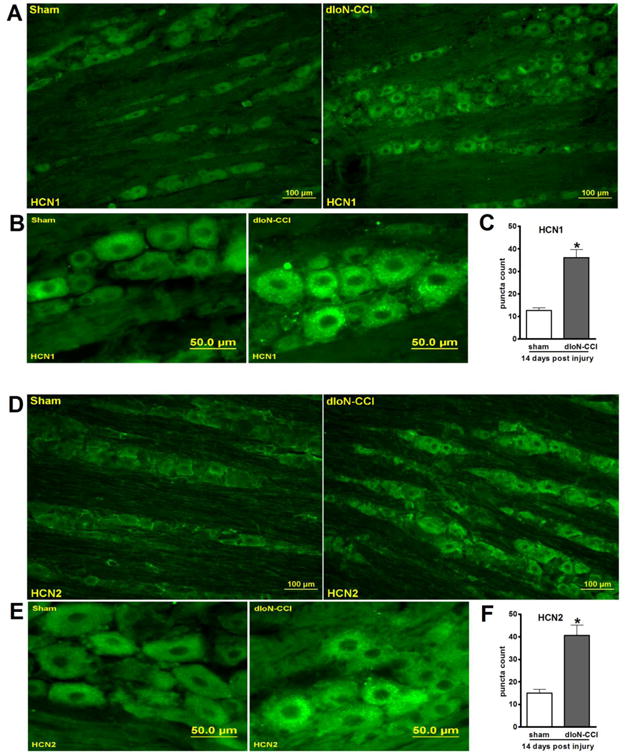
Representative micrographs of the ipsilateral GG isolated from sham or dIoN-CCI (14 days) rats were stained with anti-HCN1 (A-B) or anti-HCN2 (D-E) antibody. The ipsilateral GG of dIoN-CCI rat showed increase in HCN1 (C) and HCN2 (F) immuno-positive puncta counts. *p<0.001, n=4
4. Discussion
Damage to the trigeminal nerve causes debilitating orofacial pain, which is known to be more resistant to treatment than other types of neuropathic pain. We report that microinjection of the HCN blocker ZD7288 or ivabradine into the GG site ameliorated both evoked and non-evoked nociceptive behaviors in dIoN-CCI rats with trigeminal neuropathic pain. Our behavioral data suggested that clinically available HCN channel blocker ivabradine had a strong anti-nociceptive effect. Among four HCN isoforms, we found that expression levels of HCN1 and HCN2 isoforms were higher than those of HCN3 and HCN4 isoforms in the GG. We also observed increase in HCN1 and HCN2 immuno-positive puncta counts in the ipsilateral GG of dIoN-CCI rats.
Spinal nerve injury or inflammation induced HCN channel dysfunction, which has been associated with spontaneous ectopic firing of DRG sensory neurons contributing to the development and maintenance of pain 7, 12, 27, 33. Tissue specific deletion of HCN2 in DRG sensory neurons reduced mechanical hyperalgesia in mice with CFA-induced inflammatory pain27. Despite the known presence of HCN channels in the GG8, the role of HCN channel activity in the GG in trigeminal neuropathic pain condition has not been studied. Using microinjection of HCN channel blockers into the GG, our study provides evidence that increased HCN channel activity in the GG contributes to the behavioral manifestation of chronic pain after trigeminal nerve injury.
All four HCN channel subunits (HCN1-4) have been detected in the sensory neurons in the DRG 9, 17, 23 and GG 8, 9. In DRG neurons, HCN1and HCN2 are the dominant isoforms17, 23 related to pain after nerve injury7, 27. In this study, we found that the expression of HCN1 and HCN2 mRNA was much higher than that of HCN3 and HCN4 in the rat GG. To quantify the observed visual difference in HCN1 and HCN2 immunostaining of the GG sections between sham and dIoN-CCI rats, we performed HCN1 and HCN2 immuno-positive puncta counting for the statistical analysis. To get a count, ImageJ's FindMaxima command was employed with a tolerance of 700. A tolerance or threshold of 700 was found to be less sensitive to variation in the final intensity of acquired immunostaining micrograph in our case. Compared to sham, dIoN-CCI neurons had significant higher counts of HCN1 or HCN2 immune-positive puntae (both p<0.001). The puncta counting is considered more suitable for quantifying the immunostaining to reflect nerve injury induced changes in HCN channels in the GG in this study. First, the majority of the punctae of HCN1 and HCN2 immunostaining signal were of similar size and intensity, suggesting that each puncta represents a uniform complex. In a report by Fox PD et al 14, a K+ channel similar to HCN, was expressed in HEK cells and the punctae of immunostain were found to represent single channels. So our puncta counts may represent the quantity of the active form of the channel more appropriately than a simple intensity metric. Second, the lack of a proper internal control, which should be consistent and reliable, prevents an accurate normalization of the immunofluorescence signal. Without a good normalization, staining efficiency and photobleaching associated variation in immunosignal rendered the integrated pixel intensity method far less sensitive than desirable.
We compared the anti-nociceptive effect of two HCN channel blockers ZD7288 and ivabradine. ZD7288 has been widely used as a selective HCN blocker to study the role of HCN channel activity in heart tissue and neurons. The analgesic effect of ZD7288 is consistent with a role of HCN activity in pain condition. However, one study suggested that ZD7288 may also block Na+ channel in the DRG (ex vivo) and in vitro 35, suggesting that inhibition of Na+ channel could partially contribute to the anti-nociceptive effect of ZD7288. Ivabradine is a clinical drug that selectively and specifically blocks HCH current of cardiac sinoatrial node (SAN) 6, which is mainly HCN4 current. Studies indicate that ivabradine is also very effect in blockage of HCN1 current 6. Our data showed anti-nociceptive effect of both ZD7288 and ivabradine in dIoN-CCI rats when administered directly to the GG site, supporting the notion that increased HCN channel activity in the GG is an underlying mechanism of trigeminal neuropathic pain. Of interest to note that ivabradine exhibited prolonged analgesic effects in dIoN-CCI rats. The differences in molecular mechanism of HCN channel blockage between ZD7288 28 and ivabradine 6 may contribute to their differences in analgesic effect for neuropathic pain.
All known HCN blockers cause bradycardia resulting from blocking the sinoatrial HCN46. Thus, broad HCN channel blockers may not be useful when applied systemically in neuropathic pain management. In contrast, administering a drug at the sites of peripheral pain transmission may avoid the side effects of systemic delivery. A recent study demonstrated that under the guidance of computed tomography (CT), a drug can be delivered precisely around the DRGs to decrease pain transmission in swine5. Our study suggests that microinjection of an HCN blocker around the GG site might provide an alternative treatment of trigeminal neuropathic pain because the GG is enclosed in the Meckel's cave which is readily accessible via needle access. This approach, coupled with the development of specific blockers of HCN1 and HCN2, would be clinically beneficial to patients with trigeminal neuropathic pain.
Highlights.
Infraorbital nerve injury (dIoN-CCI) induced trigeminal neuropathic pain (TNP).
HCN1 and HCN2 were the major HCN isoforms in the gasserian ganglion (GG).
Inhibition of HCN channel activity in the GG ameliorated TNP.
HCN blocker ivabradine showed a prolonged analgesic effect.
HCN1 and HCN2 expression was increased in the GG after dIoN-CCI.
Acknowledgments
This work was supported by NIH grants R01 DE022901 and R01 DE018214 (J.M.) and National Natural Science Foundation of China 81771194 (S.Z.).
Footnotes
Disclosures: The authors declare no competing financial interests.
Author contributions: Z.Y. and J.M. conceived the project, designed the experiments and wrote the manuscript. W.D., Z.Y., S.S., L.G., and J.D. performed the experiments and collected the data. J.Y. performed statistical and image analysis. S.Z, Y.Z., and L.C. helped with manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acosta C, McMullan S, Djouhri L, Gao L, Watkins R, Berry C, Dempsey K, Lawson SN. HCN1 and HCN2 in Rat DRG neurons: levels in nociceptors and non-nociceptors, NT3-dependence and influence of CFA-induced skin inflammation on HCN2 and NT3 expression. PloS one. 2012;7:e50442. doi: 10.1371/journal.pone.0050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117:280–291. doi: 10.1016/j.pain.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Bae JY, Kim JH, Cho YS, Mah W, Bae YC. Quantitative analysis of afferents expressing substance P, calcitonin gene-related peptide, isolectin B4, neurofilament 200, and Peripherin in the sensory root of the rat trigeminal ganglion. The Journal of comparative neurology. 2015;523:126–138. doi: 10.1002/cne.23672. [DOI] [PubMed] [Google Scholar]
- 4.Barros Vde M, Seraidarian PI, Cortes MI, de Paula LV. The impact of orofacial pain on the quality of life of patients with temporomandibular disorder. Journal of orofacial pain. 2009;23:28–37. [PubMed] [Google Scholar]
- 5.Brown JD, Saeed M, Do L, Braz J, Basbaum AI, Iadarola MJ, Wilson DM, Dillon WP. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Science translational medicine. 2015;7:305ra145. doi: 10.1126/scitranslmed.aac6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucchi A, Tognati A, Milanesi R, Baruscotti M, DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. The Journal of physiology. 2006;572:335–346. doi: 10.1113/jphysiol.2005.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HJ, Furness JB, Jennings EA. Postnatal maturation of the hyperpolarization-activated cation current, I(h), in trigeminal sensory neurons. Journal of neurophysiology. 2011;106:2045–2056. doi: 10.1152/jn.00798.2010. [DOI] [PubMed] [Google Scholar]
- 9.Cho HJ, Staikopoulos V, Ivanusic JJ, Jennings EA. Hyperpolarization-activated cyclic-nucleotide gated 4 (HCN4) protein is expressed in a subset of rat dorsal root and trigeminal ganglion neurons. Cell and tissue research. 2009;338:171–177. doi: 10.1007/s00441-009-0869-8. [DOI] [PubMed] [Google Scholar]
- 10.Ding W, You Z, Shen S, Chen L, Zhu S, Mao J. Inhibition of HCN channel activity in the thalamus attenuates chronic pain in rats. Neuroscience letters. 2016;631:97–103. doi: 10.1016/j.neulet.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding W, You Z, Shen S, Yang J, Lim G, Doheny JT, Chen L, Zhu S, Mao J. An Improved Rodent Model of Trigeminal Neuropathic Pain by Unilateral Chronic Constriction Injury of Distal Infraorbital Nerve. The journal of pain : official journal of the American Pain Society. 2017 doi: 10.1016/j.jpain.2017.02.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djouhri L, Al Otaibi M, Kahlat K, Smith T, Sathish J, Weng X. Persistent hindlimb inflammation induces changes in activation properties of hyperpolarization-activated current (Ih) in rat C-fiber nociceptors in vivo. Neuroscience. 2015;301:121–133. doi: 10.1016/j.neuroscience.2015.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Flegel C, Schobel N, Altmuller J, Becker C, Tannapfel A, Hatt H, Gisselmann G. RNA-Seq Analysis of Human Trigeminal and Dorsal Root Ganglia with a Focus on Chemoreceptors. PloS one. 2015;10:e0128951. doi: 10.1371/journal.pone.0128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox PD, Loftus RJ, Tamkun MM. Regulation of Kv2.1 K(+) conductance by cell surface channel density. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1259–1270. doi: 10.1523/JNEUROSCI.3008-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao LL, McMullan S, Djouhri L, Acosta C, Harper AA, Lawson SN. Expression and properties of hyperpolarization-activated current in rat dorsal root ganglion neurons with known sensory function. The Journal of physiology. 2012;590:4691–4705. doi: 10.1113/jphysiol.2012.238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katagiri A, Shinoda M, Honda K, Toyofuku A, Sessle BJ, Iwata K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Molecular pain. 2012;8:23. doi: 10.1186/1744-8069-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouranova EV, Strassle BW, Ring RH, Bowlby MR, Vasilyev DV. Hyperpolarization-activated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: correlation with hyperpolarization-activated current gating. Neuroscience. 2008;153:1008–1019. doi: 10.1016/j.neuroscience.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Kubo A, Shinoda M, Katagiri A, Takeda M, Suzuki T, Asaka J, Yeomans DC, Iwata K. Oxytocin alleviates orofacial mechanical hypersensitivity associated with infraorbital nerve injury through vasopressin-1A receptors of the rat trigeminal ganglia. Pain. 2017;158:649–659. doi: 10.1097/j.pain.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 19.Manteniotis S, Lehmann R, Flegel C, Vogel F, Hofreuter A, Schreiner BS, Altmuller J, Becker C, Schobel N, Hatt H, Gisselmann G. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PloS one. 2013;8:e79523. doi: 10.1371/journal.pone.0079523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michot B, Bourgoin S, Viguier F, Hamon M, Kayser V. Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain. 2012;153:1939–1948. doi: 10.1016/j.pain.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Michot B, Deumens R, Hermans E. Immunohistochemical comparison of astrocytic mGluR5 upregulation in infraorbital nerve- versus sciatic nerve-ligated rat. Neuroscience letters. 2017;653:113–119. doi: 10.1016/j.neulet.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Momin A, Cadiou H, Mason A, McNaughton PA. Role of the hyperpolarization-activated current Ih in somatosensory neurons. The Journal of physiology. 2008;586:5911–5929. doi: 10.1113/jphysiol.2008.163154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. European journal of biochemistry. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- 24.Noh S, Kumar N, Bukhanova N, Chen Y, Stemkowsi PL, Smith PA. The heart-rate-reducing agent, ivabradine, reduces mechanical allodynia in a rodent model of neuropathic pain. European journal of pain. 2014;18:1139–1147. doi: 10.1002/j.1532-2149.2014.00460.x. [DOI] [PubMed] [Google Scholar]
- 25.Obermann M. Treatment options in trigeminal neuralgia. Therapeutic advances in neurological disorders. 2010;3:107–115. doi: 10.1177/1756285609359317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2007 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 27.Schnorr S, Eberhardt M, Kistner K, Rajab H, Kasser J, Hess A, Reeh P, Ludwig A, Herrmann S. HCN2 channels account for mechanical (but not heat) hyperalgesia during long-standing inflammation. Pain. 2014;155:1079–1090. doi: 10.1016/j.pain.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Shin KS, Rothberg BS, Yellen G. Blocker state dependence and trapping in hyperpolarization-activated cation channels: evidence for an intracellular activation gate. The Journal of general physiology. 2001;117:91–101. doi: 10.1085/jgp.117.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takasu K, Ono H, Tanabe M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain. Pain. 2010;151:87–96. doi: 10.1016/j.pain.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 31.Voisin DL, Domejean-Orliaguet S, Chalus M, Dallel R, Woda A. Ascending connections from the caudal part to the oral part of the spinal trigeminal nucleus in the rat. Neuroscience. 2002;109:183–193. doi: 10.1016/s0306-4522(01)00456-0. [DOI] [PubMed] [Google Scholar]
- 32.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan Y. Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain. Sheng Li Xue Bao. 2008;60:579–580. [PubMed] [Google Scholar]
- 34.Weng X, Smith T, Sathish J, Djouhri L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Adelta-nociceptors. Pain. 2012;153:900–914. doi: 10.1016/j.pain.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Liao L, Liu X, Luo F, Yang T, Li C. Is ZD7288 a selective blocker of hyperpolarization-activated cyclic nucleotide-gated channel currents? Channels. 2012;6:438–442. doi: 10.4161/chan.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zakrzewska JM. Multi-dimensionality of chronic pain of the oral cavity and face. The journal of headache and pain. 2013;14:37. doi: 10.1186/1129-2377-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, You Z, Wang S, Yang J, Yang L, Sun Y, Mi W, Yang L, McCabe MF, Shen S, Chen L, Mao J. Neuropeptide S modulates the amygdaloidal HCN activities (I) in rats: Implication in chronic pain. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.02.004. [DOI] [PubMed] [Google Scholar]


