Abstract
American Indian communities are at greater risk of hypertension and cardiovascular disease than the general US population and are exposed to greater cadmium levels. However, cadmium's effect on blood pressure is unclear. This study assesses the association between baseline urinary cadmium and longitudinal changes in blood pressure in American Indian communities. Cadmium was measured in 3047 baseline urine samples from Strong Heart Study participants from three geographic areas. Longitudinal changes in blood pressure across three study visits (1989–1999) were modeled using linear mixed models by baseline log urinary cadmium to creatinine ratio. Hypertension risk was evaluated using interval-censored survival analysis. Higher levels of urinary cadmium at baseline were associated with faster rates of increase in diastolic and systolic blood pressure (P [trend] = .001 and .02, respectively). The estimated change in diastolic and systolic blood pressures per year was 0.18 mm Hg (0.05–0.31) and 0.62 mm Hg (0.37–0.87) in the upper quintile of cadmium level compared with −0.11 mm Hg (−0.24 to 0.02) and 0.21 mm Hg (−0.04 to 0.46) in the lowest, respectively. A one-unit increase in log-transformed urinary cadmium was associated with 10% greater hypertension risk (95% confidence interval: 1.01-1.20). In conclusion, blood pressure of individuals with greater baseline levels of urinary cadmium increased at a faster rate relative to those with lower levels.
Keywords: Heavy metals, high blood pressure, indigenous population
Introduction
Since 2000, approximately a quarter of the adult population worldwide had hypertension, defined as diastolic blood pressure (DBP) measurement of ≥90 mm Hg or systolic blood pressure (SBP) measurements of ≥140 mm Hg.1 The prevalence of hypertension in the United States is higher at 29.1%. Hypertension is a leading cause of cardiovascular disease (CVD)3 and chronic kidney disease.4 Although smoking, body mass index (BMI), diet, and physical inactivity are known to contribute to hypertension, an increasing body of evidence supports that metalloids and metals, such as arsenic, lead, and cadmium, may play a role.5,6
Cadmium is a heavy metal known to have a toxic effect on human kidneys and the skeletal and respiratory systems.7 Sources of human exposure include active and passive smoking, diet, and occupational exposures, including iron and steel production and phosphate fertilizers.8,9 Primarily, cadmium accumulates in the kidneys where it has a half-life of 10–35 years.10 This accumulation can lead to renal tubular dysfunction.11 Cadmium exposure is also associated with an increased CVD risk, including peripheral arterial disease,12 heart failure,13 stroke,13 and myocardial infarction.14
American Indian communities are exposed to higher cadmium levels than the US population.15 Furthermore, they are at greater risk of CVD16 and other metabolic disorders including diabetes and obesity, than the general US population,17,18 highlighting these individuals as an at-risk population.
Previous studies have identified an adverse effect of metals on blood pressure (BP) and hypertension risk, including cadmium.19,20 However, findings have been inconsistent. A recent meta-analysis showed a positive association between blood cadmium and BP in women, but the study was limited by a small sample of population-based studies.21 Some studies have not found evidence of a relationship between cadmium and subsequent BP levels,22 whereas ours and other studies have identified an association, but lacked temporality due to a cross-sectional design.23,24
Within this context, we studied data collected from American Indian communities from the Northern and Southern plains and the Southwest and sought to address the nature of the relationship between urinary cadmium, an established biomarker of cadmium exposure,25 and both longitudinal BP and hypertension risk.
Materials and Methods
Study Population
The Strong Heart Study (SHS) is a prospective cohort study of American Indian men and women. The study commenced from 1989 to 1991, recruiting men and women aged 45–75 years from American Indian communities based in Arizona, Oklahoma, and North and South Dakota. All eligible individuals in the communities in Arizona and Oklahoma were invited to participate, whereas a cluster sampling technique was used to invite participants from North and South Dakota communities.26 Three thousand five hundred sixteen individuals (78% of those invited) consented to take part in the study and were included in this analysis, and 468 individuals were excluded as they lacked either a urinary cadmium or creatinine measurement. A further 200 individuals were excluded from the BP analysis, and 183 individuals were excluded from the hypertension analysis, due to missing covariate data and either BP measurements or hypertension data, respectively. This resulted in a cohort of 2853 in the BP analyses and 2865 in the hypertension analyses. All participants provided written and oral informed consent. The protocol for the SHS was approved by the Institutional Indian Health Service Review Boards, institutional review boards and by the participating communities.
Data Collection
Participants reported sociodemographic factors, smoking status, and medical history in a baseline questionnaire. A physical examination provided anthropometric measures, including height, weight, and BP, with a fasting blood test and spot urine collection. Participants were followed up between 1993 and 1995, and between 1998 and 1999. Deaths occurring during the follow-up were confirmed through the Indian Health Service or private hospital records and through direct contact by study personnel with participants' families or other informants, and included medical records, autopsy reports, and informant interviews; all materials were independently reviewed by physician members of the SHS study's morbidity and mortality committees.27 Fatal coronary heart disease was defined by the occurrence of fatal myocardial infarction or sudden death. Other fatal events were classified as definitive or possible fatal stroke, or definitive or possible heart failure. Follow-up went from 1989 to 1991 through December 31, 2006.
Urinary Cadmium Measurement
The methodology has been described previously.15 Briefly, spot urine samples were collected in polypropylene tubes in the morning of the baseline visit and frozen within 2 hours. After defrosting, dilution, and centrifugation, the cadmium concentration was measured using inductively coupled plasma mass spectrometry (Agilent 7700× ICP-MS; Agilent Technologies, Waldbronn, Germany). The “Seronorm Trace Elements Urine Blank” (SERO AS, Bill-ingstad, Norway) was used for quality control. The limit of detection for urinary cadmium was 0.015 μg/L; three samples were below the limit of detection. The intra-assay and interassay coefficients of variation for cadmium in the SHS were 1.3% and 8.7%, respectively.
To account for kidney function differences, automated alkaline picrate methodology was used to assay urinary creatinine.26 Creatinine-adjusted urinary cadmium levels were calculated from the ratio of urinary cadmium to creatinine. Quintiles of log-transformed creatinine-adjusted urinary cadmium were calculated, with the lowest quintile as the reference group, and when using continuous measures of cadmium exposure, the logarithm was taken to normalize the distribution.
BP Measurement and Hypertension Definition
During the physical examination at each visit, brachial artery BP (first and fifth Korotkoff sounds) was measured three consecutive times by a mercury sphygmomanometer (WA Baum Co). Participants were seated and rested for five minutes before the measurement. An appropriately sized cuff was placed on the right arm, pulse occlusion pressure was determined, and the cuff was inflated to 20 mm Hg above that pressure. The mean of the last two measurements was used to estimate the BP.
Hypertension was defined as the use of antihypertension medication, or a SBP of 140 mm Hg or greater or a DBP of 90 mm Hg or greater. A constant was added to the BP of individuals using antihypertensive medication, an established method to adjust for medication use, that has less bias and greater power than other methods.28 A 10 mm Hg constant was added to SBP and 5 mm Hg was added to DBP.
Covariates
Smoking was defined as ever, never, or current. Current smokers were defined as those who reported smoking more than 100 cigarettes in their lifetime and stated they currently smoked. BMI was calculated from weight in kilograms divided by the square of height in meters. Years of education and physical activity, through walking and leisure activity, were self-reported at baseline. From the latter, the metabolic equivalent of the task per week was calculated.29
Diabetes was defined by use of diabetic medication, a fasting glucose measurement of ≥126 mg/dL, or HbA1c measurement of ≥6.5%. Individuals were recorded as having a history of prior heart conditions if they reported a previous heart attack, heart failure, heart catheterization, or another heart condition.
Triglycerides, total cholesterol and high-density lipoprotein cholesterol (HDL-c), were measured from blood samples. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease-Epidemiology Collaboration creatinine equation based on serum creatinine, measured by an alkaline picrate rate method, by sex, and age.30
Urine arsenic species were used to assess long-term arsenic exposure using thawed urine samples, using up to 1 mL of urine, in 2007 (Trace Element Laboratory, Graz University, Graz, Austria). The analytical methods and quality control for arsenic analysis have been described previously.31 The sum of inorganic and methylated arsenic species concentrations were determined by high-performance liquid chromatography linked with inductively coupled mass spectrometry.
Statistical Analyses
Participant characteristics were summarized by quintiles of creatinine-adjusted urinary cadmium measurement. Continuous variables were presented by median and interquartile range and compared by using Kruskal-Wallis tests. Categorical variables were presented as number and percentage, and compared by using χ2 tests. SBP, DBP, and pulse pressure (PP), adjusted for antihypertensive treatment, were summarized separately for each visit.
Mixed effects linear models were used to estimate the linear trends over time for SBP, DBP, and PP separately in relation to log-transformed creatinine-adjusted cadmium at baseline, modeling the exposure both in quintiles and continuously. As less than 5% of individuals had missing data on relevant variables, there was minimal data loss, and, as we have no reason to believe the missing data are not missing at random, all models were constructed using individuals with complete data.
The first model included baseline age, centered at 44 years, the square of that term, recruiting center, and an interaction between creatinine-adjusted urinary cadmium in quintiles and time (duration since first visit) as fixed effects. The same terms were included in the fully adjusted model along with baseline measurements of: eGFR, BMI, duration of education, diabetes, smoking history, alcohol consumption status, physical activity during the previous week (measured in metabolic equivalent of the task), tri-glycerides, total cholesterol, and HDL-c levels. In both models, time was included as a random effect in addition to the random intercept for each subject.
The estimated annual changes in SBP, DBP, and PP were calculated separately for each quintile, and the mean and 95% confidence interval (CI) predicted at 5-year intervals were estimated for each quintile from the mixed linear models and the results displayed graphically.
Hypertension was assessed prospectively, and risk was modeled using a Cox regression model. Censoring occurred at intervals as hypertension was only ascertained at visits. To take into account this censoring, an interval-censored survival analysis program was used. The time scale was duration since the first visit, the event of interest was hypertension, and individuals were censored at the last visit if they were never hypertensive. Urinary cadmium levels were both categorized into quintiles, with the lowest quintile as the reference group, and treated continuously using the log-transformed creatinine-adjusted cadmium.
Using individuals with complete data on the relevant covariates, associations with hypertension were initially assessed in unadjusted models. A subsequent model (model 1) adjusting for sex, age, and center was constructed. Model 2 further adjusted for eGFR, BMI, education level, alcohol consumption, diabetes status, triglycerides, total cholesterol, HDL-c, and physical activity level.
As smoking is a common determinant of both cadmium level and hypertension, in both the unadjusted and fully adjusted models, the change in the association with and without adjustment for smoking was compared using only individuals with complete data on smoking and all relevant covariates from model 2.
Sensitivity analyses were conducted to assess the consistency of the BP findings. The analyses were stratified by smoking status, center, and sex. A Bonferroni correction was applied to account for multiple sensitivity analyses that were conducted. Five further analyses, evaluating (1) BP unadjusted for reported antihypertensive treatment, (2) exclusion of individuals with a history of heart conditions, (3) exclusion of individuals with baseline hypertension, (4) adjusting for packs per year to reflect the quantity of cigarettes smoked, and (5) adjusting for the sum of inorganic and methylated urinary arsenic, were also conducted.
All analyses were conducted on STATA release 14.2 (Stata Corp, College Station, TX, USA).
Results
Descriptive Statistics
The characteristics of the 3047 SHS participants are shown by quintile of baseline urinary cadmium measurement (Table 1). Several demographic, lifestyle, and clinical characteristics varied by quintile of urinary cadmium except for SBP in visit 3, PP in visit 1 and 3, and total cholesterol levels. When examining characteristics by visit, we found no evidence that duration of education, leisure activity, and the proportion of individuals at each center varied over time, although all other characteristics did (eTable 1).
Table 1. Descriptive characteristics by baseline quintiles of creatinine-adjusted urinary cadmium.
| Quintiles of creatinine-adjusted urinary cadmium | All | Q1 | Q2 | Q3 | Q4 | Q5 | P-Value |
|---|---|---|---|---|---|---|---|
| N (%) | 3047 (100%) | 599 (19.80%) | 613 (20.10%) | 607 (19.90%) | 618 (20.30%) | 610 (20.00%) | |
| Systolic blood pressure (adjusted) —mm Hg (IQR) | |||||||
| Visit 1 | 126 (114–140) | 128 (118–141) | 128 (117–142) | 125 (113–138) | 125 (112–140) | 123 (111–138) | <0.01 |
| Visit 2 | 129 (116–144) | 131 (118–144) | 131 (119–145) | 129 (115–145) | 126 (113–141) | 127 (114–144) | <0.01 |
| Visit 3 | 133 (120–148) | 133 (122–149) | 134 (121–148) | 133 (119–148) | 132 (120–147) | 131 (118–146) | 0.15 |
| Diastolic blood pressure (adjusted)— mm Hg (IQR) | |||||||
| Visit 1 | 77 (70–84) | 80 (73–87) | 78 (71–85) | 76 (70–84) | 76 (69–84) | 74 (68–81) | <0.01 |
| Visit 2 | 75 (69–83) | 78 (72–87) | 76 (70–84) | 75 (69–82) | 75 (68–82) | 73 (67–80) | <0.01 |
| Visit 3 | 76 (69–83) | 78 (70.5–85) | 75 (69–84) | 75 (69–83) | 75 (69–82) | 75 (68–80) | <0.01 |
| Pulse pressure (adjusted)—mm Hg (IQR) | |||||||
| Visit 1 | 48 (40–59) | 48 (40–59) | 49 (41–60) | 48 (40–59) | 49 (40–60) | 48 (40–59) | 0.66 |
| Visit 2 | 52 (43–64) | 51 (42–63) | 54 (44–65) | 53 (44–65) | 51 (42–63) | 53 (43–68) | 0.03 |
| Visit 3 | 57 (47–69) | 56 (46–67) | 57 (48–69) | 57 (47–69) | 57 (46–68) | 56 (46–69) | 0.67 |
| Age—y (IQR) | 55.3 (49.4–62.6) | 54 (48.0–61.5) | 55.2 (49.4–62.9) | 55.2 (29.4–62.9) | 56 (49.8–63.5) | 55.9 (50.1–62.4) | <0.01 |
| Body mass index—kg/m2 (IQR) | 29.7 (25.0–33.8) | 30.6 (27.6–35.1) | 30.4 (27.5–33.9) | 29.7 (25.7–33.3) | 29.4 (25.7–33.3) | 28.4 (25.0–32.7) | <0.01 |
| Urinary cadmium—mg/g (IQR) | 1.1 (0.6–1.8) | 0.6 (0.4–0.8) | 0.9 (0.6–1.2) | 1.1 (0.7–1.6) | 1.4 (0.9–2.1) | 2.3 (1.4–3.6) | <0.01 |
| Log creatinine-adjusted urinary cadmium level—mg/g (IQR) | 0 (−;0.5 to 0.4) | −;0.9 (−;1.1 to −;0.7) | −;0.4 (−;0.5 to 0.3) | 0 (−;0.1 to 0.0) | 0.3 (0.2–0.4) | 0.8 (0.6–1.1) | <0.01 |
| Duration of education—y (IQR) | 12 (10–14) | 12 (11–14) | 12 (10–14) | 12 (10–13) | 12 (10–12) | 11 (9–12) | <0.01 |
| eGFR—mL/min/1.73 m2 (IQR) | 73.2 (61.3–86.7) | 65 (56.1–75.4) | 71.6 (59.6–84.7) | 74.3 (62.3–86.4) | 75.6 (64.1–88.6) | 79.9 (67.7–94.7) | <0.01 |
| Triglycerides—mg/dL (IQR) | 119 (83–172) | 130 (94–185) | 119.5 (88–166) | 118 (81–172.5) | 116 (79–164) | 113 (76–169) | <0.01 |
| Total cholesterol—mg/dL (IQR) | 193 (169–218) | 192.5 (168–217) | 194 (169–216) | 192 (170.5–220) | 191 (168–218) | 194 (170–219) | 0.87 |
| HDL cholesterol—mg/dL (IQR) | 44 (37–52) | 41 (35–49) | 43 (37–51) | 44 (37–53) | 45 (38–54) | 45 (38–55) | <0.01 |
| Leisure activity per wk—METs (IQR) | 3.5 (0–18) | 7 (0–24.5) | 5.4 (0–20.0) | 3.5 (0–17.5) | 3.5 (0–17.5) | 3.5 (0–14.0) | <0.01 |
| Smoking status—n (%) | |||||||
| Ex-smoker | 1021 (100.00) | 259 (25.40) | 242 (23.70) | 201 (19.70) | 169 (16.60) | 150 (14.70) | <0.01 |
| Nonsmoker | 885 (100.00) | 225 (25.40) | 179 (20.20) | 177 (20.00) | 162 (18.30) | 142 (16.10) | |
| Smoker | 1139 (100.00) | 115 (10.10) | 191 (16.80) | 229 (20.10) | 287 (25.20) | 317 (27.80) | |
| Diabetes—n (%) | 1289 (100.00) | 285 (22.10) | 276 (21.40) | 262 (20.30) | 223 (17.30) | 243 (18.90) | <0.01 |
| Hypertension—n (%) | 1146 (100.00) | 267 (21.60) | 248 (18.40) | 211 (18.40) | 227 (19.80) | 193 (16.80) | <0.01 |
| Alcohol consumption—n (%) | |||||||
| Ever drank | 1307 (100.00) | 272 (20.80) | 269 (20.60) | 258 (19.70) | 255 (19.50) | 253 (19.40) | <0.01 |
| Never drank | 452 (100.00) | 72 (15.90) | 85 (18.80) | 87 (19.30) | 101 (22.40) | 107 (23.70) | |
| Current drinker | 1279 (100.00) | 251 (19.60) | 258 (20.20) | 261 (20.40) | 262 (20.50) | 247 (19.30) | |
| Male—n (%) | 1278 (100.00) | 423 (33.10) | 297 (23.20) | 250 (19.60) | 202 (15.80) | 106 (8.30) | <0.01 |
eGFR, estimated glomerular filtration rate; HDL cholesterol, high-density lipoprotein cholesterol; IQR, interquartile range.
Cadmium and Longitudinal BP
There was little difference in baseline SBP between cadmium quintiles in either the minimally or fully adjusted linear mixed models (Figures 1A and B). Over 10 years, SBP increased within all quintiles. However, in the fully adjusted model, the increase was greatest in the upper quintile of cadmium, with an average increase per year of 0.62 mm Hg (0.37–0.87) in SBP, and least in the lowest quintile, 0.21 mm Hg (−0.04 to 0.46) in SBP (P-value for difference across all quintiles = 0.044) (Table 2). At the end of follow-up, the mean SBP for individuals was estimated to be 135.00 mm Hg (132.93–137.07) in the upper quintile and 132.54 mm Hg (130.48–134.60) for individuals in the lowest quintile of cadmium (Table 3).
Figure 1.
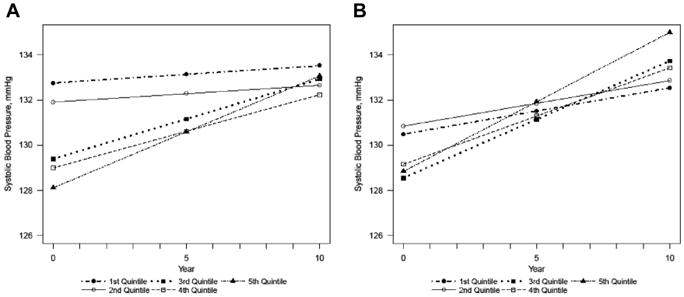
Estimated systolic blood pressure over time by quintiles of creatinine-adjusted urinary cadmium, in the (A) minimally adjusted and (B) fully adjusted models. Minimally adjusted for sex, age, age2, center. P-value for change over time: .055. Fully adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity. P-value for change over time: .044.
Table 2. Estimated average blood pressure changes per year by quintiles of creatinine-adjusted urinary cadmium, n = 2853.
| Quintiles of creatinine-adjusted urinary cadmium | Q1 | Q2 | Q3 | Q4 | Q5 | P-Value |
|---|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | ||||||
| Minimally adjusteda | 0.08 (−0.17 to 0.33) | 0.07 (−0.17 to 0.32) | 0.35 (0.11–0.60) | 0.32 (0.08–0.57) | 0.50 (0.24–0.75) | 0.06 |
| Fully adjustedb | 0.21 (−0.04 to 0.46) | 0.20 (−0.04 to 0.45) | 0.52 (0.27–0.77) | 0.43 (0.18–0.67) | 0.62 (0.37–0.87) | 0.04 |
| Diastolic blood pressure (mm Hg) | ||||||
| Minimally adjusteda | −0.14 (−0.27 to -0.01) | −0.18 (−0.31 to −0.05) | 0.04 (−0.09 to 0.18) | −0.01 (−0.14 to 0.12) | 0.15 (0.02–0.28) | <0.01 |
| Fully adjustedb | −0.11 (−0.24 to 0.02) | −0.14 (−0.27 to −0.01) | 0.10 (−0.12 to 0.14) | 0.01 (−0.12 to 0.14) | 0.18 (0.05–0.31) | <0.01 |
| Pulse pressure (mm Hg) | ||||||
| Minimally adjusteda | 0.21 (0.02–0.41) | 0.25 (0.06–0.44) | 0.31 (0.11–0.50) | 0.33 (0.13–0.52) | 0.35 (0.15–0.55) | 0.85 |
| Fully adjustedb | 0.31 (0.11–0.51) | 0.34 (0.15–0.53) | 0.42 (0.22–0.61) | 0.42 (0.22–0.61) | 0.42 (0.24–0.63) | 0.86 |
181 individuals were excluded due to missing covariates.
Adjusted for age, age2, sex, and recruiting center.
Adjusted for age, age, sex, recruiting center, estimated glomerular filtration rate, body mass index, duration of education, diabetes, smoking history, alcohol consumption status, metabolic equivalent of task of leisure physical activity during the previous week, triglycerides, total cholesterol, and high-density lipoprotein cholesterol levels.
Table 3. Estimated average blood pressure measurement by quintiles of creatinine-adjusted urinary cadmium in fully adjusted model, n = 2853.
| Quintiles of creatinine-adjusted urinary cadmium | Q1 | Q2 | Q3 | Q4 | Q5 |
|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | |||||
| 0 y | 130.48 (128.65-132.31) | 130.84 (129.13-132.55) | 128.54 (126.84-130.24) | 129.15 (127.46-130.84) | 128.84 (127.05-130.62) |
| 5 y | 131.51 (130.02-133.01) | 131.85 (130.48-133.22) | 131.14 (129.76-132.51) | 131.29 (129.90-132.67) | 131.92 (130.45-133.39) |
| 10 y | 132.54 (130.48-134.60) | 132.86 (130.91-134.81) | 133.73 (131.76-135.71) | 133.43 (131.43-135.42) | 135.00 (132.93-137.07) |
| Diastolic blood pressure (mm Hg) | |||||
| 0 y | 77.59 (76.62-78.56) | 78.09 (76.74-78.55) | 75.96 (75.06-76.87) | 76.92 (76.02-77.81) | 75.51 (74.57-76.46) |
| 5 y | 77.04 (76.30-77.79) | 77.21 (76.26-77.62) | 76.47 (75.79-77.15) | 76.95 (76.26-77.64) | 76.41 (75.68-77.14) |
| 10 y | 76.49 (75.47-77.51) | 76.33 (75.27-77.20) | 76.98 (76.00-77.96) | 76.98 (75.99-77.97) | 77.31 (76.28-78.34) |
| Pulse pressure (mm Hg) | |||||
| 0 y | 52.91 (51.52-54.30) | 53.25 (51.95-54.55) | 52.57 (51.28-53.87) | 52.26 (50.97-53.54) | 53.25 (51.90-54.61) |
| 5 y | 54.47 (53.34-55.59) | 54.95 (53.91-55.98) | 54.65 (53.61-55.68) | 54.34 (53.29-55.38) | 55.43 (54.31-56.54) |
| 10 y | 56.02 (54.42-57.62) | 56.64 (55.12-58.16) | 56.72 (55.18-58.27) | 56.41 (54.86-57.97) | 57.60 (55.99-59.21) |
Adjusted for age, age2, sex, recruiting center, estimated glomerular filtration rate, body mass index, duration of education, diabetes, smoking history, alcohol consumption status, metabolic equivalent of task of leisure physical activity during the previous week, triglycerides, total cholesterol, and high-density lipoprotein cholesterol levels. 181 individuals were excluded due to missing covariates.
When treated continuously, the per unit increase of log-transformed, creatinine-adjusted urinary cadmium was associated with an estimated increase of 0.19 mm Hg (0.04–0.34) in SBP per year in the fully adjusted model, P = .015.
Baseline DBP measurements were higher for individuals in the lower quintiles, and lower for individuals in the upper quintiles of cadmium regardless of adjustment (Figures 2A and B). After 10 years of follow-up, estimated DBP was lower for individuals in the lower quintile, whereas they increased in the upper quintile of cadmium. In the fully adjusted model, the estimated increase in DBP per year in the upper quintile was 0.18 mm Hg (0.05–0.31), whereas the DBP measurement decreased per year in the lowest quintile, −0.11 mm Hg (−0.24 to 0.02) (P-value for difference across all quintiles = 0.001) (Table 2).
Figure 2.
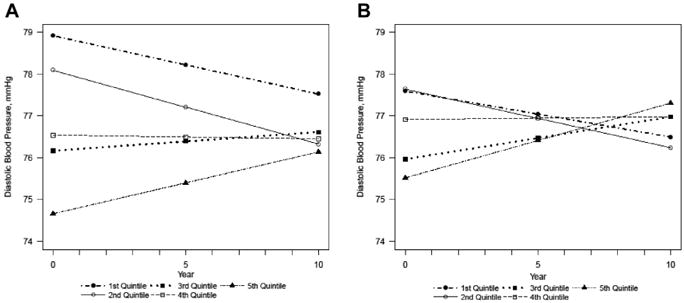
Estimated diastolic blood pressure over time by quintiles of creatinine-adjusted urinary cadmium, in the (A) minimally adjusted and (B) fully adjusted models. Minimally adjusted for sex, age, age2, center. P-value for change over time: .002. Fully adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity. P-value for change over time: .001.
At the end of follow-up, the estimated DBP in the lowest quintile was 76.49 mm Hg (75.47–77.51), and it was 77.31 mm Hg (76.28–78.34) in the upper quintile (Table 3). Consistent with this finding, per unit the log-transformed, creatinine-adjusted urinary cadmium was associated with an estimated increase in DBP of 0.13 mm Hg per year (0.05–0.21) (P = .001).
There were no consistent differences in PP between the quintiles at baseline (Figure 3A and B). At the end of the follow-up, the greatest estimated increase in PP was found in the upper quintile, 0.42 mm Hg (0.24–0.63), and the smallest change was estimated in the lowest quintile of cadmium, 0.31 mm Hg (0.11–0.51) (P = .858) in the fully adjusted model. The PP in the lowest quintile was estimated to be 56.02 mm Hg (54.42–57.62) at the end of the study, whereas the PP in the upper quintile was 57.60 mm Hg (55.99–59.21). Similarly, no evidence of a change in PP was found per year with log-transformed, creatinine-adjusted urinary cadmium, 0.06 mm Hg (−0.06 to 0.18) (P = .35).
Figure 3.
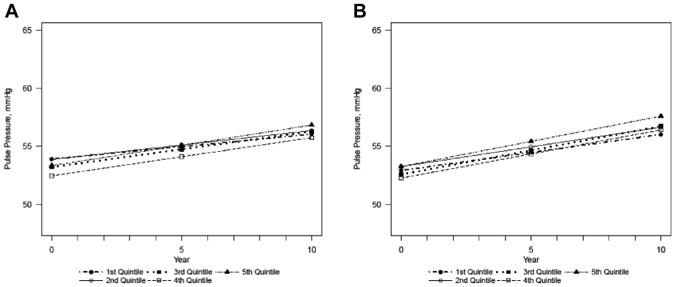
Estimated pulse pressure over time by quintiles of creatinine-adjusted urinary cadmium, in the (A) minimally adjusted and (B) fully adjusted models. Minimally adjusted for sex, age, age2, center. P-value for change over time: .848. Fully adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption and physical activity. P-value for change over time: .858.
Cadmium and Hypertension
The hazard ratio (HR) and 95% CI for hypertension per one unit increase in log-transformed urinary cadmium in the unadjusted interval-censored Cox regression model was 1.02 (0.95–1.10). After adjustment for additional covariates, the HR increased and became statistically significant, HR = 1.10 (1.01–1.20) (Table 4).
Table 4. Hazard ratios and 95% confidence intervals for the association between creatinine-adjusted urinary cadmium level and risk of hypertension n = 2865.
| Incident Cases/N | Unadjusted | Model 1 | Model 2 | |
|---|---|---|---|---|
| Continuous | 626/2865 | 1.02 (0.95–1.10) | 1.09 (1.01–1.19) | 1.10 (1.01–1.20) |
| First quintile | 114/564 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Second quintile | 132/582 | 0.99 (0.85–1.15) | 1.03 (0.89–1.21) | 1.04 (0.89–1.22) |
| Third quintile | 141/583 | 0.94 (0.81–1.10) | 1.02 (0.87–1.20) | 1.04 (0.89–1.23) |
| Fourth quintile | 106/580 | 1.03 (0.88–1.21) | 1.12 (0.95–1.32) | 1.15 (0.97–1.36) |
| Fifth quintile | 133/556 | 1.02 (0.87–1.20) | 1.15 (0.97–1.37) | 1.17 (0.97–1.40) |
Model 1: Sex, age, and center.
Model 2: Model 1 + estimated glomerular filtration rate, body mass index, education level, alcohol consumption, diabetes status, triglycerides, total cholesterol, high-density lipoprotein cholesterol, physical activity level. 182 individuals excluded due to missing covariates.
Hypertension risk was greatest in the fourth and fifth quintiles, 1.15 (0.97–1.36) and 1.17 (0.97–1.40), respectively, relative to the 1st quintile, but none of the HRs were statistically significant, which was consistent regardless of adjustment level (Table 4) and there was no statistical evidence of differences between quintiles (P = .91 for the test for differences across all quintiles). Further adjustment for smoking had minimal impact on the magnitude of the association when evaluating cadmium levels either continuously or categorically (eTable 2).
Sensitivity Analyses
After applying a Bonferroni correction, there was little evidence that the estimated temporal changes in SBP, DBP, and PP varied by sex, center, or smoking status (eFigures 1–3, eTables 3–5). In addition, exclusion of individuals with a history of heart conditions, those with baseline hypertension, conducting the BP analyses unadjusted for hypertension treatment, and adjustment for packs per year did not materially alter our findings (eFigures 4 and 5, eTables 6–9). Finally, further adjustment for the sum of inorganic and methylated arsenic had negligible impact on the results.
Discussion
Main Findings
A positive independent relationship was found between creatinine-adjusted urinary cadmium and longitudinal SBP and DBP. Likewise, we found evidence that suggests that the risk of hypertension increased with greater levels of urinary cadmium.
SBP increased for individuals in all quintiles, reflecting age-dependent increases in BP. Across the 10 years of the study, the average SBP for individuals in the upper quintile increased by 6.16 mm Hg. For individuals in the lowest quintile, the increase was smaller, 2.06 mm Hg. Increased SBP is associated with coronary heart disease events, stroke, and all-cause mortality. For each 3 mm Hg difference in SBP, it has been estimated that there is a 5% difference in coronary mortality, an 8% difference in stroke mortality, and a 4% difference in all deaths, while a 5 mm Hg lower SBP lowers mortality by 9% for coronary deaths, 14% for stroke deaths, and 7% for all deaths.32 Therefore, the described increases in SBP over time in upper quintiles of urinary cadmium are of public health importance.
There was little absolute difference in DBP across the 10 years of the study. DBP in the lowest quintile decreased from 77.59 mm Hg (76.62–78.56) to 76.49 mm Hg (75.47– 77.51), an average decrease of 1.10 mm Hg. There was a small increase in DBP in the upper quintile, from 75.51 mm Hg (74.57–76.46) to 77.31 mm Hg (76.28– 78.34), a change of 1.80 mm Hg. A reduction in DBP, as occurs in the lower quintiles, has been estimated to decrease the prevalence of CVD. A 2 mm Hg reduction in the population average of DBP for the white US residents, 35–64 years old, would lead to a 17% reduction in the prevalence of hypertension, a 14% reduction in stroke and transient ischemic attacks risk, and a 6% reduction in the coronary heart disease risk.33 We did not find significant differences in PP, which could occur when SBP increases with minimal changes in DBP.
Interpretation in Light of Other Evidence
Previous studies have reported mixed results; some have found evidence for raised SBP or DBP,34–37 whereas other studies have found no association.20,22,38–41 A previous meta-analysis focusing on occupational exposure found an increased risk of hypertension relative to those without occupational exposure, odds ratio = 1.81 (1.03–3.20), and an increase in SBP and DBP.19 Similarly, animal models have demonstrated an increased prevalence of hypertension on cadmium exposure,42 whereas an ecological study found a negative association.43 In addition, in a cross-sectional study, a stronger association was noted in women,23 which was not replicated in the current analysis.
The diversity of previous findings may result from differing methodologies, as reflected by the large amount of between-study heterogeneity that was noted in the previous meta-analyses.19,21 Heterogeneity may arise from multiple sources, including the method used to measure cadmium exposure. Cadmium can be measured in a range of biological samples including, blood, urine, nails, and hair. Null findings have been reported with hair40 and toenail measurements,20 but the validity of using toenails as a biomarker has been questioned, as in some studies cadmium toenail measurements have not be found to vary by smoking status.20
The study populations also differed, ranging from white elderly men20 to preschool children41 and the general Korean population.37 Cadmium exposure levels ranged from low20 to high.40 Differences in participants' age will also have affected the prevalence of hypertension already found in the population as well as the prevalence of other factors that affect BP. Participants' age varied from a mean of 72 years20 to a mean of 45 years19 and a minimum age of 20 years22 in studies of hypertension.
Importantly, some previous studies only used a single BP measure, rather than taking the average of several measures at a single time point,20 which may have resulted in nondifferential bias, attenuating any association.
In spite of the inconsistencies in methodology and findings in previous studies, there are a number of mechanisms that may underpin any relationship between cadmium exposure and raised BP. Animal studies have provided evidence for cadmium's adverse role in DNA methylation,44 oxidative stress,45 inflammation,46 endothelial dysfunction, increased vasoconstriction,47 activation of the sympathetic nervous system,47 and reduced coronary flow,48 whereas studies of in vitro human cells have provided further evidence for endothelial dysfunction,49 renal tubular damage, and sodium retention.11
Strengths and Limitations
This study has a number of strengths. It focuses on an underserved ethnic group of American Indians. This is an important population, who have high rates of CVD,50 dia-betes51, and chronic kidney disease,52 as well as greater than population average body burden of metals.53 In addition, this is a population-based study of individuals who are exposed to lower levels of metals than individuals with occupational exposure, thus providing a relevant public health estimate of risk and evidence that cadmium exposure remains a risk factor for hypertension at lower levels.
Methodological strengths include repeat standardized BP measurement. The study also uses BP measured longitudinally, ensuing temporality between exposure and outcome. In addition, with 10 years of follow-up available and an average recruitment age of 55.2 years, the study duration was sufficient to identify hypertension cases. This, combined with a large sample size, ensured sufficient power to not only assess the direct relationship but also conduct sensitivity analyses by subsections of the population.
This study is limited by a single measure of cadmium obtained at baseline visit, and fluctuations may have occurred over the 10 years of the study. However, urinary cadmium reflects body burden, and the biological half-life of cadmium in the urine has been estimated to be 13.6 years (9.0–28.2) for men and 13.9 years (11.2–19.4) for women, making this less of a concern.54
In addition, it was difficult to assess the impact of mortality as a competing interest when modeling hypertension risk.
Individuals diagnosed with hypertension after baseline may have altered their behavior, changing their dietary habits or smoking status, based on the diagnosis, which may have affected BP levels. However, based on the information from multiple visits, the majority of individuals diagnosed with hypertension after the study's onset did not alter their smoking behavior (10.3% of smokers who reported a diagnosis of hypertension or initiation antihypertensives stopped smoking).
It was not possible to evaluate the role of specific sources of cadmium exposure within this analysis. Multiple sources of cadmium exposure are thought to be involved in this population, including a diet high in processed meat8 and smoking. Although in some American Indian communities the prevalence of smoking is higher than the general population,55,56 the frequency of smoking is much lower,55 which suggests that smoking is unlikely to be sole sources of exposure given the greater levels of cadmium in this population relative to other US ethnic groups. Other studies of American Indian communities have suggested that, small-scale motor vehicle repair,57 surface dust in jewelry-making homes,58 and nearby mining and contaminating factories59,60 may be sources of exposure.
Future Research
Given the inconsistency of previous findings, subsequent research needs to evaluate the association in large, diverse prospective cohorts to robustly confirm these findings within both the larger population, and populations exposed to non-occupational raised cadmium levels.
It is also important to assess cadmium exposure in the light of other metals and metalloids. Additional adjustment for the sum of inorganic and methylated arsenic, also measured in the urine samples of this cohort, had negligible impact on the results. However, the correlation between the sum of inorganic and methylated arsenic and creatinine-adjusted cadmium in the SHS is low, whereas correlations between cadmium and both zinc and manganese have been found in some countries or communities,61 suggesting evaluation of cadmium exposure in conjunction with zinc and manganese may be worth investigating further.
Conclusion
We have identified a positive relationship between creatinine-adjusted urinary cadmium and longitudinal SBP and DBP in American Indians communities, already at greater risk of CVD. Urinary cadmium was also associated with incident hypertension risk, although this increased risk was not statistically significant. These findings have important public health implications about reducing cadmium exposure in general populations. Further research should focus on confirming these findings within the wider population.
Supplementary Material
eFigure 1. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, in upper and lower quintiles of creatinine-adjusted urinary cadmium by sex, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .728; (B) P for interaction: .876; (C) P for interaction: .758. N: males—Q1 = 394, Q2 = 284, Q3 = 233, Q4 = 189, Q5 = 93; females—Q1 = 169, Q2 = 297, Q3 = 346, Q4 = 389, Q5 = 459. Adjusted for age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 2. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, in upper and lower quintiles of creatinine-adjusted urinary cadmium by recruiting center, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .054; (B) P for interaction: .017; (C) P for interaction: .335. N: Arizona— Q1 = 98, Q2 = 100, Q3 = 80, Q4 = 59, Q5 = 57; Oklahoma— Q1 = 303, Q2 = 294, Q3 = 239, Q4 = 259, Q5 = 157; South Dakota— Q1 = 162, Q2 = 187, Q3 = 260, Q4 = 260, Q5 = 338. Adjusted for sex, age, age2, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 3. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .043; (B) P for interaction: .996; (C) P for interaction: .014. N: ever—Q1 = 243, Q2 = 233, Q3 = 191, Q4 = 157, Q5 = 132; never— Q1 = 211, Q2 = 168, Q3 = 173, Q4 = 154, Q5 = 124; current—Q1 = 109, Q2 = 180, Q3 = 215, Q4 = 267, Q5 = 296. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 4. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, not adjusted for antihypertensive medication, in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .077; (B) P for interaction: .002; (C) P for interaction: .915. N: Q1 = 563, Q2 = 581, Q3 = 579, Q4 = 578, Q5 = 552. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption and physical activity.
eFigure 5. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with a history of heart conditions, fully adjusted, n = 2784. (A) P for interaction (across all quintiles): .422; (B) P for interaction: .017; (C) P for interaction: .573. N: Q1 = 424, Q2 = 431, Q3 = 242, Q4 = 440, Q5 = 426. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 6. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with baseline hypertension, fully adjusted, n = 1790. (A) P for interaction (across all quintiles): .138; (B) P for interaction: .015; (C) P for interaction: .730. N: Q1 = 314, Q2 = 347, Q3 = 380, Q4 = 366, Q5 = 383. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 7. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, adjusted for packs per year, fully adjusted, n = 1905 (A) P for interaction (across all quintiles): .511; (B) P for interaction: .105; (C) P for interaction: .594 N: Q1 = 318, Q2 = 389, Q3 = 385, Q4 = 401, Q5 = 412. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, physical activity, and packs per year.
eTable 1. Descriptive characteristics of strong heart study participants by visit
eTable 2. Hazard ratios and 95% confidence intervals for the association between creatinine-adjusted urinary cadmium level and risk of hypertension, only including individuals with smoking data, n = 2864
eTable 3. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary. cadmium by sex, fully adjusted, n = 2853
eTable 4. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by recruiting center, fully. adjusted, n = 2853
eTable 5. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853
eTable 6. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure, not adjusted for antihypertensive medication, in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853
eTable 7. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with a history of heart conditions, fully adjusted, n = 2784
eTable 8. Estimated systolic blood pressure, diastolic blood pressure, pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with baseline hypertension, fully adjusted, n = 1790
eTable 9. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, adjusted for packs per year, fully adjusted, n = 1905
Acknowledgments
We are grateful to all of the staff and participants of the Strong Heart Study, without whom this analysis would not be possible.
This work was supported by cooperative agreement grants U01-HL41642, U01-HL41652, U01-HL41654, U01-HL65520, and U01-HL65521 and research grants R01-HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319 from the National Heart, Lung, and Blood Institute, Bethesda, MD, as well as by the National Institute of Environmental Health Sciences (grant number R01ES021367, 1R01ES025216, 5P30ES009089, P30ES010126, and P42ES010349), a collaborative partnership award made jointly by the University of Cambridge and the UNC Gillings School of Global Public Health, the British Heart Foundation Cambridge Centre of Excellence, (RE/13/6/30180), and Homerton College, University of Cambridge. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data for replication can be obtained from Strong Heart Study conditional on appropriate Strong Heart Study Steering Committee approval and institutional and ethics approval from the Institutional Indian Health Service Review Boards. The opinions expressed in this article are those of the author(s) and do not necessarily reflect the views of the Indian Health Service.
Footnotes
Conflict of interest: None.
Supplemental Material can be found at www.ashjournal.com.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. [Accessed November 11, 2015];End-stage renal disease patients, by selected characteristics: United States, selected years 1980–2010. Available at. http://www.cdc.gov/nchs/data/hus/2011/051.pdf.
- 5.Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J Toxicol. 2011;2011:870125. doi: 10.1155/2011/870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alghasham AA, Meki ARMA, Ismail HAS. Association of blood lead level with elevated blood pressure in hypertensive patients. Int J Health Sci (Qassim) 2011;5:17–27. [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organisation. Exposure to Cadmium: A Major Public Health Concern. Geneva, Switzerland: [Accessed November 11, 2015]. Available at. http://www.who.int/ipcs/features/cadmium.pdf. [Google Scholar]
- 8.Olmedo P, Grau-Perez M, Fretts A, Tellez-Plaza M, Gil F, Yeh F, et al. Dietary determinants of cadmium exposure in the Strong Heart Family Study. Food Chem Toxicol. 2017;100:239–46. doi: 10.1016/j.fct.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.J€arup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organisation. Guidelines for drinking-water quality, 3rd edition incorporating 1st and 2nd addenda Vol 1 Recommendations. Geneva, Switzerland: [Accessed November 11, 2015]. Cadmium. Available at. http://www.who.int/water_sanitation_health/dwq/GDW12rev1and2.pdf. [Google Scholar]
- 11.Satarug S, Nishijo M, Lasker JM, Edwards RJ, Moore MR. Kidney dysfunction and hypertension: role for cadmium, p450 and heme oxygenases? Tohoku J Exp Med. 2006;208:179–202. doi: 10.1620/tjem.208.179. [DOI] [PubMed] [Google Scholar]
- 12.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 13.Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environ Res. 2008;106:284–6. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–9. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) [Accessed November 11, 2015];American Indian and Alaska Native Heart Disease and Stroke Fact Sheet. Available at. http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/docs/fs_aian.pdf.
- 17.American Diabetes Association. [Accessed November 11, 2015];American Diabetes Association: Native American programs. Available at. http://www.diabetes.org/in-my-community/awareness-programs/american-indian-programs/
- 18.Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 19.Caciari T, Sancini A, Fioravanti M, Capozzella A, Casale T, Montuori L, et al. Cadmium and hypertension in exposed workers: a meta-analysis. Int J Occup Med Environ Health. 2013;26:440–56. doi: 10.2478/s13382-013-0111-5. [DOI] [PubMed] [Google Scholar]
- 20.Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect. 2012;120:98–104. doi: 10.1289/ehp.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:1676–84. doi: 10.1289/ehp.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters JL, Fabian MP, Levy JI. Combined impact of lead, cadmium, polychlorinated biphenyls and non-chemical risk factors on blood pressure in NHANES. Environ Res. 2014;132:93–9. doi: 10.1016/j.envres.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Zhu G, Lei L, Jin T. The association between blood pressure and blood cadmium in a Chinese population living in cadmium polluted area. Environ Toxicol Pharmacol. 2013;36:595–9. doi: 10.1016/j.etap.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Franceschini N, Fry R, Balakrishnan P, Navas-Acien A, Oliver-Williams C, Howard AG, et al. Cadmium body burden and increased blood pressure in middle-aged American Indians: the Strong Heart Study. J Hum Hypertens. 2016;31:225–30. doi: 10.1038/jhh.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordberg G, Nogawa K, Nordberg M, Friberg L. Cadmium. In: Nordberg G, Fowler B, Nordberg M, Friberg L, editors. Handbook on the toxicology of metals. Amsterdam: Elsevier; 2007. pp. 445–86. [Google Scholar]
- 26.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The Strong Heart Study, A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 27.Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopik A, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45-74 years, 1984-1988. The Strong Heart Study. Am J Epidemiol. 1998;147:995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 28.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–35. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 29.Jette M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–65. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. Ann Intern Med. 2013;159:649–59. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamler R. Implications of the INTERSALT study. Hypertens (Dallas, Tex 1979) 1991;17:I16–20. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]
- 33.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–9. [PubMed] [Google Scholar]
- 34.Satarug S, Nishijo M, Ujjin P, Vanavanitkun Y, Moore MR. Cadmium-induced nephropathy in the development of high blood pressure. Toxicol Lett. 2005;157:57–68. doi: 10.1016/j.toxlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition examination Survey (NHANES) Environ Health Perspect. 2008;116:51–6. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee MS, Park SK, Hu H, Lee S. Cadmium exposure and cardiovascular disease in the 2005 Korea National health and Nutrition examination Survey. Environ Res. 2011;111:171–6. doi: 10.1016/j.envres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee BK, Kim Y. Association of blood cadmium with hypertension in the Korean general population: analysis of the 2008-2010 Korean National Health and Nutrition Examination Survey data. Am J Ind Med. 2012;55:1060–7. doi: 10.1002/ajim.22078. [DOI] [PubMed] [Google Scholar]
- 38.Staessen JA, Kuznetsova T, Roels HA, Emelianov D, Fagard R. Exposure to cadmium and conventional and ambulatory blood pressures in a prospective population study. Public Health and Environmental Exposure to Cadmium Study Group. Am J Hypertens. 2000;13:146–56. doi: 10.1016/s0895-7061(99)00187-9. [DOI] [PubMed] [Google Scholar]
- 39.Staessen J, Amery A, Bernard A, Bruaux P, Buchet JP, Bulpitt CJ, et al. Blood pressure, the prevalence of cardiovascular diseases, and exposure to cadmium: a population study. Am J Epidemiol. 1991;134:257–67. doi: 10.1093/oxfordjournals.aje.a116079. [DOI] [PubMed] [Google Scholar]
- 40.Hermann U, Kaulich TW, Schweinsberg F. Correlation of blood pressure and cadmium and lead content of the hair in nonsmoking males. Zentralbl Hyg Umweltmed. 1989;188:240–53. [PubMed] [Google Scholar]
- 41.Skroder H, Hawkesworth S, Kippler M, El Arifeen S, Wagatsuma Y, Moore SE, et al. Kidney function and blood pressure in preschool-aged children exposed to cadmium and arsenic-potential alleviation by selenium. Environ Res. 2015;140:205–13. doi: 10.1016/j.envres.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder H, Vinton W. Hypertension induced in rats by small doses of cadmium. Am J Physiol. 1962;202:515–8. doi: 10.1152/ajplegacy.1962.202.3.515. [DOI] [PubMed] [Google Scholar]
- 43.Kagamimori S, Watanabe M, Nakagawa H, Okumura Y, Kawano S. Case-control study on cardiovascular function in females with a history of heavy exposure to cadmium. Bull Environ Contam Toxicol. 1986;36:484–90. doi: 10.1007/BF01623539. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Hernandez A, Kuo C, Rentero-Garrido P, Tang W, Redon J, Ordovas JM, et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin Epigenetics. 2015;7:55. doi: 10.1186/s13148-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zikic RV, Stajn AS, Ognjanovic BI, Saicic ZS, Kostic MM, Pavlovic SZ, et al. The effect of cadmium and selenium on the antioxidant enzyme activities in rat heart. J Environ Pathol Toxicol Oncol. 1998;17:259–64. [PubMed] [Google Scholar]
- 46.Kayama F, Yoshida T, Elwell MR, Luster MI. Cadmium-induced renal damage and proinflammatory cytokines: possible role of IL-6 in tubular epithelial cell regeneration. Toxicol Appl Pharmacol. 1995;134:26–34. doi: 10.1006/taap.1995.1165. [DOI] [PubMed] [Google Scholar]
- 47.Varoni MV, Palomba D, Gianorso S, Anania V. Cadmium as an environmental factor of hypertension in animals: new perspectives on mechanisms. Vet Res Commun. 2003;27(Suppl 1):807–10. doi: 10.1023/b:verc.0000014277.06785.6f. [DOI] [PubMed] [Google Scholar]
- 48.Kisling GM, Kopp SJ, Paulson DJ, Tow JP. Cadmium-induced attenuation of coronary blood flow in the perfused rat. Toxicol Appl Pharmacol. 1993;118:58–64. doi: 10.1006/taap.1993.1009. [DOI] [PubMed] [Google Scholar]
- 49.Prozialeck WC, Edwards JR, Woods JM. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006;79:1493–506. doi: 10.1016/j.lfs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–95. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention (CDC) Diagnosed diabetes among American Indians and Alaska Natives aged <35 years-United States, 1994-2004. MMWR Morb Mortal Wkly Rep. 2006;55:1201–3. [PubMed] [Google Scholar]
- 52.Narva AS, Sequist TD. Reducing health Disparities in American Indians with chronic kidney disease. Semin Nephrol. 2010;30:19–25. doi: 10.1016/j.semnephrol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Environmental Protection Agency. Investigation of potential health, geographic, and environmental issues of abandoned uranium mines. Washington (DC): [Accessed November 11, 2015]. Technical report on technologically enhanced naturally occurring radioactive materials from uranium mining: Volume 2. Available at. http://www2.epa.gov/sites/production/files/2015-05/documents/402-r-08-005-v2.pdf. [Google Scholar]
- 54.Suwazono Y, Kido T, Nakagawa H, Nishijo M, Honda R, Kobayashi E, et al. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers. 2009;14:77–81. doi: 10.1080/13547500902730698. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. From the centers for disease control and prevention. Cigarette smoking among American Indians, Alaskan Natives– behavioral risk factor surveillance system, 1987-1991. JAMA. 1992;3052:268. 3055. [PubMed] [Google Scholar]
- 56.Fabsitz RR, Sidawy AN, Go O, Lee ET, Welty TK, Devereux RB, et al. Prevalence of peripheral arterial disease and associated risk factors in American Indians: the Strong Heart Study. Am J Epidemiol. 1999;149:330–8. doi: 10.1093/oxfordjournals.aje.a009817. [DOI] [PubMed] [Google Scholar]
- 57.Yassin AS, Martonik JF. Urinary cadmium levels in the U S working population, 1988-1994. J Occup Environ Hyg. 2004;1:324–33. doi: 10.1080/15459620490445499. [DOI] [PubMed] [Google Scholar]
- 58.Gonzales M, Shah V, Bobelu A, Qualls C, Natachu K, Bobelu J, et al. Concentrations of surface-dust metals in native American jewelry-making homes in Zuni Pueblo, New Mexico. Arch Environ Health. 2004;59:245–9. doi: 10.3200/AEOH.59.5.245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moon J, Smith TJ, Tamaro S, Enarson D, Fadl S, Davison AJ, et al. Trace metals in scalp hair of children and adults in three Alberta Indian villages. Sci Total Environ. 1986;54:107–25. doi: 10.1016/0048-9697(86)90259-7. [DOI] [PubMed] [Google Scholar]
- 60.Schmitt CJ, Brumbaugh WG, Linder GL, Hinck JE. A screening-level assessment of lead, cadmium, and zinc in fish and crayfish from Northeastern Oklahoma, USA. Environ Geochem Health. 2006;28:445–71. doi: 10.1007/s10653-006-9050-4. [DOI] [PubMed] [Google Scholar]
- 61.Andra SS, Makris KC, Charisiadis P, Costa CN. Cooccurrence profiles of trace elements in potable water systems: a case study. Environ Monit Assess. 2014;186:7307–20. doi: 10.1007/s10661-014-3928-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, in upper and lower quintiles of creatinine-adjusted urinary cadmium by sex, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .728; (B) P for interaction: .876; (C) P for interaction: .758. N: males—Q1 = 394, Q2 = 284, Q3 = 233, Q4 = 189, Q5 = 93; females—Q1 = 169, Q2 = 297, Q3 = 346, Q4 = 389, Q5 = 459. Adjusted for age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 2. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, in upper and lower quintiles of creatinine-adjusted urinary cadmium by recruiting center, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .054; (B) P for interaction: .017; (C) P for interaction: .335. N: Arizona— Q1 = 98, Q2 = 100, Q3 = 80, Q4 = 59, Q5 = 57; Oklahoma— Q1 = 303, Q2 = 294, Q3 = 239, Q4 = 259, Q5 = 157; South Dakota— Q1 = 162, Q2 = 187, Q3 = 260, Q4 = 260, Q5 = 338. Adjusted for sex, age, age2, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 3. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .043; (B) P for interaction: .996; (C) P for interaction: .014. N: ever—Q1 = 243, Q2 = 233, Q3 = 191, Q4 = 157, Q5 = 132; never— Q1 = 211, Q2 = 168, Q3 = 173, Q4 = 154, Q5 = 124; current—Q1 = 109, Q2 = 180, Q3 = 215, Q4 = 267, Q5 = 296. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 4. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure, not adjusted for antihypertensive medication, in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853. (A) P for interaction (across all quintiles): .077; (B) P for interaction: .002; (C) P for interaction: .915. N: Q1 = 563, Q2 = 581, Q3 = 579, Q4 = 578, Q5 = 552. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption and physical activity.
eFigure 5. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with a history of heart conditions, fully adjusted, n = 2784. (A) P for interaction (across all quintiles): .422; (B) P for interaction: .017; (C) P for interaction: .573. N: Q1 = 424, Q2 = 431, Q3 = 242, Q4 = 440, Q5 = 426. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 6. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with baseline hypertension, fully adjusted, n = 1790. (A) P for interaction (across all quintiles): .138; (B) P for interaction: .015; (C) P for interaction: .730. N: Q1 = 314, Q2 = 347, Q3 = 380, Q4 = 366, Q5 = 383. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, and physical activity.
eFigure 7. Estimated (A) systolic blood pressure, (B) diastolic blood pressure, (C) pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, adjusted for packs per year, fully adjusted, n = 1905 (A) P for interaction (across all quintiles): .511; (B) P for interaction: .105; (C) P for interaction: .594 N: Q1 = 318, Q2 = 389, Q3 = 385, Q4 = 401, Q5 = 412. Adjusted for sex, age, age2, center, estimated glomerular filtration rate, body mass index, smoking status, education duration, history of diabetes, triglyceride level, total cholesterol level, high-density lipoprotein cholesterol level, alcohol consumption, physical activity, and packs per year.
eTable 1. Descriptive characteristics of strong heart study participants by visit
eTable 2. Hazard ratios and 95% confidence intervals for the association between creatinine-adjusted urinary cadmium level and risk of hypertension, only including individuals with smoking data, n = 2864
eTable 3. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary. cadmium by sex, fully adjusted, n = 2853
eTable 4. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by recruiting center, fully. adjusted, n = 2853
eTable 5. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853
eTable 6. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure, not adjusted for antihypertensive medication, in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, fully adjusted, n = 2853
eTable 7. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with a history of heart conditions, fully adjusted, n = 2784
eTable 8. Estimated systolic blood pressure, diastolic blood pressure, pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, excluding individuals with baseline hypertension, fully adjusted, n = 1790
eTable 9. Estimated systolic blood pressure, diastolic blood pressure, and pulse pressure in upper and lower quintiles of creatinine-adjusted urinary cadmium by smoking status, adjusted for packs per year, fully adjusted, n = 1905


