Abstract
Controlled delivery systems play a critical role in the success of bone morphogenetic proteins (i.e., BMP2 and BMP7) for challenged bone repair. Instead of single-drug release that is currently and commonly prevalent, dual-drug delivery strategies are highly desired to achieve effective bone regeneration because natural bone repair process is driven by multiple factors. Particularly, angiogenesis is essential for osteogenesis and requires more than just one factor (e.g., Vascular Endothelial Growth Factor, VEGF). Therefore, we developed a novel mesoporous silicate nanoparticles (MSNs) incorporated-3D nanofibrous gelatin (GF) scaffold for dual-delivery of BMP2 and deferoxamine (DFO). DFO is a hypoxia-mimetic drug that can activate hypoxia- inducible factor-1 alpha (HIF-1α), and trigger subsequent angiogenesis. Sustained BMP2 release system was achieved through encapsulation into large-pored MSNs, while the relative short-term release of DFO was engineered through covalent conjugation with chitosan to reduce its cytotoxicity and elongate its half- life. Both MSNs and DFO were incorporated onto a porous 3D GF scaffold to serve as a biomimetic osteogenic microenvironment. Our data indicated that DFO and BMP2 were released from a scaffold at different release rates (10 vs 28 days) yet maintained their angiogenic and osteogenic ability, respectively. Importantly, our data indicated that the released DFO significantly improved BMP2-induced osteogenic differentiation where the dose/duration was important for its effects in both mouse and human stem cell models. Thus, we developed a novel and tunable MSNs/GF 3D scaffold-mediated dual-drug delivery system and studied the potential application of the both FDA-approved DFO and BMP2 for bone tissue engineering.
Keywords: Dual release system, Mesoporous silicate nanoparticles, Nanofibrous scaffold, Angiogenesis, Osteogenesis, Deferoxamine
Graphical abstract

1. Introduction
Reparation of large bone defects remains a significant clinical challenge. Biomaterials-delivered bone morphogenetic proteins (i.e. BMP2 and BMP7) are FDA-approved and most widely studied alternative to improve challenged bone repair because BMPs are so far the most potent osteogenic factors [1]. However, the clinical applications of BMPs are impeded by super-high dosage requirement, short half- life, high costs, and undesirable side effects, such as an inflammatory reaction, neoplasia and ectopic bone formation [2, 3]. To address these challenges, one promising strategy is to develop a scaffold-based controlled release system for locally and sustained release of BMPs with improved bone formation at relatively low dose [4, 5]. Most current delivery systems target single drug release however, natural bone regeneration is a complex and well-orchestrated physiological process, which at least includes inflammation, fibrocartilage callus formation, bony formation, and remodeling phases [6, 7]. Various bioactive molecules are involved and required in these processes [8, 9]. Therefore, multiple/dual-drug delivery strategies are highly desired to achieve effective bone regeneration by mimicking the natural bone repair process [10].
Among all the bioactive factors involved in the bone repair, angiogenic factor, such as Vascular Endothelial Growth Factor (VEGF), the primary growth factor regulating both vasculogenesis and angiogenesis [11], plays a pivotal role for successful bone healing because the bone is a highly vascularized tissue [12, 13]. Although several lines have suggested the synergistic effect of VEGF and BMP2 co-delivery on bone regeneration [13–15], it is difficult to promote angiogenesis in engineered tissues by delivering just one or two growth factors (e.g., VEGF, and fibroblast growth factor, FGF) because it’s a multi- factor system [16, 17]. Hypoxia- inducible factor-1 alpha (HIF-1α), a key upstream transcription factor response to hypoxia stress, plays essential roles in bone regeneration [18–20] by generating VEGF [18, 21], Stromal cell-Derived Factor-1 (SDF-1), and other reparative signals for angiogenesis and progenitor cells recruitment [22, 23]. Deferoxamine (DFO), a FDA-approved iron chelator for iron overload diseases, is a potent hypoxia-mimetic agent by inhibiting the activity of Prolyl Hydroxylase enzyme (PHD), which is the key enzyme responsible for the degradation of HIF-1α [24, 25]. DFO has shown promise in promoting bone repair by improving angiogenesis [19, 20, 26]. However, high cytotoxicity, off- target effects, and the short half- life of DFO have significantly limited its further applications [27, 28]. To address these challenges, we recently developed a novel strategy to locally deliver DFO through a polymer conjugation method [29]. The DFO-conjugated gelatin nanofibrous (GF) scaffold not only demonstrated largely decreased cytotoxicity on both Human Umbilical Vein Endothelial Cells (HUVECs) and human Mesenchymal Stem Cells (hMSCs) but also significantly improved VEGF expression and endogenous bone formation in a mouse cranial bone defect model [29]. Nevertheless, it is still elusive if and how DFO can be co-delivered with BMP2 to improve stem cells osteogenic differentiation as well as bone formation.
Biomimetic 3D GF scaffolds with defined macro-pore and nanofiber structure, fabricated by combining the Thermally Induced Phase Separation method with the particle leach technique (TIPS & P), are advantageous for bone regeneration because they mimic both the physical structure and the chemical composition of the native bone collagen matrix [30, 31]. One important missing piece for biomimetic 3D GF scaffold’s further application in bone tissue engineering is an appropriate drug release carrier that can be tuned for sustained and controlled release of bioactive factors for bone regeneration. It is noted that GF scaffold is an ideal platform for a high amount of drug/nanoparticles incorporation due to the abundant functional groups and the high surface area of nanofibrous structures [29, 32]. Among numerous nanoparticles used for drug delivery, mesoporous silica nanoparticle (MSNs) is a promising drug vehicle because of their unique mesoporous structure, good biocompatibility and chemical stability, excellent surface functionality, and potent targeted/controlled release capability [33–35]. Although most applications of MSNs are limited to the systemic route of drug administration so far (e.g., cancer therapy) [36–39], recent exploratory works [40–42] clearly suggest that the MSN/3D porous scaffold is a versatile/potent local and controlled release system for bone tissue engineering. Similar to bioceramics, silica-based MSNs are particularly promising for bone tissue repair due to excellent bioactivity (apatite formation) of these materials [43].
Therefore, we developed a novel MSNs incorporated-GF dual-drug delivery system to the controlled release of the macromolecule BMP2 as well as the small molecule drug DFO at distinct kinetics. For the first time, we studied the potential application of co-delivery of the FDA-approved DFO and BMP2 for bone tissue engineering with targeting both angiogenesis and osteogenesis.
2. Materials and methods
2.1. Materials
Chitosan (CTS), tetraethyl orthosilicate (TEOS), cetyltrimethylammonium bromide (CTAB), Mesitylene, Genipin, 1, 4-dioxane and Iron (III) Chloride Hexahydrate (FeCl3) were purchased from Sigma-Aldrich Trading Co., Ltd. (St. Louis MO, USA). Ethanol, hexane and cyclohexane were purchased from Fisher Scientific (New Jersey, USA). Hydrochloric acid (HCl) was purchased from J.T. Baker (Center Valley, PA, USA). Triton X-100 was purchased from Fisher Scientific (New Jersey, USA).
Gelatin B (from bovine skin, 225 Bloom), Bovine Serum Albumin (BSA) was purchased from Sigma-Aldrich Trading Co., Ltd. (St. Louis MO, USA). Deferoxamine mesylate was purchased from Abcam (Abcam, Cambridge, MA, USA). Recombinant human BMP2 (rhBMP2) was purchased from Peprotech (New Jersey, USA).
2.2. Preparation of three-dimensional (3D) gelatin nanofibrous (GF) scaffolds
GF scaffolds were fabricated by using TIPS&P technique as previously described [29, 32, 44]. The main procedure was summarized in Fig.1. Briefly, gelatin B was dissolved in 50% of ethanol/water solvent at 37 °C to make a 7.5 wt% homogeneous gelatin solution. Gelatin solution was added into paraffin spheres and then transferred into −80 °C freezer for phase separation overnight. After that, the samples were immersed in ethanol at −20 °C for 24 h. Then the samples were transferred into 1, 4-dioxane solution for another 24 h. At last, the samples were frozen at −20 °C overnight and lyophilized for salt-ice bath 48 h. The scaffolds were cut into Φ 5 mm x 2 mm discs by using biopsy punch (Premier Medical Product Company, PA, USA) and blade. The freeze-dried gelatin-paraffin scaffolds were then immersed in hexane to remove paraffin spheres. The samples were freeze-dried in the salt-ice bath for 48 h. The chemical crosslinking of 3D GF scaffolds were carried out in an MES buffer (pH 5.3, 0.05 M) with EDC and NHS as cross linker at 4 °C for 24 h. After crosslinking, the GF scaffolds were washed with De-Ionized (DI) water and freeze-dried for 48 h.
Fig. 1.
Schematic illustration of synthesis procedure of 3D GF scaffolds.
2.3. Synthesis and characterization of mesoporous silica nanoparticles (MSNs)
The MSNs were synthesized as previously reported [45]. The main procedure was summarized in Fig.2. Briefly, 1.0 g of Cetyltrimethylammonium bromide (CTAB) was dissolved in 480 mL water and 3.5 mL 2.0 M NaOH with stirring at 80 °C. 7.0 mL Mesitylene was then added to the solution and stirred vigorously at 80 °C for 2 h. 5.0 mL Tetraethyl orthosilicate (TEOS) was then added dropwise at a rate of 1 mL per min to the solution and stirred vigorously at 80 °C for another 2 h. The resulting white precipitate was isolated by filtration, washed with ethanol for three times, and dried under vacuum at 100 °C overnight. After that, 1.0 g of the prepared material was immersed in 200 mL acetic ethanol solution and stirred for 48 h at 50 °C to remove CTAB and mesitylene. The template-removed solid product was then isolated via filtration, washed several times with ethanol and dried at 80 °C.
Fig. 2.
Schematic illustration of synthesis procedure of MSNs.
Tecnai G2 transmission electron microscope (TEM) operated at 120 kV acceleration voltage was used to acquire TEM images of the samples. The samples were dispersed in dry hexane and sonicated before they were loaded on a carbon coated copper grid. The small angle X-ray diffraction (SAXRD) pattern of the samples was recorded using Rigaku Ultima IV diffractometer operated at 1.76 kW (44 kV and 40 mA). The diffraction pattern was recorded using Cu Kα (λ = 1.5408 Å) for 2θ range from 0.5 o to 6 o with a step width of 0.02°. Samples were tightly packed with flat surface on a glass sample holder for the acquisition of data. The nitrogen physisorption isotherms were recorded at 77 K using NOVA 2200e surface area analyzer (Quantachrome Inc). Oven dried samples were degassed at 100 °C overnight under vacuum prior to obtaining the isotherms. The specific surface area of materials was obtained using Brunauer-Emmett-Teller (BET) method in the relative pressure (P/P0) range of 0.05–0.30, pore volume was recorded at highest relative pressure of 0.99 and pore size was calculated by applying Barrett-Joyner-Halenda (BJH) method to desorption isotherm.
2.4. Preparation of MSNs-incorporated GF scaffold
To prepare GF/MSNs scaffold, 10 mg of MSNs were first dispersed in 100 μL PBS, 15 μL of MSN suspension was added into GF scaffold. To prepare GF/MSN-BMP2 scaffold, 10 mg of MSNs was mixed with 60 μL of 100 μg/mL BMP2/PBS solution with shaking at 4 °C for 2 h. The BMP2- loaded MSNs were collected after centrifuging (3381 RCF, Eppendorf, USA) and about 50 μL supernatant was collected. About 3 μg BMP2 was loaded into MSNs. Next, the BMP2 loaded MSN was redistributed in 100 μL of PBS solution, the BMP2 concentration was 30 μg/mL. 15 μL MSN-BMP2 was then added onto GF scaffold (450 ng BMP2 was loaded on each GF/MSN-BMP2). To prepare GF/MSN-BMP2/CTS scaffold, 10 μL of 0.1% genipin crosslinked CTS solution was added to GF/MSN-BMP2 scaffold and freeze dried. To prepare GF/DFO sample, 10 μL of 10 mM DFO solution was added into GF scaffold. To prepare GF/MSN/CTS-DFO scaffold, 10 mM DFO and 0.1% (wt%) chitosan (CTS, 1% acetic acid) were mixed with Genipin and crosslinked at 4 °C for 24 h. After crosslinking, 10 μL of CTS-DFO was added into GF and GF/MSN-BMP2 scaffolds. To prepare GF/MSN-BMP2/CTS-DFO scaffold, 15 μL MSN-BMP2 solution was first added into GF scaffold, 10 μL CTS-DFO solution was then added to GF/MSN-BMP2 scaffold. The prepared scaffolds were then frozen and freeze dried (Labconco Freezone Freeze Dryers, Kansas City, USA).
2.5. BMP2 and DFO release
GF/MSN-BMP2 and GF/MSN-BMP2/CTS scaffolds were studied for in vitro BMP2 release. The freeze-dried scaffolds were then immersed in 1 mL PBS solution at 37 °C. Each sample was incubated in an orbital shaker (Talboys Standard 1000, Thorofare, USA)at a speed of 90 rpm. After 28 days of release, the amount of BMP2 released was determined by a BMP2 ELISA kit as per our previous report [32].
GF/CTS-DFO and GF absorbed DFO (GF/DFO) scaffolds were studied for in vitro DFO release study. For DFO-loaded GF scaffolds, 10 mM DFO was crosslinked with CTS overnight at room temperature using 0.1% genipinas crosslinker. After that, 10 μL of CTS-DFO was load onto GF/MSN/CTS-DFO and GF/MSN-BMP2/CTS-DFO scaffolds (about 100 nmol DFO was loaded onto each scaffold). The freeze-dried scaffolds were then immersed in 1 mL pure water solution at 37 °C and incubated in an orbital shaker at 90 rpm. The amount of DFO released was measured by integrating with a certain amount of FeCl3 and determined by using a UV spectrophotometer at 485 nm, as carried out in previous studies [46]. After 10 days of release, the remained DFO on the GF scaffold was measured by adding 400 μL FeCl3 solution and incubated at room temperature for 10 min.
2.6. Measurement of VEGF expression in hMSCs
hMSCs (5×104 cells per well) were seeded into 24 well plate and incubated overnight at 37 °C and 5% CO2. GF/MSN, GF/MSN/CTS-DFO, GF/MSN-BMP2 and GF/MSN-BMP2/CTS-DFO scaffolds were added to each well and cultured for another 24 h. After that, the amount of VEGF in the supernatant was measured by using human VEGF ELISA Development Kit (Peprotech, Rocky Hill, NJ, USA) according to the manufacturer’s protocols. Microplate reader (Infinite M200, Tecan) was used to measure absorbance at 405 nm with wavelength correction set at 650 nm.
2.7. In vitro cell study
The multipotent C2C12 cell was a generous gift from Dr. Yifan Li at the University of South Dakota. The cells were cultured in a Dulbecco’s modification of Eagle’s medium (DMEM, Gibco, USA), containing 10% fetal bovine serum (FBS, Gibco, USA) and 100 U/mL penicillin, 100 mg/mL streptomycin sulfate (Gibco, Grand Island, NY). All the cells were cultured under a humidified atmosphere with 5% CO2 at 37 °C. The medium was changed every 2 or 3 days. Cells were passaged when they reached around 90% confluency. Passages between 10–15 were used for this study. 5% FBS was used for osteogenic differentiation while 10% FBS used for the subculture. hMSCs were obtained from Lonza (Walkersville, MD, USA) and were cultured in a Minimum Essential Medium Alpha Medium (α-MEM, Gibco, USA), containing 10% FBS, and 100 U/mL penicillin, and 100 mg/mL streptomycin sulfate (Gibco, Grand Island, NY) (growth medium). The hMSC prior to passage 10 were used in this study. To induce osteoblastic differentiation, the hMSC were cultured in an osteogenic medium (the above growth medium supplemented with 50 mg/ml L-ascorbic acid, 10mM glycerophosphate and 100 nM dexamethasone (Sigma, St. Louis, MO)).
2.7.1. Cell viability
Cell viability was quantitatively analyzed using Cell Titer 96 AQueous One Solution Cell Proliferation Assay (MTS, Promega, USA) according to the manufacture’s instruction. Briefly, 1×104 C2C12 cells were seeded into GF/MSN, GF/MSN/CTS-DFO, GF/MSN/BMP2 and GF/MSN-BMP2/CTS-DFO scaffolds, after culturing for 1 and 4 days, the culture medium was removed; fresh medium with 10% MTS was then added, and incubated at 37 °C with 5% CO 2 in dark for 1 h. The absorbance was measured at 490 nm using a microplate reader (Infinite M200, Tecan, USA). The relative cell viability (%) was expressed as percentage relative to the control group.
hMSCs morphologies on GF, GF/MSN and GF/MSN/CTS scaffolds were visualized by staining with Texas red-X Phalloidin (Life Technologies, OR, USA) and DAPI (Southern Biotech, Birmingham, AL), which could label F-actin and cell nuclear, respectively [29]. Briefly, cell- seeded scaffolds were fixed in 3.7% paraformaldehyde for 10 min and the permeabilized with 0.1% Triton X-100 for another 5 min. Thereafter, the samples were blocked with 1% bovine serum albumin for 30 min before they were stained with Texas red and DAPI for 20 and 5 min, respectively. The cells/scaffolds were examined by using a confocal laser scanning microscope (CLSM, FV1200, Olympus, Japan).
2.7.2 ALP activity and calcium content
5×104 C2C12 cells were seeded into 24-well plate and incubated overnight at 37 °C and 5% CO2. GF/MSN, GF/MSN/CTS-DFO, GF/MSN-BMP2 and GF/MSN-BMP2/CTS-DFO scaffolds were then added to each well. After 7 days of culture, ALP activity was carried out using an EnzoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA), as was previously described [47] with some minor modifications. ALP activity was measured at 405 nm and normalized against total protein content. The total protein content was measured with a BCA kit ( Thermo Scientific™, Waltham, MA) according to the manufacture’s instruction.
The samples were also examined for calcium deposition by using a total calcium LiquiColor® kit (Stanbio laboratory, TX). After 3 weeks of culture, cells were rinsed with PBS. The calcium was extracted by using 1 mL 6 M hydrochloric acid for 6 h. Thereafter, 10 μL extraction solution or 10 μL standard solution was added into 1 mL working solution prepared according to the manufacturer’s instruction. The absorbance was measured at 550 nm, and the calcium content was calculated from the following equation:
where Au and As are the absorbance values of sample and standard, respectively.
2.7.3. Gene expression analysis
Quantitative gene expression analysis was carried out as we previously described [48]. Briefly, 1×105 C2C12 cells were seeded into GF/MSN, GF/MSN/CTS-DFO, GF/MSN-BMP2 and GF/MSN-BMP2/CTS-DFO scaffolds. After 7 days of incubation, total RNA was extracted using the GeneJET RNA Purification Kit (Thermo Scientific, Waltham, MA) by following the manufacturer’s instruction. An equivalent amount of RNA was processed to generate cDNA by using the High Capacity cDNA Reverse Transcript kit purchased from Applied Biosystems (Forster City, CA). Quantitative PCR was performed with Taqman gene expression assays (Applied Biosystems, Forster City, CA) using the Applied Biosystems 7500 Fast Real- Time PCR System (Applied Biosystems, Carlsbad, CA). TaqMan® Gene Expression Assays of GAPDH (Mm99999915), RUNX2 (Mm00501584), BSP (Mm00436767) and OCN (Mm03413826) were purchased from Applied Biosystems (Forster City, CA).
2.7.4. Effects of DFO and BMP2 on Osteogenic Differentiation
To study the influence of DFO on the differentiation of hMSCs, the ALP activity and mineralization were detected. After cultured for 10 days, the ALP staining was carried out using a Leukocyte Alkaline Phosphatase Kit (Sigma, USA) by following the manufacturer’s instruction. Briefly, the samples were washed with PBS twice, fixed for 30 s, washed with DI water for 45 s and then stained for 15 min. For the mineralization test, after 21 days of cell culture, the samples were fixed in 4% paraformaldehyde solution for 1 h at 4 °C, washed with PBS twice and staining using 40 mM Alizarin Red S solution (pH 4.2) for 10 min at room temperature. In addition, we also studied the influence of DFO on the expression of stemness-related genes (Nanog, POU5F, and ITGA6) of hMSC by using real-time PCR assay as described above.
2.7.5 Statistical Analysis and Image Editing
To determine statistical significance of observed differences between the study groups, a two-tailed homoscedastic t-test was applied. A value of p < 0.05 was considered to be statistically significant while 0.05 < p < 0.10 was considered to represent a nonsignificant, but clear trend in cell or tissue response. Values are reported as the mean ± standard deviation (SD). Brightness and contrast were adjusted equally across all images for improved visibility.
3. Results
3.1. Characterization of MSN
The TEM images indicated the monodispersion of MSNs has an average diameter of 66±11 nm (Fig. 3a) and the typical hexagonal array of mesoporous channels can be seen (Fig. 3b). The phase of MSNs was determined by Small Angle XRD (SAXRD). As shown in Fig. 3c, the SAXRD pattern of MSNs showed four peaks corresponding to diffraction of (100), (110), (200), and (210) planes, which are the characteristic peaks of MCM-41 type MSNs, indicating that the synthesized MSNs have hexagonal type of mesoporous structure [40, 49] that supported the TEM results discussed previously. Three measurements were taken of the samples. Around 50 mg of samples were used for pore size and surface area analysis. The BJH pore diameter and BET surface area of MSNs were 4.5±0.8 nm and 1066±225 m2/g, respectively. These data indicated that our prepared MSNs have relatively large pore size and volume that are critical for protein loading and delivery [45].
Fig. 3. Physicochemical characterization of MSNs.
(a, b) Transmission electron micrographs, (c) Small-angle X-ray diffraction patterns, and (d) Pore size distribution of MSNs.
3.2. Morphologies of GF, GF/MSN and GF/MSN/CTS scaffolds
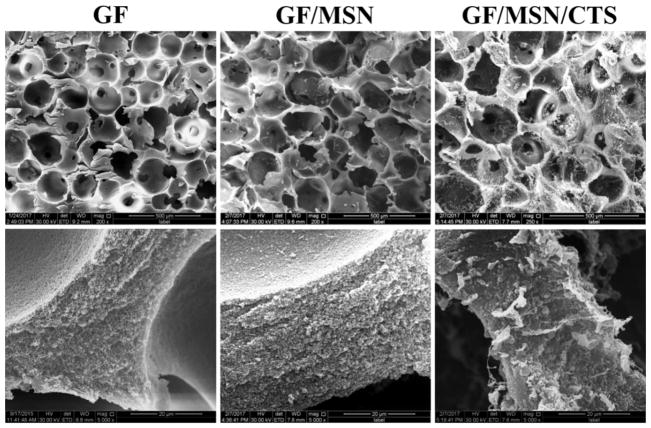
The 3D GF scaffolds with interconnected macroporous structure (pore size was between 150 to 300 μm, the top panels of Fig.4) and typical nanofibrous microstructure (bottom panels of Fig.4) were observed by SEM, which were similar to the previous reports [29, 32]. After MSNs loading, the interconnected macroporous structure was maintained, whereas the nanofibrous struts were covered by a layer of MSNs (middle column, GF/MSN). Moreover, coating of 0.1% CTS did not block macro-pores although the obvious solid CTS polymers were observed throughout the GF scaffolds after freeze drying treatment (right column, GF/MSN/CTS).
Fig. 4. Morphologies of prepared scaffolds.
SEM images of GF, GF/MSN, and GF/MSN/CTS at low (upper panel, scale bars = 500 μm) and high (lower panel, scale bars = 20 μm) magnifications, respectively.
3.3. DFO and BMP2 release from GF scaffolds
The release profiles of BMP2 from GF/MSN-BMP2 and GF/MSN-BMP2/CTS are shown in Fig. 5(a). An initial burst release was observed in both groups, and this is followed by a sustained release during the whole test period, 28 days. Although the overall release patterns of the two groups were similar, significantly higher amounts of BMP2 (~108 ng vs 160 ng) were detected from GF/MSN-BMP2/CTS scaffolds than the GF/MSN-BMP2 group. After 28-day of release, no more BMP2 on the scaffolds could be detected by ELISA. These data indicated that the mesoporous structure of MSNs could support the long-term sustained release of BMP2 from the 3D scaffolds. Moreover, the coating of the CTS on the surface of MSNs and GF scaffolds was able to provide further protection for the protein from fast degradation since same amounts of BMP2 were loaded in both groups (450 ng per scaffold). To achieve the relative fast release of DFO, DFO either was directly absorbed on GF scaffolds or incorporated into GF scaffolds via covalent conjugation with CTS. As the data indicated (Fig. 5b), the absorbed DFO was completed released from the scaffolds in less than 48 h (GF/DFO), whereas the GF/CTS-DFO group showed a sustained release of DFO over a period of 10 days. The total amounts of DFO from the cumulative release for the GF/DFO and GF/CTS-DFO scaffolds were 51.59 and 91.90 nmol, respectively, while same amounts of DFO were originally loaded to each group (100 nmol per scaffold). Therefore, these results suggested that the crosslink of DFO with CTS could not only elongate the release of DFO but also protect DFO from degradation compared to physical absorption (GF/DFO).
Fig. 5. Drug release profiles from scaffolds.
(a) BMP2 and (b) DFO release behavior from GF scaffolds.
3.4. Cell morphology on scaffolds
To study the effects of MSNs and CTS coating on cell attachment and viability on the 3D scaffolds, we firstly investigated the C2C12 cells morphologies on GF, GF/MSN and GF/MSN/CTS scaffolds after 1 day of culture. Relatively fewer C2C12 cells were observed on the GF/MSN scaffolds (Fig. 6b) compared to GF (Fig. 6a) or GF/MSN/CTS scaffolds (Fig. 6c). Moreover, the 3D constructed z-stacked images (Fig. 6 lower panel) indicates that cells grew inside the pores as well and not just on the surface of the 3D scaffolds. These data suggested that the MSNs/CTS incorporation do not interfere with the penetration of cells in the scaffolds. This further supported the fact that the interconnected pores of 3D GF scaffolds were not blocked as observed SEM images showed (Fig. 4).
Fig. 6. Cells morphologies on scaffolds.
hMSCs morphologies on (a) GF, (b) GF/MSN, and (c)and GF/MSN/CTS imaged by CLSM. Upper and lower panels are a top and z -stacked view, respectively. Scale bars = 400 μm.
3.5. Cell viability and VEGF expression of hMSCs on scaffolds
To study the cytotoxicity of the DFO/BMP2 loaded scaffolds, the cell viabilities of C2C12 cells on GF/MSN, GF/MSN/CTS-DFO, GF/MSN-BMP2 and GF/MSN-BMP2/CTS-DFO scaffolds were quantitatively measured by MTS assay. As the data shown, cells on different scaffolds exhibited similar viability after 1 and 4 days of culture (Fig. 7a). These data indicated that the cytotoxicity of DFO was largely reduced after conjugation with CTS because significant cytotoxicity of free DFO on cell viability was noticed even at a low concentration as we previously reported [29]. Consistent with our previous report, these data suggested covalent crosslinking with the polymer was a valid method to eliminate the high cytotoxicity of DFO.
Fig. 7. Cell viability and VEGF expression of hMSCs on scaffolds.
(a) Cell viability of C2C12 cells on scaffolds and (b) VEGF expression in hMSCs on scaffolds. Data are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01.
To study the bioactivity of incorporated-DFO on angiogenesis, we used the hMSCs as the cell model to study the VEGF expression because hMSCs are more responsible for DFO-induced VEGF expression compared to other cell types, e.g. HUVECs according to our previous findings [29]. As the ELISA data shown, hMSCs on both DFO loaded scaffolds (GF/MSN/CTS-DFO and GF/MSN-BMP2/CTS-DFO groups) expressed a significantly higher level of VEGF compared with GF/MSN and GF/MSN-BMP2 groups after 24 h of culture (Fig. 7b). These data indicated that DFO maintained its bioactivity after covalent conjugation with CTS. Additionally, it was interesting to note that the presence of BMP2 in the scaffolds significantly improved DFO-stimulated VEGF expression in hMSCs (GF/MSN-BMP2/CTS-DFO) while BMP2 alone did not elevate the expression level of VEGF (GF/MSN-BMP2).
3.6. Effects of DFO on BMP2-induced osteogenic differentiation
To study the effects of the released BMP2 and DFO on osteogenic differentiation of the cells seeded on the scaffolds, we measured the osteogenic gene expressions, ALP activity, and total calcium content for different cells types. Firstly, real-time PCR results indicated that C2C12 cells, a multipotential mouse cell line with high responsiveness to BMP2 treatment [32], expressed the highest level of RUNX2 on GF/MSN-BMP2 scaffolds compared to other groups. The lower level of RUNX2 expression in GF/MSN-BMP2/CTS-DFO group compared to the GF/MSN-BMP2 group suggested that DFO inhibited the early osteogenic gene expression (Fig. 8a). It was interesting that the expression of the mature osteogenic marker OCN was significantly improved by DFO release alone (GF/MSN/CTS-DFO). Moreover, the highest level of OCN was observed in the combination group (GF/MSN-BMP2/CTS-DFO) (Fig. 8b).
Fig. 8. Stem cells osteogenic differentiation on scaffolds.
(a) RUNX2, (b) OCN (b) expression in C2C12 cells were studied by real-time PCR assay on GF/MSN, GF/MSN/CTS-DFO, GF/MSN-BMP2 and GF/MSN-BMP2/CTS-DFO scaffolds after 7 days of culture. (c)ALP activity, and (d) total calcium content produced by C2C12 and hMSCs cultured on scaffolds, respectively. Data are expressed as mean ± SD (n = 3).* P < 0.05, **P< 0.01, *** P< 0.001.
In addition to gene expression, the ALP activity of C2C12 cells cultured on different scaffolds was investigated as well. After 7 days, both GF/MSN-BMP2 and GF/MSN-BMP2/CTS-DFO groups demonstrated significantly higher ALP activity compared to GF/MSN group. Intriguingly, similar to OCN gene expression, the cells on GF/MSN-BMP2/CTS-DFO scaffolds exhibited significantly higher ALP activity than GF/MSN-BMP2 group although the DFO alone (GF/MSN/CTS-DFO) did not elevate the ALP (Fig. 8c). To further study the effects of released DFO from the scaffolds on osteogenic differentiation, we measured the total calcium content produced by hMSCs after 3-weeks of culture on scaffolds. Consistent with OCN expression and ALP activity in C2C12, considerably higher calcium content was produced by hMSCs on the GF/MSN-BMP2/CTS-DFO scaffolds compared to that from the other scaffolds (Fig. 8d).
To further understand the effects of DFO on hMSCs osteogenic differentiation, we used the standard petri dish culture and the free- form DFO in addition to our incorporated-DFO on 3D GF scaffold model. In contrast to the improved osteogenic differentiation in C2C12 and mineralization in hMSCs by CTS-DFO treatment on 3D GF scaffolds, the free DFO consistently and significantly improved the stemness-related gene expression, including Nanog, POU5F (Oct4) and ITGA6 (CD49f) of hMSCs in a dose-dependent manner (Fig. 9a, b, and, c). Furthermore, the free DFO indicated significant inhibition of hMSCs osteogenic differentiation, including ALP activity (Fig. 9d left panel) and mineralization (Fig. 9d right panel) on petri dish culture. This remarkable difference strongly suggests that the polymer-conjugated DFO/3D GF scaffolds played an important role in DFO-improved osteogenic differentiation.
Fig. 9. Effect of free DFO on stem cells osteogenic differentiation.
(a–c) stemness gene expressions (Nanog, POU5F, ITAG6) in hMSCs after 1-day culture of DFO treatment. (d) ALP staining (left panel), and Alizarin Red S staining (right panel) of hMSCs after treated with different doses of DFO in osteogenic medium. Data are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P< 0.001.
4. Discussion
One important advantage of MSN as a drug delivery system is its favorable textural properties (such as high specific surface area and pore volume, and large pore size) that allow for high loading and tunability, enabling drug release to be tightly controlled from days to weeks by tailoring MSN features such as surface chemistry, pore size, and polymer coating [50, 51]. Pore size and pore volume play an important role in the determination of drug release rate and loading capacity. Generally, small pore size and pore volume result in relatively low drug loading and slow release rate mainly because of the slow solvent diffusion rate into the mesopores with a smaller diameter [52]. The typical pore size of MSN produced by the CTAB-templated method is less than 3 nm, which is suitable for small molecule drug applications while not large enough for encapsulating macromolecules (e.g., DNA, RNA, and protein) [34, 52]. To address this challenge, we adopted a modified method [45] to prepare MSNs with larger pore diameter (5 nm) and volumes (1.34 cm3/g) by adding a pore-expanding agent (mesitylene) to the CTAB-templated for loading large amount of high molecule weight protein [45]. Our release data indicated that the release of BMP2 from MSNs/GF scaffolds lasted for more than 28 days, suggesting BMP2 was loaded into MSNs through physical encapsulation and the sustained release was obtained by virtue of the mesoporous structure and high surface area. It is reported that polymer-coated mesoporous materials (e.g., silk and poly (ethylene glycol), PEG) can considerably reduce burst release and achieve long sustained release for both small molecules (i.e., dexamethasone (DEX)) [53] and large proteins (i.e., trypsin inhibitor) [54]. Interestingly, coating with the low concentration of CTS significantly improved the accumulative amount of BMP2 released from the MSNs/GF scaffolds and more continuous release was achieved when compared with the material that did not contain CTS coating group in our studies. This unexpected effect on BMP2 release was possible because CTS coating provided additional protection for BMP2 from fast degradation. Moreover, the CTS coating also helped with the incorporation of MSNs onto the 3D GF scaffolds which not only restricted MSNs locally and avoided the off-target effects, but also improved sustained drug release as we previously reported [55].
DFO showed encouraging results for bone repair in a variety of preclinical animal studies [19, 26, 56]. However, it is critical to understand the roles of DFO in BMP2- induced bone regeneration and thereby develop appropriate delivery strategies for further applications. As a potent HIF-1α activator, DFO strongly promotes angiogenesis through producing angiogenic factors [18, 23], which is thought to be the main contribution of DFO to improved bone regeneration [26, 56, 57]. The common strategy for dual-delivery of BMPs and angiogenic factors is relatively short/fast/early release for angiogenic factors while sustained/slow/late release for BMPs because angiogenesis and vascularization are prerequisite s for bone healing process [58, 59]. Therefore, we developed the MSNs/GF scaffolds for sustained release of BMP2 (over 28 days) and CTS /GF scaffolds for fast release of DFO (around 10 days). Consistent to our previous study [29], covalent conjugation of DFO to the polymer (e.g., CTS and gelatin) was not only able to control the release of DFO (about 1 day through adsorption) but also significantly reduced its cytotoxicity. In addition to angiogenesis, our previous work also suggested that DFO could promote osteogenesis through stimulating high- level BMP2 expression especially in endothelial cells [29]. This finding was confirmed by an in vivo study where vascular tissues were a primary source of BMP2 expression during bone formation in a mouse model [60].
Furthermore, many conflicting results were reported on whether hypoxic conditions were able to directly modulate osteogenic differentiation [61]. Our data indicated that conjugated DFO could significantly improve BMP2- induced C2C12 osteogenic differentiation on 3D GF scaffolds. Our finding was supported by the previous report that BMP2- induced ALP activity in C2C12 was significantly improved by free DFO treatment [62]. Interestingly, dual-released DFO and BMP2 could improve mineralization of hMSCs on 3D GF scaffolds while free DFO significantly inhibited hMSCs osteogenic differentiation. Instead, free DFO largely improved the stemness markers on a petri dish in a dose-dependent manner. These different effects of DFO on BMP2- induced osteogenic differentiation could be due to many reasons. In addition to the different cell type (i.e., mouse C2C12 vs human MSCs) and culture condition (i.e., 2D petri dish vs 3D GF scaffolds), our data also suggested that dosage and release rate of DFO played an important role in the determination of its effects on osteogenesis. This complexity may explain why most of the reported studies are still focusing on the application of DFO alone. As far as we know, only one study attempted to apply DFO to improve BMP2- induced bone healing in a rat long bone defect model. Their result indicated DFO did not significantly improve BMP2-induced bone formation although vascularity was increased [63]. Therefore, more extensive studies on how DFO affects BMP 2- induced osteogenesis are needed for its further application. Our novel and tunable MSNs/GF scaffold will provide a potent dual-drug release system to study these effects both in vitro and in vivo.
5. Conclusion
Overall, we developed a novel mesoporous silicate nanoparticles incorporated-3D nanofibrous gelatin scaffold for dual-delivery of osteogenic protein (BMP2) and small compound(DFO) for HIF-1α activation. Both MSNs and DFO were incorporated into a porous 3D GF scaffold to serve as a biomimetic osteogenic microenvironment. Our data indicated that MSNs/GF have the ability for controlled dual-release of DFO and BMP2 at distinct release rate while maintaining their angiogenic and osteogenic abilities, respectively. Importantly, our data indicated that DFO release significantly improved BMP2- induced osteogenic differentiation although the dose/duration was important for its effects in both mouse and human stem cell models. Thus, we developed a novel and tunable MSNs/GF 3D scaffold-based dual-drug delivery system and we studied the potential application of both FDA-approved DFO and BMP2 for bone tissue engineering.
Acknowledgments
This material is based upon work supported by the National Science Foundation/EPSCoR Cooperative Agreement #IIA-1355423 and by the State of South Dakota. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This study was also supported by NIH/NIDCR R03 DE027491 (H.S.), South Dakota Board of Regents Competitive Research Grant(CRG) award (Award# UP1500172) and by three MRI grants from the National Science Foundation (Award Nos. CHE-1337707, CHE-0840507, and CHE-0722632). Financial support for this work from the Chinese National Nature Science Foundation (31600773) is acknowledged. The authors want to thank the assistance provided by Sanford Research Imaging Core and Molecular Pathology Core, which are supported by the National Institutes of Health COBRE grants (P20 GM103620 and P20 GM103548). The authors thank Drs. Xiaohua Liu and Erin B. Harmon for their outstanding technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krishnakumar GS, Roffi A, Reale D, Kon E, Filardo G. Clinical application of bone morphogenetic proteins for bone healing: a systematic review. Int Orthop. 2017:1–11. doi: 10.1007/s00264-017-3471-9. [DOI] [PubMed] [Google Scholar]
- 2.Lad SP, Bagley JH, Karikari IO, Babu R, Ugiliweneza B, Kong M, Isaacs RE, Bagley CA, Gottfried ON, Patil CG. Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery. 2013;73:440–449. doi: 10.1227/NEU.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 3.Gothard D, Smith E, Kanczler J, Rashidi H, Qutachi O, Henstock J, Rotherham M, El Haj A, Shakesheff K, Oreffo R. Tissue engineered bone using select growth factors: a comprehensive review of animal studies and clinical translation studies in man. Eur Cells Mater. 2014;28:166–208. doi: 10.22203/ecm.v028a13. [DOI] [PubMed] [Google Scholar]
- 4.Biondi M, Ungaro F, Quaglia F, Netti PA. Controlled drug delivery in tissue engineering. Adv Drug Deliver Rev. 2008;60:229–242. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 5.Bessa PC, Casal M, Reis R. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 6.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng Part B Rev. 2015;21:354–364. doi: 10.1089/ten.teb.2014.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YH, Tabata Y. Dual-controlled release system of drugs for bone regeneration. Adv Drug Deliver Rev. 2015;94:28–40. doi: 10.1016/j.addr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramazanoglu M, Lutz R, Rusche P, Trabzon L, Kose GT, Prechtl C, Schlegel KA. Bone response to biomimetic implants delivering BMP-2 and VEGF: an immunohistochemical study. J Cranio Maxill Surg. 2013;41:826–835. doi: 10.1016/j.jcms.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Z, Nie C, Si Z, Ma Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α. Diabetes Res Clin Pr. 2013;101:62–71. doi: 10.1016/j.diabres.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8:358–366. doi: 10.1038/nrrheum.2012.36. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC. The hypoxia- inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan Y, Zhang Y, Yao S, Shi H, Huang X, Li Y, Wei Y, Lin S. The translation initiation factor eIF3i up-regulates vascular endothelial growth factor A, accelerates cell proliferation, and promotes angiogenesis in embryonic development and tumorigenesis. J Biol Chem. 2014;289:28310–28323. doi: 10.1074/jbc.M114.571356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovas Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 24.Brittenham GM. Iron-chelating therapy for transfusional iron overload. New Engl J Med. 2011;364:146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- 26.Guzey S, Ozturk S, Aykan A, Avsever H, Karslioglu Y, Ertan A. The Effects of Desferroxamine on Bone and Bone Graft Healing in Critical-Size Bone Defects. Plast Reconstr Surg. 2014;134:62–63. doi: 10.1097/SAP.0000000000000679. [DOI] [PubMed] [Google Scholar]
- 27.Hallaway PE, Eaton JW, Panter SS, Hedlund BE. Modulation of deferoxamine toxicity and clearance by covalent attachment to biocompatible polymers. Proc Natl Acad Sci U S A. 1989;86:10108–10112. doi: 10.1073/pnas.86.24.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imranul-haq M, Hamilton JL, Lai BF, Shenoi RA, Horte S, Constantinescu I, Leitch HA, Kizhakkedathu JN. Design of long circulating nontoxic dendritic polymers for the removal of iron in vivo. ACS nano. 2013;7:10704–10716. doi: 10.1021/nn4035074. [DOI] [PubMed] [Google Scholar]
- 29.Yao Q, Liu Y, Tao J, Baumgarten KM, Sun H. Hypoxia-mimicking nanofibrous scaffolds promote endogenous bone regeneration. ACS Appl Mater Inter. 2016;8:32450–32459. doi: 10.1021/acsami.6b10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30:4094–4103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Jiang Y, Liu Q, Gao T, Feng JQ, Dechow P, D’Souza RN, Qin C, Liu X. Biomimetic engineering of nanofibrous gelatin scaffolds with noncollagenous proteins for enhanced bone regeneration. Tissue Eng Part A. 2013;19:1754–1763. doi: 10.1089/ten.tea.2012.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Q, Sandhurst E, Liu Y, Sun H. BBP-Functionalized Biomimetic Nanofibrous Scaffold Can Capture BMP2 and Promote Osteogenic Differentiation. J Mater Chem B. 2017;5:5195–5205. doi: 10.1039/C7TB00744B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallet-Regí M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Edit. 2007;46:7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 34.Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliver Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Kwon S, Singh RK, Perez RA, Abou Neel EA, Kim H-W, Chrzanowski W. Silica-based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng. 2013;4:2041731413503357. doi: 10.1177/2041731413503357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mekaru H, Lu J, Tamanoi F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliver Rev. 2015;95:40–49. doi: 10.1016/j.addr.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Q, Shi J. MSN Anti-Cancer Nanomedicines: Chemotherapy Enhancement, Overcoming of Drug Resistance, and Metastasis Inhibition. Adv Mater. 2014;26:391–411. doi: 10.1002/adma.201303123. [DOI] [PubMed] [Google Scholar]
- 38.Baeza A, Colilla M, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin Drug Del. 2015;12:319–337. doi: 10.1517/17425247.2014.953051. [DOI] [PubMed] [Google Scholar]
- 39.Fu C, Liu T, Li L, Liu H, Chen D, Tang F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials. 2013;34:2565–2575. doi: 10.1016/j.biomaterials.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Feng W, Qiu K, Chen L, Wang W, Nie W, Mo X, He C. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl Mater Inter. 2015;7:15777–15789. doi: 10.1021/acsami.5b02636. [DOI] [PubMed] [Google Scholar]
- 41.Shi M, Chen Z, Farnaghi S, Friis T, Mao X, Xiao Y, Wu C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016;30:334–344. doi: 10.1016/j.actbio.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 42.Shi X, Wang Y, Ren L, Zhao N, Gong Y, Wang DA. Novel mesoporous silica-based antibiotic releasing scaffold for bone repair. Acta Biomater. 2009;5:1697–1707. doi: 10.1016/j.actbio.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Shadjou N, Hasanzadeh M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress. Mater Sci Eng C. 2015;55:401–409. doi: 10.1016/j.msec.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Smith LA, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30:2252–2258. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slowing II, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles for intracellular delivery of membrane- impermeable proteins. J Am Chem Soc. 2007;129:8845–8849. doi: 10.1021/ja0719780. [DOI] [PubMed] [Google Scholar]
- 46.Jia P, Chen H, Kang H, Qi J, Zhao P, Jiang M, Guo L, Zhou Q, Qian ND, Zhou HB. Deferoxamine released from poly (lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J Biomed Mater Res Part A. 2016;104:2515–2527. doi: 10.1002/jbm.a.35793. [DOI] [PubMed] [Google Scholar]
- 47.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31:1133–1139. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao Q, Cosme JG, Xu T, Miszuk JM, Picciani PH, Fong H, Sun H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siavashani AZ, Nazarpak MH, Fayyazbakhsh F, Toliyat T, McInnes SJP, Solati-Hashjin M. Effect of amino- functionalization on insulin delivery and cell viability for two types of silica mesoporous structures. J Mater Sci. 2016;51:10897–10909. [Google Scholar]
- 50.Natarajan SK, Selvaraj S. Mesoporous silica nanoparticles: importance of surface modifications and its role in drug delivery. RSC Adv. 2014;4:14328–14334. [Google Scholar]
- 51.Qu H, Bhattacharyya S, Ducheyne P. Silicon oxide based materials for controlled release in orthopedic procedures. Adv Drug Deliver Rev. 2015;94:96–115. doi: 10.1016/j.addr.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Knežević NŽ, Durand J-O. Large pore mesoporous silica nanomaterials for application in delivery of biomolecules. Nanoscale. 2015;7:2199–2209. doi: 10.1039/c4nr06114d. [DOI] [PubMed] [Google Scholar]
- 53.Wu C, Zhang Y, Zhu Y, Friis T, Xiao Y. Structure–property relationships of silk-modified mesoporous bioglass scaffolds. Biomaterials. 2010;31:3429–3438. doi: 10.1016/j.biomaterials.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 54.Bhattacharyya S, Wang H, Ducheyne P. Polymer-coated mesoporous silica nanoparticles for the controlled release of macromolecules. Acta Biomater. 2012;8:3429–3435. doi: 10.1016/j.actbio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. Novel antibacterial nanofibrous PLLA scaffolds. J Control Release. 2010;146:363–369. doi: 10.1016/j.jconrel.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donneys A, Weiss DM, Deshpande SS, Ahsan S, Tchanque-Fossuo CN, Sarhaddi D, Levi B, Goldstein SA, Buchman SR. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 2013;52:318–325. doi: 10.1016/j.bone.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen X, Wan C, Ramaswamy G, Mavalli M, Wang Y, Duvall CL, Deng LF, Guldberg RE, Eberhardt A, Clemens TL. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharmin F, McDermott C, Lieberman J, Sanjay A, Khan Y. Dual growth factor delivery from biofunctionalized allografts: Sequential VEGF and BMP-2 release to stimulate allograft remodeling. J Orthop Res. 2017;35:1086–1095. doi: 10.1002/jor.23287. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Wang X, Wang S, Zhao J, Xu L, Zhu C, Zeng D, Chen J, Zhang Z, Kaplan DL. The use of injectable sonication- induced silk hydrogel for VEGF 165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials. 2011;32:9415–9424. doi: 10.1016/j.biomaterials.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51:168–180. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drager J, Harvey EJ, Barralet J. Hypoxia signalling manipulation for bone regeneration. Expert Rev Mol Med. 2015;17:e6. doi: 10.1017/erm.2015.4. [DOI] [PubMed] [Google Scholar]
- 62.Yang Q, Jian J, Abramson SB, Huang X. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res. 2011;26:1188–1196. doi: 10.1002/jbmr.337. [DOI] [PubMed] [Google Scholar]
- 63.Stewart R, Goldstein J, Eberhardt A, Chu TMG, Gilbert S. Increasing vascularity to improve healing of a segmental defect of the rat femur. J Orthop Trauma. 2011;25:472. doi: 10.1097/BOT.0b013e31822588d8. [DOI] [PMC free article] [PubMed] [Google Scholar]