Abstract
Conditioned pain modulation (CPM), a psychophysical paradigm that is commonly used to infer the integrity of endogenous pain-altering systems by observation of the effect of one noxious stimulus on another, has previously identified deficient endogenous analgesia in fibromyalgia (FM) and other chronic pain conditions. The mechanisms underlying this deficiency, be they insufficient inhibition and/or active facilitation, are largely unknown. The present cross sectional study used a combination of behavioral CPM testing, voxel based morphometry (VBM), and resting state functional connectivity to identify neural correlates of CPM in healthy controls (HC; n=14) and FM patients (n=15), and to probe for differences that could explain the pain-facilitative CPM that was observed in our patient sample. VBM identified a cluster encompassing the periaqueductal gray (PAG) that contained significantly less gray matter volume in FM patients. Higher resting connectivity between this cluster and cortical pain processing regions was associated with more efficient inhibitory CPM in both groups, whereas PAG connectivity with the dorsal pons was associated with greater CPM inhibition only in HC. Greater PAG connectivity to the caudal pons/rostral medulla, which was pain-inhibitory in HC, was associated with pain facilitation in FM.
Perspective
These findings indicate that variation in the strength of the PAG's resting functional connectivity can explain some of the normal variability in CPM. In addition, pain-facilitative CPM observed in FM patients likely involves both attenuation of pain inhibitory and amplification of pain facilitative processes in the central nervous system.
Keywords: endogenous pain modulation, diffuse noxious inhibitory controls, descending analgesia, functional magnetic resonance imaging, quantitative sensory testing
1. Introduction
Conditioned pain modulation (CPM) is a psychophysical paradigm in which two noxious stimuli are simultaneously applied to remote body locations 71. In healthy individuals, the noxious conditioning stimulus often reduces the painfulness of the noxious test stimulus. There are numerous factors that can facilitate or inhibit pain and determine the magnitude of pain modulation in CPM, like age 14, sex 20, expectation 4, and experimental paradigm 48. A lack of inhibitory CPM or even test pain facilitation can occur in healthy individuals 5, 55, 60, 73, 74 but is more likely to occur in individuals with chronic pain 44, including fibromyalgia (FM) 34, 38, 41, 51, 55, 60. CPM inhibition is thought to be mediated, at least in part, by diffuse noxious inhibitory controls (DNIC) 42, which dampen ascending nociceptive signals in the spinal cord via descending inhibitory projections from the brainstem 67.
Studies measuring pain-evoked brain activity using functional magnetic resonance imaging (fMRI) have indicated that the CPM paradigm engages multiple descending pain-inhibitory networks including the anterior cingulate cortex (ACC), as well as brainstem regions including the periaqueductal gray (PAG) 5, 53, 63, 73, 74. These descending inhibitory pathways are shared with other forms of endogenous analgesia, including long-term pain habituation 3 and placebo analgesia 2, but are possibly distinct from those filtering pain temporally 47. However, it is still unclear what brain abnormalities might be responsible for deficient CPM inhibition in FM patients.
FM is a chronic condition characterized by widespread musculoskeletal pain, fatigue, sleep disturbances, and memory deficits 9. Research has begun to uncover the central pathophysiology of FM 31, 50, 57, including deficiencies in the endogenous analgesic circuitry. For example, subjectively-equated noxious pressure evokes significantly less activity in the rostral ACC of FM patients 32. Furthermore, we recently showed that regional dysfunction in the μ-opioid system in FM is associated with less pain-evoked activity in numerous anti-nociceptive brain regions 61, which is important considering the known role of endogenous opioids in CPM 36, 63, 69. Finally, there is ample evidence from preclinical research that normally pain-inhibitory brainstem regions like the PAG can shift their descending output to actively facilitating incoming pain signals in pathological states 25, 26, 54, 64. It is plausible that pain-facilitating descending output from these brainstem regions contributes to pain hypersensitivity in humans, but direct confirmation of this in humans has been scarce [28,41] and in FM not yet existent.
In the present study, we examined the neurobiology of endogenous pain modulation across its entire range, from strong inhibition to robust facilitation. Next, we assessed the extent to which the abnormal pain modulation in chronic pain patients is the result of a lack of the normal inhibition, a pathological facilitation of pain, or a combination of the two. Finally, we conducted exploratory mediation analyses to test whether our results are compatible with models of descending pain modulation. We hypothesized that the strength of functional connections with pain-inhibitory regions would be associated with CPM inhibition in healthy individuals, but that these connections (or others) might lead to pain facilitation in patients.
2. Methods
2.1. Participants
23 female patients diagnosed with FM and 15 age- and sex-matched healthy subjects were enrolled in this investigation. FM patients were recruited through attendance at a Chronic Pain Education Seminar hosted by the University of Michigan Chronic Pain and Fatigue Research Center and through a patient registry. The study was originally powered to identify mechanisms of action of the drug milnacipran. Healthy subjects were recruited subsequently by means of community fliers. Inclusion criteria for patients were: 1) meeting the American College of Rheumatology 1990 criteria for FM 70 and having chronic widespread pain for at least six months; 2) 18 – 70 years of age; 3) capable of giving written informed consent; 4) right handed; 5) self-reported pain rated between 40 and 90 mm (inclusive) on a 100 mm pain Visual Analogue Scale; 6) willing to withdraw from CNS-active therapies marketed as antidepressants (monoamine oxidase inhibitors, tricyclics, tetracyclics, selective serotonin reuptake inhibitors, norepinephrine reuptake inhibitors, SNRIs), stimulants, anorectic agents, or anticonvulsants. Exclusion criteria were: 1) significant risk of suicide; 2) medical conditions including cardiac diseases, glaucoma, autoimmune disease, systemic infections (e.g., human immunodeficiency virus, hepatitis), active cancer, pulmonary disease or dysfunction, unstable endocrine disease (must be stable at least 3 months prior to study enrolment), unstable diabetes, unstable thyroid disease; 3) pregnant or lactating; 4) any other severe, acute, or chronic medical or psychiatric conditions that could increase risk or interfere with trial results; 5) body mass index greater than 36; 6) contraindications with MRI procedures.
Inclusion criteria for the healthy subjects were: 1) 18 – 75 years old; 2) right handed; 3) being capable of giving written informed consent; 4) willing to complete all study procedures, and 5) pain-free at the time of screening. Exclusion criteria were: 1) having any chronic medical illness, including a psychiatric disorder; 2) diagnosed with a chronic pain disorder; or 3) pregnant or lactating. Subjects were asked to abstain from taking over-the-counter analgesics at least 8 hours prior to a study visit.
All study participants gave written informed consent. The study protocol and informed consent documents were approved by the University of Michigan Institutional Review Board (Ann Arbor, Michigan, USA) and Forest Laboratories (New York, New York, USA). All clinical data were verified for accuracy and the database was locked before analysis. All imaging data were stored, validated, analyzed, and assessed for quality at the University of Michigan independent of Forest personnel. In the present study, only data obtained at baseline for a larger treatment study of milnacipran was analyzed, and we do not report on any effects of milnacipran here. Results correlating pre-treatment fMRI scans with changes in clinical pain, as well as results pertaining to how milnacipran effects resting state connectivity, were previously published in a separate manuscript 56.
From the 23 FM patients, eight were excluded for not completing all of the study procedures. The detailed reasons for their exclusion and the medication lists of the patients whose data were analyzed have been previously provided 56. Thus, 15 patients were included in the VBM analysis, along with 14 age-matched healthy individuals. One healthy subject was excluded from resting state analyses due to excessive head motion while in the scanner. Finally, behavioral CPM data from 2 healthy subjects and 1 FM patient was unusable due to the participants stopping the procedure early, and experimenter error in the case of 1 FM participant. Thus, the psychophysical data are based on that of 13 patients and 12 healthy subjects.
The demographics of the two groups were largely similar, with the main difference being their reported levels of clinical pain. See Table 1.
Table 1. Participant demographics.
| Group Means (StdDev) | Inferential Statistics | |||
|---|---|---|---|---|
|
| ||||
| Healthy (n=14) | Fibromyalgia (n=15) | t Value | p Value | |
| Age | 40.7 (11.5) | 40.7 (10.2) | -0.01 | .996 |
| VAS | 8.4 (12.5) | 68.3 (13.4) | -12.42 | < .001 |
| BPI Sev | 0.5 (1.0) | 5.6 (1.5) | -10.78 | < .001 |
| BPI Int | 0.1 (0.5) | 5.0 (2.5) | -7.16 | < .001 |
| HADS Dep | 2.2 (2.8) | 4.9 (3.3) | -2.31 | .029 |
| HADS Anx | 4.4 (3.1) | 6.5 (3.5) | -1.71 | .099 |
Note: VAS=visual analogue scale rating of clinical pain; BPI=brief pain inventory; Sev=severity; Int=interference; HADS=hospital anxiety and depression subscale; Dep=depression; Anx=anxiety; bold text=p<.05 group difference using independent samples t-test
2.2. Procedure
2.2.1. Clinical pain and psychological assessment
Clinical pain was assessed with the Short Form of the Brief Pain Inventory, which captures both pain severity (BPI Sev) and interference due to pain (BPI Int) over the course of the previous week 10. Participants also reported their level of current clinical pain intensity using a 100mm visual analogue scale (VAS), where 0mm means “no pain” and 100mm means “the most intense pain imaginable”. The Hospital Anxiety and Depression Scale (HADS) was also administered to participants, to obtain indications of depression and anxiety in the two groups 76.
2.2.2. Conditioned pain modulation (CPM)
CPM was assessed within 72 hours prior to neuroimaging. The test and conditioning stimuli were noxious pressure applied to the right and left thumbnails, respectively, using a multimodal automated sensory testing (MAST; Arbor Medical Innovations, Ann Arbor, MI) device 24 (Figure 1a). There were two runs, a baseline run in which the test stimulus was presented alone and a conditioning run in which the test stimulus was presented in the presence of the conditioning stimulus. This protocol was derived from the work of Yarnitsky 72 except modified for mechanical instead of thermal stimulation. It was previously used by our group to assess pain modulation in breast cancer survivors 27 and fibromyalgia patients 60. Before CPM testing, the subject experienced a series of brief pressure pulses on the dominant (right) thumb to determine a pressure intensity that evokes a moderate intensity pain, defined as a rating of 40-50/100 on a numerical rating scale (NRS) for use in the procedure. To this end, the MAST system was used to deliver an ascending series of discrete pressures (5-s duration; 4 kg/cm2/s ramp rate) to the dominant thumbnail at 20-s intervals, beginning at 0.50 kg/cm2 and increasing in 0.50 kg/cm2 steps. Pain intensity was rated after each stimulus on a digital 0-100 NRS (with 0 indicating “no pain” and 100 indicating “most intense pain imaginable”) displayed on a touchscreen monitor. The test was terminated when subjects reached their maximum tolerable pain level and requested the test to stop, a pain intensity rating of ≥ 80/100 was recorded, or the maximum allowable pressure of 10 kg/cm2 was reached. In the CPM baseline run, the test stimulus was applied by the MAST system continuously for 30-s to the dominant thumbnail at each participant's moderate pain pressure intensity. Participants verbally rated the intensity of the test stimulus at 10-, 20-, and 30-s on a NRS from 0 to 100. After the baseline run, CPM was induced 10 min later by applying 60-s of continuous moderate pain pressure to the non-dominant (left) thumbnail via a second MAST device as a conditioning stimulus. Parallel to the last 30-s of conditioning, the moderate pain test stimulus was reapplied to the dominant thumbnail for 30-s during the conditioning run and the participants were asked to verbally rate the intensity of the test stimulus at 40-, 50-, and 60-s (Figure 1b).
Figure 1.
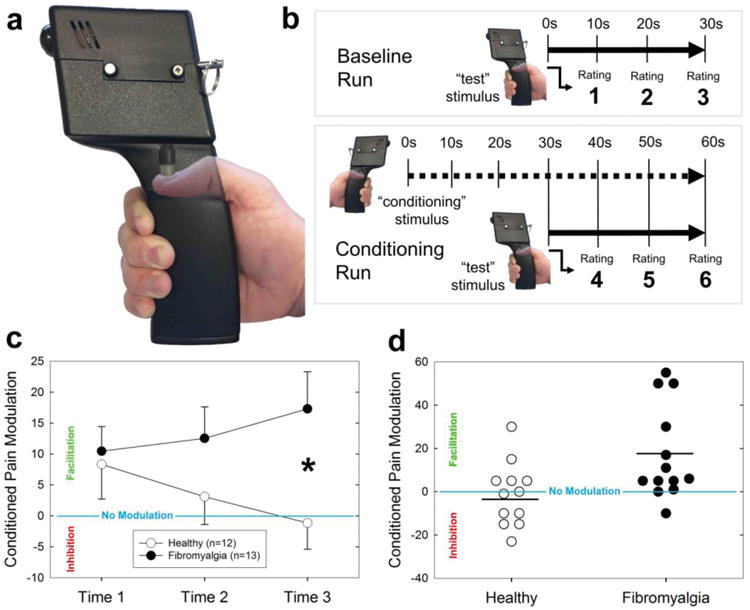
Behavioral measurement of conditioned pain modulation (CPM). a) The device used to measure pressure pain sensitivity and CPM. The piston pressed down onto the middle of the thumbnail at a pressure intended to produce a pain intensity rating of 40-50 out of 100. b) First, subjects underwent a baseline run with the test stimulus alone, followed by a conditioning run where the test stimulus was presented concurrently with a noxious pressure applied to the contralateral thumb. The thick solid line indicates time when the test stimulus was applied and the thick dotted line indicates when the conditioning stimulus was applied. Subjects rated the painfulness of the test stimulus 3 times per run. c) The three time points represent the painfulness of the test stimulus in the conditioning run subtracted from its painfulness at the corresponding time in the baseline run (e.g. Time 1 = Rating 4 – Rating 1); thus, negative numbers indicate inhibition of the test pain and positive numbers indicate test pain facilitation. Error bars represent +/- 1 SEM. *p<.05. d) Individual pain modulation scores at Time 3, separated by group. Horizontal black lines represent group means.
2.2.3. Neuroimaging
All participants were scanned on a 3 Tesla General Electric, SIGNA scanner. T1-weighted gradient echo sequences (TR=12.3ms, TE=3.4ms, flip angle=25°, FOV=256 × 256, yielding 106 sagittal slices with acquired voxel size of 0.94 × 0.94 × 1.5mm and reconstructed to 1 × 1 × 1) using an Eclipse 3.0 T 94 quadrature head coil. T1-weighted images were used in the voxel-based morphometry (VBM) analysis. Inspection of them revealed no gross morphological abnormality for any participant.
Resting state functional connectivity data were acquired using a T2*-weighted spiral sequence encompassing the whole brain (TR = 2.0 s, TE = 30 ms, FA = 90°, matrix size 64 × 64 with 43 ascending sequential axial slices aligned to the anterior commissure – posterior commissure line, FOV = 20 cm and 3.12 × 3.12 × 3 mm voxels, with inhomogeneity field map correction), using the same scanner as for T1 images. During the 6 min acquisition period (180 scans), subjects were asked to remain awake with their eyes open and to stare at a motionless cross presented on the screen.
2.3. Data Analysis
2.3.1. Conditioned pain modulation (CPM)
CPM magnitude was calculated as the difference in pain evoked by test stimulus across the baseline and conditioning runs. Thus, the three ratings of test stimulus painfulness during the conditioning run were subtracted from the three corresponding ratings during the baseline run (i.e. 40/10, 50/20, and 60/30 sec) to generate difference scores at 3 time points. Negative CPM numbers therefore represent inhibitory pain modulation, or a reduction in the painfulness of the test stimulus by the conditioning stimulus, whereas positive numbers represent facilitative pain modulation. A mixed- model analysis of variance (ANOVA) was employed to determine the effects of Rating Number (i.e. the 3 pain modulation difference scores) and the between-subjects effect of Cohort (i.e. healthy or chronic pain). Planned comparisons (independent samples t-tests) were conducted to determine the extent of group differences in CPM at the three time points. In addition, we examined (post-hoc) whether the change in CPM over time was significant for either group separately using one-way ANOVAs. Finally, scores from the 3 time points were averaged for each subject, to give a more stable indication of CPM magnitude, for use in correlational analyses with brain attributes. Post-hoc t-tests were also used on these scores within each group to determine whether there was a significant overall CPM effect in either group. SPSS version 23 (IBM, Armonk, NY, USA) was used for analysis with alpha level set to 0.05, two-tailed.
2.3.2. Voxel-based morphometry
Data were processed using Statistical Parametric Mapping software version 8 (SPM8; Functional Imaging Laboratories, London, UK). Structural scans were segmented into gray matter, white matter, and CSF using the New Segment tool in SPM8. Templates were created based on average characteristics of the study participants' gray and white matter maps using the diffeometric anatomical registration through exponentiated lie algebra (DARTEL) toolbox 1 in SPM, with default values. Gray matter volumetric maps were normalized to Montreal Neurological Institute space using the DARTEL-generated template. Images were smoothed using a kernel size of 8mm FWHM.
An independent-samples t-test was performed in SPM, using the whole-brain high-resolution gray matter volume maps, testing for regional differences in gray matter volume between fibromyalgia patients and controls. Significance was set at the voxel-level to p<.001, and significance was determined by utilizing a small volume correction based on the average location of the periaqueductal gray (PAG) reported in over 40 previous studies on pain 46. Age and total intracranial volume were used as regressors of no interest.
2.3.3. Resting state functional connectivity
The first 6 images of the resting state scan were discarded from the dataset and not analyzed in order to avoid equilibration effects. Data were pre-processed and analyzed using FSL version 3.2b (http://www.fmrib.ox.ac.uk/fsl) and SPM8, as well as the functional connectivity Conn toolbox (Cognitive and Affective Neuroscience Laboratory, Massachusetts Institute of Technology, Cambridge, USA) running under Matlab 7.5b (Mathworks, Sherborn, MA, USA). Upon collection of the functional data, cardiorespiratory artifacts were corrected for using the RETROICOR 29, 52 algorithm in FSL. Pre-processing steps included brain extraction using the default parameters of the BET function in FSL, slice time correction using the 10th slice as a reference, motion correction (realignment to the first image of the time series), normalization to the standard SPM–EPI template (generating 2 × 2 × 2 mm resolution images) and smoothing (convolution with an 8 mm FWHM Gaussian Kernel). Participant head motion was assessed by evaluating three translations and three rotations for each scan. Translational thresholds were set to ±2 mm, while rotational thresholds were limited to ±1°. A subject was excluded from the analysis if head motion exceeded either of these thresholds.
Regions showing differences in gray matter volume between chronic pain patients and healthy participants were used as seeds for resting functional connectivity. Within the Conn toolbox, the seed region's time-series was extracted; white matter, cerebrospinal fluid, and realignment parameters were entered into the analysis as covariates of no interest. A band-pass filter (frequency window: 0.01–0.1 Hz) was applied, thus removing linear drift artifacts and high frequency noise. Single sample t-tests were performed using conditioned pain modulation magnitude as a regressor of interest, first in all subjects together to determine common correlates of regardless of clinical status. Second, connectivity was compared with pain modulation scores separately in the two groups. Third, an interaction was performed, where connectivity was compared across the two groups; in this case, the test was for clusters whose connectivity had a differential effect on conditioned pain modulation across groups (e.g., pain inhibition in one group versus pain facilitation in the other). Significance was set at p<.001 voxel-level, and p<.05 cluster-level FWE-corrected. Areas of interest, including the perigenual anterior cingulate cortex (pgACC) and the rostroventral medial medulla (RVM), were probed using small volume corrections, based on a previous study of resting state connectivity of the PAG in 100 healthy subjects 37. Four regions of interest were used for small volume correction analyses, and were defined by creating a 6 mm3 with the origin of each sphere set to the peak MNI coordinate defined in Table 4. Significance was set at p <.05 cluster-level FWE-corrected. Finally, significant clusters were extracted and further analyzed in SPSS.
Table 4. Regions used for small volume corrections.
| Brain Region | Paper | MNI Coordinates | Previous Study Findings | Significance of present result with 6mm sphere (p value) |
|---|---|---|---|---|
| Gray matter volume | ||||
| PAG | Linnman et al. 2012 | 1,-29,-10 | Average peak voxel of PAG reported in 40 studies (n=703) on pain. | .006* |
| Kong et al. 2010 | 4,-26,-14 | Ventrolateral PAG seed region that was used in resting functional connectivity analysis | .011* | |
|
| ||||
| Resting state functional connectivity correlations with endogenous pain modulation | ||||
| pgACC | Kong et al. 2010 | 4,38,6 | Significant positive resting functional connectivity between the PAG and this region in sample of 100 healthy subjects | .015* |
| Pons / RVM | Kong et al. 2010 | -4,-34,-40 | (see above) | .009* |
Note: MNI=Montreal Neurological Institute; PAG=periaqueductal gray; pgACC=perigenual anterior cingulate cortex; RVM=rostral ventromedial medulla;
=p<.05
2.3.4. Assessment of relationships with clinical pain levels
Relationships between clinical pain measured on the day of the scan (using a VAS) and the behavioral and neuroimaging outcomes were also assessed. First, a series of partial correlations between CPM and functional connectivity findings was conducted, controlling for clinical pain, to examine whether clinical pain levels explained some of these relationships. Second, we examined whether clinical pain was correlated with functional connectivity of the PAG to clusters identified in the analysis and with CPM magnitude.
2.3.5. Mediation analyses
To test the hypothesis that functional connectivity descending from the cortex to the brainstem through the PAG is differentially associated with the magnitude of CPM in healthy participants and chronic pain patients, mediation analyses were conducted using MPLUS version 5.2. Models were tested in which the degree of connectivity between the PAG and insula was both directly associated with the magnitude of CPM (direct effect) and indirectly associated (indirect mediated effects) with the magnitude of CPM through PAG-to-LC connectivity, and PAG-to-RVM/pons connectivity. Models were estimated separately for the two groups using maximum likelihood. Indirect effects were evaluated using bias-corrected bootstrapped (20,000 resamples) 95% confidence intervals. Models using PAG-insula connectivity and PAG-ACC connectivity as independent variables were both evaluated.
3. Results
3.1. Behavioral conditioned pain modulation (CPM)
The average conditioning stimulus pressures were 3.33 kg (SD=1.18) in healthy controls and 2.69 kg (0.86) in FM patients. An independent-samples t-test showed that these values were not statistically different across groups [t(22)=1.56,p=.13]. The average painfulness of the conditioning stimulus (rated at the end of the 1-min conditioning run) was 72.7 (18.1) and 63.9 (23.8) in HC and FM participants, respectively, which was also not significantly different across groups [t(23)=1.03,p=.32].
Pain modulation magnitudes were compared using a mixed-model analysis of variance (ANOVA) with Rating Number (i.e. 1–3) as a within-subject variable and Cohort (healthy participants versus FM patients) as the between-subject variable. The Cohort × Rating Number interaction was significant [F(2,46)=5.07,p=.01], indicating a significant shift towards pain inhibition over time in healthy subjects contrasted with a shift towards pain facilitation in chronic pain patients (Fig. 1c). Post-hoc one-way ANOVAs showed that the change in CPM over time was significant in FM patients [F(2,24)=4.5,p=.02], but not in HCs [F(2,22)=2.03,p=.16]. The main effect of Cohort on CPM magnitude was not statistically significant [F(1,23)=2.46,p=.13], but was in the direction of our hypothesis: increased pain facilitation in chronic pain patients. The main effect of Rating Number was not significant [F(2,46)=0.22,p=.80], indicating no overall shift in pain modulatory magnitude over time. Post hoc analysis revealed that compared to healthy controls, pain modulation was significantly more facilitative in chronic pain patients at Time 3 [t(23)=2.47, p=.02].
The between-group difference in CPM between groups is further illustrated by examining the spread of individual participants in each that experienced inhibition versus facilitation of the test pain at the end of the test stimulus (Fig. 1d). Six healthy participants (50%) experienced inhibition of the test pain at the 3rd rating point, whereas only one FM patient (7.7%) experienced inhibition.
The mean test stimulus pain ratings in the two conditions and the overall CPM magnitude of the 3 time points, which was used for the imaging correlations, are provided in Table 2. Post-hoc t-tests show that the change in pain between conditions was significant in FM patients (i.e. facilitative CPM effect) but not in HCs (i.e. no overall CPM effect).
Table 2.
Test stimulus ratings (mean of three ratings +/- SD) at baseline and during conditioning stimulation, and overall CPM magnitude score (conditioning run minus baseline run.
| Baseline Run | Conditioning Run | CPM Magnitude | p values* | |
|---|---|---|---|---|
| Healthy Control | 52.5 (19.0) | 55.9 (23.3) | 3.4 (13.8) | .408 |
| Fibromyalgia | 38.9 (19.7) | 52.3 (21.7) | 13.4 (17.7) | .018 |
| p values# | .092 | .694 | .130 |
Note:
Healthy Control vs. Fibromyalgia;
Baseline Run vs. Conditioning Run
3.2. Voxel based morphometry (VBM)
The results of a whole-brain voxel-to-voxel comparison of the two groups showed a midbrain region encompassing the PAG that contained greater gray matter volume in healthy participants compared with individuals with FM (Fig. 2; Table 3), statistically significant with a small volume correction (SVC) using either average coordinates from a PAG meta-analysis 46 or those from a previous resting functional connectivity analysis that were situated more in the ventrolateral subdivision of the PAG 37 (Table 4). This result was similar when the images were smoothed using smaller Gaussian kernels (e.g. 2, 4mm) that are more often utilized in brainstem analyses (data not shown). No other significant differences in gray matter volume between the groups were detected in either direction.
Figure 2.
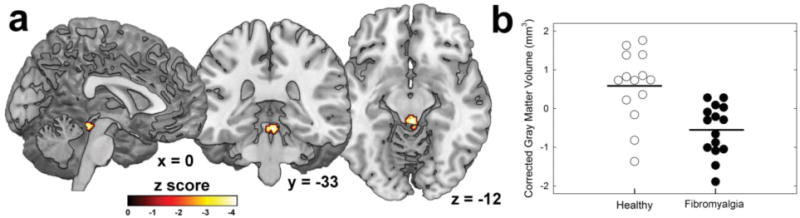
Reduced gray matter volume of periaqueductal gray (PAG) in chronic pain patients. a) Sagittal, coronal, and axial views of the PAG cluster. b) Scatterplot of PAG gray matter volume in healthy participants (n=14) and chronic pain patients (n=15), corrected for total intracranial volume and age. Horizontal black lines indicate group means.
Table 3. Significant neuroimaging findings.
| Brain Region | Coordinates (MNI) | Z Score | Finding | Cluster Size (mm3) | Significance (p value) |
|---|---|---|---|---|---|
|
| |||||
| Gray matter volume | |||||
| PAG | 2,-31,-14 | 3.52 | HC > FM | 395 | .011† |
| PAG connectivity negatively correlated with CPM (i.e. higher connectivity = more pain inhibition) | |||||
| pgACC | 8,46,2 | 4.14 | HC & FM | 400 | .015† |
| L Insula | -36,-6,-16 | 4.04 | HC & FM | 784 | .044* |
| -46,2,-16 | 3.56 | ||||
| DPons | 6,-32,-26 | 4.53 | HC Only | 736 | .007* |
| 6,-18,-20 | 3.66 | ||||
| 12,-22,-30 | 3.47 | ||||
| CPons / RVM | -4,-28,-34 | 3.71 | HC > FM# | 584 | .009† |
| -12,-20,-26 | 3.42 | ||||
Note:
p<.05 familywise error corrected for multiple comparisons, derived from voxel-level threshold of p<.001;
p<.05 small volume corrected (See Table 4); PAG=periaqueductal gray; pgACC=perigenual anterior cingulate cortex; L=left; DPons=dorsal pons; CPons=caudal pons; RVM=rostral ventromedial medulla;
For this result, higher PAG connectivity was associated with more pain inhibition (i.e. correlation was more negative) in healthy subjects compared to a more facilitative effect of higher PAG connectivity on pain (i.e. correlation was more positive) in patients.
3.3. Resting state functional connectivity of the PAG
3.3.1. Similarities across groups
Since the PAG is known to be an important region for the descending modulation of pain and because it contained significantly less gray matter in chronic pain patients, we asked whether the strength of PAG functional connectivity is associated with individual differences in endogenous pain modulation. We focused on the PAG's connectivity to brain regions that were previously identified as having strong positive functional connections with the PAG in a larger sample of 100 healthy participants 37, including the pgACC and the RVM, and because of their well-established roles in the PAG's pain modulatory pathway 2, 3, 26, 32, 33, 54. See Table 4. We also conducted whole-brain exploratory analyses using p<.001 uncorrected voxel-level thresholds and cluster-level corrections for multiple comparisons at p<.05.
First, all subjects were analyzed together, probing common patterns of PAG connectivity that relate to endogenous pain modulation irrespective of chronic pain status. A PAG-to-whole-brain analysis revealed a large cluster hitting the left mid insula, where the strength of functional connectivity was significantly negatively associated with CPM. In addition, connectivity between the PAG and a cluster in the pgACC was also significantly negatively correlated with CPM magnitude. Since negative CPM values reflect inhibition, these results mean that increased strength of functional connectivity between the PAG and these cortical regions was associated with more pronounced endogenous pain inhibition (or less endogenous pain facilitation; Fig. 3).
Figure 3.
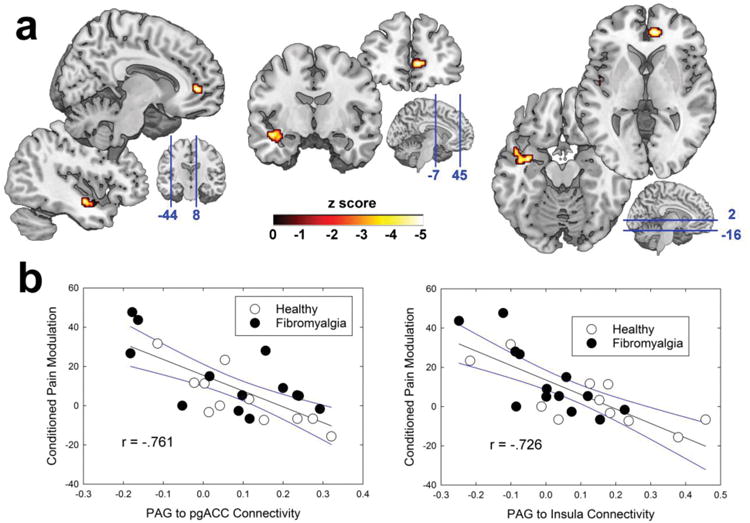
Functional resting connectivity between the periaqueductal gray (PAG) and cortical structures explains variance in conditioned pain modulation (CPM). a) Sagittal, coronal, and axial brain slices are depicted left-to-right, with the left insula cluster and the perigenual anterior cingulate cortex (pgACC) cluster pictured bottom-left and top-right of each, respectively. The blue lines on the three bottom-right brains indicate the slice locations, with the coordinates listed in MNI space. b) Left panel shows the scatterplot of PAG-to-pgACC connectivity versus pain modulation, while the right panel shows PAG-to-Insula connectivity's relationship. Black lines show the best-fit regression and blue lines indicate 95% confidence intervals. R values were derived from Pearson's correlations between extracted connectivity values.
3.3.2. Differences between groups
There were no significant overall differences between the groups in resting connectivity of the PAG. To study potential pathology related to ongoing widespread pain, separate PAG seed-to-whole-brain analyses were conducted for the two groups of participants. In healthy individuals, there was a strong negative correlation between endogenous pain modulation and PAG connectivity to a brainstem region encompassing the LC, where higher connectivity was associated with more endogenous pain inhibition / less facilitation (Fig. 4a,b). No such relationship between pain modulation and PAG to LC connectivity was present in individuals with chronic pain (Fig. 4c), and PAG connectivity was not significantly correlated with pain modulation in the whole-brain search using only this group.
Figure 4.
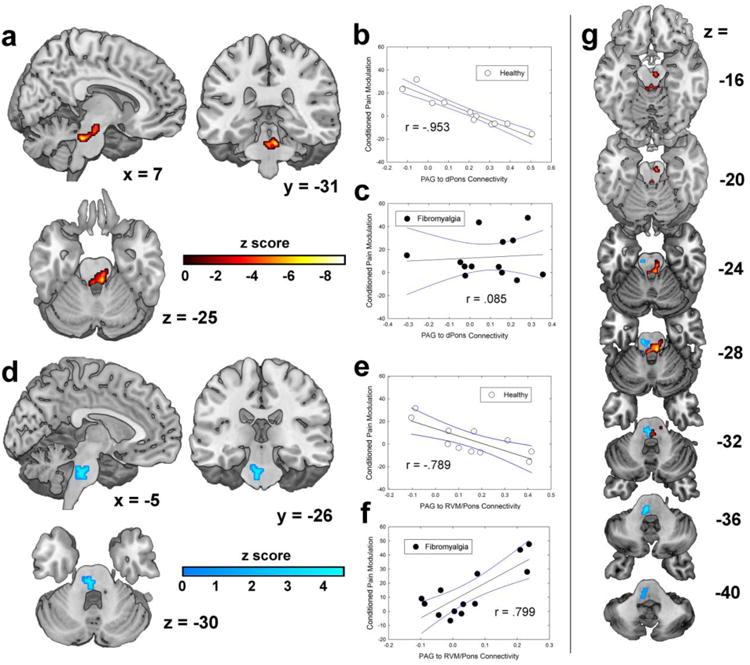
PAG connectivity with the brainstem explains variance in conditioned pain modulation (CPM). a) Significant cluster in the dorsal pons (DPons) that was obtained by correlating pain modulation scores with PAG-to-whole-brain connectivity only in healthy participants. Significance is indicated using the warm-colored bar. b) Relationship between DPons connectivity and CPM in healthy participants. c) DPons relationship with CPM in FM patients. Black lines show best-fit linear regressions and blue lines show 95% confidence intervals. d) Significant cluster that extends from the caudal pons into the rostral ventromedial medulla (RVM), obtained by an interaction model to probe differences in PAG connectivity's association with CPM that differed across groups. Greater PAG connectivity to the cluster depicted was associated with pain inhibition in healthy participants but pain facilitation in chronic fibromyalgia patients. Scatterplots show the relationships in e) healthy subjects and f) FM patients. Linear regression lines and 95% confidence intervals shown. R values reported in panels b, c, e, and f were obtained from Pearson's correlations using extracted connectivity values. g) Descending axial slices through the brainstem illustrate the locations of the two clusters in the same space.
Finally, the potential pathology of chronic pain was examined by conducting an interaction between the groups in terms of PAG connectivity's relationship with pain modulation. Specifically, this tested whether any PAG connectivity had differential effects on pain modulation across groups (e.g. pain inhibition in healthy individuals and pain facilitation in chronic pain patients). The results revealed a significant cluster encompassing the RVM and portions of pons (Fig. 4d), wherein increased PAG connectivity was associated with endogenous pain inhibition in healthy subjects (Fig. 4e) but endogenous pain facilitation in chronic pain patients (Fig. 4f).
The two brainstem clusters detected in the above analyses only partially overlapped, the LC cluster being more rostral and primarily in the left brainstem and the RVM/pons cluster being more caudal and primarily in the right brainstem (Fig. 4g). Together, these results strongly suggest that chronic pain patients lack some of the normal pain inhibitory mechanisms and that there is a shift to active pain-facilitative mechanisms in the brainstem.
3.4 Correlations with clinical pain levels
We considered the possibility that the relationships between CPM and resting functional connectivity could be explained, at least in part, by differences in clinical pain. Controlling for current pain levels using a series of partial correlations revealed that clinical pain levels were unrelated to the observed relationships between CPM and brain connectivity (i.e. the robustness and significance of the reported effects did not change when controlling for clinical pain). Clinical pain levels in FM patients were, however, positively associated with resting PAG-to-Insula connectivity [r=.66, p=.014], but not PAG connectivity to other identified regions (p>.10 for all). Finally, clinical pain in FM was not significantly related to CPM, though there was a trend towards higher pain being associated with less facilitation (or more inhibition) [r=-.52, p=.07]. However, this marginal relationship between CPM and clinical pain is abolished [r=.02, p=.96] when PAG-to-Insula connectivity is controlled for, suggesting that the shared variance between them is best explained by their relationship with brain connectivity, rather than a direct causal link between the two.
3.5. Mediation analyses
Next we conducted exploratory mediation analyses using bootstrapped confidence intervals to assess the significance of indirect effects. The primary hypothesis was that the association between cortex-to-PAG connectivity and endogenous pain modulation would be mediated by the degree of PAG connectivity to regions of the brainstem. In these analyses, the dependent variable was endogenous pain modulation, the independent variable was cortex (i.e. either insula or pgACC)-to-PAG connectivity, and the simultaneous mediators were PAG-to-LC and PAG-to-RVM/Pons connectivity. By using the two mediators together, we were able to probe whether the cortical effects on pain modulation were preferentially mediated by one of the two brainstem pathways. Path analysis was conducted for all models and produced standardized effect estimates for the measured relationships.
In healthy subjects, insula-to-PAG connectivity was no longer directly associated with the magnitude of endogenous pain modulation (p = .54) after accounting for the indirect effect through the LC (β= -.616, 95% CI = -1.056, -.176). In this model, there was no significant indirect effect through the RVM/Pons (β= .056, 95% CI = -.612, .725). See Figure 5a. In chronic pain patients, insula-to-PAG connectivity was no longer directly related to endogenous pain modulation (p =.13), after accounting for the significant indirect effect through the RVM/Pons (β= -.367, 95% CI = -.727, -.008), and the indirect effect through the LC was also not significant (β= -.001, 95% CI = -.309, .310). See Figure 5b. For healthy subjects, a 1-SD increase in insula-to-PAG connectivity equates to a ∼9-point reduction in pain (out of 100) during CPM via the indirect effect of increased PAG-to-LC connectivity. For FM patients, a 1-SD increase in insula-to-PAG connectivity would result in an approximately 7-point drop in pain via decreased PAG-to-RVM connectivity. Results of mediation analyses using pgACC-to-PAG connectivity as the independent variable were similar in healthy participants (Fig. S1), but in patients neither indirect pathway effect met statistical significance (Fig. S2).
Figure 5.
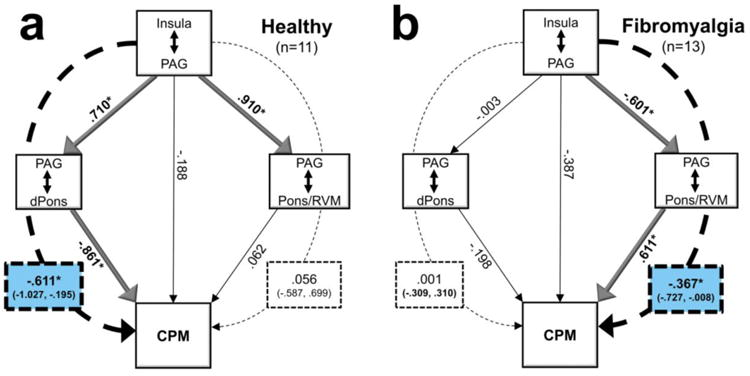
Mediation analyses suggest that the cortical associations with CPM are mediated through the brainstem. a) In healthy individuals, increased Insula-to-PAG connectivity is associated with increased PAG-to-dorsal pons (dPons) connectivity, which is associated with pain inhibition. The inhibitory effect of PAG-to-Pons / rostral ventromedial medulla (RVM) connectivity is not significant in this model. b) In fibromyalgia patients, the effect of Insula-to-PAG connectivity is mediated by PAG-to-Pons/RVM connectivity. As Insula-to-PAG connectivity increases, PAG-to-Pons/RVM connectivity decreases, which would reduce the facilitative effect on pain in this group. Thus, both mediations show inhibitory effects on pain, via increasing pain-inhibitory brainstem connectivity in controls and decreasing pain-facilitative brainstem connectivity in FM. Statistically significant effects are indicated by bold text and lines. Dotted lines indicate mediation effects; significant mediation statistics are highlighted in blue. *p<.05
4. Discussion
The present results show that increased resting connectivity between the PAG and cortical regions is associated with more effective inhibitory pain control both in healthy individuals and those with FM. PAG connectivity with the brainstem is associated with greater pain inhibition in healthy participants, but not in FM patients. In the case of PAG-to-dPons connectivity, the healthy relationship with CPM inhibition is lacking in the FM group. The pain-facilitative PAG-to-Pons/RVM effect observed in FM that is functionally opposite that seen in controls is consistent with decades of preclinical research in animals showing active pain facilitation in pathological states 25, 54. Mediation analyses support the idea that these functional connections help set the gain control of the descending pain-modulatory brain circuitry.
4.1. Understanding the normal variability in CPM
The CPM paradigm can produce highly variable results in healthy individuals ranging from robust analgesia to hyperalgesia 55. Numerous factors that help explain this variability in CPM have been uncovered, including expectation 4, 19, mood 13, personality traits 49, stress level 18, genetic polymorphisms 45, and pain-evoked brain activity and functional connectivity during measurement of CPM 5, 53, 63, 73, 74.
The neural correlates of CPM have also been examined in healthy participants, by measuring the pain-evoked BOLD responses to both the CPM conditioning and test stimuli. In general, the results have shown decreased activation in pro-nociceptive brain regions (e.g. insula) in response to the test stimulus during CPM, compared with the test stimulus response alone 5, 53, 63. Stronger conditioning stimulus-evoked activations of descending pain-inhibitory network regions 5 and higher pain-evoked connectivity between these regions have been found to be associated with stronger CPM inhibition 73. The present study adds to our understanding of individual variability in CPM by revealing that the strength of connectivity between pain-modulatory brain regions at rest may reflect the potential of the pain-modulatory system to engage during noxious stimulation, and specifically during CPM.
Our results are in agreement with the idea that the CPM paradigm engages the descending pain-modulatory circuitry. Pain-inhibitory CPM effects are thought to be largely mediated by the descending DNIC pathway, which has been well delineated in animal research 6, 7, 42. While the PAG is not directly involved in the DNIC circuitry 6, it can play a modulatory role 8. Furthermore, CPM in humans is known to involve multiple cerebral mechanisms in addition to DNIC 53, 63, which might also involve the PAG. Descending projections from the PAG, which is activated by noxious stimuli 22, 28, synapse in more caudal areas of the brainstem including the RVM, which in turn sends its projections predominately to the dorsal horn, where ascending afferents first synapse 26. The significant and robust mediations of the cortical-to-PAG effects on CPM by the PAG's connectivity with the brainstem, particularly the dorsal pons in healthy subjects, are consistent with the idea that we have measured the strength of functional connections in the descending pain-modulatory system.
4.2. Possible Underpinnings of Aberrant CPM in FM
This study corroborates previous investigations showing deficient inhibitory (or pain-facilitative) CPM effects in FM 32, 34, 38, 41, 55, 60 and shows that it can be explained, in part, by aberrant PAG-to-brainstem connectivity. In healthy participants, the strength of resting PAG-to-dPons connectivity was strongly associated with CPM pain inhibition, but no such relationship was uncovered in FM. The dorsal pons contains a number of nuclei involved with pain modulation including the locus coeruleus (LC), which is a noradrenergic nucleus that contains ascending projections to numerous cortical regions and descending connections to the dorsal horn where incoming pain signals can be modulated 64. The measurement of functional connectivity does not provide an index of directionality of the effect, so PAG-to-dPons connectivity could have both ascending and descending influences on CPM. The lack of this relationship in FM patients suggests that their deficient inhibitory CPM might result from disruption of the normal pain-inhibitory processes that contribute to inhibitory CPM in healthy individuals.
Finally, the PAG-to-Pons/RVM connectivity results suggest that facilitative CPM in FM not only involves lack of normal inhibitory mechanisms, but also active pain facilitative mechanisms. The descending pain modulatory pathway from the PAG through the RVM to the spinal cord has been well characterized in animals 54. The normally pain-inhibitory RVM can become pain-facilitative following nerve injury or inflammation, as evidenced by the reduction in pain behaviors in injured animals when the RVM is inactivated 35, 66. For individuals with FM, the long-term effects of experiencing pain and activating the pain modulatory system may shift the balance of the RVM output to pain-facilitative. It can be difficult to interpret whether a behaviorally measured increase in pain (e.g. hyperalgesia) is due to reduced pain inhibition or to increased pain facilitation using neuroimaging, since increased cortical BOLD activation could result from either. However, these results add to a growing body of human neuroimaging literature, pioneered by Tracey and colleagues 12, 22, 30, 43, 65, 68, 75, strongly suggesting that active pain-facilitative central mechanisms play a role in chronic pain.
Given these results, we conclude that FM is associated with changes in the pain-regulatory system that involve both a loss of the normal inhibition, as seen with the dorsal pons, and active facilitation, as seen with the caudal pons / rostral medulla 54, 64 (Fig. 6). Chronic pain may also change PAG morphology as well since patients had less PAG gray matter volume, a result that is in agreement with previous studies showing brainstem decreases in gray matter volume in chronic pain patients 16, 58, 59, 62. Alternatively, it could be that individual differences in PAG morphology and connectivity contribute to one's propensity to develop chronic pain (and to deficient CPM inhibition). This possibility is supported by a study showing that deficient inhibitory CPM predicts propensity to develop chronic pain following surgery 72.
Figure 6.
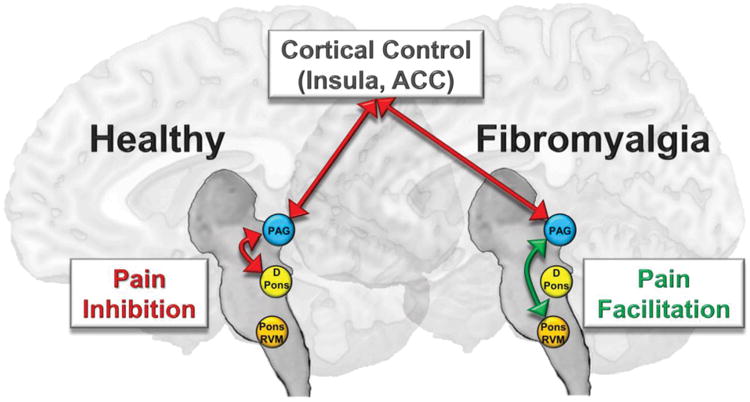
Simplified model depicting the effects of periaqueductal gray (PAG) functional connectivity on endogenous pain modulation. Cortical areas including the insula and the anterior cingulate cortex (ACC) are functionally connected with the PAG and can signal to brainstem regions including regions within the dorsal pons (DPons) and caudal pons / rostral ventromedial medulla (Pons/RVM), which other studies have shown send descending inhibitory signals to the level of the spinal cord to inhibit incoming pain signals. Our results show that resting connectivity between the PAG and DPons is more important for pain inhibition than PAG-to-Pons/RVM connectivity in healthy individuals, despite the fact that the Pons/RVM was found to be more significantly more pain-inhibitory in healthy participants in the interaction analysis with patients. In fibromyalgia patients, cortical pain-inhibitory influences on the PAG seem to be intact, but the strength of PAG-to-DPons connectivity is not associated with pain inhibition as it is in healthy participants. In addition, PAG-to-Pons/RVM connectivity is associated with pain facilitation in these patients, an effect that is in agreement with previous findings in animal models of chronic pain. Mechanisms such as these may help explain why individuals with chronic pain are less able to regulate and dampen incoming pain signals, which instead become perceptually amplified.
The fact that increased cortical connectivity with the PAG was associated with stronger pain inhibition regardless of clinical status suggests that these cortical mechanisms of endogenous pain control are still functionally intact, though perhaps attenuated, in FM. Previous imaging studies have observed less pronounced pain-evoked activity and dysregulated opioidergic signaling in anti-nociceptive brain regions 23, 32, 33, 61 in FM. Thus, while the present results suggest that higher resting cortex-to-PAG connectivity is associated with greater CPM inhibition in FM patients (as it is in pain-free individuals), our data do not rule out the possibility that a failure to properly engage this part of the descending circuit during experimental pain contributes to deficient inhibitory CPM in FM. Furthermore, the interpretation of this result is complicated by the fact that Insula-to-PAG connectivity was positively associated with clinical pain in FM, suggesting that the descending pain-modulatory system's measured activity at rest might be influenced by a patient's level of ongoing, spontaneous pain.
4.3. Limitations
The study is somewhat limited by a relatively small sample size, inclusion of only female participants, and a lack of an overall inhibitory CPM effect in healthy controls. The lack of overall inhibitory CPM, which is not unique to our study 73, 74, in our all-female healthy sample (average age = 40.7 years) could be due to the known tendency for CPM to be less inhibitory (or more facilitative) in women and with increasing age 14, 20, 21, 39, 40. Nevertheless, the significant group differences in CPM provide good evidence that the CPM scores reflect meaningful variation in endogenous pain modulatory mechanisms.
Finally, our imaging scans were not optimized for the brainstem, and thus there may have been some loss of signal especially for some of the more caudal regions reported. Our PAG cluster appears to be situated more in the dorsal rather than the ventrolateral (vl)PAG, the latter of which is thought to be more directly involved in the descending antinociceptive system. The PAG subdivisions are known to interact 17, and the present results do not rule out the possibility of vlPAG mediation of these effects. The brainstem nuclei (e.g. LC) and the subdivisions of the PAG, which can have different functions and anatomical connections 11, 15, are generally too small to be precisely localized given our MRI scanner strength and acquisition parameters, so we are unable to say with much certainty which specific subcortical regions underlie the effects reported herein. Future imaging studies of CPM in FM could optimize parameters to more precisely examine subdivisions of the PAG and the brainstem, as was recently done in healthy subjects 74.
4.4. Conclusions
This study confirms a common finding in the literature: FM patients are tuned more towards experiencing pain facilitation in a CPM paradigm compared to control subjects. We have extended this by 1) identifying differences in the descending analgesic circuitry that help explain the normal variability of CPM in healthy participants, and 2) showing that disruptions in this circuitry are associated with pain facilitation in FM. More specifically, it appears that pain-facilitative CPM in FM likely involves both decreases in pain inhibitory mechanisms and increases in brainstem pain facilitative mechanisms. More research is needed to determine whether these same mechanisms are disrupted in other chronic pain conditions and to what extent the deficiencies in these systems can help guide treatment of chronic pain patients.
Supplementary Material
Supplemental Figure 1. In healthy participants, the results of the periaqueductal gray (PAG) mediation analysis using the anterior cingulate cortex (ACC) were largely similar to those using the insula as the independent variable. The direct effect of ACC-to-PAG connectivity on pain modulation was not significant in the model. Instead, the effect was significantly mediated by PAG-to-dorsal pons (DPons) connectivity, but not PAG-to-Pons/RVM connectivity. Statistically significant effects are indicated by bold text and lines. Dotted lines indicate mediation effects; significant mediation statistics are highlighted in blue. *p<.05
Supplemental Figure 2. In individuals with chronic pain, the results of the periaqueductal gray (PAG) mediation analysis using the anterior cingulate cortex (ACC) differed somewhat from those using the insula as the independent variable. The direct effect of ACC-to-PAG connectivity on pain modulation was not significant in the model (similar to the insula model in this group), though in this model (in contrast to the insula model) the indirect effect of the ACC through the Pons/RVM failed to reach statistical significance.
Highlights.
Conditioned pain modulation (CPM) facilitation observed in fibromyalgia patients
Smaller periaqueducal gray (PAG) volume observed in fibromyalgia patients
PAG resting connectivity with cortical areas associated with pain inhibition
PAG-to-brainstem connectivity helps explain CPM facilitation in fibromyalgia
Consistent with pathological pain-facilitative brainstem mechanisms found in animals
Acknowledgments
The authors would like to thank Keith Newnham for his technical expertise with MRI acquisition and Gabriela Ramirez for her help with subject recruitment and data collection.
Dr. Harper is supported by NIDCR grant K99 DE026810. Tobias Schmidt-Wilcke was supported by the Deutsche Forschungsgemeinschaft (SFB874 A8).
Disclosures: This was an investigator initiated study funded by Forest Laboratories (MD-SAV-09). Dr. Clauw has consulted for Forest Laboratories, Pfizer, Inc., Cerephex Corporation, Eli Lilly and Company, Merck & Co., Nuvo Research Inc., Tonix Pharmaceuticals, Johnson & Johnson, Pierre Fabre, Cypress Biosciences, Wyeth Pharmaceuticals, UCB, AstraZeneca, Jazz Pharmaceuticals, Abbott Laboratories, and Iroko Pharmaceuticals. Dr. Harris has consulted for Pfizer, Inc. Dr. Harte has received research funding from Aptinyx, Cerephex, Eli Lily, Forest Laboratories, and Merck; and served as a consultant for Pfizer, Analgesic Solutions, Aptinyx, and deCode Genetics. He is also a member of Arbor Medical Innovations, LLC (Ann Arbor, MI), current licensee of the MAST pain testing device from the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Bingel U, Schoell E, Herken W, Buchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131:21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkedal E, Flaten MA. Expectations of increased and decreased pain explain the effect of conditioned pain modulation in females. J Pain Res. 2012;5:289–300. doi: 10.2147/JPR.S33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdanov VB, Vigano A, Noirhomme Q, Bogdanova OV, Guy N, Laureys S, Renshaw PF, Dallel R, Phillips C, Schoenen J. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behav Brain Res. 2015;281:187–198. doi: 10.1016/j.bbr.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouhassira D, Bing Z, Le Bars D. Studies of the brain structures involved in diffuse noxious inhibitory controls: the mesencephalon. J Neurophysiol. 1990;64:1712–1723. doi: 10.1152/jn.1990.64.6.1712. [DOI] [PubMed] [Google Scholar]
- 7.Bouhassira D, Villanueva L, Bing Z, le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res. 1992;595:353–357. doi: 10.1016/0006-8993(92)91071-l. [DOI] [PubMed] [Google Scholar]
- 8.Bouhassira D, Villanueva L, Le Bars D. Intracerebroventricular morphine decreases descending inhibitions acting on lumbar dorsal horn neuronal activities related to pain in the rat. J Pharmacol Exp Ther. 1988;247:332–342. [PubMed] [Google Scholar]
- 9.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 11.Coulombe MA, Erpelding N, Kucyi A, Davis KD. Intrinsic functional connectivity of periaqueductal gray subregions in humans. Hum Brain Mapp. 2016;37:1514–1530. doi: 10.1002/hbm.23117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards RR, Dolman AJ, Michna E, Katz JN, Nedeljkovic SS, Janfaza D, Isaac Z, Martel MO, Jamison RN, Wasan AD. Changes in Pain Sensitivity and Pain Modulation During Oral Opioid Treatment: The Impact of Negative Affect. Pain Med. 2016 doi: 10.1093/pm/pnw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 15.Ezra M, Faull OK, Jbabdi S, Pattinson KT. Connectivity-based segmentation of the periaqueductal gray matter in human with brainstem optimized diffusion MRI. Hum Brain Mapp. 2015;36:3459–3471. doi: 10.1002/hbm.22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon N, Alghamdi J, Chiu Y, Sluming V, Nurmikko T, Stancak A. Structural alterations in brainstem of fibromyalgia syndrome patients correlate with sensitivity to mechanical pressure. Neuroimage Clin. 2013;3:163–170. doi: 10.1016/j.nicl.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanselow MS, Decola JP, Deoca BM, Landeirafernandez J. Ventral and Dorsolateral Regions of the Midbrain Periaqueductal Gray (Pag) Control Different Stages of Defensive Behavior - Dorsolateral Pag Lesions Enhance the Defensive Freezing Produced by Massed and Immediate Shock. Aggressive Behavior. 1995;21:63–77. [Google Scholar]
- 18.Geva N, Pruessner J, Defrin R. Acute psychosocial stress reduces pain modulation capabilities in healthy men. Pain. 2014;155:2418–2425. doi: 10.1016/j.pain.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Goffaux P, de Souza JB, Potvin S, Marchand S. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients. Pain. 2009;145:18–23. doi: 10.1016/j.pain.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:142–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Grashorn W, Sprenger C, Forkmann K, Wrobel N, Bingel U. Age-dependent decline of endogenous pain control: exploring the effect of expectation and depression. PloS one. 2013;8:e75629. doi: 10.1371/journal.pone.0075629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis and rheumatism. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 23.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harte SE, Mitra M, Ichesco EA, Halvorson ME, Clauw DJ, Shih AJ, Kruger GH. Development and validation of a pressure-type automated quantitative sensory testing system for point-of-care pain assessment. Med Biol Eng Comput. 2013;51:633–644. doi: 10.1007/s11517-013-1033-x. [DOI] [PubMed] [Google Scholar]
- 25.Heinricher MM. Pain Modulation and the Transition from Acute to Chronic Pain. In: Ma C, Huang Y, editors. Translational Research in Pain and Itch. Springer; Netherlands, Dordrecht: 2016. pp. 105–115. [DOI] [PubMed] [Google Scholar]
- 26.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry NL, Conlon A, Kidwell KM, Griffith K, Smerage JB, Schott AF, Hayes DF, Williams DA, Clauw DJ, Harte SE. Effect of estrogen depletion on pain sensitivity in aromatase inhibitor-treated women with early-stage breast cancer. J Pain. 2014;15:468–475. doi: 10.1016/j.jpain.2014.01.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996;64:303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- 30.Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain. 2014;15:815–826 e811. doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144:95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Molecular pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. The Journal of Neuroscience. 1991;11:1433. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King CD, Goodin B, Kindler LL, Caudle RM, Edwards RR, Gravenstein N, Riley JL, 3rd, Fillingim RB. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. J Behav Med. 2013;36:315–327. doi: 10.1007/s10865-012-9424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 39.Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. The Clinical journal of pain. 2007;23:506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 40.Lautenbacher S, Kunz M, Burkhardt S. The effects of DNIC-type inhibition on temporal summation compared to single pulse processing: does sex matter? Pain. 2008;140:429–435. doi: 10.1016/j.pain.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. The Clinical journal of pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 43.Lee MC, Zambreanu L, Menon DK, Tracey I. Identifying brain activity specifically related to the maintenance and perceptual consequence of central sensitization in humans. J Neurosci. 2008;28:11642–11649. doi: 10.1523/JNEUROSCI.2638-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Lindstedt F, Berrebi J, Greayer E, Lonsdorf TB, Schalling M, Ingvar M, Kosek E. Conditioned pain modulation is associated with common polymorphisms in the serotonin transporter gene. PloS one. 2011;6:e18252. doi: 10.1371/journal.pone.0018252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain. 2014;155:2491–2501. doi: 10.1016/j.pain.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nahman-Averbuch H, Nir RR, Sprecher E, Yarnitsky D. Psychological Factors and Conditioned Pain Modulation: A Meta-Analysis. The Clinical journal of pain. 2016;32:541–554. doi: 10.1097/AJP.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 49.Nahman-Averbuch H, Yarnitsky D, Sprecher E, Granovsky Y, Granot M. Relationship between Personality Traits and Endogenous Analgesia: The Role of Harm Avoidance. Pain practice : the official journal of World Institute of Pain. 2016;16:38–45. doi: 10.1111/papr.12256. [DOI] [PubMed] [Google Scholar]
- 50.Napadow V, Lacount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and rheumatism. 2010 doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Normand E, Potvin S, Gaumond I, Cloutier G, Corbin JF, Marchand S. Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. The Journal of clinical psychiatry. 2011;72:219–224. doi: 10.4088/JCP.08m04969blu. [DOI] [PubMed] [Google Scholar]
- 52.Pfeuffer J, Van de Moortele PF, Ugurbil K, Hu X, Glover GH. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magn Reson Med. 2002;47:344–353. doi: 10.1002/mrm.10065. [DOI] [PubMed] [Google Scholar]
- 53.Piche M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci. 2009;29:14236–14246. doi: 10.1523/JNEUROSCI.2341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 55.Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain. 2016;157:1704–1710. doi: 10.1097/j.pain.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt-Wilcke T, Ichesco E, Hampson JP, Kairys A, Peltier S, Harte S, Clauw DJ, Harris RE. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 2014;6:252–261. doi: 10.1016/j.nicl.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt-Wilcke T, Kairys A, Ichesco E, Fernandez-Sanchez ML, Barjola P, Heitzeg M, Harris RE, Clauw DJ, Glass J, Williams DA. Changes in clinical pain in fibromyalgia patients correlate with changes in brain activation in the cingulate cortex in a response inhibition task. Pain Med. 2014;15:1346–1358. doi: 10.1111/pme.12460. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 60.Schoen CJ, Ablin JN, Ichesco E, Bhavsar RJ, Kochlefl L, Harris RE, Clauw DJ, Gracely RH, Harte SE. A novel paradigm to evaluate conditioned pain modulation in fibromyalgia. J Pain Res. 2016;9:711–719. doi: 10.2147/JPR.S115193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta JK, Clauw DJ, Harris RE. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. 2016;157:2217–2225. doi: 10.1097/j.pain.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain. 2013;14:663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprenger C, Bingel U, Buchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain. 2011;152:428–439. doi: 10.1016/j.pain.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 64.Taylor BK, Westlund KN. The noradrenergic locus coeruleus as a chronic pain generator. J Neurosci Res. 2016 doi: 10.1002/jnr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tracey I. Neuroimaging mechanisms in pain: from discovery to translation. Pain. 2017;158(1):S115–s122. doi: 10.1097/j.pain.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 66.Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proceedings of the National Academy of Sciences. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: diffuse noxious inhibitory controls. Biol Res. 1995;28:113–125. [PubMed] [Google Scholar]
- 68.Wanigasekera V, Lee MC, Rogers R, Hu P, Tracey I. Neural correlates of an injury-free model of central sensitization induced by opioid withdrawal in humans. J Neurosci. 2011;31:2835–2842. doi: 10.1523/JNEUROSCI.5412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. Eur J Pharmacol. 1990;182:347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- 70.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 71.Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD, Wilder-Smith OH. Recommendations on practice of conditioned pain modulation (CPM) testing. European journal of pain (London, England) 2015;19:805–806. doi: 10.1002/ejp.605. [DOI] [PubMed] [Google Scholar]
- 72.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 73.Youssef AM, Macefield VG, Henderson LA. Cortical influences on brainstem circuitry responsible for conditioned pain modulation in humans. Hum Brain Mapp. 2016;37:2630–2644. doi: 10.1002/hbm.23199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Youssef AM, Macefield VG, Henderson LA. Pain inhibits pain; human brainstem mechanisms. Neuroimage. 2016;124:54–62. doi: 10.1016/j.neuroimage.2015.08.060. [DOI] [PubMed] [Google Scholar]
- 75.Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. In healthy participants, the results of the periaqueductal gray (PAG) mediation analysis using the anterior cingulate cortex (ACC) were largely similar to those using the insula as the independent variable. The direct effect of ACC-to-PAG connectivity on pain modulation was not significant in the model. Instead, the effect was significantly mediated by PAG-to-dorsal pons (DPons) connectivity, but not PAG-to-Pons/RVM connectivity. Statistically significant effects are indicated by bold text and lines. Dotted lines indicate mediation effects; significant mediation statistics are highlighted in blue. *p<.05
Supplemental Figure 2. In individuals with chronic pain, the results of the periaqueductal gray (PAG) mediation analysis using the anterior cingulate cortex (ACC) differed somewhat from those using the insula as the independent variable. The direct effect of ACC-to-PAG connectivity on pain modulation was not significant in the model (similar to the insula model in this group), though in this model (in contrast to the insula model) the indirect effect of the ACC through the Pons/RVM failed to reach statistical significance.


