Abstract
The peripherally projecting axons of dorsal root ganglion (DRG) neurons readily regenerate after damage while their centrally projecting branches do not regenerate to the same degree after injury. One important reason for this inconsistency is the lack of pro-regeneration gene expression that occurs in DRG neurons after central injury relative to peripheral damage. The transcription factor SRY-Box containing gene 11 (Sox11) may be a crucial player in the regenerative capacity of axons as previous evidence has shown that it is highly upregulated after peripheral axon damage but not after central injury. Studies have also shown that overexpression or inhibition of Sox11 after peripheral nerve damage can promote or block axon regeneration, respectively. To further understand the mechanisms of how Sox11 regulates axon growth, we artificially overexpressed Sox11 in DRG neurons in vitro to determine if increased levels of this transcription factor could enhance neurite growth. We found that Sox11 overexpression significantly enhanced neurite branching in vitro, and specifically induced the expression of glial cell line-derived neurotrophic factor (GDNF) family receptors, GFRα1 and GFRα3. The upregulation of these receptors by Sox11 overproduction altered the neurite growth patterns of DRG neurons alone and in response to growth factors GDNF and artemin; ligands for GFRα1 and GFRα3, respectively. These data support the role of Sox11 to promote neurite growth by altering responsiveness of neurotrophic factors and may provide mechanistic insight as to why peripheral axons of sensory neurons readily regenerate after injury, but the central projections do not have an extensive regenerative capacity.
Keywords: dorsal root ganglia, neurite growth, regeneration, neurotrophic factor, transcription factor, molecular biology
INTRODUCTION
Peripheral axons of sensory neurons regenerate after injury significantly better than their central projections, due to several discrepancies including Wallerian Degeneration, changes in gene expression and neurotrophic factor production in the injured nerve and periphery (Dodla et al 2011; Jankowski et al 2009b; Fu and Gordon, 1997; Trupp et al., 1995; Funakoshi et al., 1993; Meyer et al., 1992). Among these, dorsal root ganglion (DRG) neurons display significant inconsistencies in transcriptional output of pro-regeneration genes between peripheral and central axon injury (Kury et al., 2001).
Although several genes could play a role in this difference in regenerative ability, the changes in gene expression in the transcription factor SRY-box containing gene 11 (Sox11) after axon damage may contribute to this inconsistency and subsequent differences in regenerative capacity of peripherally and centrally projecting DRG axons. This high mobility group (HMG)-box transcription factor is a member of the group C family along with both Sox4 and Sox12 (Lefebvre et al., 2007). Group C proteins have been shown to be critical in the establishment of pan-neuronal gene expression and the progression of neurogenesis during development (Bergsland et al., 2011). In fact, Sox11 knockout mice present developmental abnormalities in the spinal cord and DRGs (Sock et al., 2004), suggesting a role in neuronal maturation, neuron survival and neurite growth (Jankowski et al., 2006; Hargrave et al., 1997). In addition to its developmental role, Sox11 was recently found to be largely enhanced in adult DRGs after both dissociation of neurons in vitro and after nerve transection in vivo (Jankowski et al., 2009a, 2006). Subsequent experiments have shown that inhibition of the dissociation or injury-induced increase of Sox11 blocks neurite growth in culture and axon regeneration in vivo (Jankowski et al., 2009a, 2006), while overexpression of Sox11 in axotomized nerves promotes regeneration (Jing et al., 2015; Wang et al., 2015).
Although levels of Sox11 appear to regulate neurite growth, the mechanisms by which it may act are not clear. In order to determine the role of enhanced Sox11 levels on neurite growth, we transfected primary DRG neurons with modified plasmids designed to transfect neurons in vitro at high efficiency. Plasmids were modified with Penetratin-1, a lipophilic peptide that was previously shown to facilitate the movement of negatively charged molecules like DNA across cell membranes of heterologous cells (Dom et al., 2003), and was also successfully utilized for neuronal transfection with siRNAs (Jankowski et al., 2006; Davidson et al., 2004). As axonal growth typically requires neurotrophic factor responsiveness (Richner et al., 2014; Jankowski et al., 2009b; Mandai et al., 2009; Priestley et al., 2002), and various neurotrophic factors are released by glial cells in vitro and upregulated at the nerve injury sites in vivo (Baloh et al 2000; Trupp et al., 1995; Funakoshi et al 1993; Meyer et al 1992), here we tested the hypothesis that Sox11 promotes neurite growth by regulating the sensitivity of neurons to neurotrophic factors.
EXPERIMENTAL PROCEDURES
Animals
Adult male Swiss Webster mice approximately 2–3 months of age were used in all studies. Mice were housed in group cages and given food and water ad libitum under a 12-hour light/dark cycle. All procedures were performed in accordance with US and NIH approved policies for use of animals in laboratory research and institutional IACUC approved practices. A total of 131 animals were used in these studies.
Primary DRG cultures
Mice were anesthetized with a mixture of ketamine (90mg/kg) and xylazine (10mg/kg) and intracardially perfused with 25 mL of ice cold Hank’s balanced salt solution (HBSS; Gibco). DRGs were collected in HBSS and then incubated in a solution containing 60U of activated papain for 10 min at 37°C/5%CO2. The papain solution was removed and DRGs were digested in 12mg of collagenase Type II in HBSS for 10 min at 37°C/5%CO2. Cells were then washed in F12 media, containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (F12 complete), reconstituted in 1mL of F12 complete media, triturated with fire polished glass Pasteur pipettes and plated onto laminin and poly-d-lysine coated wells or glass coverslips in either a six- or 12-well culture dish. Cells were incubated for 1–2 hours at 37°C/5%CO2 and then flooded with F12 complete media and/or transfected/treated as described below.
Penetratin-1 modified plasmids, siRNA transfections and growth factor treatments
Plasmids containing Sox11 driven by a CMV promoter and containing a GFP reporter separated by an internal ribosomal entry site (IRES) were generated by subcloning full length Sox11 (IMAGE Clone ID#: 6329841) into the pIRES-eGFP vector (Invitrogen). Penetratin-1 is a 16-amino acid peptide that has been found to allow rapid and highly efficient uptake of conjugated cargos by neurons in vitro (Derossi et al., 1998; Davidson et al., 2004; Jankowski et al., 2006) and in vivo (Jankowski et al., 2009a). Penetratin-1 is also known to be a high affinity DNA binding protein that can facilitate transfection of heterologous cell lines with DNA (Dom et al., 2003). Plasmid DNA (1μg/μL, based on dose-response analysis not shown) was therefore mixed with activated Penetratin-1 (MP Biomedicals, Ohio) at a molar ratio of 1:8 and allowed to incubate at room temperature for 30 minutes prior to transfection. Primary DRG neurons were transfected dropwise as a 2X solution in F12 complete media with Penetratin-modified control plasmids (PenIRES), Sox11 containing plasmids (PenSOX), or untreated and allowed to incubate in vitro at 37°C/5%CO2 for 2–3 days.
For siRNA transfections, siRNAs were first conjugated to Penetratin-1 as described previously (Davidson et al., 2004; Jankowski et al., 2006). 80nM Penetratin conjugated non-targeting control (PenCON), GFRα3 targeting (Penα3) or GFRα1 (Penα1) targeting siRNAs were then transfected into primary DRG neurons as described previously (Jankowski et al., 2006) but one hour prior to plasmid co-transfection. For growth factor treatments, cells were treated with 100ng/mL nerve growth factor (NGF), GDNF, or artemin at the time of plasmid transfection but one hour after siRNA transfection.
Immunocytochemistry
At the times described, cells taken from the appropriate conditions were gently washed in 0.1M phosphate buffer (PB) and then fixed for 5 minutes in 4% paraformaldehyde in 0.1M PB at room temperature. Cells were then rinsed in PB and blocked in 0.25% triton X-100 with 5% normal horse serum in 0.1M PB for 30 minutes and incubated in primary antibodies, rabbit anti-PGP9.5 (1:2000), mouse anti-NeuN (1:1000) or chicken anti-GFP (1:2000) overnight at room temperature. Cells were then rinsed and incubated for 2 hours in appropriate secondary antibody (1:200, CY3-conjugated donkey anti-rabbit, CY3-conjugated donkey anti-mouse, CY2-conjugated donkey anti-chicken). Cells were then rinsed with PB, coverslipped in glycerol using photoetched gridded coverslips (Electron Microscopy Sciences, Hatfield, PA) and analyzed using a Leica fluorescence microscope. Images were chosen from each condition by randomly selecting five squares in the grid at 20×. For each condition, 10–30 labeled cells were analyzed offline for neurite branching and length (Jankowski et al., 2006).
Transfection Efficiency and Neurite Quantification
Transfection efficiency was performed by calculating the percentage of GFP positive/NeuN positive cells in vitro for PenIRES transfected cells, PenSox transfected cells, and untreated cells at three days. 112 PenSox transfected cells, 382 transfected PenIRES cells, and 96 untreated cells were analyzed. Neurons were chosen randomly using grid selections as described above. Images of cells were analyzed offline using NIH Image J for numbers of primary neurites, branch points on primary neurites and maximum neurite length in cells that were either untreated, transfected with control (PenIRES) or PenSox containing plasmids at the aforementioned time points that were additionally treated with or without various growth factors or siRNAs. The experimenter was blinded to the conditions for all neurite quantifications. A total of 1454 cells obtained from a minimum of three mice per condition, were quantified for average neurite branching and length at the time points outlined herein. Primary neurites were identified as branches projecting directly from the cell body. Secondary neurites were identified as branches projecting directly from primary branches as well as branches projecting from other secondary branches, and were quantified for each neuron. Maximum neurite length was analyzed by measuring the longest neurite branch beginning at the cell body using Image J software.
RNA isolation and realtime polymerase chain reaction (PCR) analysis
RNA isolation from cells was performed using Qiagen RNeasy mini kits using the supplied protocol. 1 μg of total RNA was then treated with DNase I (Invitrogen), annealed to random hexamer primers, and reverse transcribed using Superscript II reverse transcriptase (Invitrogen). 20ng of cDNA was used in SYRB green real time PCR reactions and run in triplicate on an Applied Biosystems Imager. Ct values were normalized to neuronal specific enolase (NSE). The ΔΔCt value was calculated after normalization and the fold change was described as 2ΔΔCt (Applied Biosystems). Values are presented as a percent change where a 2-fold change equals 100% change.
Protein isolation and western blotting
At both 2 and 3 days post-plating, neurons from similarly treated wells of a 6-well plate were pooled and homogenized in lysis buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris–HCl (pH 7.4) and protease inhibitors (1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mM sodium orthovanadate). Samples were centrifuged, supernatant boiled for 10 minutes in a denaturing buffer containing β-mercaptoethanol and SDS, separated on a 10% polyacrylamide SDS-PAGE gel and transferred to a PVDF membrane that was blocked in either 5% milk in 0.1M Tris-buffered saline with 0.1% Tween 20 or a 1:1 mixture of 0.1M phosphate buffered saline (PBS) and LiCor Blocking Buffer (LiCor) and then incubated with primary antibodies overnight at 4 °C (rabbit anti-Sox11, 1:1000, goat anti-GFRα3, 1:100 or 1:200, rabbit anti-actin, 1:5000, chicken anti-GAPDH, 1:1000). Antibody binding was visualized using horseradish peroxidase-conjugated goat anti-rabbit, or donkey anti-goat, secondary antibodies (1:5000) and chemiluminescent detection for standard western blotting or infrared conjugated secondary bodies for use on the LiCor Odyssey Protein Imager (donkey anti-rabbit IR800, donkey anti-goat IR680; donkey anti-chicken IR800; 1:10000). Completed scans were sized and imaged curves were adjusted to gamma of 1.0. Intensity was set to 5.0 for membranes, and was changed if enhanced intensity was needed for accurate band detection. Protein expression was analyzed offline by densitometery analysis using Image J for each condition and timepoint.
Data Analysis
Statistical significance was determined for all neurite quantification experiments and RT-PCR comparisons using t-tests, one-way ANOVA and Tukey’s post hoc test or Kruskal-Wallis with Dunn’s post hoc (when appropriate) with p value ≤ 0.05. Normal distributions and equal variances were confirmed prior to performing parametric statistics. For non-normal data, non-parametric tests were used. Statistical outliers defined as greater than 2 standard deviations from the mean were excluded.
RESULTS
Sox11 overexpression enhances neurite branching
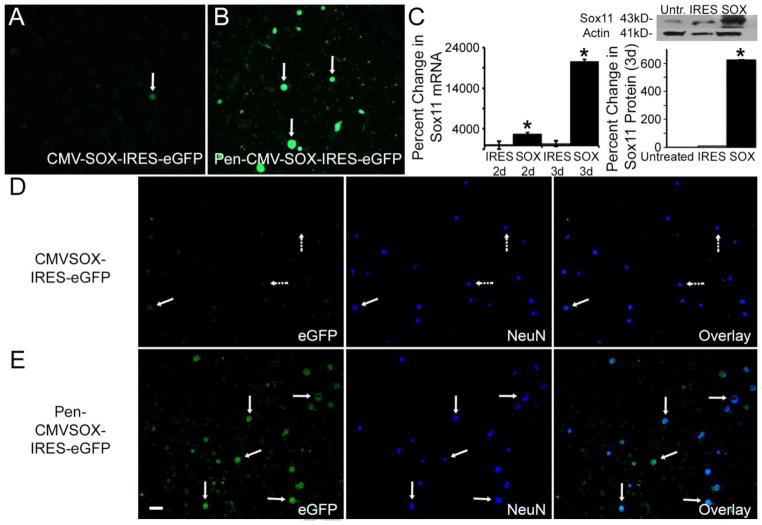
We hypothesized that Penetratin bound plasmids could be taken up by DRG neurons in vitro with similar efficiency as previously reported using Penetratin-modified siRNAs (e.g. Jankowski et al 2006). We confirmed that Penetratin could significantly enhance the transfection of plasmids into DRG neurons without the use of any other transfection reagents (Figure 1). Using the green fluorescent protein (GFP) reporter, transfection efficiency of primary DRG neurons (NeuN positive) was found to be 91.5% for Penetratin modified vector control (PenIRES) transfected cells and 93.8% for neurons transfected with Penetratin modified Sox11 (PenSOX) containing plasmids, using these methods. Some NeuN negative cells also appeared to take up the plasmids in these cultures. RNA and protein were then isolated to determine if this technique could enhance gene expression in vitro. We found that Sox11 mRNA was significantly enhanced in DRG cultures (2d: IRES: −39.1% ± 1014%; SOX: 2743% ± 410%*; F2, 6 = 8.3, *p<0.03, relative to untreated cells; 3d: IRES: 415% ± 674%; SOX: 20622% ± 493%*, F2, 6= 9.5, *p<0.02, relative to untreated cells) at both two and three days, which was validated at the protein level (Figure 1). Other detectable bands using western blotting suggest that some variants, degradation products or protein aggregates are also present. This could be due to a technical issue during isolation that may not separate all proteins from Sox11 which can bind to other proteins with high affinity. This may be more readily detectable in the Sox11 overexpressed conditions; however, this was not confirmed here.
Figure 1. Penetratin-1 enhances the transfection efficiency of primary DRG neurons and promotes increased protein expression in vitro.
A: Few primary DRG neurons express a GFP reporter when transfected with naked CMV-Sox-IRES-eGFP plasmids (arrow). B: In contrast, binding plasmids with Penetratin-1 greatly enhances the transfection of primary DRG neurons (arrows). C: Sox11 mRNA is substantially enhanced in DRG cultures compared to IRES control at both two and three days in vitro. Sox11 protein expression is also significantly increased compared to Untreated and IRES controls. *p<0.001; n=3 mice per condition. D: Co-expression of eGFP and NeuN is minimal (arrows) in CMV-Sox-IRES-eGFP treated cultures (n=3 mice). Most NeuN positive cells do not contain eGFP (dashed arrows) E: Extensive co-labeling of NeuN and eGFP (arrows) is detected in Penetratin bound Sox11 containing plasmids (n=3 mice). Scale Bar, 100μm (all panels).
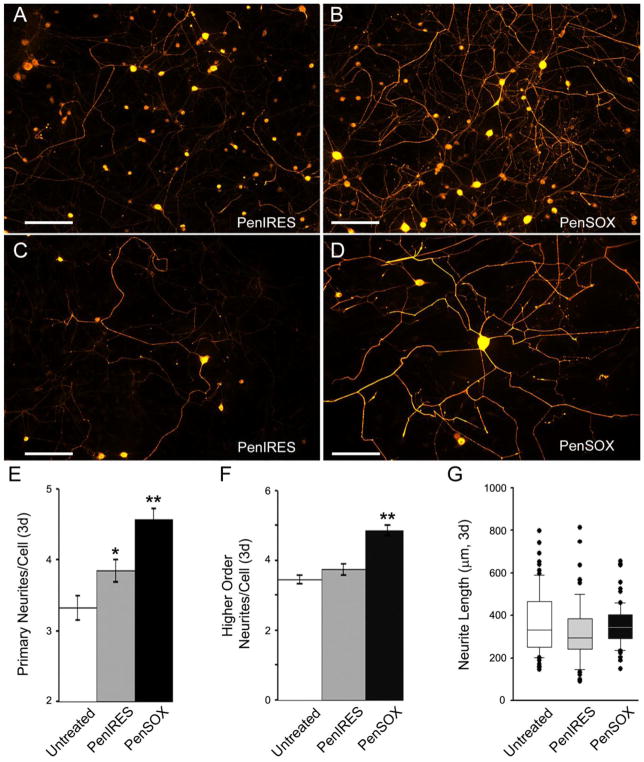
DRG neurons were then transfected with modified Sox11 containing (PenSOX) or vector control (PenIRES) plasmids and allowed to grow for two or three days in vitro for quantification of neurite growth. Light microscopic examination of PGP9.5 positive cells showed there was a significant change in higher order neurite branching (Untreated: 3.1 ± 0.8; PenIRES: 3.7 ± 0.2; PenSOX: 4.9 ± 0.3*, F2, 196 = 20.3, *p<0.001) as well as primary neurite branching (Untreated: 3.1 ± 0.2; PenIRES: 3.5 ± 0.2; PenSOX: 4.1 ± 0.2*, F2, 196 = 20.3, *p<0.04) at the two-day time point in Sox11 transfected neurons. PenSOX transfected cells also had a significant increase in neurite elongation vs. untreated cells, but this was not statistically different than PenIRES transfected cells at this time point (Untreated: 313.0 ± 17.6μm; PenIRES: 449.3 ± 25.9μm*; PenSOX: 479.1 ± 26.6*; H93, 65, 65= 31.8, *p<0.001 related to untreated but p<1.0 vs each other). At the three-day time point, Sox11 transfected neurons exhibited significant changes in primary (F2, 241= 16.7, *p< 0.05) and higher order neurites (F2, 241= 28.1, *p<0.05), but not a significant change in maximum neurite length (H88, 87, 87= 6.1, p>0.05; Figure 2). PenIRES transfected cultures showed a small but statistically significant increase in primary neurite branching relative to untreated cultures (F2, 241= 16.7, p<0.05). However, this was the only parameter in which we observed any effects of vector control treatment.
Figure 2. Overexpression of Sox11 enhances neurite branching but not maximum neurite length in DRG neurons in vitro.
A: Vector control (PenIRES) transfected neurons immunoreactive for PGP9.5 exhibit some branching and neurite elongation after three days in vitro. B: Sox11 transfected neurons (PenSOX) immunoreactive for PGP9.5 display significant branching at three days. Scale Bar (A and B), 200μm. Higher magnification images of neurons in cultures treated with PenIRES (C) and PenSOX (D). Scale Bar (C and D), 100μm. E: The number of primary neurites three days in vitro in Sox11 transfected neurons (PenSOX) was greater relative to vector control transfected neurons and untreated neurons (**p<0.05). Number of primary neurites in the PenIRES condition was also significantly increased from untreated (*p<0.05). F: The number of secondary neurites three days in vitro in Sox11 transfected neurons (PenSOX) was also greater relative to vector control transfected neurons (PenIRES) and untreated neurons (**p<0.05). G: Maximum neurite length three days in vitro in Sox11 transfected neurons (PenSOX) however, was not significantly different relative to vector control transfected neurons (PenIRES) or untreated neurons. n=4 mice per condition.
Overexpression of Sox11 alters neurotrophic factor responsiveness in vitro
To better understand the mechanisms of Sox11, we then performed realtime PCR analysis on neuronal cultures with enhanced Sox11 for genes thought to be associated with neurite growth. We found no change between PenIRES transfected cultures and PenSOX transfected cells for activating transcription factor 3 (ATF3), leucine rich repeat immunoglobin family member, Linx, or growth associated protein 43 (GAP43). However, we did observe a significant upregulation of transcription factor c-Jun, and GDNF family neurotrophic factor receptors GFRα1 and GFRα3. We interestingly observed a slight decrease in the NGF receptor, trkA (F2, 6, *p< 0.05; n=3 for each condition; Table 1).
Table 1.
Percent change in gene expression in Sox11 transfected cells at two and three days in vitro relative to untreated cells.
| 2d | 3d | |||
|---|---|---|---|---|
| IRES | Sox11 | IRES | Sox11 | |
| Sox11 | 3.5 ± 6.6 | 15692 ± 2.0* | 5.2 ± 6.6 | 20722 ± 5.0# |
| cJun | 103.5 ± 9.0 | 262 ± 80* | 89 ± 6.5 | 187.5 ± 47.5# |
| ATF3 | 55 ± 34.5 | 39.5 ± 28.5 | 25 ± 88.5 | 55.5 ± 11.5 |
| Linx | 19.5 ± 24.5 | 213 ± 142 | 70.5 ± 92.5 | 159 ± 86.5 |
| GFRα1 | 23.5 ± 53.5 | 618 ± 185.5* | 78.5 ± 44.5 | 99.5 ± 26.5 |
| GFRα3 | 55 ± 5.5 | 140.5 ± 41.5* | 88.5 ± 9 | 89 ± 41.5 |
| GAP43 | 112 ± 58 | 91.5 ± 23.5 | 62.5 ± 41.5 | 45.5 ± 38.5 |
| trkA | 38.5 ± 14.5 | 16 ± 23.5 | 88.9 ± 13.2 | 65.5 ± 5.5# |
p value < 0.05, compared to IRES 2d
p value < 0.05, compared to IRES 3d
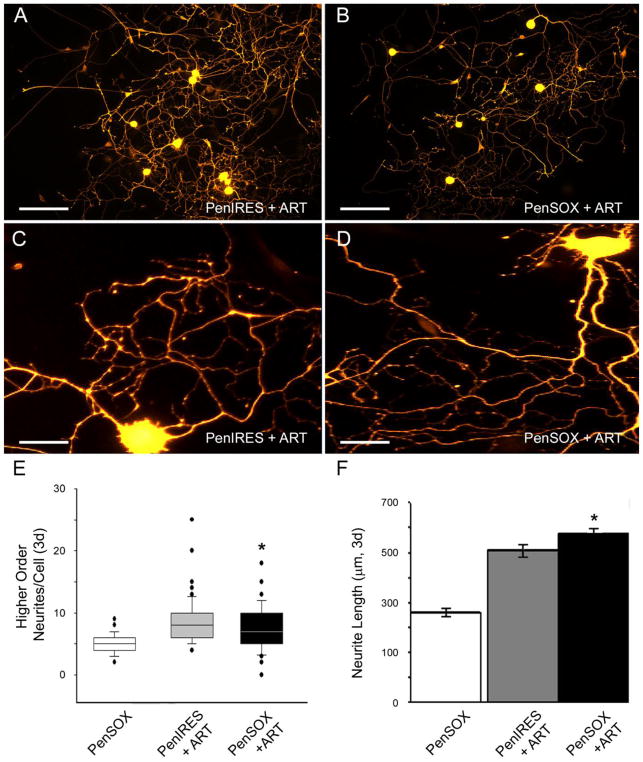
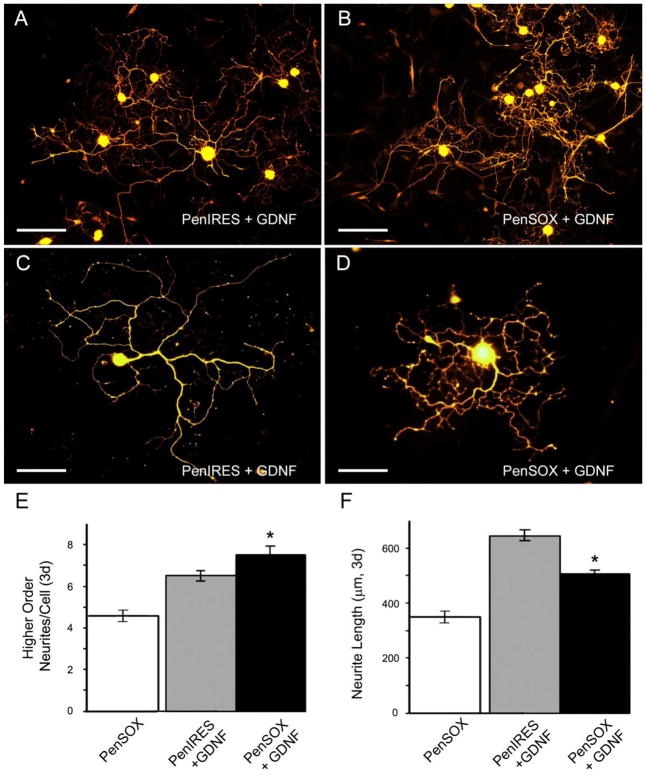
Since various growth factors are known to be produced by glial cells and can regulate regeneration (Dolda et al 2010; Fu and Gordon, 1997; Meyer et al., 1992; Trupp et al., 1995; Funakoshi et al., 1993; Park and Hong 2006) and the GDNF family member artemin functions through signaling of the GFRα3-RET receptor complex while GDNF itself functions through GFRα1-RET (Baloh et al., 2000), we analyzed whether increased Sox11 would facilitate changes in artemin and GDNF responsiveness. Transfection of cells with Sox11 containing plasmids altered the responsiveness to artemin compared to vector controls as DRG neurons displayed a significant decrease in higher order branching (t132, = 2.1, p< 0.04) and became substantially longer (t113= −2.2, p< 0.03; Figure 3). Likewise, we analyzed GDNF responsiveness following overexpression of Sox11, and found that this transcription factor substantially reduced neurite elongation in the presence of GDNF (t125= 5.99, p< 0.01) but increased the amount of higher order branching (t150= −1.98, p< 0.05; Figure 4) relative to GDNF treated, vector controls. Neurons treated with nerve growth factor (NGF) and PenIRES or PenSOX plasmids displayed no significant change between neurite branching (Primary branching: PenIRES: 1.8 ± 0.1; PenSOX: 1.9 ± 0.1, U115,116= 6405, p< 0.58; Secondary branching: PenIRES: 4.6 ± 0.2; PenSOX: 4.5 ± 0.2, t229= 0.8, p< 0.40) or neurite length (PenIRES: 536.7 ± 16.9μm; PenSOX: 520.1 ± 15.9μm, t192= 0.8, p< 0.43; n=6 mice for each condition).
Figure 3. Sox11 enhances responsiveness to the neurotrophic factor artemin.
A: Primary DRG neurons immunopositive for PGP9.5 transfected with vector control plasmids and treated with artemin (PenIRES+ART) exhibit extensive branching. B: Primary DRG neurons transfected with Sox11 containing plasmids and treated with artemin (PenSOX+ART) display less higher order branching, but have longer neurites. Scale Bar (A and B), 200μm. Higher magnification images of neurons in cultures treated with PenIRES+ART (C) and PenSOX+ART (D). Scale Bar (C and D), 40μm. E: Quantification of higher order branching in primary DRG neurons treated with Sox11 containing plasmids and artemin (PenSOX+ART) are significantly reduced relative to control plasmid transfected cells treated with artemin (PenIRES+ART). F: Maximum neurite length is substantially increased with overexpression of Sox11 in the presence of artemin (PenSOX+ART) in primary DRG neurons relative to control conditions (PenIRES+ART). Data from PenSOX treated cultures alone is provided for reference. *p<0.05; n=6 mice per condition.
Figure 4. Sox11 alters responsiveness to the neurotrophic factor GDNF.
A: Primary DRG neurons immunopositive for PGP9.5 transfected with vector control plasmids and treated with GDNF (PenIRES+GDNF) exhibit some branching and a few long neurites. B: Primary DRG neurons transfected with Sox11 containing plasmids and treated with GDNF (PenSOX+GDNF) however, display more branching, but shorter neurites. Scale Bar (A and B), 200μm. Higher magnification images of neurons in cultures treated with PenIRES+GDNF (C) and PenSOX+GDNF (D). Scale Bar (C and D), 40μm. E: Quantification of higher order branching in primary DRG neurons treated with Sox11 containing plasmids and GDNF (PenSOX+GDNF) are significantly increased relative to control plasmid transfected cells treated with GDNF (PenIRES+GDNF). F: Maximum neurite length is substantially decreased with overexpression of Sox11 in the presence of GDNF (PenSOX+GDNF) in primary DRG neurons relative to control conditions (PenIRES+GDNF). Data from PenSOX treated cultures alone is provided for reference. *p<0.05; n=6 mice per condition.
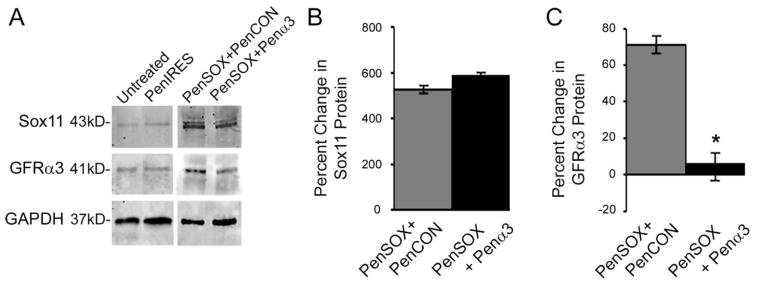
To confirm that enhanced Sox11 levels were altering artemin and GDNF responsiveness through their respective receptors (GFRα3 and GFRα1), we then transfected primary DRG neurons with either non targeting control (PenCON) siRNAs, GFRα3 targeting (Penα3) or GFRα1 targeting (Penα1) siRNAs in cultures that had enhanced levels of Sox11. We next performed neurite analyses in cultures treated with and without exogenous growth factors. We first verified that Sox11 mRNA levels were consistently elevated with enhanced Sox11 and non-targeting (PenCON), or targeting (Penα3) siRNAs (PenSOX+PenCON: 16948% ± 10522%; PenSOX+Penα3: 19186% ± 1403%, relative to untreated cultures). We then verified the knockdown of these receptors in DRG neurons in vitro. We found that transfection of DRG cultures with Penα3 siRNAs significantly knocked down the expression of GFRα3 mRNA (PenSOX+PenCON: 103.5% ± 28.3%*; PenSOX+Penα3: 5.6% ± 24.9%, F2,13= 5.81, *p< 0.05, relative to untreated) and transfection with Penα1 siRNAs significantly inhibited the expression of GFRα1 (PenSOX+PenCON: 2076% ± 89.4%*; PenSOX+Penα1: 314.6% ± 60.0%, F2,9= 6.0, *p< 0.02, relative to untreated). Protein expression was also confirmed for Penα3 transfected cells to verify that mRNA correlated with protein (Figure 5).
Figure 5. Transfection of Sox11 plasmid treated DRG cultures with GFRα3 targeting siRNAs significantly inhibits the expression of GFRα3 protein.
A: Examples of western blot images for Sox11, GFRα3 and GAPDH obtained from cultures treated with Penetrain-bound Sox11 containing plasmids (PenSOX) and either Penetratin-linked control (PenCON) or GFRα3 targeting siRNAs (Penα3). Untreated and PenIRES transfected control cultures are provided for reference. B: Increased expression of Sox11 is verified at the protein level under both conditions. C: Sox11-induced increase of GFRα3 protein is significantly reduced with Penα3 siRNAs. F2, 6= 9.7, p<0.02; n=3–4 mice per condition.
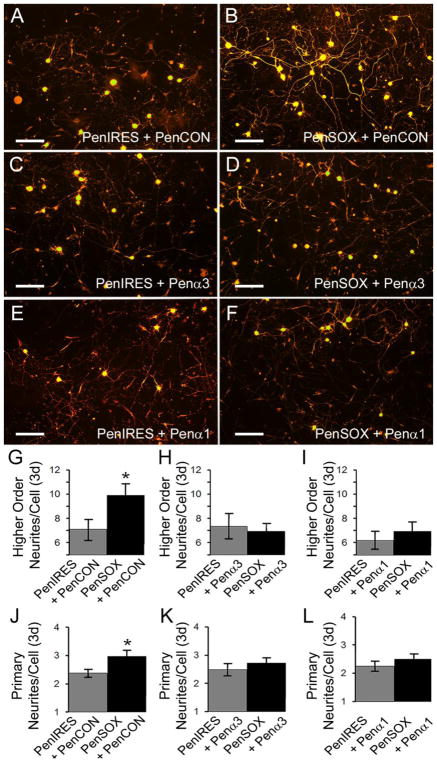
We then found that similar to cutlures treated with plasmids alone (Figure 2), that cells transfected with PenCON siRNAs and Sox11 containing plasmids displayed significantly increased primary (F1, 84= 5.2, p<0.03) and higher order neurite branching (F1, 84= 4.6, p<0.04) compared to vector plasmid controls. No effects were observed on maximum neurite length (PenIRES+PenCON: 225.4 ± 21.2 vs. PenSOX+PenCON: 218.8 ± 21.8; F1, 73= 0.04, p<0.8). However transfection of cutlures with either GFRα3 or GFRα1 targeting siRNAs in combination with Sox11 overexpression, was found to block the observed increases in primary (F1,84= 5.2, p<0.05 (PenCON); F1, 76= 0.7 (GFRα3), p<0.4; F1, 82= 0.9, p<0.4 (GFRα1)) and higher order neurite branching (F1,84= 5.2, p<0.05 (PenCON); F1, 76= 0.7 (GFRα3), p<0.4; F1, 82= 0.91, p<0.4 (GFRα1)) compared to vector treated controls (Figure 6). Again, no alterations in neurite length were observed under these conditions (PenIRES+Penα1 197.6 ± 14.0 vs PenSOX+ Penα1: 231.8 ± 19.4; F1, 91= 2.0, p<0.2; PenIRES+Penα3: 251.8 ± 20.4 vs. PenSOX+Penα3: 231.1 ± 14.1; F1, 76= 0.7, p<0.4).
Figure 6. siRNA-mediated inhibition of the GDNF family receptors GFRα3 and GFRα1 blocks the effects of overexpressing Sox11 in vitro.
A: Cells treated with vector control plasmids (PenIRES) and non-targeting control siRNAs (PenCON) display moderate branching in vitro. B: PenCON transfected cultures with Sox11 overexpression (PenSOX) display increased neurite branching. C: Knockdown of GFRα3 (Pena3) in vector control treated cultures does not significantly alter neurite growth. D: Inhibition of GFRα3 (Pena3) in cells co-transfected with Sox11 containing plasmids (PenSOX) show reduced branching. E: Similar to GFRα3 knockdown, inhibition of GFRα1 (Penα1) in vector control treated cultures does not significantly alter neurite growth. F: However, knockdown of GFRα1 in cells with enhaced Sox11 also reduces neurite branching. Scale Bars, 200μm. G–I: Higher order branching in vector control treated cells that are also treated with PenCON siRNAs is not altered by knockdown of either GFRα3 or GFRα1. However, the Sox11 induced increased in branching in PenCON treated conditions is blocked by GFRα1 and GFRα1 treatment. J–L: Similar effects of GFRα3 and GFRα1 targeting siRNAs are found in regard to primary neurite numbers whereby the Sox11 induced increase in primary neurites observed in control conditions of blocked by knockdown of GFRα3 and GFRα1. *p<0.05 vs. controls; n=3 mice per condition.
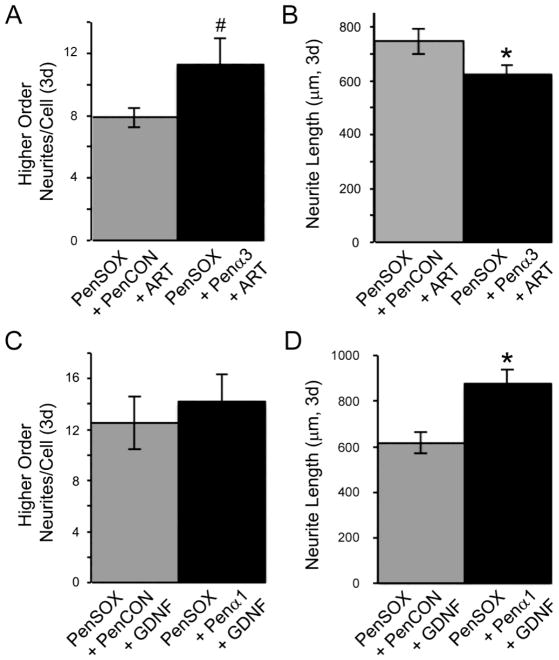
We then found that higher order neurites and neurite length possessed somewhat opposing effects in DRG neurons transfected with targeting siRNAs in the presence of artemin and GDNF compared to neurons transfected with PenCON siRNAs (Figure 7). When neurons were treated with GFRα3 siRNAs in addition to Sox11 plasmids and artemin, higher order branching was slightly increased (t 60= −1.8, p<0.08) yet elongation was significantly reduced (t65= 2.0, p<0.05) compared to neurons treated with control siRNAs, Sox11 plasmids and artemin. Likewise, when neurons were treated with GFRα1 siRNAs, Sox11 plasmids and GDNF, neurite elongation was substantially increased (t63= −3.2, p<0.002) compared to siRNA control neurons. However, neurite branching was not statistically changed with GFRα1 knockdown under these conditions (t54= −1.1, p<0.3).
Figure 7. Inhibition of the Sox11-induced expression of GDNF family receptors GFRα3 and GFRα1 blocks the effects of artemin and GDNF on neurite growth, respectively.
A: Higher order branching in primary DRG neurons transfected with Sox11 containing plasmids and treated with artemin display more branching with knockdown of GFRα3 (PenSOX+Penα3+ART) compared to control siRNA transfected cells (PenSOX+PenCON+ART). *p value < 0.05. B: Maximum neurite length is substantially decreased when cells are transfected with Sox11 containing plasmids and treated with artemin and GFRα3 siRNA (PenSOX+Penα3+ART) compared to treatment with control siRNA (PenSOX+PenCON+ART). #p<0.08; *p<0.05. C: Higher order branching in primary DRG neurons transfected with Sox11 containing plasmids and treated with GDNF and GFRα1 siRNA (PenSOX+Penα1+GDNF) exhibit more branching compared to control siRNA transfected cells (PenSOX+PenCON+GDNF), although not statistically significant. D: Maximum neurite length is substantially increased when transfected with Sox11 containing plasmids and treated with GDNF and GFRα1 siRNA (PenSOX+Penα1+GDNF) compared to controls (PenSOX+PenCON+GDNF). *p value < 0.002; n=6 mice per condition (artemin treated) and n=5 mice per condition (GDNF treated).
DISCUSSION
Enhanced Sox11 increases neurite branching and GDNF family neurotrophic factor responsiveness
Recent evidence suggested that manipulation of Sox11 regulates neurite growth and axon regeneration (Jing et al 2015; Jankowski et al., 2009a, 2006). Here we sought to determine possible mechanisms of how enhanced levels of Sox11 could facilitate neurite growth in sensory neurons. We found that cultured DRG neurons with enhanced levels of Sox11 had increased primary and higher order branching but not significantly altered neurite length. We also found that Sox11 overexpression enhanced neurotrophic factor receptor expression; specifically GDNF family member receptors GFRα3 and GFRα1. Because of these upregulated levels, we found that overexpression of Sox11 caused changes in neurite growth when in the presence of the GDNF family neurotrophic factors, but not NGF. Sox11 treated cells displayed fewer, yet longer, neurite branches after treatment with artemin, while Sox11 transfected cells treated with GDNF showed shorter but more numerous branches. These results suggest that one potential mechanism by which enhanced Sox11 enhances the regenerative capacity of DRG neurons is by increasing GDNF family neurotrophic factor responsiveness.
Sox11 is a selective regulator of axonal growth
Enhanced neurite growth caused by increased Sox11 may translate into a greater degree of regeneration in vivo, perhaps by influencing neurotrophic factor signaling. Although other studies involving the Sox family have shown significant effects on embryonic development and maturation, stem cell maintenance, neuronal differentiation, and lineage allocation (Lee and Saint-Jeannet, 2011; Hargrave et al., 1997), Sox11 appears to have a distinct function on neurite growth in DRG neurons. Alone, increased Sox11 appears to only enhance branching and not maximum neurite length. However, in the presence of growth factors such as artemin, Sox11 can significantly enhance elongation in DRG neurons. In the presence of GDNF however, the increased branching effects of Sox11 appear to be enhanced. Thus, the context by which Sox11 can enhance the capacity of DRG axons to grow seems to be dependent on the neurotrophic factor present. This is supported by the results showing a significant increase in both GFRα3 and GFRα1, and the changes in neurite growth when these receptors are altered. It is important to note that we did not perform a rescue of GFRα3 or GFRα1 expression in our siRNA experiments. However, we have previously reported that GFRα3 siRNAs and several other Penetratin linked siRNAs do not induce any unwanted off-target effects (Jankowski et al 2010, 2012, 2009a), thus it is likely that results presented here display specific effects of manipulating GFRα3 and GFRα1 under conditions of enhanced Sox11 levels.
Under basal conditions, not all DRG neurons possess GFRα3 and GFRα1 (Priestley et al., 2002; Molliver and Snider, 1997). However, in the context of axonal injury, the expression of these two receptors, along with Sox11, is significanly enhanced in the DRGs (Jankowski et al 2009a; Mills et al 2007; Bennett et al., 2000; 1998), suggesting that there could be a strong role for Sox11 and these pathways in mediating axon growth or regeneration. Although we did not assess the expression of GFRα1 or GFRα3 in our previous reports in which Sox11 knockdown reduced neurite growth (Jankowski et al 2006) and axon regeneration (Jankowski et all 2009a) of all cell/axon types, data from the current study support a role for Sox11 mediating its effects on axon growth at least in part through GDNF/artemin signaling in neurons via GFRα1/GFRα3 (Figs. 3–7). It is important to note however that the neurotrophic factor receptor levels at the three day time point are reduced compared to their levels at two days (Table 1), which may further reveal an interesting time dependency of Sox11. This will have to be specifically tested in future experiments.
One possible interpretation of these data may be that Sox11 increases the ability of artemin to speed up neurite elongation after injury, while it may allow GDNF to facilitate target innervation by enhancing branching. Our in vitro data show a 15–20% change in neurite branching or maximum neurite length in our various conditions. Although this may be perceived to be relatively minimal change, altering axonal receptive field innervation or rate of axon growth by this amount could be quite significant in vivo. GDNF responsive axons appear to have target innervation deficits when downstream mediators of GFRα1/Ret signaling are altered in vivo (Mandai et al., 2009), thus supporting the notion that GDNF alters branching and innervation. Systemic artemin treatment however, appears to cause regeneration of damaged axons in the central nervous sytem, allowing long-lasting restoration of function (Wang et al., 2008) and thereby supports the concept of artemin promoting neurite elongation. In the context of nerve regeneration in vivo, when axons are regenerating back towards their target, it would be beneficial to induce elongation and minimize branching through the neurotrophic factor artemin, and upon reaching the target it may be beneficial to stimulate branching through GDNF. Previous work has shown that both artemin and GDNF are upregulated in the skin after nerve injury (Jankowski et al., 2009b), and are likely altered in injured nerves in vivo (Dolda et al 2010; Baloh et al 2000; Fu and Gordon, 1997; Trupp et al., 1995; Funakoshi et al., 1993; Meyer et al., 1992), suggesting that regenerating axons would be stimulated to both elongate and branch upon reaching the periphery (Keast et al., 2010).
In addition to GDNF and artemin, it has been shown that other neurotrophic factors such as NGF and BDNF are upregulated at the site of axon damge in the periphery (e.g. Richner et al., 2014; Dolda et al 2010; Zochodne and Cheng, 2000; Baloh et al 2000; Fu and Gordon, 1997; Trupp et al., 1995; Funakoshi et al., 1993; Meyer et al., 1992) and these factors along with GDNF have been shown to promote axon regeneration (Hoyng et al., 2014). Artemin has recently been linked to long-distance regeneration after dorsal root crush (Wong et al., 2015) and may provide an alternative approach to central regeneration though the mechanisms described here. It will thus be important to determine the time depandency and localization of GDNF and Artemin upregulation after axon injury to better understand how Sox11 may regulate neurotrophic factor responsiveness to alter axon regeneration both peripherally and centrally.
It is important to note that our assays were performed on mixed cultures containing DRG neurons and non-neuronal cells. Our previous report (Jankowski et al 2009) found that Sox11 was not expressed in peripheral nerves and thus many non-neuronal cells in our cultures may not express Sox11. However, GFRα1 has been reported to be expressed in peripheral glia (Hase et al 2005), while GFRα3 is expressed at a much lower level postnatally in these non-neuronal cells (Piirsoo et al 2010). Artificially overexpressing Sox11 in non-neuronal cells could potentially alter their function through similar means described here, but this would need to be confirmed in future experimentation. In addition, although similar ranges of our growth factor doses have been used in many other reports to assess sensory neuron function and neurite growth (e.g. Fjell et al 1999; Malin et al 2007; Madduri et al 2009), doses could be percieved as slightly high. However, it has been shown that treatment of cultures with GDNF for example, at various concentrations ranging from 2–40ng/mL, did not show substantial alterations in neurite growth when compared with each other (Mills et al 2007). Thus, it is unlikely that using a lower dose of growth factors in the current report would yield different results. Nevertheless, this should be confirmed in the future.
Sox11 does not upregulate all factors associated with neurite growth
Sox11 overexpression did not seem to affect expression of classic factors associated with neurite growth such as ATF3 or GAP43 (Table 1; Aigner et al., 1995; Seijffers et al., 2006, 2007). ATF3 has been found to increase DRG neurite elongation and appears to contribute to an increase in the intrinsic growth state of injured neurons in mice (Seijffers et al., 2007). Other evidence however showed ATF3 knockdown occurs when Sox11 is inhibited (Jankowski et al., 2009a), although a lack of enhanced expression of ATF3 with Sox11 overexpression was not observed here. This may suggest that Sox11 is a permissive, but not an inductive factor in ATF3 expression, or Sox11 perhaps may already be maximally activating ATF3 expression and thus cannot increase its expression any further due to a ceiling effect. Further examination of the interactions between Sox11 and ATF3 may be beneficial to understand the interactions of these two transcription factors in regard to axon regeneration.
GAP43 overexpression has been shown to induce sprouting in the nervous system of transgenic mice, also suggesting a significant role in neurite growth (Aigner et al., 1995). The fact that this molecule was not affected with overexpression of Sox11 despite its involvement in regulating neurite growth either suggests that this factor functions in a different pathway, or may perhaps require Sox11 expression prior to its actions. These scenarios will also need to be investigated in more detail in the future.
Interestingly, enhanced Sox11 expression caused upregulation of the transcription factor c-Jun. Previous research showed a reduction of axonal outgrowth in DRG neurons in vitro following inhibition of c-Jun prosphorylation (Lindwall et al., 2004). This study also showed that transection of the vagus nerve elicited a significant increase in c-Jun phorphorylation, suggesting that c-Jun may also be an important contributer to axonal outgrowth of sensory neurons (Lindwall et al., 2004). c-Jun further appears to promote apoptosis in dissociated sympathetic neurons, yet stimulates axonal growth in sensory neurons, further displaying a selective type of regulation (Lindwall and Kanje, 2005). Although c-Jun phosphorylation appears to occur either before or at the same time as the induction of Sox11 after nerve injury (Jankowski et al., 2009a; Lindwall et al., 2004), the fact that enhanced Sox11 alters the expression of this transcription factor suggest that it may be able to facilitate the effects of c-Jun or even work in conjunction with this pro-regeneration gene to accelerate axon growth. Sox11 may also regulate PGP9.5 itself as cultures with Sox11 overexpression qualitatively have more intense staining. This could be due to the fact that overexpression of Sox11 increases neurite branching. Neverheless, future tests will be important to confirm these notions.
In summary, these results suggest that enhanced levels of Sox11 may alter neurite growth by regulating GDNF family neurotrophic factor responsiveness. A more detailed analysis of the regulation of gene expression by Sox11, as well as Sox11 overexpression in vivo may also highlight more of the discrepancies in regeneration observed following central and peripheral nerve injuries. It will be interesting in the future to determine whether artificial overexpression of Sox11 in DRG neurons after dorsal root injury will facilitate axon regeneration into the spinal cord in vivo. Nevertheless, these results provide a better understanding of the possible actions of Sox11 and its potential promotion of axon regeneration. These results may provide insight into the feasibility of enhancing Sox11 in order to promote axon regeneration in vivo for patients that experience central nerve lesions.
HIGHLIGHTS.
Overexpression of Sox11 in sensory neurons promotes neurite growth.
Sox11 enhances the responsiveness of DRG neurons to GDNF and artemin but not NGF.
Results suggest that sox11 may promote nerve regeneration by altering their responsiveness to GDNF family ligands.
Acknowledgments
This work was supported by grants from the NIH to HRK (R01NS023725) and MPJ (R56NS103179). We would also like to thank Ms. Collene Anderson and Ms. Katrina Ekmann for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors - implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, Phillips HS, McMahon SB, Shelton DL. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DLMG, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestly JV. A distinct subgroup of small DRG cells express GDNFF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland M, Ramskold D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25:2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, Troy CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- Dodla MC, Mukhatyar VJ, Bellamkonda RV. Principles of Regenerative Medicine. 2. Acad. Press; 2011. Peripheral Nerve Regeneration; pp. 1047–1063. [Google Scholar]
- Dom G, Shaw-Jackson C, Matis C, Bouffioux O, Picard JJ, Prochiantz A, Mingeot-Leclercq MP, Brasseur R, Rezsohazy R. Cellular uptake of Antennapedia Penetratin peptides is a two-step process in which phase transfer precedes a tryptophan-dependent translocation. Nucleic Acids Res. 2003;31:556–561. doi: 10.1093/nar/gkg160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res. 1999;67:267–82. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneraiton. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbanv G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave M, Wright E, Kun J, Emery J, Cooper L, Koopman P. Expression of the Sox11 gene in mouse embryos suggests roles in neuronal maturation and epithelio-mesenchymal induction. Dev Dyn. 1997;210:79–86. doi: 10.1002/(SICI)1097-0177(199710)210:2<79::AID-AJA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hase A, Saito F, Yamada H, Arai K, Shimizu T, Matsumura K. Characterization of glial cell line-derived neurotrophic factor family receptor alpha-1 in peripheral nerve Schwann cells. J Neurochem. 2005;95:537–543. doi: 10.1111/j.1471-4159.2005.03391.x. [DOI] [PubMed] [Google Scholar]
- Hoyng SA, De Winter F, Gnavi S, de Boer R, Boon LI, Korvers LM, Tannemaat MR, Malessy MJ, Verhaagen J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol. 2014;261:578–593. doi: 10.1016/j.expneurol.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 2006;143:501–514. doi: 10.1016/j.neuroscience.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009a;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Lawson JJ, McIlwrath SL, Rau KK, Anderson CE, Albers KM, Koerber HR. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009b;29:1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Anderson CE, Koerber HR. Enhanced artemin/GFRα3 levels regulate mechanically insensitive, heat sensitive C-fiber recruitment after axotomy and regeneration. J Neurosci. 2010;30:16272–83. doi: 10.1523/JNEUROSCI.2195-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Anderson CE, Molliver DC, Koerber HR. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal sensitivity during peripheral inflammation. Pain. 2012;153:410–9. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, Wang T, Huang S, Glorioso JC, Albers KM. The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprra1. Exp Neurol. 2015;233:221–232. doi: 10.1016/j.expneurol.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR, Forrest SL, Osborne PB. Sciatic nerve injury in adult rats causes distinct changes in the central projections of sensory neurons expressing different glial cell line-derived neurotrophic factor family receptors. J Comp Neurol. 2010;518:3024–3045. doi: 10.1002/cne.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kury P, Stoll G, Muller HW. Molecular mechanisms of cellular interactions in peripheral nerve regeneration. Curr Opin Neurol. 2001;14:635–639. doi: 10.1097/00019052-200110000-00013. [DOI] [PubMed] [Google Scholar]
- Lee YH, Saint-Jeannet JP. Sox9 function in craniofacial development and disease. Genesis. 2011;49:200–208. doi: 10.1002/dvg.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39:2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Kanje M. The Janus role of c-Jun: cell death versus survival and regeneration of neonatal sympathetic and sensory neurons. Exp Neurol. 2005;196:184–194. doi: 10.1016/j.expneurol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Dahlin L, Lundborg G, Kanje M. Inhibition of c-Jun phosphorylation reduces axonal outgrowth of adult rat nodose ganglia and dorsal root ganglia sensory neurons. Mol Cell Neurosci. 2004;27:267–279. doi: 10.1016/j.mcn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Madduri S, Papaloïzos M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65:88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–60. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Mandai K, Guo T, St Hillaire C, Meabon JS, Kanning KC, Bothwell M, Ginty DD. LIG family receptor tyrosine kinase-associated proteins modulate growth factor signals during neural development. Neuron. 2009;63:614–627. doi: 10.1016/j.neuron.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olsen L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Allchorne AJ, Griffin RS, Woolf CJ, Costigan M. GDNF selectively promotes regeneration of injured-primed sensory neurons in the lesioned spinal cord. Mol Cell Neurosci. 2007;36:185–194. doi: 10.1016/j.mcn.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Snider WD. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J Comp Neurol. 1997;381:428–438. doi: 10.1002/(sici)1096-9861(19970519)381:4<428::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Park S, Hong YW. Transcriptional regulation of artemin is related to neurite outgrowth and actin polymerization in mature DRG neurons. Neurosci Lett. 2006;404:61–66. doi: 10.1016/j.neulet.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Piirsoo M, Kaljas A, Tamm K, Timmusk T. Expression of NGF and GDNF family members and their receptors during peripheral nerve development and differentiation of Schwann cells in vitro. Neurosci Lett. 2010;469:135–140. doi: 10.1016/j.neulet.2009.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. doi: 10.1139/y02-034. [DOI] [PubMed] [Google Scholar]
- Richner M, Ulrichsen M, Elmegaard SL, Dieu R, Pallesen LT, Vaegter CB. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol. 2014;50:945–970. doi: 10.1007/s12035-014-8706-9. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sock E, Rettig SD, Enderich J, Bosl MR, Tamm ER, Wegner M. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol. 2004;24:6635–6644. doi: 10.1128/MCB.24.15.6635-6644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Rydén M, Jörnvall H, Funakoshi H, Timmusk T, Arenas E, Ibáñez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, Cariani P, Frank E, Sah DW, Porreca F. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11:488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Reynolds A, Kirry A, Nienhaus C, Blackmore MG. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J Neurosci. 2015;35:3139–3145. doi: 10.1523/JNEUROSCI.2832-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LE, Gibson ME, Arnold HM, Pepinsky B, Frank E. Artemin promotes functional long-distance axonal regeneration to the brainstem after dorsal root crush. PNAS. 2015;112:6170–6175. doi: 10.1073/pnas.1502057112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW, Cheng C. Neurotrophins and other growth factors in the regenerative milieu of proximal nerve stump tips. J Anat. 2000;196(Pt 2):279–283. doi: 10.1046/j.1469-7580.2000.19620279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]