Abstract
A halophilic bacterial consortium was enriched from Red Sea saline water and sediment samples collected from Abhor, Jeddah, Saudi Arabia. The consortium potentially degraded different low (above 90% for phenanthrene and fluorene) and high (69 ± 1.4 and 56 ± 1.8% at 50 and 100 mg/L of pyrene) molecular weight polycyclic aromatic hydrocarbons (PAHs) at different concentrations under saline condition (40 g/L NaCl concentration). The cell hydrophobicity (91° ± 1°) and biosurfactant production (30 mN/m) confirmed potential bacterial cell interaction with PAHs to facilitate biodegradation process. Co-metabolic study with phenanthrene as co-substrate during pyrene degradation recorded 90% degradation in 12 days. The consortium in continuous stirred tank reactor with petroleum refinery wastewater showed complete and 90% degradation of low and high molecular weight PAHs, respectively. The reactor study also revealed 94 ± 1.8% chemical oxygen demand removal by the halophilic consortium under saline condition (40 g/L NaCl concentration). The halophilic bacterial strains present in the consortium were identified as Ochrobactrum halosaudis strain CEES1 (KX377976), Stenotrophomonas maltophilia strain CEES2 (KX377977), Achromobacter xylosoxidans strain CEES3 (KX377978) and Mesorhizobium halosaudis strain CEES4 (KX377979). Thus, the promising halophilic consortium was highly recommended to be employed in petroleum saline wastewater treatment process.
Keywords: Halophiles, Biodegradation, Petroleum wastewater, PAHs, Co-metabolism, Continuous stirred tank reactor
Introduction
Marine environment is greatly affected by petroleum hydrocarbons during exploration, spillage via transportation, effluent discharge from industrial treatment plants (Mnif et al. 2009; Nogales et al. 2011). Polycyclic aromatic hydrocarbons are toxic compounds (carcinogenic, mutagenic effects) that persist in the environment for longer period due to their hydrophobic nature (Marston et al. 2001; Mukherjee et al. 2010). Piubeli et al. (2012) reported equal volume of saline wastewater is produced during crude oil exploration, which leads to a great challenge for effluent treatment. Several treatment methods for petroleum hydrocarbon degradation such as solubilization, evaporation, photochemical decomposition and microbial degradation were reported (Fingas 2013; Liu et al. 2016; Lamichhane et al. 2017). Among the methods microbial degradation attracted more attention and numerous research was performed at normal condition for almost three decades. Saudi Arabia exports nearly 10 million barrels of crude oil every day which alternatively results in release of high amount of petroleum-contaminated saline wastewater during exploration and refining process. The produced water from different petrochemical refineries leads a major threat to the marine ecosystem directly or indirectly. High salinity act as major hindering factor for biodegradation process, which greatly influence the treatment of petroleum wastewater under saline condition (Lefebvre and Moletta 2006). The rate of biodegradation is limited by high salinity and availability of nutrients (nitrogen and phosphorus) in the marine environment (Atagana et al. 2003). Thus the studies on halophilic bacterial strains to treat petroleum hydrocarbons in wastewater observe to be more important. Our previous research to overcome nutrient depletion by addition of nutrients along with PAHs under saline condition accelerated the mineralization process and reduced the time taken for biodegradation (Pugazhendi et al. 2017). Limited studies were performed under saline conditions by the researchers (Mnif et al. 2014; Ghosal et al. 2016). This area of research using extremophiles in wastewater treatment attracts many researchers around the world. Promising PAHs degrading bacterial consortium was obtained from sediment samples near the cruise parking which release ballast water with hydrocarbon contamination into the marine environment. Low molecular weight PAHs such as anthracene, phenanthrene and fluorene with bay and K region in structure represent the PAH contamination in marine environment (Mallick et al. 2011). The present study focused on extremophilic (halophilic) bacterial consortium capable of degrading different selected low and high molecular weight PAHs and to treat petroleum refinery wastewater under saline condition. The role of additional nutrients in biodegradation was also detailed in comparison with our previous research.
Materials and methods
Sample collection
The saline sea water and sediment samples were collected from Abhor, Red Sea, Jeddah, Saudi Arabia. Halophilic mineral salt medium (HSM) with phenanthrene as sole carbon source was used for the enrichment of the consortium from mixture of marine water and sediment samples. Enrichment of the halophilic consortium was achieved by 3–4 identical transfers.
Chemicals
All the chemicals used in the study are analytical grade. High pure PAHs were purchased from Sigma (> 99.8% purity). High performance liquid chromatography (HPLC) and gas chromatograph mass spectrometry (GCMS) was used for analyzing biodegradation of PAHs by the halophilic consortium. HPLC grade chemicals were used for PAHs extraction process and analysis.
Halophilic mineral salt medium
The carbon-free HSM contained (g/L) NH4Cl-2.5, Na2HPO4-4.76, KH2PO4-5.46, MgSO4-0.20 and NaCl-40. The final pH of the medium was adjusted to 7.4 ± 0.2 with NaOH (0.1 N), and the medium was sterilized in an autoclave (JSR, South Korea) at 121 °C for 15 min. Phenanthrene (PHN) and fluorene (FLU) was selected from low molecular weight PAHs. Pyrene (PY) represented high molecular weight PAHs in the present study. PAHs stock solution at the concentration of 5000 mg/L was prepared and stored for experimental use.
Biodegradation of PAHs
Preliminary study to screen the potential PAHs degradation by the bacterial consortium (BC) was performed in HSM agar medium coated with PHN as sole carbon source. PAHs clearing zone by the consortium was performed as detailed by Kiyohara et al. (1982). The biodegradation experimental set up consist of two controls (HSM + BC and HSM + PAH) and a test sample (HSM + BC + PAH). All the experiments were performed in duplicates. Degradation of PHN was studied at different saline conditions ranging from 40, 80, 120, 160 g/L of NaCl concentration. The optimized saline condition for degradation of PAHs was 40 g/L of NaCl concentration. Co-metabolism was studied with PHN (100 mg/L) and PY (50 mg/L) at 40 g/L of NaCl concentration. Urea was used instead of ammonium chloride in HSM at 160 g/L of NaCl concentration to enhance the biodegradation of PAHs. Yeast extract (100 mg/L) was employed as additional substrate along with FLU (100 mg/L) under extreme saline condition (160 g/L of NaCl concentration). After acidification of the experimental flask to pH 2.5 with 1 N HCl, ethyl acetate (v/v) was used for extraction of the PAHs. The extraction was performed twice for high PAHs recovery (91–95%). Further, the extract passed through anhydrous sodium sulphate to remove the aqueous phase completely (Arulazhagan and Vasudevan 2009). Filtered extract was condensed to 1 mL for HPLC (Agilent, USA) analysis. Prior to HPLC analysis the samples were filtered using syringe filter (0.2 µm). C18 general column (4.6 µm, 150 mm × 5 µ, Zorbax Eclipse plus) was used to analyze the residual hydrocarbon for evaluating the potential of biodegradation process. Acetonitrile at the flow rate of 1 mL/min was used as the mobile phase with 40 °C column oven temperature. High pure PAH standards (99.9% purity, Sigma, USA) were used as a reference in HPLC.
Respirometric analysis
CO2 evolution during biodegradation of PHN was analyzed in respirometer (Bioscience, USA) at 30 °C. CO2 evolved in test and control bottles were analyzed every 8 h time interval. KOH (45%) was filled in the CO2 collection tube and the sensor was filled with H2SO4 (1 N). CO2 trapped in the collection tube by KOH was mixed with 5 mL BaCl2. The mixture (KOH + BaCl2) was titrated against HCl (0.25 N) with phenolphthalein as indicator solution. Disappearance of pink color was noted as end point and correspondingly the O2 uptake was recorded in the respirometer sensor connected with a computer (Pugazhendi et al. 2017).
GCMS analysis
GCMS was used to identify the metabolites formed during PAH biodegradation by the halophilic consortium. GCMS with fuse-silica capillary column (30 m × 0.25 mm ID × 0.25 µm) was used for PAHs metabolites analysis. The temperature program (1 min holding at 100 °C, 160 °C by 15 °C/min and 7 min at 300 °C by 5 °C/min) was used with helium as carrier gas representing the mobile phase (Pugazhendi et al. 2017). The injector temperature under splitless mode was held at 280 °C for 3 min. GCMS standards (PAHs and metabolites) obtained from Sigma Aldrich and GCMS internal library search was used to identify the metabolites formed during PAHs degradation.
Cell hydrophobicity analysis
Contact angle, zeta potential measurement and biosurfactant production
The halophilic bacterial consortium grown with PHN as sole carbon source was used for contact angle and zeta potential study. 50 mL of the halophilic culture was taken and centrifuged at 3000 rpm for 10 min at 20 °C. After centrifugation the supernatant was decanted, to the pellet 50 mL of 10 mM KNO3 was added and washed twice. The pellet was mixed with 1 mL of KNO3 as the final volume of sample for analysis and vortexed gently for complete mixing of the sample. From the sample mixture 100 µL was added with 20 mL of KNO3 and filtered through a 0.45-µm membrane filter paper. The filter paper consisted of enough bacterial cells that was allowed to dry for 2 h at 30 °C. After drying, the membrane filter was analyzed on drop shape analyzer (Kruss, Germany) using the software available in the instrument. For zeta potential measurements, 50 µL of the final sample was added to 10 mL of KNO3 that was analyzed in zeta potential analyzer (Malvern, UK). Biosurfactant production by the consortium was analyzed using surface tensiometer (Kruss, Germany).
Lab-scale reactor study
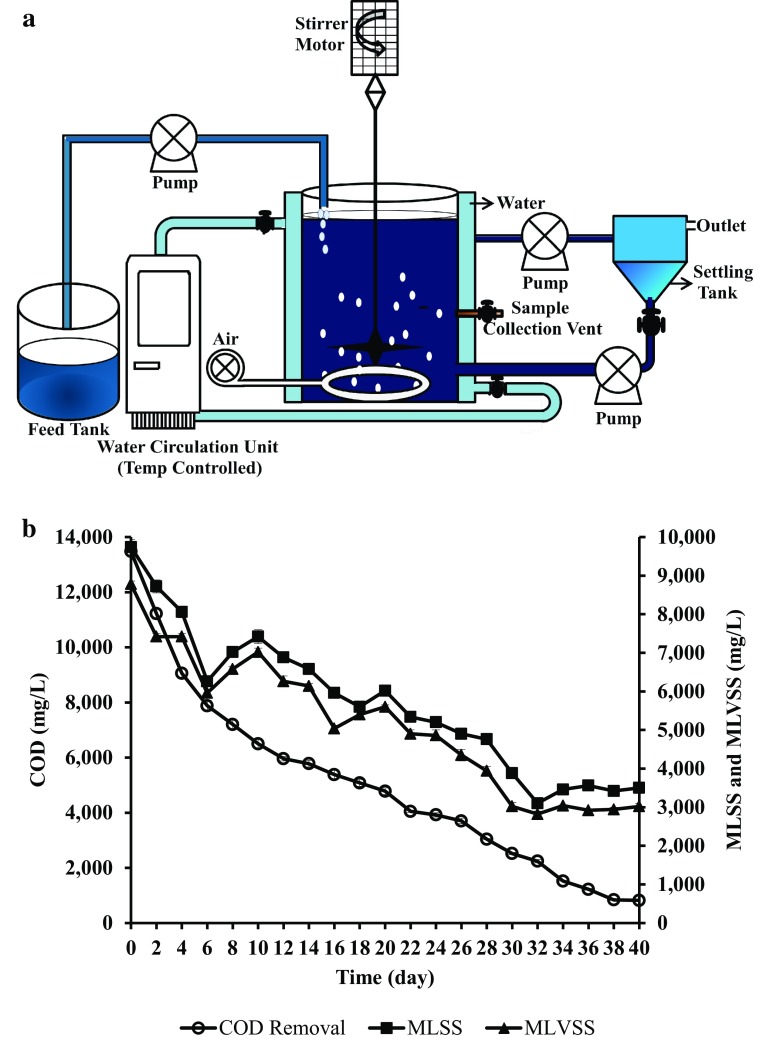
The lab scale reactor study was executed in a temperature controlled bioreactor (continuous stirred tank reactor) with total volume of 10 L and a working volume of 7 L capacity. Petroleum refinery wastewater was collected from Petro Rabigh, Jeddah, Saudi Arabia. The reactor was operated under continuous stirred condition with 6.5 L of wastewater and 0.5 L of bacterial consortium. Optimization of CSTR operating condition was pertained by executing the study at different organic loading rate (OLR) such as 0.128, 0.106, 0.08 and 0.064 kg COD/m3 day, where the optimized OLR was found to be 0.106 kg COD/m3 day. When OLR was increased the results revealed an inverse relationship between OLR and COD removal by the bacterial consortium. Also decrease in HRT (less than 10 days) influenced the COD removal. The PAHs present in the wastewater analyzed in HPLC showed the presence of different PAHs such as naphthalene (354.7 ± 1.5 mg/L), PHN (126.5 ± 2.1 mg/L), FLU (140.7 ± 1.4 mg/L), anthracene (54.8 ± 1.7 mg/L) and PY (742.7 ± 2.2 mg/L). Total nitrogen (TN) and total phosphate (TP) content in the petroleum wastewater was 47 ± 1 and 18 ± 0.5 mg/L. Initial concentration of TN and TP inside the reactor with the consortium was 62 ± 1 and 244 ± 0.8 mg/L, respectively. MLSS and MLVSS concentration was 9.74 and 8.78 g/L in the reactor on 0th day. Wastewater treatment parameters such as COD, mixed liquor suspended solids (MLSS), mixed liquor volatile suspended solids (MLVSS), total phosphate and total nitrogen were analyzed as stated in standard methods by American Public Health Association (Rice et al. 2005).
Phylogenetic analysis
Qiagen DNA isolation kit was used to extract the bacterial DNA of the halophilic consortium present in different degradation experiments and reactor study. The potential halophilic bacterial strains present in the consortium was identified using high through put sequencing techniques. DNA samples from different degradation experiments and reactor study were analyzed to picture the changes in halophilic bacterial community. Primer set (515-532U 5′-GTGYCAGCMGCCGCGGTA-3′ and 909-928U 5′-CCCCGYCAATTCMTTTRAGT-3′) targeting at V4–V5 region of 16S rRNA was used in the high through put sequencing (Wang and Qian 2009). The high-throughput sequencing reactions were performed as detailed by Pugazhendi et al. (2017). BLASTN search confirms the genus of the bacterial strain, further neighborhood relationship was obtained by evolutionary analyses using MEGA v5 (Molecular Evolutionary Genetic Analysis).
Results and discussion
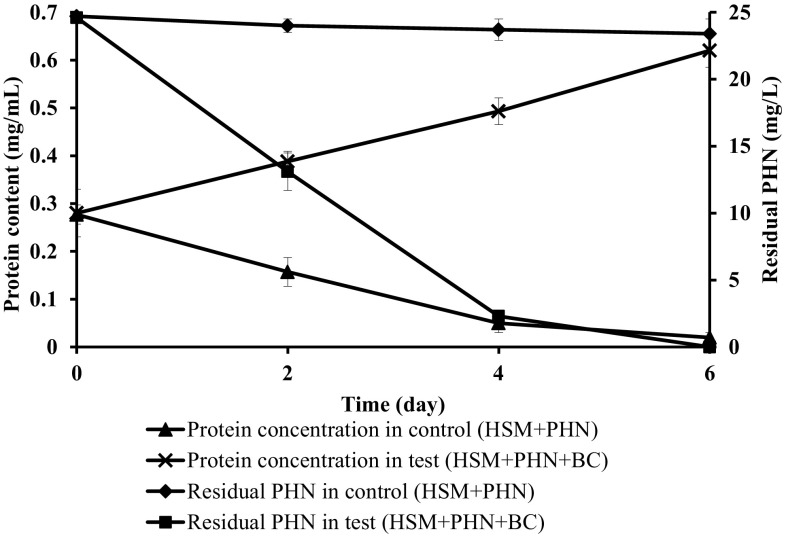
The halophilic bacterial consortium enriched from water and sediment samples from Red Sea, Jeddah, Saudi Arabia, utilized phenanthrene as the sole carbon source under 40 g/L of NaCl concentration. Preliminary study on PAHs degradation by the bacterial consortium performed in HSM agar medium with PHN as sole carbon source revealed zone of clearance around the disc with halophilic consortium. Thus the consortium potentially mineralized the PAHs in agar medium under saline condition as sole carbon source. Initial study on PHN (25 mg/L) in HSM confirmed biodegradation potential of the bacterial consortium with 91 ± 0.5% and complete degradation in 4 and 6 days, respectively (Fig. 1).
Fig. 1.
PHN degradation at 25 mg/L under 40 g/L NaCl concentration. Error bars indicate the relative standard deviation (n = 2)
Mineralization of PAHs
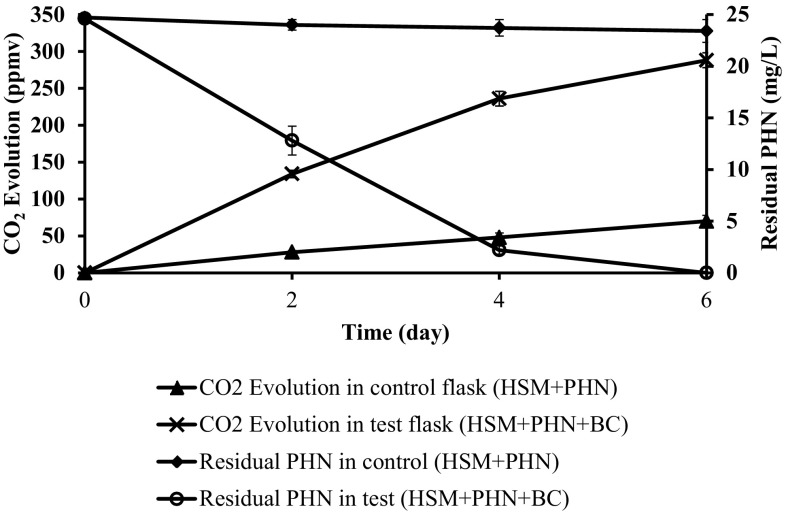
Release of carbon dioxide during biodegradation confirmed the complete mineralization of PHN to CO2 under saline condition. The consortium released 82 ± 1.8% of CO2 during mineralization of PHN (25 mg/L) as sole carbon source in mineral salt medium under saline condition (Fig. 2).
Fig. 2.
Respirometric (CO2) analysis during biodegradation of PHN. Error bars indicate the relative standard deviation (n = 2)
The metabolites formed during the biodegradation of PHN confirmed complete mineralization of PHN to harmless end products. Metabolites formed during LMW (PHN and FLU) and HMW PAHs were benzene-1,2-dicarboxylic acid and benzoic acid, respectively. The metabolites enter TCA (tricarboxylic acid) cycle and results in complete mineralization of PAHs to CO2 and H2O. PAHs were mostly oxidized by the bacterial strains to dihydroxy compounds and enter the ortho- or meta-cleavage pathways (Gao et al. 2013). The metabolites formed in the present study were in agreement with previous reports by Zeinali et al. (2008), Tsai et al. (2009), Roy et al. (2012) and Pugazhendi et al. (2017). Mineralization of PAHs to non-toxic form is an important criterion in all the biodegradation process, since the by-products formed by metabolization and no further degradation results in high toxicity impact than the parent compounds (Elgh-Dalgren et al. 2011).
Bacterial cell hydrophobicity
The halophilic bacterial consortium cell hydrophobicity and biosurfactant production was confirmed mainly using contact angle measurement (91° ± 1°), Zeta potential (− 27 mv) and surface tensiometer (30 mN/m) analysis. The cell hydrophobicity study confirmed the presence of the consortium with moderately hydrophobic cell surface nature which facilitates PAHs adherence and degradation. The hydrophobic stability of the consortium potentially enhanced the bacterial cell interaction with PAHs by reducing the distance between them (Johnsen et al. 2005; Harms et al. 2010). The biosurfactant production facilitates the attraction of hydrophobic hydrocarbons resulted in mineralization of hydrocarbons (Vasudevan et al. 2007; Gomes et al. 2018). Thus the studies confirm the attachment of PAHs to the cell surface of the halophilic consortium for potential degradation.
Degradation of PAHs at different concentrations
Low molecular weight PAHs
PHN was studied at different concentrations (50, 100, 200 and 500 mg/L) under saline condition (40 g/L of NaCl concentration). The halophilic bacterial consortium degraded 95 ± 0.8% of PHN (50 mg/L) in 8 days (Fig. 3a). Increase in PHN concentration to 100 and 200 mg/L showed 97 ± 0.7 and 98 ± 1.6% degradation by the halophilic consortium in 8 and 10 days, respectively (Fig. 3b, c). Further increase in PHN concentration to 500 mg/L recorded 92 ± 2.1% degradation in 12 days under saline condition (Fig. 3d). Increase in PAHs concentration influenced the bacterial growth which correspondingly resulted in decrease of percent degradation under saline condition.
Fig. 3.
PHN degradation at different concentrations a 50 mg/L, b 100 mg/L, c 200 mg/L and d 500 mg/L by halophilic consortium. Error bars indicate the relative standard deviation (n = 2)
FLU degradation by the halophilic bacterial consortium was also studied at similar concentrations (50, 100, 200 and 500 mg/L) of PHN under saline condition (40 g/L of NaCl concentration). At 50 mg/L complete degradation was achieved by the consortium in 8 days (Fig. 4a). When FLU concentration increased to 100 and 200 mg/L the degradation was 98 ± 1.6% in 8 and 10 days, respectively (Fig. 4b, c). FLU concentration at 500 mg/L recorded 96 ± 0.4% degradation by the consortium in 12 days under saline condition (Fig. 4d). As compared to PHN, the consortium degraded FLU at high rate as sole carbon source under saline condition.
Fig. 4.
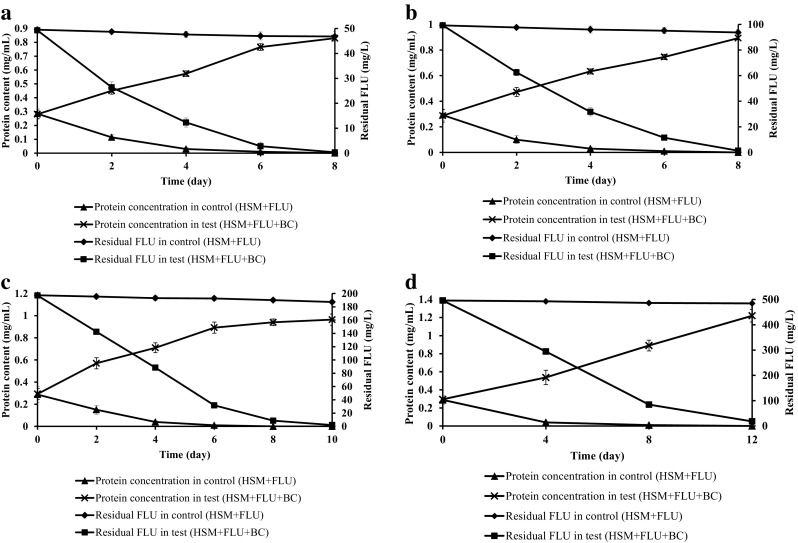
FLU degradation at different concentrations a 50 mg/L, b 100 mg/L, c 200 mg/L and d 500 mg/L by halophilic consortium. Error bars indicate the relative standard deviation (n = 2)
High molecular weight PAHs
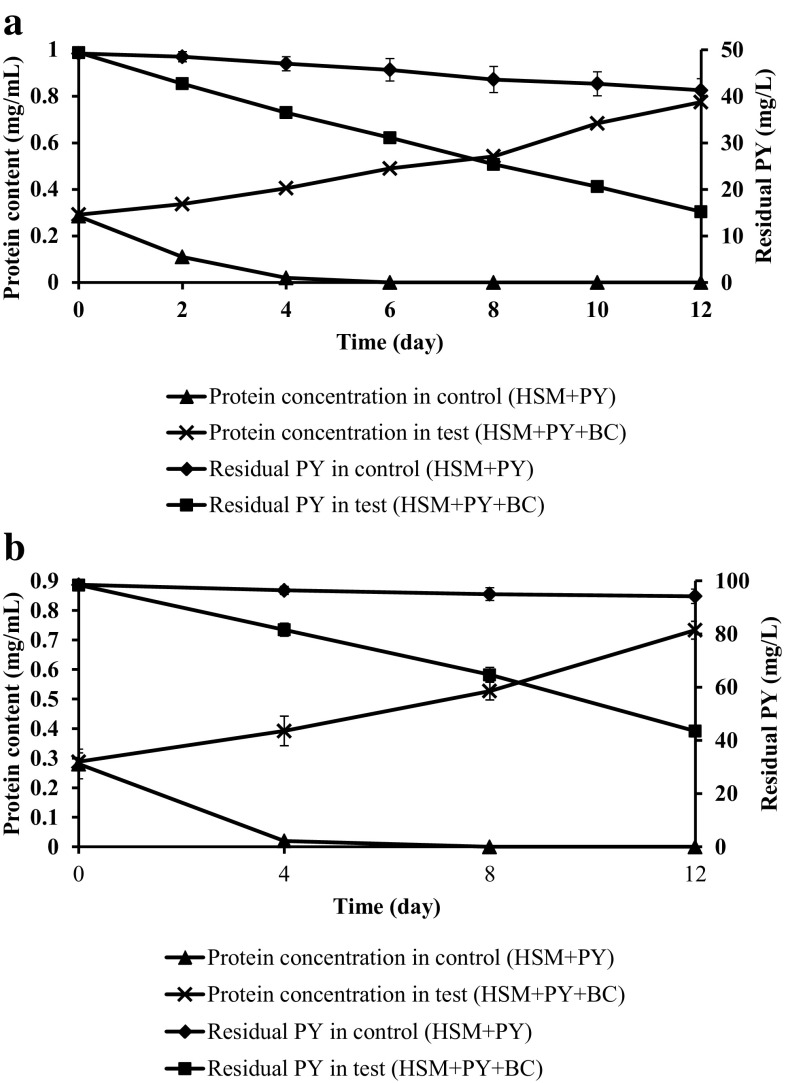
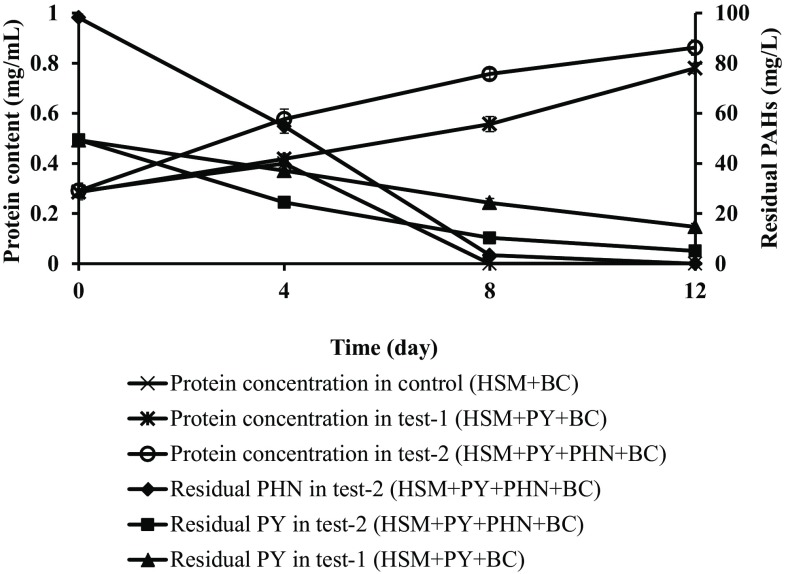
PY was used as the model compound under high molecular weight PAHs, where two different concentrations (50 and 100 mg/L) was used to study the degradation potential by the bacterial consortium. The consortium showed 69 ± 1.4 and 56 ± 1.8% degradation at 50 and 100 mg/L concentration of PY under saline condition (Fig. 5a, b). The results confirmed the consortium under saline condition struggled to degrade PY. To overcome this problem, PHN 100 mg/L was added which will enhance co-metabolism under saline condition (Arulazhagan et al. 2014). The consortium initially utilized PHN and after potential growth simultaneously consumed PY. The consortium potently utilized PHN and PY under co-metabolism study with complete degradation of PHN and 90 ± 1.5% of PY degradation in 12 days (Fig. 6).
Fig. 5.
Degradation of PY at a 50 mg/L and b 100 mg/L concentration by halophilic consortium. Error bars indicate the relative standard deviation (n = 2)
Fig. 6.
Co-metabolism study using PHN with PY. Error bars indicate the relative standard deviation (n = 2)
Degradation of PAHs at different salinity
Degradation potential of the halophilic consortium was analyzed at different salinity with FLU (100 mg/L) as sole carbon source. Salinities such as 80, 120 and 160 g/L were used in the study. The degradation of FLU at 80 and 120 g/L salinity was 92 ± 2.1 and 84 ± 2.4%, respectively. The consortium was able to degrade FLU potentially upto 120 g/L. At 160 g/L salinity the consortium reached saturation and the percent degradation decreased drastically to 27 ± 2.2% (Table 1). Thus increase in salinity highly influenced the biodegradation of FLU. This may be due to the absence of nutrients under high saline conditions or in marine environment. Previous reports also confirmed the importance of nutrients during biodegradation under saline condition (Atagana et al. 2003; Arulazhagan and Vasudevan 2011; Pugazhendi et al. 2017). To overcome this problem, ammonium chloride in HSM was replaced with urea as nitrogen source and yeast extract (100 mg/L) to enhance bacterial growth. This served as an additional substrate that potentially increased the percent degradation of FLU (78 ± 2.7%) at 160 g/L salinity. Yeast extract composed of nitrogen, vitamins, carbon and amino acids to support bacterial growth under saline condition (Arulazhagan et al. 2013, 2014). Thus yeast extract was employed as additional substrate which increased the bacterial growth and recorded corresponding PAH degradation. Previous studies at high salinity above 5% limited the bacterial growth and reduced the percent degradation of PHN (Bonfa et al. 2011; Dastgheib et al. 2012; Guo et al. 2016). In the present study the bacterial consortium recorded potential degradation of FLU as sole carbon source upto 120 g/L (12%) salinity.
Table 1.
Degradation of FLU at different saline concentrations
| Salinity (g/L) | Degradation of FLU (%) | Time (days) | Degradation of FLU with yeast extract (%) | Time (days) |
|---|---|---|---|---|
| 40 | 98 ± 1.6 | 8 | 98 ± 1.1 | 5 |
| 80 | 92 ± 2.1 | 8 | 97 ± 1.7 | 6 |
| 120 | 84 ± 2.4 | 12 | 92 ± 1.5 | 6 |
| 160 | 27 ± 2.2 | 16 | 78 ± 2.7 | 12 |
Lab-scale reactor study
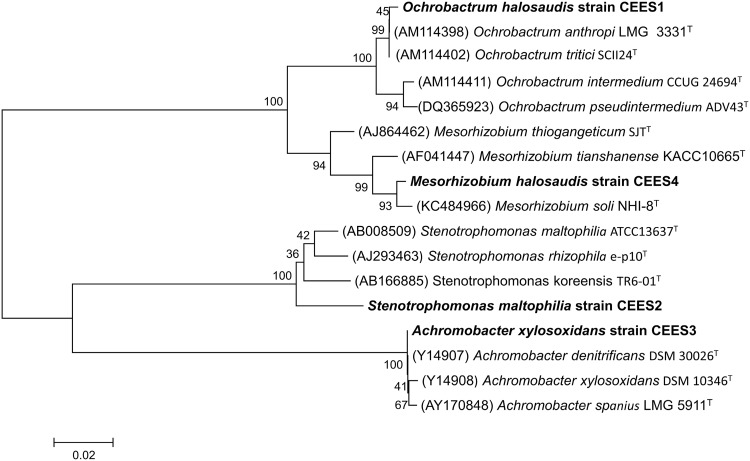
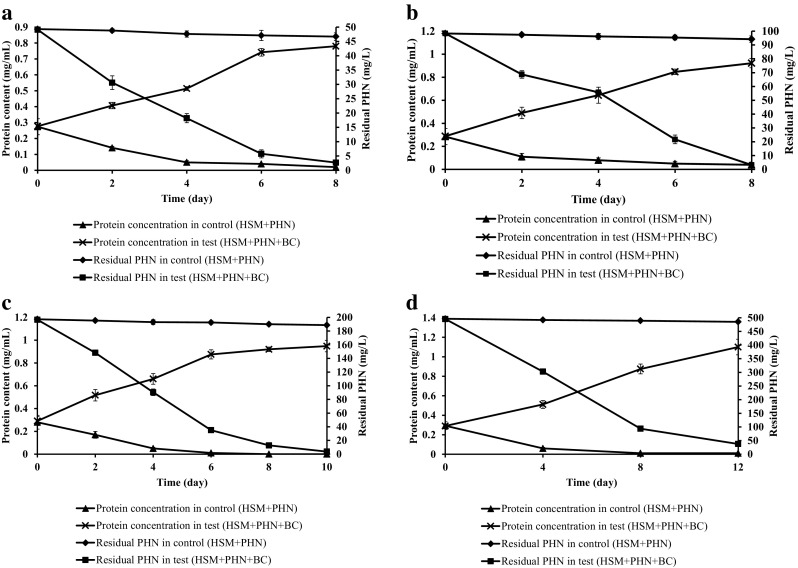
Petroleum contaminated saline wastewater collected from petroleum refinery was used in the present study. The bacterial consortium potentially degraded different petroleum hydrocarbons present in the wastewater along with COD reduction. The halophilic consortium degraded both LMW and HMW PAHs under saline condition in CSTR (Fig. 7a). The reactor was operated under optimized OLR (0.106 kg/m3 day) and corresponding flow rate with hydraulic retention time (HRT) of 10 days under saline condition. Figure 7b clearly depicts 94 ± 1.8% COD removal in 40 days by the halophilic bacterial consortium and corresponding MLSS and MLVSS concentration was maintained in the reactor. During potential petroleum wastewater treatment MLSS and MLVSS was maintained at the range of 3.5–5 and 3–4 g/L, respectively (Fig. 7b). The initial concentration of total nitrogen and phosphate content in the reactor was 62 ± 1 and 244 ± 0.8 mg/L. The reactor study showed increase in MLVSS concentration with reduction in total nitrogen and phosphate content, which revealed the utilization of the nutrients (nitrogen and phosphate) by the bacterial consortium (Arulazhagan and Vasudevan 2011). Phylogenetic analysis with BLASTN similarity search confirmed 99% similarity to the strains of Ochrobactrum, Stenotrophomonas maltophilia, Mesorhizobium sp. and 100% for Achromobacter xylosoxidans. The presence of four different bacterial strains namely Ochrobactrum halosaudis strain CEES1 (KX377976), Stenotrophomonas maltophilia strain CEES2 (KX377977), Achromobacter xylosoxidans strain CEES3 (KX377978) and Mesorhizobium halosaudis strain CEES 4 (KX377979) in all the degradation experiments and reactor study was confirmed. Ochrobactrum halosaudis observed to dominate the consortium with 60–70% occupancy followed by other strains. Previous reports also confirmed Ochrobactrum as potential PAHs and phenol degrading strain (Arulazhagan et al. 2014; Pugazhendi et al. 2017; Chandrasekaran et al. 2018). The neighborhood distance relationship of the PAHs degrading potential halophilic strains was detailed, using MEGA v5 (Fig. 8). Thus the study confirmed that the potential halophilic bacterial consortium can be employed in the treatment of petroleum-contaminated saline wastewater. Further studies are planned for large-scale application of the halophilic bacterial consortium in secondary treatment process to treat saline wastewater from petroleum refinery.
Fig. 7.
a Pictorial representation of CSTR, b treatment of petroleum refinery saline wastewater by halophilic consortium. Error bars indicate the relative standard deviation (n = 2)
Fig. 8.
Phylogenetic analysis of halophilic bacterial strains in the halophilic consortium using nucleotide sequence with neighborhood relationship
Conclusion
The present study provided complete details on degradation of different PAHs under saline condition. The research study established the importance of nutrients availability during biodegradation of PAHs under high saline condition by the halophilic bacterial consortium. The study also revealed the role of the halophilic consortium in treating petroleum hydrocarbons contaminated saline wastewater. Thus the consortium acts as a potential candidate to be employed in the treatment of saline refinery wastewater.
Acknowledgements
This project was funded by Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under Grant no. G-297-150-38. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Arulazhagan P, Vasudevan N. Role of moderately halophilic bacterial consortium in biodegradation of polyaromatic hydrocarbons. Mar Pollut Bull. 2009;58:256–262. doi: 10.1016/j.marpolbul.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Arulazhagan P, Vasudevan N. Biodegradation of polycyclic aromatic hydrocarbons by a halotolerant bacterial strain Ochrobactrum sp. VA1. Mar Pollut Bull. 2011;62:388–394. doi: 10.1016/j.marpolbul.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Arulazhagan P, Yeom IT, Sivaraman C, Srikanth M, Rajesh Banu J. Role of nutrients on biodegradation of 1,4-dioxane by a bacterial consortium enriched from industrial sludge. Adv Environ Biol. 2013;7:2081–2090. [Google Scholar]
- Arulazhagan P, Sivaraman C, Adish Kumar S, Aslam M, Rajesh Banu J. Co-metabolic degradation of benzo(e)pyrene by halophilic bacterial consortium at different saline conditions. J Environ Biol. 2014;35:445–452. [PubMed] [Google Scholar]
- Atagana HI, Haynes RJ, Wallis FM. Optimization of soil physical and chemical conditions for the bioremediation of creosote-contaminated soil. Biodegradation. 2003;14:297–307. doi: 10.1023/A:1024730722751. [DOI] [PubMed] [Google Scholar]
- Bonfa MRL, Grossman MJ, Mellado E, Durrant LR. Biodegradation of aromatic hydrocarbons by haloarchaea and their use for the reduction of the chemical oxygen demand of hypersaline petroleum produced water. Chemosphere. 2011;84(11):1671–1676. doi: 10.1016/j.chemosphere.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S, Pugazhendi A, Banu RJ, Ismail IMI, Qari HA. Biodegradation of phenol by a moderately halophilic bacterial consortium. Environ Prog Sustain Energy. 2018 [Google Scholar]
- Dastgheib SMM, Amoozegar MA, Khajeh K, Shavandi M, Ventosa A. Biodegradation of polycyclic aromatic hydrocarbons by a halophilic microbial consortium. Appl Microbiol Biotechnol. 2012;95(3):789–798. doi: 10.1007/s00253-011-3706-4. [DOI] [PubMed] [Google Scholar]
- Elgh-Dalgren K, Arwidsson Z, Ribé V, Waara S, von Kronhelm T, van Hees PAW. Bioremediation of a soil industrially contaminated by wood preservatives-degradation of polycyclic aromatic hydrocarbons and monitoring of coupled arsenic translocation. Water Air Soil Pollut. 2011;214(1–4):275–285. doi: 10.1007/s11270-010-0422-0. [DOI] [Google Scholar]
- Fingas MF. Modeling oil and petroleum evaporation. J Pet Sci Res. 2013;2(3):104–115. [Google Scholar]
- Gao S, Seo JS, Wang J, Keum YS, Li J, Li QX. Multiple degradation pathways of phenanthrene by Stenotrophomonas maltophilia C6. Int Biodeterior Biodegrad. 2013;79:98–104. doi: 10.1016/j.ibiod.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D, Ghosh S, Dutta TK, Ahn Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Front Microbiol. 2016;7:1369. doi: 10.3389/fmicb.2016.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MB, Gonzales-Limache EE, Sousa STP, Dellagnezze BM, Sartoratto A, et al. Exploring the potential of halophilic bacteria from oil terminal environments for biosurfactant production and hydrocarbon degradation under high-salinity conditions. Int Biodeterior Biodegrad. 2018;126:231–242. doi: 10.1016/j.ibiod.2016.08.014. [DOI] [Google Scholar]
- Guo G, He F, Tian F, Huang Y, Wang H. Effect of salt contents on enzymatic activities and halophilic microbial community structure during phenanthrene degradation. Int Biodeterior Biodegrad. 2016;110:8–15. doi: 10.1016/j.ibiod.2016.02.007. [DOI] [Google Scholar]
- Harms H, Smith KEC, Wick LY. Microorganism–hydrophobic compound interactions. In: Timmis KN, McGenity TJ, van der Meer JR, de Lorenzo V, editors. Handbook of hydrocarbon and lipid microbiology. Berlin: Springer; 2010. pp. 1479–1490. [Google Scholar]
- Johnsen AR, Wick LY, Harms H. Principles of microbial PAH degradation in soil. Environ Pollut. 2005;133:71–84. doi: 10.1016/j.envpol.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Kiyohara H, Nagao K, Yana K. Rapid screen for bacteria degrading water insoluble, solid hydrocarbons on agar plates. Appl Environ Microbiol. 1982;43:454–457. doi: 10.1128/aem.43.2.454-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane S, Bal Krishna KC, Sarukkalige R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: a review. J Environ Manag. 2017;199:46–61. doi: 10.1016/j.jenvman.2017.05.037. [DOI] [PubMed] [Google Scholar]
- Lefebvre O, Moletta R. Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res. 2006;40:3671–3682. doi: 10.1016/j.watres.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen B, Zhang BY, Jing L, Zhang H, Lee K. Photocatalytic degradation of polycyclic aromatic hydrocarbons in offshore produced water: effects of water matrix. J Environ Eng. 2016;142(11):04016054. doi: 10.1061/(ASCE)EE.1943-7870.0001135. [DOI] [Google Scholar]
- Mallick S, Chakraborty J, Dutta TK. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: a review. Crit Rev Microbiol. 2011;37:64–90. doi: 10.3109/1040841X.2010.512268. [DOI] [PubMed] [Google Scholar]
- Marston CP, Pereira ZC, Ferguson J, Fischer L, Hedstrom O, Dashwood WM, Baird WM. Effect of a complex environmental mixture from coal tar containing polycyclic aromatic hydrocarbons (PAH) on tumor initiation, PAH DNA binding and metabolic activation of carcinogenic PAH in mouse epidermis. Carcinogenesis. 2001;22:1077–1086. doi: 10.1093/carcin/22.7.1077. [DOI] [PubMed] [Google Scholar]
- Mnif S, Chamkha M, Sayadi S. Isolation and characterization of Halomonas sp. Strain C2SS100, a hydrocarbon degrading bacterium under hypersaline conditions. J Appl Microbiol. 2009;107:785–794. doi: 10.1111/j.1365-2672.2009.04251.x. [DOI] [PubMed] [Google Scholar]
- Mnif S, Sayadi S, Chamkha M. Biodegradative potential and characterization of a novel aromatic-degrading bacterium isolated from a geothermal oil field under saline and thermophilic conditions. Int Biodeterior Biodegrad. 2014;86:258–264. doi: 10.1016/j.ibiod.2013.09.015. [DOI] [Google Scholar]
- Mukherjee S, Bardolui NK, Karim S, Patnaik VV, Nandy RK, Bag PK. Isolation and characterization of a monoaromatic hydrocarbon-degrading bacterium, Pseudomonas aeruginosa from crude oil. J Environ Sci Health A Toxic Hazard Subst Environ Eng. 2010;45:1048–1053. doi: 10.1080/10934529.2010.486328. [DOI] [PubMed] [Google Scholar]
- Nogales B, Lanfranconi M, Pina-Villalonga JM, Bosch R. Anthropogenic perturbations in marine microbial communities. FEMS Microbiol Rev. 2011;35:275–298. doi: 10.1111/j.1574-6976.2010.00248.x. [DOI] [PubMed] [Google Scholar]
- Piubeli F, Grossman MJ, Fantinatti-Garboggini F, Durrant LR. Identification and characterization of aromatic degrading Halomonas in hypersaline produced water and COD reduction by bioremediation by the indigenous microbial population using nutrient addition. Chem Eng Trans. 2012;27:385–390. [Google Scholar]
- Pugazhendi A, Qari J, Basahi JMA, Godon JJ, Dhavamani J. Role of a halothermophilic bacterial consortium for the biodegradation of PAHs and the treatment of petroleum wastewater at extreme conditions. Int Biodeterior Biodegrad. 2017;121:44–54. doi: 10.1016/j.ibiod.2017.03.015. [DOI] [Google Scholar]
- Rice EW, Baird RB, Eaton AD, Clesceri LS, editors. Standard methods for the examination of water and wastewater. 21. Washington: American Public Health Association, APHA; 2005. [Google Scholar]
- Roy M, Khara P, Dutta TK. meta-Cleavage of hydroxynaphthoic acids in the degradation of phenanthrene by Sphingobium sp. strain PNB. Microbiology. 2012;158:685–695. doi: 10.1099/mic.0.053363-0. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Kumar M, Lin JG. Anaerobic biotransformation of fluorene and phenanthrene by sulfate-reducing bacteria and identification of biotransformation pathway. J Hazard Mater. 2009;164(2–3):847–855. doi: 10.1016/j.jhazmat.2008.08.101. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Bharathi S, Arulazhagan P. Role of plasmid in the degradation of petroleum hydrocarbon by Pseudomonas fluorescens NS1. J Environ Sci Health Part A. 2007;42(8):1141–1146. doi: 10.1080/10934520701418649. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qian PY. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One. 2009;4:e7401. doi: 10.1371/journal.pone.0007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinali M, Vossoughi M, Ardestani SK. Degradation of phenanthrene and anthracene by Nocardia otitidiscaviarum strain TSH1, a moderately thermophilic bacterium. J Appl Microbiol. 2008;105(2):398–406. doi: 10.1111/j.1365-2672.2008.03753.x. [DOI] [PubMed] [Google Scholar]