Abstract
Purpose
Conventional grayscale ultrasound (US) is accurate in the diagnosis of gallbladder disease (GD), but in some cases, it is not decisive. Contrast-enhanced ultrasound (CEUS) improves the diagnostic accuracy of US. The primary objective of this study is to assess the reliability of CEUS in the diagnosis of sludge; the secondary objective is to assess the ability of CEUS to diagnose cancer.
Methods
We retrospectively reviewed the US of 4137 patients positive for GD. In 43/4137 (1.04%), the use of could not discriminate between sludge and neoplasms. Then, we evaluated CEUS in only 39 of these patients, and in 4/43 (9%) cases it was not performable. After CEUS, the absence of enhancement was considered diagnostic for sludge, while contrast washout within 60 s diagnosed malignant lesions.
Results
Among the 39 patients, 16 had biliary sludge and 23 had lesions of the gallbladder wall; 9 of these were carcinomas and 14 were benign tumors. The absence of enhancement was present in 16/16 patients with sludge and in 0/23 patients with lesions of the gallbladder (sensitivity and specificity 100%). Washout was within 60 s in 9/9 gallbladder carcinomas and 2/14 benign lesions (sensitivity 100%; specificity 85%).
Conclusions
US is confirmed to be accurate in the diagnosis of GD. In doubtful cases, CEUS is very accurate in biliary sludge diagnosis. An intralesional washout at 60 s is a pattern of malignancy that can orient towards a correct diagnosis, but it is limited by the presence of false positive results, especially for smaller lesions.
Keywords: Gallbladder, Ultrasound, CEUS, Sludge, Benign disease, Malignant disease
Sommario
Scopo
L’ecografia convenzionale in scala dei grigi (US) è accurata nella diagnosi delle malattie colecistiche (GD) ma in alcuni casi non è decisiva. L’ecografia con mezzo di contrasto (CEUS), migliora l’affidabilità diagnostica dell’US. Obiettivo primario di questo studio è quello di determinare l’affidabilità della CEUS nella diagnosi di fango biliare; obiettivo secondario definire l’affidabilità della CEUS nella diagnosi di carcinoma della colecisti.
Metodi
Abbiamo analizzato retrospettivamente 4137 ecografie di pazienti positive per GD. In 43/4137 (1.04%) l’ecografia non era capace di discriminare tra fango e neoplasie. La CEUS era valutata in soli 39 pazienti, in 4/43 (9%) dei casi non era eseguibile. L’assenza di enhancement alla CEUS era considerato diagnostico per sludge, mentre un washout del contrasto entro 60 secondi era diagnostico per malattie maligne. L’assenza di enhancement era presente in 16/16 pazienti con fango e in 0/23 pazienti con lesioni colecistiche (sensibilità e specificità 100%). Il wash-out era presente a 60 sec in 9/9 tumori colecistiti e in 2/14 lesioni benigne (sensibilità 100%, specificità 85%).
Conclusioni
L’ecografia si conferma accurata nella diagnosi delle GD. Nei casi dubbi la CEUS è veramente accurata nella diagnosi di sludge. Un wash out intralesionale a 60 secondi è un pattern di malignità che può orientare verso una corretta diagnosi, ma è limitato dalla presenza di risultati falsi positivi, specialmente nelle lesioni più piccole.
Introduction
Conventional gray-scale ultrasound (US) is very accurate in the diagnosis of gallbladder disease, but in some cases, it is not decisive. Among the major limitations of this tool is its inability to differentiate between dense biliary sludge and tumors [1–11].
In US, sludge appears as low-level echoes that layer in the dependent portion of the gallbladder without acoustic shadowing; they generally shift slowly with changes in the patient’s position [12, 13]. When sludge is deposited in the lower wall of the gallbladder or in the infundibulum or fundus and is too dense, no movement can be observed, which can be misleading.
In some cases, it may be exchanged for a polypoid lesion or it may mask a malignancy, since sludge does not allow for proper study of the underlying gallbladder wall. In these cases, other imaging techniques are useful, such as CT, MRI, Magnetic resonance cholangiography and EUS scans, which offer better reliability and specificity. However, the preoperative diagnosis of gallbladder cancer can be often difficult [14, 15].
In recent years, the introduction of contrast-enhanced ultrasonography (CEUS) has made significant contributions to the diagnosis of many abdominal diseases [16–19]. This is also true for gallbladder diseases. Since, unlike tumors, sludge is not vascularized, CEUS can actually improve the diagnostic accuracy of US in differentiating tumors from sludge. In fact, using Levovist, Inoue showed that the absence of enhancement has a 100% sensitivity for sludge diagnosis and a specificity of 98% [20]. Xiao, using SonoVue (a second-generation contrast medium), reported a CEUS sensitivity of 100%, but a lower specificity [21]. Regarding washout time there is no agreement; in fact, Xiao found that malignant tumors present a faster washout time (35 s), while Badea found 41.4 s [21, 22].
In the diagnosis of the gallbladder, carcinoma data are not unique. In fact, while the EFSUMB guidelines include CEUS among the methods useful for gallbladder disease, they do not consider it to have a clear role in diagnosis [23, 24]. Since then, numerous studies and a meta-analysis have been published, but they have not yet clarified the real role of CEUS [25–28]. Moreover, CEUS has currently no role in differentiating benign from malignant gallbladder polyps.
Aims of the study
The primary objective of this retrospective study was to assess the reliability of CEUS in the diagnosis of sludge in a population of patients where grayscale US was not discriminatory.
The secondary objective was to assess the ability of CEUS to differentiate benign from malignant lesions.
Materials and methods
We retrospectively reviewed the sonograms of 4137 patients (2653 F, 1484 M) positive for gallbladder disease, which were performed between March 2013 and December 2015 at the Diagnostic and Interventional Ultrasound section, Department of Internal Medicine and Transplantation, University of Bologna (Hospital St. Orsola Malpighi). In 43/4137 patients (1.04%)—(17 M, 26 F), mean age 54 ± 12 years—US using classic criteria (formation within the gallbladder, mobile with changes in body position) did not discriminate between sludge and neoplasm.
The ultrasound examinations were performed with a Philips IU 22 US system. A 1–5 MHz convex transducer and 3–9 MHz linear transducer were used.
Scans were performed on patients in the morning after fasting for at least 10 h; CEUS was performed by an operator (CS) with more than 10 years of experience who also performed the preliminary conventional ultrasound for each patient.
CEUS: SonoVue (Bracco, Italy) is an SF6-filled microbubble contrast agent stabilized by phospholipids. A contrast bolus of 2.4 ml was injected into an antecubital vein via a 20-gauge cannula followed by a 10-ml normal saline flush. Real-time examination was performed for up to 5 min after contrast injection.
The enhancement process of gallbladder lesions using CEUS is classified in two vascular phases: the “early/arterial phase” (10–30 s after contrast injection) and the “late/venous phase” (31–180 s after contrast injection), since the blood supply of the gallbladder is entirely arterial. Contrast in the late phase persists for a shorter time compared with liver parenchyma, so this must be kept in mind to avoid a false positive diagnosis of malignancy, since it could be misconceived as a washout [17, 23]. Digital images and clips including B-Mode, Color-Doppler and CEUS in all vascular phases were routinely recorded for each examination. The CEUS diagnosis was compared with the patient’s surgical specimen when surgery was indicated. In other cases, patients were enrolled in a follow-up program for a minimum of 24 months, monitoring every 6 months.
In 4/43 (9%) cases, CEUS was not available due to one case with the presence of stones with a large shadow that prevented a good view of the whole lumen of the gallbladder, one case of porcelain gallbladder, one case of meteorism and finally one case of poor collaboration.
The lesions were classified as follows:
-
A.Grayscale examination:
- Polypoid lesions: sessile or with pedicle protruding into the gallbladder lumen. Echogenicity was divided into: (a) hyperechogenic; (b) hypoechogenic; (c) hysoechogenic if, respectively, greater/less than or like the liver; (d) inhomogeneous (with inhomogeneous internal texture).
- Wall thickening with irregular surface: when the wall of the gallbladder appeared focally and irregularly thickened with irregular surface and non-homogeneous internal texture.
-
B.CEUS examination:
- Avascular lesions: without enhancement.
- Vascular lesions: with enhancement.
Findings were defined as:
Sludge: lesions protruding into the gallbladder or thickening without evidence of enhancement or infiltration into the adjacent liver.
Lesions: lesions in the gallbladder or thickening with evidence of enhancement.
These in turn were defined as:
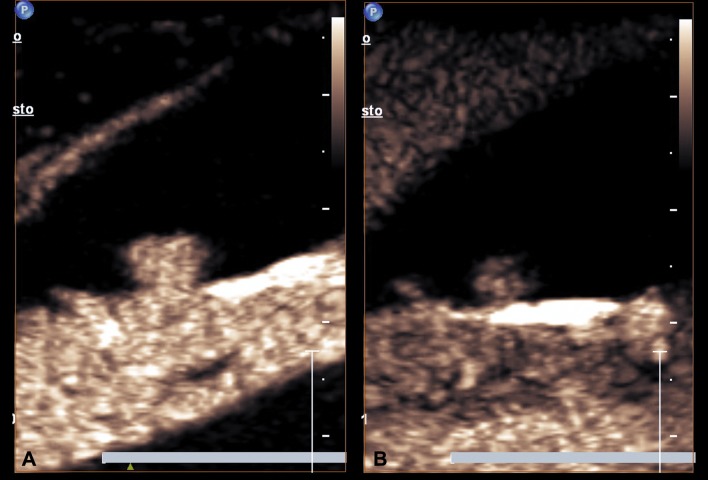
Benign lesions: lesions protruding into the gallbladder or thickening without washout within 60 s and infiltration into the adjacent liver (Fig. 1) [22].
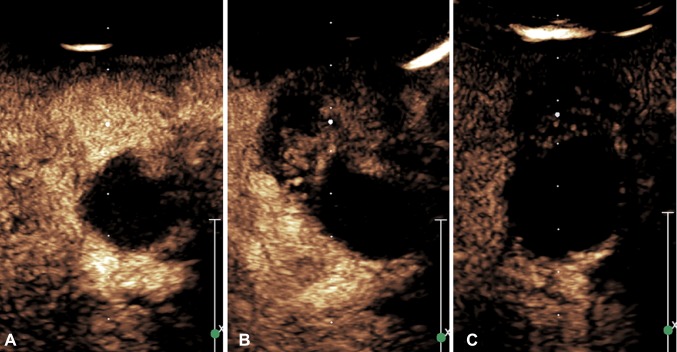
Cancer: lesions protruding into the gallbladder or thickening with washout within 60 s in the presence or absence of infiltration into the adjacent liver (Figs. 2 and 3) [22].
Fig. 1.
a At CEUS homogeneous enhancement of the polypoid mass protruding into the GB lumen during the arterial phase, b the same lesion shows a persistent enhancement in the late phase (60 s)
Fig. 2.
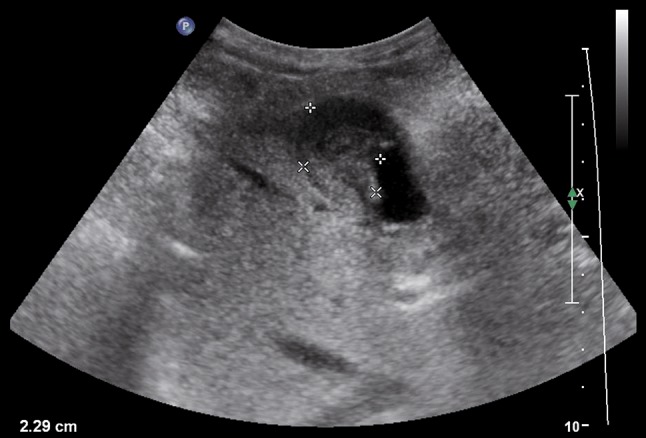
B-mode ultrasound demonstrates hypoechoic lesion 2.29 cm in diameter in the gallbladder and without clear cleavage with the liver
Fig. 3.
a In the early phase of CEUS (23 s), the gallbladder lesion (arrow) showed homogeneous hyper-enhancement, b the lesion (arrow) showed hypo-enhancement 40 s after contrast agent injection; c the lesion showed continuous hypo-enhancement during the late phase (120 s)
The choice of submitting patients to follow-up or surgery was made in agreement with the data in the literature on the natural history of this disease [29, 30]:
Follow-up: lesions ≤ 1 cm, without clinical, laboratory and US signs of cholestasis.
- Surgery:
- lesions of any size with clinical, ultrasound or laboratory signs of cholestasis,
- lesions > 1 cm, even without signs of cholestasis,
- increase in lesion size during follow-up > 1 cm;
Diagnosis of benign/malignant lesions was obtained:
by surgery;
by the absence of signs of gallbladder cancer in contrast-enhanced computed tomography (CT);
by the appearance of typical signs of cholelithiasis on US during follow-up;
or by no changes in the shapes and sizes of the lesions during follow-up.
The study was carried out under informed consent according to protocols approved by Department of Organ Insufficiency and Transplantation.
Statistical analysis
CEUS’ diagnostic reliability was evaluated by calculating sensitivity and specificity with standard formulas. When the data distribution was Gaussian, values were expressed as mean ± standard deviation and their differences were calculated using Student’s t test; otherwise, data were expressed as the median and range and analyzed with the Mann–Whitney U test. Fisher’s exact and χ2 tests and Spearman’s rank correlations were used where appropriate.
Results
Diagnosis of sludge
Table 1 shows some US and CEUS characteristics of the study population with lesions or sludge.
Table 1.
Main US and CEUS characteristics of the study population patients with lesions or sludge
| Lesions n = 23 |
Sludge n = 16 |
P | |
|---|---|---|---|
| Age | 63.3 ± 10 | 43.2 ± 12 | 0.0001 |
| M/F | 8/15 | 7/9 | ns |
| Size (mm) | 10 (8–60) | 14 (10–24) | ns |
| Shape Polypoid lesions/wall thickening |
17/6 | 14/2 | ns |
| Echogenicity | |||
| Hypoechoic | 7 (30.4%) | 2 (12.5%) | ns |
| Hysoechoic | 0 (0%) | 2 (12.5% | ns |
| Hyperechoic | 16 (43.4%) | 12 (75%) | ns |
| Texture | |||
| Inhomogeneous | 6 (21.4%) | 3 (18.7%) | ns |
| Enhancement | 23 | 0 | 0.0001 |
| Washout | 11 (48%) | – | – |
Patients with sludge had a significantly lower age (P = 0.003). There were no differences in sex or size, shape, echogenicity and texture. All lesions showed enhancement with CEUS that was absent in cases of sludge (P = 0.0001). Washout was present in 48% (11/23) of lesions. When using the absence of enhancement to define sludge, CEUS sensitivity and specificity were 100%. In fact, we did not find any false positive or negative values.
Diagnosis of benign/malignant lesions
Twenty patients were treated surgically; histopathology diagnosis revealed gallbladder carcinoma in 9 (5 with biliary sludge), adenoma in 2, cholesterol polyps in 5 and biliary sludge with microlithiasis in 4.
In the remaining patients, diagnoses were confirmed in 4 cases by the absence of contrast enhancement on CT, in 5 cases by no changes in the shapes of the lesions detected during follow-up and in 10 cases by the appearance of typical signs of cholelithiasis on US during follow-up.
Table 2 shows some US and CEUS characteristics of gallbladder cancers and benign lesions.
Table 2.
Main US and CEUS characteristics of the study population patients with carcinomas or benign lesions
| Carcinomas n = 9 |
Benign lesions n = 14 |
P | |
|---|---|---|---|
| Age | 68 ± 9 | 60 ± 11 | 0.09 |
| M/F | 3/6 | 5/9 | ns |
| Size (mm) | 20 (10–60) | 12 (10–24) | 0.001 |
| Shape | 3/6 | 14/0 | 0.0001 |
| Polypoid lesions/wall thickening | |||
| Echogenicity | |||
| Hypoechoic/hyperechoic | 7/2 | 2/12 | 0.01 |
| Hysoechoic | 0 (0%) | 0 (0%) | |
| Texture | |||
| Inhomogeneous | 5 (22.2%) | 1 (7.14%) | ns |
| Enhancement in arterial phase | 9 (100%) | 14 (100%) | ns |
| Washout | 9 (100%) | 2 (14.3%) | 0.0001 |
In patients with carcinoma, the sizes were statistically larger than were benign lesions (P = 0.001); the prevalent forms were hypoechoic (P = 0.01), with more frequent inhomogeneous textures, but this was not statistically significant. The polypoid form was prevalent in the benign lesions (P < 0.01).
All lesions showed enhancement, but washout within 60 s was present in 100% of carcinomas and in 14.3% of benign lesions. Using a washout cut-off of 60 s for the diagnosis of carcinoma of the gallbladder, CEUS sensitivity was 100% and specificity 85%, because there were 2 false positive results but no false negatives.
There were 2 invasive carcinomas in the fundus; in both cases, CEUS was able to detect their infiltrative nature, which had not been detected by US.
The two polypoid formations that were erroneously interpreted as carcinomas, due to the presence of washout, were diagnosed as adenomas on histological examination; they were 12 and 14 mm in size.
The 12 polypoid vegetations, for which CEUS showed enhancement without washout, were defined as follows: 2 polyps, 14 and 15 mm in size, were proven to be adenomas after surgery, while 10 lesions, ≤ 1 cm in size, were put in follow-up. With a median time of 14 months (12–31 months), only 2 lesions increased more than 1 cm in size, but showed no washout. Surgery revealed that they were adenomas. The remaining did not change in size. US and CEUS’ signs remained the same and thus they were considered benign.
Discussion
In some instances, chronic cholecystitis causes a thickening of the gallbladder wall that is difficult to distinguish from cancer, while in other cases a very dense sludge occupying the gallbladder creates US images that do not move or change in shape or location when the patient changes body position. In these difficult cases, the study of vascularization can be helpful for diagnosis.
Gallbladder cancer has a different vascularity from chronic cholecystitis and tumors (benign or malignant) have a blood supply, while sludge consists of cholesterol monohydrate crystals and calcium salts embedded in strands of gallbladder mucus and are not vascularized.
The limitation of US is that it does not study vascularity. For this reason, color Doppler was used in the past. However, the reliability of this method, especially for small lesions, was not satisfactory [5–7, 31].
CEUS has pathophysiological assumptions to improve US reliability in these doubtful forms. In fact, CEUS allows the viewing of microcirculation and, therefore, is able to detect vascularity, thus distinguishing between sludge (avascular) and gallbladder tumors (vascular). Moreover, thanks to enhancement, it may be helpful in differentiating chronic cholecystitis from gallbladder carcinoma [21, 32].
CEUS has provided significant improvement in the diagnostic accuracy of diseases of the abdominal organs. CEUS was accepted in the Guidelines and good clinical practice recommendations for CEUS—update 2011; however, it is not suggested for the diagnosis of gallbladder carcinoma [23].
Many authors agree on its usefulness in the diagnosis of chronic cholecystitis, but not all believe it to be reliable in distinguishing between benign and malignant lesions, while others still believe that the absence of vascularity is not a specific sign of sludge [20, 21, 33].
In our study, US proved to be a very reliable first-line imaging investigation in the diagnosis of biliary diseases. In fact, for only 43/4137 (1.04%) patients, it was not able to distinguish sludge.
The absence of CEUS enhancement always correctly diagnosed sludge, with a sensitivity and specificity of 100%, while benign or malignant lesions always showed vascularity.
In diagnosing sludge, our results are similar to those of Inoue, who used Levovist (sensitivity 100% specificity 98%), but are in contrast with those reported by Xie, who used Sonovue and obtained no enhancement in patients with gall bladder sludge nor in those with tumors; as in our study, this yielded a sensitivity of 100%, but had lower specificity [20, 21].
This variability can have several causes: (a) different study designs (retrospective and longitudinal); (b) inter-observer variability; (c) size of the tumors; (d) number of patients.
Our results indicate that the absence of enhancement with CEUS in a solid mass or a focal polypoid lesion immobile within the gallbladder should be viewed as sludge and medical treatment with bile acid, combined with an ultrasound follow-up, could be indicated.
To confirm this, in 10/23 patients with sludge, grayscale US was able to corroborate diagnosis during the follow-up period. In only four cases, patients with sludge at CEUS needed surgery because of complications, but in all cases histological diagnosis confirmed the presence of sludge.
It should be emphasized that an important factor influencing the reliability of our results is that in 9% of cases (4/43), CEUS could not be performed due to the presence of stones hampering the view of enhancement.
The secondary objective of this study was to evaluate the diagnostic accuracy of CEUS in distinguishing benign from malignant lesions.
Our study, while limited by the small number of cancers (only 9), has the distinction of having studied images in most cases < 2 cm (70%); therefore, our results are useful for assessing the reliability of CEUS for early diagnosis.
Previous studies divided gallbladder lesions into four types: Type 1, with a vascular pattern characterized by a “branch-like” arrangement; Type 2, with a “heterogeneous” vascular pattern; Type 3, lesions with a “homogeneous” vascular pattern; and Type 4, lesions without flow signals. The sensitivity and specificity of this classification system were evaluated [20]. In defining gallbladder carcinomas as Type 1 or Type 2 lesions, sensitivity and specificity were 86 and 97%, respectively. In defining sludge as a Type 4 vascular pattern, sensitivity and specificity were 100 and 98%, respectively. In defining non-neoplastic polyps as Type 3 blood flow lesions, sensitivity and specificity were 92 and 97%, respectively [20].
This classification, however, is particularly applicable to images that are larger than 2–3 cm, so these patterns would be of little use for early diagnosis, which is of great importance given the aggressiveness of gallbladder carcinoma.
Xie proposed three sonographic signs reliable for the diagnosis of carcinoma of the gallbladder: larger than 2 cm, destruction of the gallbladder wall and washout within 35 s on CEUS. Giving greater importance to the first two, Badea R found a washout time of 41.4 s [21, 22]. In the characterization of focal liver lesions, washout in the late phase is usually diagnostic. However, almost all malignant and benign tumors of the gallbladder have washout in the late phase. Therefore, Xie proposed assessing washout at 35 s, resulting in a sensitivity of 91% and a specificity of 83% in the diagnosis of carcinoma of the gallbladder [21]. In the diagnosis of carcinoma using the CEUS criteria proposed by Xie, we obtained a sensitivity of 100% and a specificity of 85%.
In our case study, a 60 s washout time allowed higher diagnostic accuracy in defining malignant tumors. Our data are in line with those recently reported by Wang and Zhuang [28, 34].
A recent meta-analysis of sixteen studies found that the sensitivity and specificity of CEUS in defining carcinoma of the gallbladder with size < 1 cm was 92 and 91%, respectively, with an AUROC of 97% (IC 95% 0.94–0.98). However, the authors conclude that further studies should be conducted to clarify the usefulness of CEUS, as the methodological quality was moderate [28].
In larger tumors than ours, Zhuang et al. detected that an irregular shape, branched intralesional vessels and hypo-enhancement in the late phase were features indicating malignant gallbladder disease. When combining any two of these three features, diagnostic specificity was 92.4%, sensitivity 90% and AUROC 0.91 [34].
In our study, the sensitivity and specificity of CEUS for defining gallbladder cancer were 100 and 85%, respectively.
However, the limitations of our study were: (1) a retrospective study; (2) the small number of cases and morphology of carcinomas, as there were only nine cases of carcinoma of the gallbladder, of which only three had polypoid morphology.
In conclusion, conventional grayscale ultrasound proved to be very reliable and accurate in the diagnosis of gallbladder disease. In doubtful cases, CEUS is very accurate in discerning biliary sludge. Early intralesional washout is a pattern of malignancy that can orient towards a correct diagnosis, but it is limited by the presence of false positive values, especially for smaller lesions.
The limited number of cases in our study did not allow us to draw reliable conclusions on the follow-up and timing of surgical treatment, especially for smaller lesions (less than 1 cm in size). Therefore, this remains an open problem to be explored.
In addition, new elastographic technology, which is increasingly emerging [35, 36], may contribute in the future to settle these doubts, as has been reported by some preliminary observations [37].
Compliance with ethical standards
Conflict of interest
Serra Carla, Felicani Cristina, Mazzotta Elena, Gabusi Veronica, Grasso Valentina, De Cinque Antonio, Giannitrapani Lydia and Soresi Maurizio, authors of this manuscript, declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Hederstrom E, Forsberg L. Ultrasonography in carcinoma of gallbladder: diagnostic difficults and pitfalls. Acta Radiol. 1987;28:715–718. doi: 10.1177/028418518702800611. [DOI] [PubMed] [Google Scholar]
- 2.Franquet T, Montes M, De Azua R, Jimenez FJ, Cozcolluela R. Primary gallbladder carcinoma: imaging findings in 50 patients with pathologic correlation. Gastrointest Radiol. 1991;16:143–148. doi: 10.1007/BF01887330. [DOI] [PubMed] [Google Scholar]
- 3.Pandey M, Sood BP, Shukla RC, Aryya NC, Singh S, Shukla VK. Carcinoma of the gallbladder: role of sonography in diagnosis and staging. J Clin Ultrasound. 2000;28:227–232. doi: 10.1002/(SICI)1097-0096(200006)28:5<227::AID-JCU4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Dalla Palma L, Rizatto G, Pozzi- Mucelli RS, Bazzocchi M. Grey scale ultrasonography in the evaluation of carcinoma of the gallbladder. Br J Radiol. 1980;53:662–667. doi: 10.1259/0007-1285-53-631-662. [DOI] [PubMed] [Google Scholar]
- 5.Hirooka Y, Naitoh Y, Goto H, Furukawa T, Ito A, Hayakawa T. Differential diagnosis of gallbladder masses using color Doppler ultrasonography. J Gastroenterol Hepatol. 1996;11:840–846. doi: 10.1111/j.1440-1746.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 6.Komatsuda T, Ishida H, Konno K, et al. Gallbladder carcinoma: color Doppler sonography. Abdom Imaging. 2000;25:194–197. doi: 10.1007/s002619910044. [DOI] [PubMed] [Google Scholar]
- 7.Ueno N, Tomiyana T, Tano S, Wada S, Kimura K. Diagnosis of gallbladder carcinoma with gallbladder carcinoma with color Doppler USG. Am J Gastroenterol. 1996;91:1647–1649. [PubMed] [Google Scholar]
- 8.Pradhan S, Shukla VK, Agrawal S, Dixit VK, Sharma OP. Sonographic and color Doppler morphology in gallbladder carcinoma. Indian J Cancer. 2002;39:143–148. [PubMed] [Google Scholar]
- 9.Hattori M, Inui K. Usefulness of contrast-enhanced ultrasonography in the differential diagnosis of polypoid gallbladder lesions. Nippon Shokakibyo Gakkai Zasshi. 2007;104(6):790–798. [PubMed] [Google Scholar]
- 10.Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130(4):301–311. doi: 10.7326/0003-4819-130-4-199902160-00016. [DOI] [PubMed] [Google Scholar]
- 11.Anastasi B, Sutherland GR. Biliary sludge ultrasonic appearance simulating neoplasm. Br J Radiol. 1981;54:679–681. doi: 10.1259/0007-1285-54-644-679. [DOI] [PubMed] [Google Scholar]
- 12.Kuo YC, Liu JY, Sheen IS, Yang CY, Lin DY, Chang Chein CS. Ultrasonographic difficulties and pitfalls in diagnosing primary carcinoma of the gallbladder. J Clin Ultrasound. 1990;18(8):639–647. [PubMed] [Google Scholar]
- 13.Filly RA, Allen B, Minton MJ, Bernhoft R, Way LW. In vitro investigation of the origin of echoes with biliary sludge. Small polypoid lesions of the gallbladder differential diagnosis and surgical indications by helical computed tomography. J Clin Ultrasound. 1980;8:193–200. doi: 10.1002/jcu.1870080302. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa H, Kosuge T, Shimada K, Yamamoto J, Kanai Y, Mukai K, Iwata R, Ushio K. Small polypoid lesions of the gallbladder differential diagnosis and surgical indications by helical computed tomography. Arch Surg. 1998;133:735–739. doi: 10.1001/archsurg.133.7.735. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Fernández A, Gómez-Río M, Medina-Benítez A, Moral JV, Ramos-Font C, Ramia-Angel JM, Llamas-Elvira JM, Ferrón-Orihuela JA, Lardelli-Claret P. Application of modern imaging methods in diagnosis of gallbladder cancer. J Surg Oncol. 2006;93:650–664. doi: 10.1002/jso.20533. [DOI] [PubMed] [Google Scholar]
- 16.Mocci G, Migaleddu V, Cabras F, Sirigu D, Scanu D, Virgilio G, Marzo M. SICUS and CEUS imaging in Crohn’s disease: an update. J Ultrasound. 2017;20(1):1–9. doi: 10.1007/s40477-016-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David E, Cantisani V, Grazhdani H, Di Marzo L, Venturini L, Fanelli F, Di Segni M, Di Leo N, Brunese L, Calliada F, Ciccariello M, Bottari A, Ascenti G, D’Ambrosio F. What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. J Ultrasound. 2016;19(4):281–287. doi: 10.1007/s40477-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci P, Cantisani V, Biancari F, Drud FM, Coniglio M, Di Filippo A, Fasoli F, Passariello R. Contrast-enhanced color Doppler US in malignant portal vein thrombosis. Acta Radiol. 2000;41(5):470–473. doi: 10.1080/028418500127345703. [DOI] [PubMed] [Google Scholar]
- 19.Cantisani V, Ricci P, Erturk M, Pagliara E, Drudi F, Calliada F, Mortele K, D’Ambrosio U, Marigliano C, Catalano C, Marin D, Di Seri M, Longo F, Passariello R. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue® low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med. 2010;31(5):500–505. doi: 10.1055/s-0028-1109751. [DOI] [PubMed] [Google Scholar]
- 20.Inoue T, Kitano M, Kuto M, et al. Diagnosis of gallbladder diseases by contrast-enhanced phase-inversion harmonic ultrasonography. Ultrasound Med Biol. 2007;33(3):353–361. doi: 10.1016/j.ultrasmedbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Xie XH, Xu HX, Xie XY, et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2010;20:239–248. doi: 10.1007/s00330-009-1538-8. [DOI] [PubMed] [Google Scholar]
- 22.Badea R, Zaro R, Opincariu I, Chiorean L (2014) Ultrasound in the examination of the gallbladder—a holistic approach: grey scale, Doppler, CEUS, elastography, and 3D. Med Ultrasonogr. 10.11152/mu.201.3.2066.164.rbrz [DOI] [PubMed]
- 23.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—update. Ultraschall Med 2008. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 24.Piscaglia F, Nolsøe C, Cf Dietrich, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33(1):33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 25.Liu LN, Xu Hx LuMd, Xy Xie, Wang W, Hu B, Yan K, Ding H, Ss Tang, Lx Qian, Bm Luo, Yl Wen. Contrast-enhanced ultrasound in the diagnosis of gallbladder diseases: a multi-center experience. PLoS One. 2012;7:e48371. doi: 10.1371/journal.pone.0048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Si Q, Qian XL, Wang F, et al. Real-time grey scale contrast-enhanced ultrasonography in diagnosis of gallbladder cancer. Ultrasound Med Biol. 2013;39:S86. doi: 10.1016/j.ultrasmedbio.2013.02.409. [DOI] [Google Scholar]
- 27.Liu L, Zhao Y, Zhang Y, Liu J. Differential diagnosis between benign and malignant gallbladder diseases with contrast-enhanced ultrasound. Ultrasound Med Biol. 2015;41:S99. doi: 10.1016/j.ultrasmedbio.2014.12.406. [DOI] [Google Scholar]
- 28.Wang W, Fei Y, Wang F. Meta-analysis of contrast-enhanced ultrasonography for the detection of gallbladder carcinoma. Med Ultrasonogr. 2016;18:281–287. doi: 10.11152/mu.2013.2066.183.wei. [DOI] [PubMed] [Google Scholar]
- 29.Moriguchi H, Tazawa J, Hayashi Y, et al. Natural history of polypoid lesions in the gallbladder. Gut. 1996;39(6):860–862. doi: 10.1136/gut.39.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheth S, Berford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy. Am J Gastrenterol. 2000;95:1402–1410. doi: 10.1111/j.1572-0241.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Dong BW, Wu YL, Yan K. Image-directed and color Doppler studies of gallbladder tumors. J Clin Ultrasound. 1994;22:551–555. doi: 10.1002/jcu.1870220906. [DOI] [PubMed] [Google Scholar]
- 32.Adamietz B, Wenkel E, Uder M, Meyer T, Schneider I, Dimmler A, Bautz W, Janka R. Contrast enhanced sonography of the gallbladder: a tool in the diagnosis of cholecystitis? Eur J Radiol. 2007;61(2):262–266. doi: 10.1016/j.ejrad.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Numata K, Oka H, Morimoto M, et al. Differential diagnosis of gallbladder diseases with contrast-enhanced harmonic gray scale ultrasonography. J Ultrasound Med. 2007;26(6):763–774. doi: 10.7863/jum.2007.26.6.763. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang B, Li W, Wang W, et al. Contrast-enhanced ultrasonography improves the diagnostic specificity for gallbladder-confined focal tumors. Abdom Radiol (NY) 2017 doi: 10.1007/s00261-017-1268-3. [DOI] [PubMed] [Google Scholar]
- 35.Ricci P, Marigliano C, Cantisani V, Porfiri A, Marcantonio A, Lodise P, D’Ambrosio U, Labbadia G, Maggini E, Mancuso E, Panzironi G, Di Segni M, Furlan C, Masciangelo R, Taliani G. Ultrasound evaluation of liver fibrosis: preliminary experience with acoustic structure quantification (ASQ) software. Radiol Med. 2013;118(6):995–1010. doi: 10.1007/s11547-013-0940-0. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (Long Version) Ultraschall Med. 2017;38(4):e16–e47. doi: 10.1055/s-0043-103952. [DOI] [PubMed] [Google Scholar]
- 37.Teber MA, Tan S, Dönmez U, İpek A, Uçar AE, Yıldırım H, Aslan A, Arslan H. The use of real-time elastography in the assessment of gallbladder polyps: preliminary observations. Med Ultrasonogr. 2014;16(4):304–308. doi: 10.11152/mu.201.3.2066.164.1mat. [DOI] [PubMed] [Google Scholar]