Abstract
Purpose
Cavernous transformation of the portal vein can be missed on color Doppler exam or arterial phase cross-sectional imaging due to their slow flow and delayed enhancement. Contrast-enhanced ultrasound (CEUS) offers many advantages over other imaging techniques and can be used to successfully detect cavernous transformations of the portal vein.
Methods
A 10-month-old female was followed for repeat episodes of hematemesis. Computed tomography angiography (CTA) and magnetic resonance arteriogram (MRA) and portal venography were performed. Color Doppler exam of the portal vein was performed followed by administration of Lumason, a microbubble US contrast agent.
Results
Magnetic resonance arteriogram, CTA, and color Doppler exam at the time of initial presentation was unremarkable without obvious vascular malformation within the limits of motion degraded exam. At 8-month follow-up, esophagogastroduodenoscopy revealed a vascular malformation in the distal esophagus which was sclerosed. At 6 month after sclerosis of the lesion, portal venography revealed occlusion of the portal vein with extensive collateralization. Color Doppler revealed subtle hyperarterialization and periportal collaterals. CEUS following color Doppler exam demonstrated extensive enhancement of periportal collaterals. Repeat color Doppler after contrast administration demonstrated extensive Doppler signal in the collateral vessels, suggestive of cavernous transformation.
Conclusions
We describe a case of cavernous transformation of the portal vein missed on initial color Doppler, CTA and MRA, but detected with contrast-enhanced ultrasound technique.
Electronic supplementary material
The online version of this article (10.1007/s40477-018-0288-3) contains supplementary material, which is available to authorized users.
Keywords: Contrast-enhanced ultrasound (CEUS), Cavernous transformation, Portal vein
Sommario
Scopo
La trasformazione cavernomatosa della vena porta può sfuggire all’esame color Doppler o nell’arterial phase cross sectional imaging a causa del flusso lento e al ritardato enhancement. L’ecografia con mezzo di contrasto (CEUS) offre molti vantaggi rispetto ad altre tecniche di imaging e può essere utilizzata per rilevare con successo i cavernomi della vena porta.
Metodi
Una bimba di 10 mesi è stata seguita per ripetuti episodi di ematemesi. Sono state eseguite angiografia con tomografia computerizzata (CTA) e angiografia con Risonanza Magnetica (MRA) e flebografia portale. È stato eseguito l’esame color Doppler della vena porta, seguito dalla somministrazione di Lumason, un agente di contrasto ecografico con microbolle.
Risultati
L’esame MRA, CTA e color Doppler al momento della presentazione iniziale era negativo senza evidenti malformazioni vascolari con i limiti della degradazione delle immagini di movimento. A 8 mesi di follow-up, l’esofagogastroduodenoscopia (EGD) ha rivelato una malformazione vascolare nell’esofago distale che veniva sclerosata. A 6 mesi dopo la sclerosi della lesione, la venografia portale ha rivelato l’occlusione della vena porta con circoli collaterali. Il color Doppler ha rivelato sottile iperarterializzazione e vasi collaterali periportali. L’esame color Doppler successivo alla CEUS ha dimostrato un ampio miglioramento dei circoli collaterali periportali. Ripetuto il color Doppler dopo la somministrazione di contrasto ha dimostrato un ampio segnale Doppler nei vasi collaterali, indicativo di trasformazione cavernosa.
Conclusioni
Descriviamo un caso di trasformazione cavernomatosa della vena porta mancata inizialmente dal color Doppler, CTA e MRA, ma rilevata con l’ecografia con mezzo di contrasto.
Introduction
Cavernous transformation of the portal vein refers to collateral vessel formation around the portal vein, resulting from long-standing non-cirrhotic portal hypertension and/or associated portal vein thrombosis. The entity can be detected by Doppler sonography, computed tomography (CT), magnetic resonance imaging (MRI), and arterial or direct portography [1]. The diagnosis of cavernous transformation of portal vein can be delayed, however, if imaging modality selected is not sensitive enough to detect slow flow collaterals formed around the portal vein which exhibit delayed enhancement relative to the arterial phase.
We present a case in which contrast-enhanced ultrasound (CEUS) successfully detected a cavernous transformation of portal vein which had been undetected by color Doppler sonography, magnetic resonance angiography (MRA), and computed tomography angiography (CTA) with 3D reconstruction. CEUS is a FDA-approved technique for evaluation of the liver in pediatric population and is a safe, easily repeatable bedside technique appropriate for pediatric imaging. Unlike CT or MRI, CEUS is not associated with ionizing radiation or lengthy exam times requiring sedation for pediatric patients. CEUS also offers dynamic enhancement information not offered by single-phase, arterial or venous, CT or MRI exams.
Case report
A 10-month-old ex-35-week premature female with twin–twin transfusion (donor) history presented with hematemesis. She had a normal lymphocyte and platelet count. An abdominal ultrasound showed no splenomegaly and patent main and right portal veins with normal waveforms. An esophagogastroduodenoscopy (EGD) showed no active bleeding and an atypical vascular lesion at the level of the lower esophageal sphincter. A CTA (Fig. 1) and motion degraded MRA performed in the arterial phase did not show a vascular malformation and was otherwise unremarkable. A repeat EGD a month later again demonstrated a slightly larger atypical vascular lesion. A CTA performed in the arterial phase with 3D reconstruction showed a possible venous vascular malformation in the distal esophagus, and a repeat abdominal ultrasound at this time showed mild splenomegaly. The patient was lost to follow-up until 18 months old when she again presented with hematemesis. She underwent an EGD with contrast injection of the lesion endoscopically. Fluoroscopic images were consistent with a venous malformation with no feeder vessels, and the vascular lesion was sclerosed. At 24 months old, she underwent repeat EGD with injection sclerosis. At this time, the vascular lesion appeared more consistent with esophageal varices. The patient had no persistent lymphopenia or thrombocytopenia, and an ultrasound showed that the main portal vein was patent with normal hepatopetal flow. Velocity of the main portal vein was unchanged from the prior study, about 40 cm/s. The right and left portal veins were patent with normal waveforms and normal flow direction. An MRI/MRA was significant for slight enhancement of the distal esophageal wall, possibly consistent with a venous malformation.
Fig. 1.
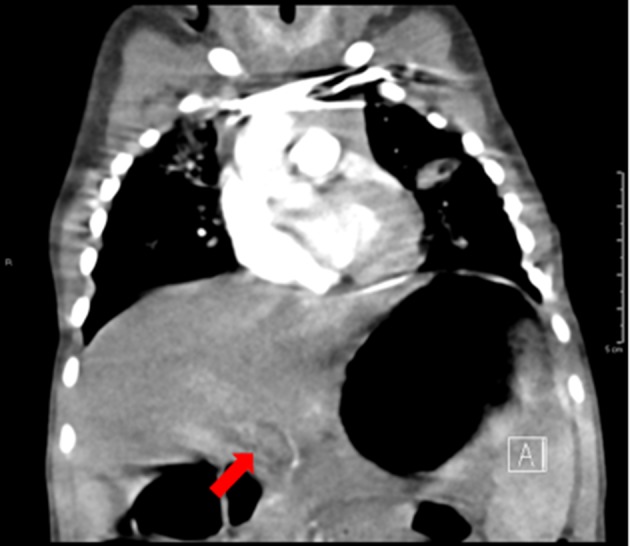
A coronal CTA image of the chest and upper abdomen demonstrates non-opacification of the main portal vein due to the arterial phase of the exam. The portal vein patency and anatomy are unclear
The patient was again lost to follow-up and represented at 30 months old with hematemesis. Laboratory tests at presentation revealed hemoglobin 9.3 g/dL [11.6–13.6 g/dL], hematocrit 27.9% [34.0–40.0%], platelet count 163 K/cu mm [150–350 K/cu mm], prothrombin time 17.3 s [22.9–30.6 s], and international normalized ratio (INR) 1.3. She underwent repeat EGD and injection sclerosis.
The patient was referred for embolization of the suspected vascular malformation. The portal venography unexpectedly revealed occlusion of the main portal vein with extensive collateralization, paraesophageal and perigastric varices (Fig. 2). Portal vein angioplasty with sclerotherapy of the paraesophageal and perigastric varices was then performed. Hepatic biopsy results were normal without portal/lobular inflammation, steatosis, or fibrosis.
Fig. 2.

A coronal subtraction image of the abdomen during portal venogram demonstrates catheterization of the portal vein with contrast opacification of extensive periportal collaterals with partial delineation of the paraesophageal and perigastric varices. Also shown is occlusion of the main portal vein and narrowing of its intrahepatic branches
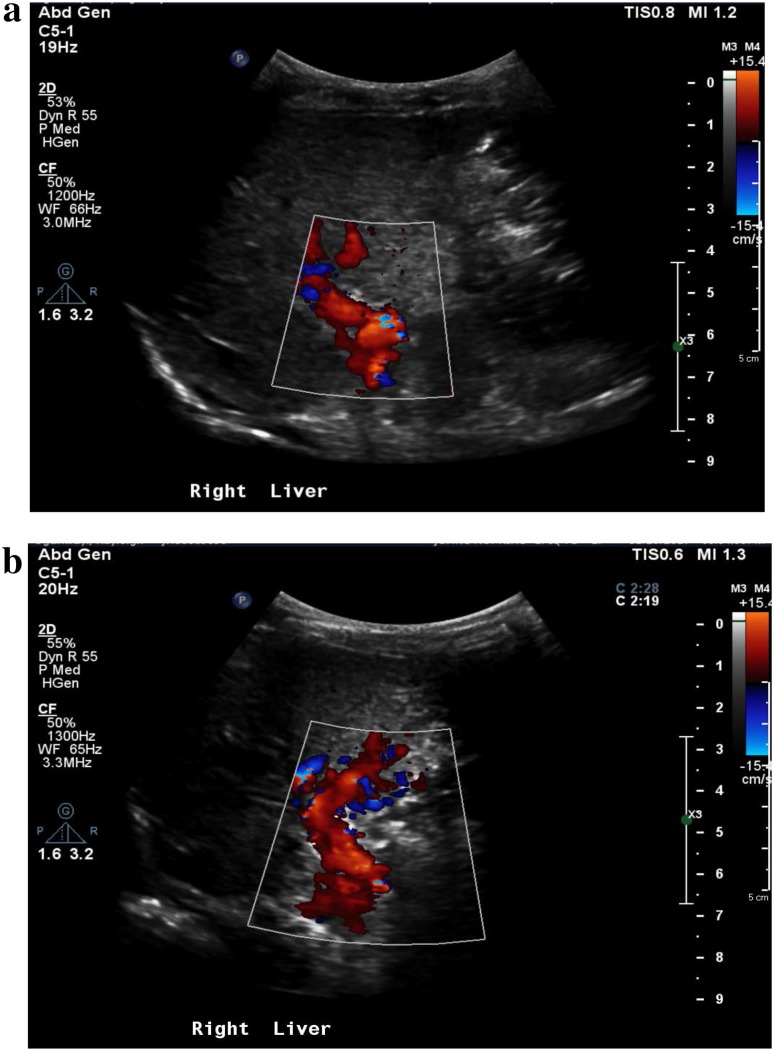
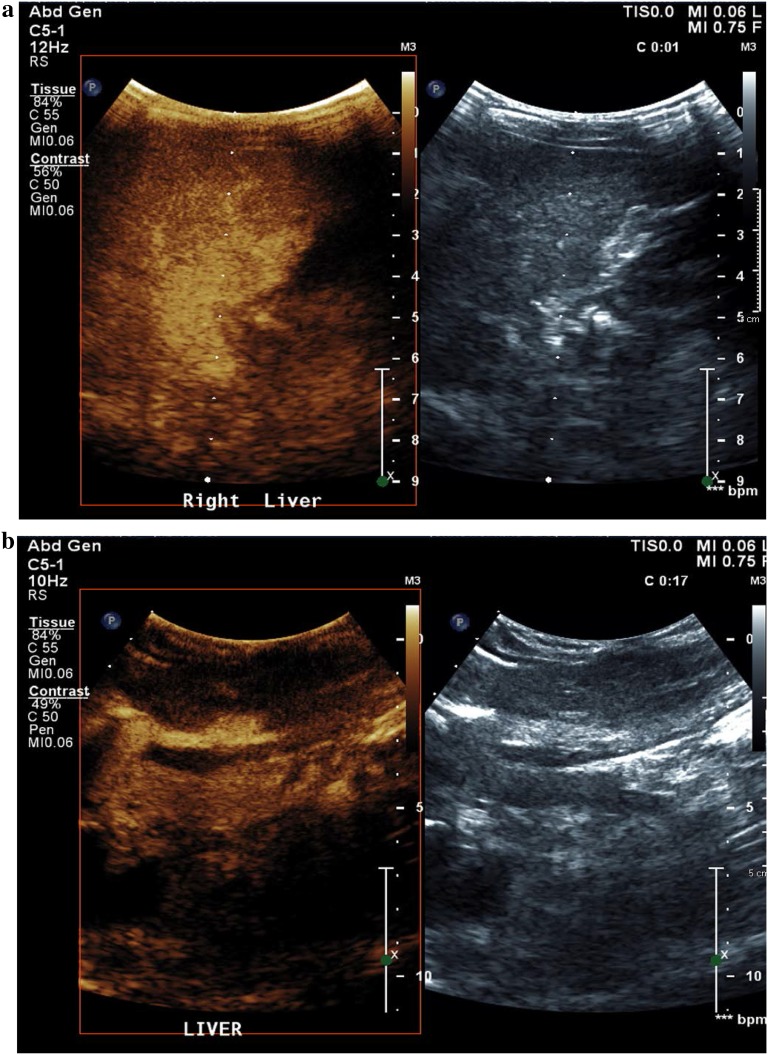
Color Doppler exam of the portal vein revealed subtle hyperarterialization and periportal collateral vessels (Fig. 3a). Repeat color Doppler imaging of the porta hepatis after Lumason injection revealed extensive Doppler signal in the collateral vessels more evident than seen on color Doppler, compatible with cavernous transformation (Fig. 3b). CEUS scan was performed to assess for patency of the main portal vein post intervention under the approval of Institutional Review Board of Johns Hopkins University using a Philips EPIQ scanner (Bothell, WA, USA) and contrast ultrasound agent (Lumason, Bracco Diagnostic Inc., NJ, USA). A plane through the portal vein was selected for 1 min cine clips during contrast enhancement. A total of two boluses of Lumason were given (0.03 mg/kg), each bolus requiring approximate 5-min scan time. Extensive enhancement in the periportal collaterals was noted during the second injection, approximately 3 min post the second injection, but not the first injection (Fig. 4a). Normal comparison is provided on Fig. 4b.
Fig. 3.
Color Doppler evaluation of the main and left portal veins pre (a) and post (b) microbubble ultrasound contrast administration. In contrast to a, the Doppler signal of the extensive periportal collaterals is well visualized post microbubble administration in b
Fig. 4.
Contrast-enhanced ultrasound image of the main portal vein and left portal vein shown, in a patient with extensive cavernous transformation of the portal vein (a) in comparison to a patient with patent, normal caliber main and left portal veins (b)
Discussion
Contrast-enhanced ultrasonography (CEUS) is a novel imaging technique where intravenously injected gas-filled microbubbles several microns in size within the intravascular compartment generate increased signal due to acoustic impedance mismatch. We demonstrate a case of extensive cavernous transformation of the portal vein missed initially on conventional grayscale and color Doppler ultrasound as well as magnetic resonance angiography (MRA) and CTA with 3D reconstruction, but seen with CEUS and portal venogram. We also demonstrate enhanced Doppler signal post ultrasound contrast administration as an incidental finding with potential clinical value.
Cavernous transformation of the portal vein refers to the formation of extensive collateral vessels. The entity may be missed on conventional grayscale and Doppler ultrasound due to slow flow, lack of dynamic imaging, or suboptimal gain settings. On CTA or MRA, moreover, the arterial phase alone will not detect delayed enhancement of the periportal collateral vessels (Fig. 1). In this regard, CEUS permits visualization of both slow flow and delayed enhancement. The technique allows visualization and quantification of dynamic intravascular contrast wash-in and wash-out [1]. The technique can also be performed at bedside, without the need for invasive catheterization, radiation, or sedation, an advantage important especially in pediatric imaging.
Extensive cavernous transformation as confirmed on portal venography (Fig. 2) was not evident during the first bolus injection of ultrasound contrast agent but well visualized approximately 3 min post second bolus injection (Fig. 4a), shown as diffuse enhancement in the region of the main and left portal veins. This confirms the need for delayed imaging for accurate evaluation of portal vein collateralization. On the portal venography, the portal vein contour is not clearly seen due to occlusion and narrowing. CEUS image of the normal caliber portal vein in a different patient can be compared (Fig. 3b).
As shown by others, we also demonstrate that ultrasound contrast agents, due to their acoustic impedance mismatch, enhances Doppler signal (Fig. 2) [2, 3]. CEUS allowed better delineation of cavernous transformation of the portal vein which was missed on the initial color Doppler evaluation obtained at the time of vascular malformation bleed. The initial miss could have occurred either due to operator dependence or development of portal vein thrombus and cavernous transformation following sclerosis of the patient’s vascular malformation. In fact, color Doppler ultrasound has been shown to be a useful technique in detecting portal vein thrombosis by others [4, 5]. Previous study has also noted ultrasound contrast agents as Doppler signal enhancers [5]. This feature of microbubbles may be of potential benefit in serial transcranial Doppler evaluation of sickle cell disease patients with closed fontanelles due to which optimal Doppler signal cannot be obtained.
The EFSUMB guidelines state the potential use of contrast ultrasound in vascular applications but do not yet list portal pathology as a FDA-approved or validated indication [6]. The application of contrast ultrasound to assess and monitor cavernous transformation of the portal vein may serve as an indication due to the improved detection of slow flow with microbubbles and the ability of contrast ultrasound to avoid dynamic, multiphase CT or sedation-requiring MRI in pediatric patients.
In conclusion, the CEUS technique may prove to be a useful diagnostic tool for enhanced detection of monitoring of vascular pathology related to the portal vein. Larger scale studies will be needed to further validate its utility and optimize the technique in this regard.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of retrospective case report, formal consent is not required.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s40477-018-0288-3) contains supplementary material, which is available to authorized users.
References
- 1.Hwang M, De Jong RM, et al. Novel contrast ultrasound evaluation in neonatal hypoxic ischemic injury: case series and future directions. J Ultrasound Med. 2016;36(11):2379–2386. doi: 10.1002/jum.14289. [DOI] [PubMed] [Google Scholar]
- 2.Di Sabatino A, Fulle I, et al. Doppler enhancement after intravenous levovist injection in Crohn’s disease. Inflamm Bowel Dis. 2002;8:251–257. doi: 10.1097/00054725-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Okura H, Yoshida K, et al. Improved transvalvular continuous-wave Doppler signal intensity after intravenous Albunex injection in patients with prosthetic aortic valves. J Am Soc Echocardiogr. 1997;10:608–612. doi: 10.1016/S0894-7317(97)70023-3. [DOI] [PubMed] [Google Scholar]
- 4.Tessler FN, Gehring BJ, et al. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. 1991;157:293–296. doi: 10.2214/ajr.157.2.1853809. [DOI] [PubMed] [Google Scholar]
- 5.Puccia F, Lombardo V, et al. Case report: acute portal vein thrombosis associated with acute cytomegalovirus infection in an immunocompetent adult. J Ultrasound. 2017;20:161–165. doi: 10.1007/s40477-016-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidhu PS, Cantisani V, et al. Role of contrast-enhanced ultrasound (CEUS) in paediatric practice: an EFSUMB position statement. Ultraschall Med. 2017;38:33–43. doi: 10.1055/s-0042-110394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.