Abstract
Background
Infectious specialist consultations (ISC) provide ever more evidence for improved outcome in Staphylococcus aureus bacteremia (SAB). Most ISC are formal (bedside). However, the impact of ISC on clinical management and prognosis lacks evaluation in aged patients with SAB.
Methods
Multicenter retrospective analysis of methicillin-sensitive (MS) SAB. Patients were stratified according to age ≥ 60 years (sub-analyses for ≥ 75 years and females) and formal (bedside) ISC given within 7 days of SAB diagnosis. The impact on management and outcome of formal ISC was explored. Statistics were performed with univariate analysis, Cox proportional hazards regression model analysis, including propensity-score adjustment, and graphic Kaplan–Meier interpretation.
Results
Altogether 617 patients were identified and 520 (84%) had formal ISC. Presence of formal ISC resulted in equivalent clinical management regardless of age over or under 60 years: localization and eradication of infection foci (80 vs. 82% and 34 vs. 36%) and use of anti-staphylococcal antibiotics (65 vs. 61%). Patients aged ≥ 60 years managed without formal ISC, compared to those with formal ISC, had less infection foci diagnosed (53 vs. 80%, p < 0.001). Lack of formal ISC in patients aged ≥ 60 years resulted in no infection eradication and absence of first-line anti-staphylococcal antibiotics. Formal ISC, compared to absence of formal ISC, lowered mortality at 90 days in patients aged ≥ 60 years (24 vs. 47%, p = 0.004). In Cox proportional regression, before and after propensity-score adjustment, formal ISC was a strong positive prognostic parameter in patients aged ≥ 60 years (HR 0.45; p = 0.004 and HR 0.44; p = 0.021), in patients aged ≥ 75 years (HR 0.18; p = 0.001 and HR 0.11; p = 0.003) and in female patients aged ≥ 75 years (HR 0.13; p = 0.005).
Conclusion
Formal ISC ensures proper active clinical management irrespective of age and improve prognosis in aged patients with MS-SAB.
Keywords: S. aureus bacteremia, High age, Infectious specialist consultation, Deep infection
Introduction
Staphylococcus aureus causes severe community- and healthcare-acquired bacteremia (SAB) [1, 2]. Despite anti-staphylococcal agents, new potent antimicrobials, and identification and eradication of infection foci [3, 4], the mortality in SAB remains high and ranges up to 30% [5]. Consultation by infectious specialist (ISC) provides evidence for improved clinical management of SAB. ISC enhances choice and duration of antibiotic treatment [6] and accelerate radiological diagnostics [7], localization [8] and eradication [9] of infection foci. Most ISC are given formal (bedside) and the superiority of formal ISC over other forms, e.g. informal (telephone) ISC, has been demonstrated [10]. However, most important, ISC improve outcome of SAB [7–10].
There is a rapid expansion of the geriatric population in the Western countries [11] with rising incidences of SAB among older persons [12, 13]. Specific characteristics have been identified: aged SAB patients are comorbid, bacteremia often hospital-acquired and the mortality high [14, 15]. However, few studies have compared clinical management and prognosis of aged versus young SAB patients and in many reports parameters that may influence prognosis, e.g. essential diagnostics, are unreported or performed rarely and deep infection foci documented scarcely or not identified [16–19]. The presence of ISC has been recorded in only three studies on aged patients with SAB reporting that patients were managed in conjunction with an ISC or that an ISC assisted in evaluating the source of SAB [14, 18], whereas one report concluded that aged patients were less likely to receive ISC guidance [19]. However, despite certification that ISC improve prognosis, the nature of ISC and the impact of ISC on clinical management and outcome have been neglected in previous reports on aged patients with SAB [14–19].
The objective was to explore how formal ISC impacts clinical management and prognosis of aged patients with methicillin-sensitive (MS) SAB. Main analyses were performed with patients aged ≥ 60 years and sub-analyses with patients aged ≥ 75 years and aged female patients. The inclusion of MS-SAB only enabled a setting where each patient received appropriate antimicrobial therapy and thus differences in empirical antibiotic therapy could be avoided.
Materials and methods
Study population
Multicenter and retrospective study with 90 days follow-up was performed. All adult patients with at least one blood culture for MS-SAB from five universities and seven central hospitals in Finland during January 1999–May 1999 and January 2000–August 2002 were recruited. Furthermore, as an extension, each adult patient with positive blood culture for S. aureus from Helsinki University Hospital in Finland in 2006–2007 was included. Two time-periods were mandatory to account for possible temporary differences in treatment management. Documentation included: gender, age, diseases, infection acquisition, illness severity, antibiotic therapy, infection foci, ISC, outcome and autopsy results. The primary outcome was mortality at 90 days. Secondary outcome measures were identification and eradication of infection foci and time to defervescence. Exclusions were: age < 18 years, pregnancy, epilepsy, bacteremia 28 days prior to the study and poly-microbial bacteremia.
Definitions
Healthcare-acquired SAB was defined as bacteremia with the first positive blood culture for S. aureus received (1) ≥ 48 h after hospital admission or (2) within 48 h of hospital admission with a preceding previous hospital discharge within 1 week. Diseases were classified with McCabe’s criteria [20]. Severe sepsis was categorized as sepsis in combination with hypotension, hypo-perfusion or organ failure [21]. Formal ISC was defined as bedside consultation within 1 week of SAB diagnosis by infectious specialist including: review of patient records and physical investigation with comprehensive written directives into patient records on clinical management.
Antibiotic therapy
The standard antibiotic therapy was anti-staphylococcal penicillin cloxacillin. Patients with penicillin contradictions received cephalosporin, either cefuroxime of ceftriaxone, as a first-line alternative therapy, or vancomycin, clindamycin or a carbapenem as second-line choice. Rifampicin and/or fluoroquinolone were provided as an adjunctive therapy. Antibiotic therapy was regarded correct when given intravenously for at least 28 days for a deep infection focus and 14 days in the absence of deep infection. Detailed information on indications, dosages and administration routes for antibiotic therapy has been provided in previous studies [22].
Statistical analyses
Categorical variables were compared with Pearson’s X2 test and non-categorical variables with Student’s t-test. Odds ratios (OR) and hazard ratios (HR) with 95% confidence intervals (CI) were calculated. Univariate factors with p < 0.1 were entered into Cox regression model (proportional hazards regression). Furthermore, a propensity-score adjusted Cox regression model analysis was performed to evaluate the explicit prognostic impact of formal ISC in aged patients. First, propensity-scores were estimated by logistic regression and variables with significant connection to ISC identified. Second, a propensity-score adjusted Cox regression analysis was performed for prognostic parameters of 90-day outcome. Tests were two-tailed and p < 0.05 considered significant. Analyses were done with SPSS 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
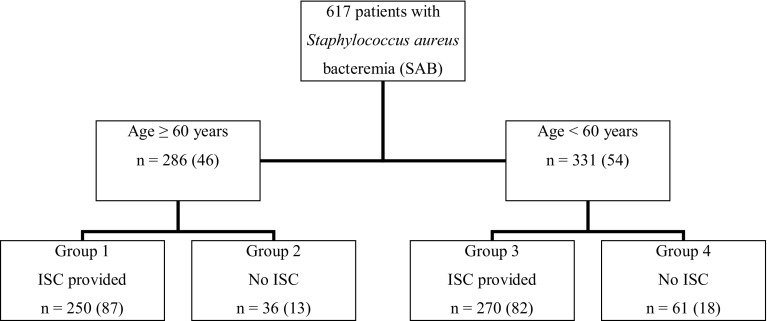
A total of 617 SAB patients were identified. Altogether 46% (286) were aged ≥ 60 years. Formal ISC was provided to 87% (250) of patients aged ≥ 60 years and 82% (270) of patients aged < 60 years (Fig. 1). When comparing patients aged ≥ 60 years according to presence of formal ISC, no differences were seen regarding gender, SAB acquisition, previous hospitalization or underlying diseases. However, among patients receiving formal ISC, those aged ≥ 60 years, compared to those < 60 years, had more healthcare-acquired SAB (63 vs. 44%), more previous hospitalization (62 vs. 42%) and less healthy or non-fatal underlying diseases (61 vs. 85%) (p < 0.001). A total of 9% (56) had severe sepsis at blood culture collection. No differences in severe sepsis were observed for patients aged over or under 60 years when compared according to presence of formal ISC (Table 1).
Fig. 1.
Study profile. Presentation of 617 methicillin-sensitive Staphylococcus aureus bacteremia patients stratified according to break-point age of 60 years and presence of formal (bedside) infectious specialist consultation (ISC) into different groups (groups 1–4)
Table 1.
Patient demographics, severity of illness, clinical management and outcome in 617 methicillin-sensitive Staphylococcus aureus bacteremia patients stratified according to break-point age of 60 years and presence of formal (bedside) infectious specialist consultation (ISC)
| Patient characteristics | Group 1 ≥ 60 years Formal ISC present n = 250 |
Group 2 ≥ 60 years Formal ISC absent n = 36 |
Group 3 < 60 years Formal ISC present n = 270 |
Group 1 vs. group 2 | Group 1 vs. group 3 | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||||
| Demographics | |||||||
| Male sex | 144 (58) | 23 (64) | 182 (67) | 0.77 (0.37–1.59) | 0.47 | 0.65 (0.46–0.94) | 0.21 |
| Age, yearsa | 72.6 ± 7.6 | 71.1 ± 7.9 | 43.0 ± 12.3 | – | 0.29 | – | < 0.001 |
| Healthcare-acquired | 157 (63) | 19 (53) | 118 (44) | 1.51 (0.75–3.05) | 0.25 | 2.18 (1.53–3.09) | < 0.001 |
| Previous hospitalizationb | 154 (62) | 25 (69) | 114 (42) | 0.71 (0.33–1.50) | 0.36 | 2.19 (1.55–3.12) | < 0.001 |
| Healthy-nonfatalc | 153 (61) | 19 (53) | 230 (85) | 1.41 (0.69–2.85) | 0.34 | 0.27 (0.18–0.42) | < 0.001 |
| Severe sepsisd | 21 (8) | 5 (14) | 18 (7) | 0.57 (0.20–1.62) | 0.28 | 1.28 (0.67–2.47) | 0.45 |
| Infection foci | |||||||
| Deep infections | 200 (80) | 19 (53) | 222 (82) | 3.58 (1.74–7.38) | < 0.001 | 0.87 (0.56–1.34) | 0.52 |
| Pneumonia | 99 (40) | 12 (33) | 103 (38) | 1.31 (0.63–2.74) | 0.47 | 1.06 (0.75–1.51) | 0.73 |
| Endocarditis | 39 (16) | 2 (6) | 47 (17) | 3.14 (0.73–13.6) | 0.11 | 0.88 (0.55–1.39) | 0.58 |
| Osteomyelitise | 94 (38) | 3 (8) | 110 (41) | 6.63 (1.98–22.2) | 0.001 | 0.87 (0.61–1.24) | 0.44 |
| Deep abscesses | 85 (34) | 2 (6) | 143 (53) | 8.76 (2.05–37.3) | 0.001 | 0.46 (0.32–0.65) | 0.001 |
| Any foreign body infection | 59 (24) | 4 (11) | 27 (10) | 2.47 (0.84–7.27) | 0.09 | 2.78 (1.69–4.55) | 0.001 |
| Infected PVC or CVCf | 45 (18) | 0 | 32 (12) | – | – | 1.48 (0.89–2.44) | 0.12 |
| Clinical management | |||||||
| TTE echocardiographyg | 165 (66) | 19 (53) | 174 (64) | 1.74 (0.86–3.51) | 0.12 | 1.07 (0.75–1.54) | 0.71 |
| TEE echocardiographyg | 55 (22) | 2 (6) | 54 (21) | 4.79 (1.12–20.6) | 0.021 | 1.13 (0.74–1.72) | 0.58 |
| Any infection foci removal | 84 (34) | 0 | 94 (36) | – | – | 0.95 (0.66–1.36) | 0.77 |
| Heart valve replacement | 2 (< 1) | 0 | 4 (1) | – | – | 0.54 (0.09–2.95) | 0.47 |
| Infected joint lavage | 7 (3) | 0 | 6 (2) | – | – | 1.27 (0.42–3.82) | 0.67 |
| Standard antibiotics | |||||||
| Cloxacillin | 163 (65) | 0 | 165 (61) | – | – | 1.19 (0.83–1.70) | 0.33 |
| Cephalosporin | 70 (28) | 27 (75) | 85 (31) | 0.13 (0.06–0.29) | < 0.001 | 0.85 (0.58–1.23) | 0.39 |
| Other antibiotics | 17 (7) | 9 (25) | 20 (7) | 4.57 (1.87–11.2) | < 0.001 | 1.09 (0.56–2.14) | 0.79 |
| Treatment durationh | 24.6 ± 13 | 19.8 ± 15 | 26.1 ± 12 | – | 0.038 | – | 0.19 |
| Adjunctive antibiotics | |||||||
| Fluoroquinolone | 129 (52) | 11 (31) | 136 (50) | 2.42 (1.14–5.14) | 0.018 | 1.05 (0.75–1.48) | 0.78 |
| Rifampicin | 152 (61) | 11 (31) | 175 (65) | 3.53 (1.66–7.49) | 0.001 | 0.84 (0.59–1.20) | 0.34 |
| Outcome | |||||||
| Hospital durationh | 38.7 ± 34 | 31.6 ± 26 | 32.7 ± 29 | – | 0.09 | – | 0.10 |
| Mortality in 28 days | 46 (18) | 11 (31) | 12 (4) | 0.51 (0.24–1.12) | 0.088 | 4.85 (2.50–9.39) | < 0.001 |
| Mortality in 90 days | 61 (24) | 17 (47) | 21 (8) | 0.36 (0.18–0.74) | 0.004 | 3.83 (2.25–6.51) | < 0.001 |
Altogether 61 (18%) patients were aged < 60 years and received no formal ISC and are omitted in the presentation. Values are expressed as number of patients (%), odds ratios (ORs) with 95% confidence intervals (CI) and mean ± standard deviation (SD)
aStudent’s t test (mean ± SD)
bWithin 2 months preceding SAB
cMcCabe’s classification [20]
dAt blood culture collection
eIncluding septic arthritis
fPeripheral or central venous catheter
gThoracic or –esophageal
hDays (mean ± SD)
Deep infection foci
Deep infection foci were identified in 75% (463) of patients. In patients aged ≥ 60 years, presence of formal ISC, compared to lack of formal ISC, resulted in more deep infection foci (80 vs. 53%, p < 0.001), osteomyelitis or septic arthritis (38 vs. 8%) and deep abscesses (34 vs. 6%) (p = 0.001) localized. However, when comparing formal ISC for patients aged ≥ 60 years with those aged < 60 years, no difference was seen in the presence of deep infection foci (80 vs. 82%) or peripheral or central venous catheters (18 vs. 12%), whereas deep abscesses were encountered less (34 vs. 53%, p = 0.001) and foreign body infections more (24 vs. 10%, p = 0.001) (Table 1).
Clinical management
Trans-thoracic and trans-esophageal echocardiography (TTE and TEE) were provided to 64% (183) and 20% (57) of patients aged ≥ 60 years. No difference in access to TTE was seen according to age over or under 60 years or presence or absence of formal ISC. However, in patients aged ≥ 60 years formal ISC, compared to lack of formal ISC, resulted in more TEE (22 vs. 6%, p = 0.021). Formal ISC resulted in active infection eradication in patients aged ≥ 60 years including infection removal (34%), heart valve replacement (< 1%) and joint lavage (3%). Formal ISC gave access to infection foci removal irrespective of age over or under 60 years. However, infection eradication was not provided to patients aged ≥ 60 years managed without formal ISC (Table 1).
Antibiotic therapy
All patients were provided with an intravenous standard antimicrobial agent effective in vitro against S. aureus blood isolate from the first day of the positive blood culture: 53% (327) received standard antibiotic cloxacillin, 37% (228) cefuroxime or ceftriaxone and 2% (12) vancomycin. The remaining received clindamycin or a carbapenem. Adjunctive rifampicin and fluoroquinolone therapy was provided to altogether 57 and 49% of patients aged ≥ 60 years, respectively. The total intravenous antibiotic duration for patients aged ≥ 60 years receiving formal ISC was 24.6 ± 13 (days ± SD) and for patients managed without formal ISC 19.8 ± 15 (days ± SD) (p = 0.038). Formal ISC resulted in equal treatment with cloxacillin, cephalosporin and adjunctive antibiotics fluoroquinolone or rifampicin for patients aged over or under 60 years. None of patients aged ≥ 60 years that were managed without formal ISC received cloxacillin as first-line antibiotics. Moreover, administration of cephalosporins or other antibiotics as first-line choice was less common among patients aged ≥ 60 receiving formal ISC compared to those managed without formal ISC (28 vs. 75% and 7 vs. 25%, p < 0.001) (Table 1).
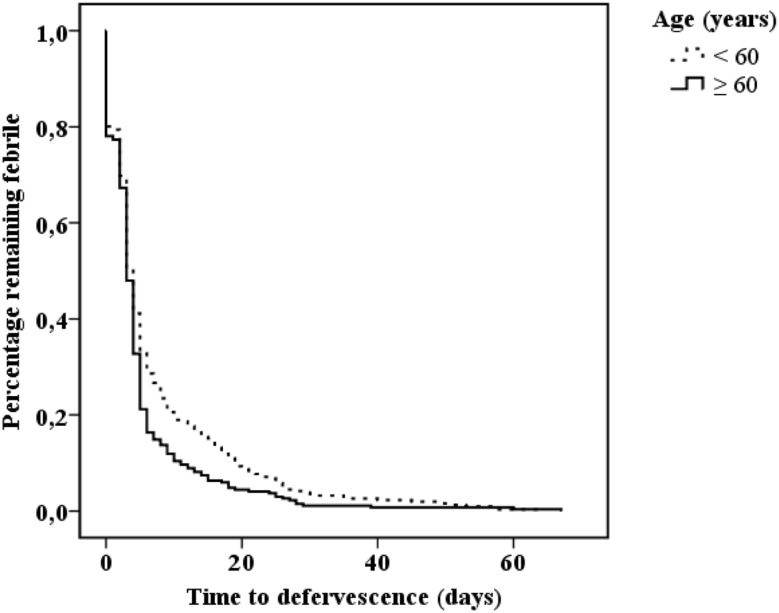
Time to defervescence
Data for time to defervescence was retrieved for 578 patients. Patients aged ≥ 60 years, compared to patients aged < 60 years, had shorter mean time to defervescence (days ± SD) (5.1 ± 7.7 vs. 7.2 ± 10.1) (p = 0.006) (Fig. 2). The impact of clinical management on defervescence in aged patients was explored by categorizing time to defervescence according to cut-off value of 7 days. However, formal ISC and localization or eradication of infection did not affect fever time in patients aged ≥ 60 years (Table 2).
Fig. 2.
Kaplan–Meier analysis for time to defervescence (days) in 578 methicillin-sensitive Staphylococcus aureus bacteremia patients categorized according to the break-point age 60 years. Log rank = 0.006
Table 2.
The impact of clinical management on time to defervescence stratified according to cut-off value of 7 days in 268 methicillin-sensitive Staphylococcus aureus bacteremia patients aged ≥ 60 years
| Clinical management | Time to defervescence | |||
|---|---|---|---|---|
| < 7 days n = 227 (85) |
≥ 7 days n = 41 (15) |
OR (95% CI) | p value | |
| Formal ISC | 209 (92) | 35 (85) | 1.99 (0.74–5.36) | 0.17 |
| Deep infection | 173 (76) | 35 (85) | 0.55 (0.22–1.38) | 0.19 |
| Endocarditis | 34 (15) | 7 (17) | 0.86 (0.35–2.09) | 0.73 |
| Pneumonia | 86 (38) | 19 (46) | 0.71 (0.36–1.38) | 0.31 |
| Osteomyelitisa | 81 (36) | 14 (34) | 1.07 (0.53–2.16) | 0.85 |
| Foreign body infection | 52 (23) | 8 (20) | 1.23 (0.53–2.82) | 0.63 |
| Surgical-radiological infection removal | 74 (33) | 10 (24) | 1.49 (0.69–3.22) | 0.29 |
| Heart valve replacement | 2 (1) | 0 | – | – |
| Infected joint lavage | 7 (3) | 0 | – | – |
Values are expressed as number of patients (%) and odds ratios (ORs) with 95% confidence intervals (CI) or mean ± standard deviation according to Student’s t test
aIncluding septic arthritis
Outcome
The total hospital duration for patients aged ≥ 60 years receiving formal ISC was 38.7 ± 34 (days ± SD) and for patients managed without formal ISC 31.6 ± 26 (days ± SD) (p value = 0.09). The overall case fatality in 617 patients was 13% at 28 days and 19% at 90 days. Patients aged ≥ 60 years receiving formal ISC, compared to those without, had lower mortality at 28 days (18 vs. 31%, p = 0.088) and at 90 days (24 vs. 47%, p = 0.004) (Table 1). Parameters for 90-days mortality in patients aged ≥ 60 years were evaluated (Table 3a, b). In univariate analysis, factors with positive prognosis were lack of underlying diseases (OR 0.27, p < 0.001), formal ISC (OR 0.36, p = 0.004) and adjunctive rifampicin therapy (OR 0.31, p < 0.001), whereas poor prognosis connected to severe sepsis (OR 2.52, p = 0.023), endocarditis (OR 2.41, p = 0.01) and pneumonia (OR 3.98, p < 0.001) (Table 3a). In Cox proportional regression analysis lack of underlying diseases (HR 0.38, p < 0.001), formal ISC (HR 0.45, p = 0.004) and adjunctive rifampicin therapy (HR 0.32, p < 0.001) were positive prognostic parameters, whereas severe sepsis (HR 1.98, p = 0.039), endocarditis (HR 2.09, p = 0.01) and pneumonia (HR 2.58, p < 0.001) were associated to poor prognosis (Table 3). When Cox proportional regression was repeated by including only patients aged ≥ 75 years or including only female patients aged ≥ 75 years, the positive prognostic impact of formal ISC remained (HR 0.18, p = 0.001 and HR 0.13, p = 0.005).
Table 3.
Cox proportional regression for prognostic factors of 90-day mortality in methicillin-sensitive Staphylococcus aureus bacteremia patients aged ≥ 60 years (n = 286)
| Patient characteristics | Outcome | Univariate analysis | Cox regression | |||
|---|---|---|---|---|---|---|
| Died n = 78 (27) |
Survived n = 208 (73) |
OR (95% CI) | p value | HR (95% CI) | p value | |
| Male sex | 46 (59) | 121 (58) | 1.03 (0.61–1.75) | NS | – | – |
| Healthcare-acquired | 48 (62) | 128 (62) | 1.00 (0.59–1.71) | NS | – | – |
| Healthy—nonfatala | 29 (37) | 143 (69) | 0.27 (0.16–0.46) | < 0.001 | 0.38 (0.24–0.61) | < 0.001 |
| Severe sepsisb | 12 (15) | 14 (7) | 2.52 (1.11–5.72) | 0.023 | 1.98 (1.04–3.79) | 0.039 |
| Formal ISCc | 61 (78) | 189 (91) | 0.36 (0.18–0.74) | 0.004 | 0.45 (0.26–0.78) | 0.004 |
| Endocarditis | 18 (23) | 23 (11) | 2.41 (1.22–4.77) | 0.01 | 2.09 (1.19–3.64) | 0.01 |
| Pneumonia | 49 (63) | 62 (30) | 3.98 (2.30–6.88) | < 0.001 | 2.58 (1.59–4.18) | < 0.001 |
| Rifampicind,e | 20 (26) | 110 (53) | 0.31 (0.17–0.55) | < 0.001 | 0.32 (0.19–0.54) | < 0.001 |
| Fluoroquinoloned | 35 (45) | 105 (50) | 0.79 (0.47–1.35) | NS | – | – |
Values are expressed as number of patients (%) and odds ratios (OR), hazards ratio (HR) and 95% confidence intervals (95% CI) are presented
NS non-significant
aMcCabe’s classification [20]
bAt blood culture collection time-point
cInfectious specialist consultation
dAdjunctive therapy
eFor at least 14 days
In propensity-score-adjusted Cox proportional analysis, parameters for 90-days outcome were: formal ISC (HR 0.44, p = 0.021), lack of underlying diseases (HR 0.37, p < 0.001), severe sepsis (HR 1.99, p = 0.039), endocarditis (HR 2.06, p = 0.014), pneumonia (HR 2.57, p < 0.001) and rifampicin adjunctive therapy (HR 0.31, p < 0.001) (Table 4). When propensity-score-adjusted Cox proportional regression was repeated by including only patients aged ≥ 75 years or including only female patients aged ≥ 75 years, the impact of formal ISC remained (HR 0.11, p = 0.003 and HR 0.13, p = 0.005).
Table 4.
Propensity-score-adjusted Cox proportional regression analysis for 90-day mortality in methicillin-sensitive Staphylococcus aureus bacteremia patients aged ≥ 60 years (n = 286) according to infectious specialist consultation
| Patient characteristics | Propensity-score-adjusted multivariate analysis HR (95% CI) | p value |
|---|---|---|
| Absence of formal ISCa | 1.0 | – |
| Presence of formal ISCa | 0.44 (0.22–0.88) | 0.021 |
| Healthy—nonfatalb | 0.37 (0.23–0.60) | < 0.001 |
| Severe sepsisc | 1.99 (1.03–3.81) | 0.039 |
| Endocarditis | 2.06 (1.16–3.67) | 0.014 |
| Pneumonia | 2.57 (1.58–4.17) | < 0.001 |
| Rifampicind,e | 0.31 (0.18–0.54) | < 0.001 |
aInfectious specialist consultation
bMcCabe’s classification [20]
cAt blood culture collection time-point
dAdjunctive therapy
eFor at least 14 days
To further evaluate differences between patients aged over or under 60 years, autopsy results were examined for altogether 57 patients. The immediate causes of death were stratified according to age. For patients aged < 60 years (n = 15), the three most common immediate causes of death were acute subarachnoid or gastrointestinal hemorrhages (43%), severe sepsis (20%) or pneumonia (20%). For patients aged ≥ 60 years (n = 42), the corresponding causes were SAB with or without a background condition (43%), acute cardio-pulmonary disease (21%) or deep infection (19%). Altogether, the immediate causes of death were infection related in 67% of patients aged ≥ 60 years and 60% in patients aged < 60 years.
Discussion
The main observations were that formal ISC ensures proper clinical management irrespective of age and formal ISC improves prognosis of aged patients with MS-SAB. Accounting for all prognostic parameters, patients aged ≥ 60 years, patients aged ≥ 75 years and female patients aged ≥ 75 years, had a more than twofold lower odds ratio for a fatal outcome due to formal ISC.
Previous reports connect ISC to improved antibiotic therapies, accelerated diagnostics and eradications of deep infection foci and better outcomes in SAB [6–10, 23]. Corresponding observations were seen in the present study. We provided formal ISC within 7 days of SAB diagnosis and, among patients aged ≥ 60 years, this resulted in echocardiography in up to 66%, deep infection foci identification in 80% and infection eradication provided to 34% of patients. Formal ISC resulted in radiological infection diagnostics, localization and eradication irrespective of age over or under 60 years. However, patients aged ≥ 60 years managed without formal ISC had less infection foci diagnosed and were provided no infection foci eradications compared to those receiving formal ISC. Similar trends were observed also for antibiotics. Patients aged ≥ 60 years managed by formal ISC had anti-staphylococcal penicillin as first-line alternative in 2/3 of cases, whereas cephalosporins or other antibiotics were used in under 1/3 and adjunctive antibiotics in over 50% of cases. Most important, formal ISC resulted in antibiotic therapies irrespective of age over or under 60 years. Contrary, no of patients aged ≥ 60 years managed without formal ISC had anti-staphylococcal penicillin as first-line antibiotics, whereas 3/4 had cephalosporins as first-line alternative and adjunctive antibiotics were provided in only 1/3 of cases.
Deep infection foci have been identified in only 14–31% of aged patients in earlier studies on SAB [14–16, 18, 24]. Previous reports have stated that aged SAB patients are less likely to receive ISC, echocardiography or infection foci identification or eradication, and extensive diagnostics have not been pursued in aged patients due to presumed poor outcome [17, 19, 24, 25]. To the best of our knowledge, only three studies report presence of ISC stating that patients were managed in conjunction with an ISC [14, 18], whereas one report concluded that aged patients were less likely to receive ISC guidance [19]. However, the content or nature of ISC or the impact of ISC on disease progression or prognosis was not evaluated [14, 18, 19]. The importance of infection identification and eradication in SAB has been confirmed [4, 26] and lack of echocardiography and undiagnosed infection foci have been suspected to connect to mortality in aged SAB patients [14, 17]. Autopsy examinations in the present study revealed that for deceased patients both bacteremia and deep infection foci account for a larger part of mortalities of aged patients compared to young ones. The present study demonstrate that formal ISC-guided clinical management result in frequent localization and eradication of deep infection with subsequent positive prognostic impact in aged patients with SAB.
Comparison of prognosis in aged and young SAB patients is challenging due to differences in age categorizations and reported follow-up time and in earlier studies. We applied break-point ages of 60 and 75 years with mortality rates for patients aged over 60 years at 28 and 90 days of 18 and 24% for patients managed by formal ISC and 31 and 47% for patients not receiving formal ISC. Previous reports have presented results according to mean ages of 63–85 years [14, 15] or break-point age of 60–65 years [16, 18, 19]. In older patients, the mortalities have varied considerably with 15% at 7 days [15], 11–36% at 30 days [18, 24], 29% at 90 days [17] and 33–56% at 6 months [14]. The present study observed higher fatalities for aged patients compared to young. This is in line with earlier reports [16, 17, 19, 24] but deviate from one study with no link between age and clinical outcome [18]. Previous reports have presented age, comorbidity, MRSA and unknown bacteremia source as independent parameters for mortality among aged patients with SAB [14, 16, 17, 19, 24]. Parameters influencing outcome in patients aged ≥ 60 years in the present study have been identified earlier in SAB patient cohorts without age specification, i.e. comorbidity [5, 6], severity of illness [7], ISC [7–10], pneumonia or endocarditis [7, 10] and rifampicin therapy [22]. Moreover, adjunctive fluoroquinolone therapy did not impact outcome and this is in line with previous observations [22].
Previous studies on aged SAB patients do not report fever [14–16, 18, 24], whereas two studies reported that aged SAB patients were more likely to be afebrile prior to SAB [17, 19]. In the present study, defervescence was significantly shorter for aged patients compared to young. We have previously shown that ISC reduced fever duration in SAB [10]. However, in the present study clinical management or formal ISC had no impact on defervescence in patients aged ≥ 60 years. Hence, time to defervescence among patients aged ≥ 60 years does not seem to correlate with treatment strategies.
MRSA weakens prognosis [27] and MRSA in previous reports on aged SAB patients has been as much as 52–100% [14–16, 18]. We included only MS-SAB. Thus, patients had proper antibiotic therapy from the first day of positive blood culture excluding bias from differences in empirical antibiotic therapy. In the present study 1/2 of patients aged ≥ 60 years received adjunctive rifampicin therapy. We have shown that rifampicin adjunctive therapy may impact prognosis positively [22, 28]. However, among patients aged ≥ 60 years, the risk of drug interactions may have resulted in a selection bias favoring rifampicin treatment in less comorbid patients and hence, the potential positive prognostic impact of rifampicin in aged patients should be interpreted with caution. The authors recommend that prospective randomized clinical trials evaluate the potential advantage of rifampicin therapy before it can be routinely recommended as part of treatment of aged patients with SAB.
There are weaknesses in the present study that have to be accounted for when interpreting results. First, the retrospective design includes risk for bias due to differences in patient groups. However, propensity-score adjustment may reduce potential bias [29]. Second, aged infectious patients may lack fever and present non-specific symptoms. Moreover, declined functional status has been connected to mortality among aged infectious patients [30]. Corresponding data were not recorded. Third, the patient cohort of the present study was originally collected to investigate trends of nosocomial SAB and prognostic impact of fluoroquinolones, rifampicin and ISC [10, 12, 22, 28]. However, the authors noticed that, although the incidence of SAB among older persons is rising, the prognostic impact of ISC has not been extensively evaluated among aged patients with SAB. Regarding the earlier time-period of the patient cohort, the question of whether the patient data in the present study is valid to current clinical practice may be raised. Management of SAB is continuously developed, however, fundamental elements of SAB management remain unchanged over the years, e.g. the importance of proper non-delayed antibiotic therapy and diagnostics of infection foci. The authors view that the high presence of formal ISC has ensured recording of relevant data and high-standard management of SAB. Hence, the authors conclude that the patient data are not outdated for current clinical practice.
Conclusion
Formal ISC ensures proper and active clinical management irrespective of age and improve prognosis in aged patients with MS-SAB. The authors encourage clinicians to manage aged patients with MS-SAB through formal ISC guidance.
Funding
The study has been supported by Grants from Helsinki University Central Hospital, the Medical Society of Finland and Svenska Kulturfonden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The study was funded by Maud Kuistila Foundation and Perkléns Foundation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
The trial was approved by The Institutional Review Board of Helsinki University Central Hospital and The Ethical Committee of Helsinki University Central Hospital.
Informed consent
For this type of study formal consent is not required.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
A correction to this article is available online at https://doi.org/10.1007/s41999-018-0054-2.
Change history
4/13/2018
Correction to: European Geriatric Medicine.
References
- 1.Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhoj P, Frimodt-Moller N. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med. 2002;162:25–32. doi: 10.1001/archinte.162.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Chong YP, Park SJ, Kim HS, Kim ES, Kim MN, Park KH, et al. Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine (Baltimore) 2013;92:98–108. doi: 10.1097/MD.0b013e318289ff1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11:208–222. doi: 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 4.Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteraemia. J Hosp Infect. 2002;52:29–36. doi: 10.1053/jhin.2002.1270. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi D, Yokota K, Takahashi O, Arioka H, Fukui T. A predictive rule for mortality of inpatients with Staphylococcus aureus bacteraemia: a classification and regression tree analysis. Eur J Intern Med. 2014;25:914–918. doi: 10.1016/j.ejim.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Pragman AA, Kuskowski MA, Abraham JM, Filice GA. Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract. 2012;20:261–267. doi: 10.1097/IPC.0b013e318255d67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieg S, Peyerl-Hoffmann G, de With K. Mortality of S. aureus bacteremia and infectious diseases specialist consultation—a study of 521 patients in Germany. J Infect. 2009;59:232–239. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Robinson JO, Pozzi-Langhi S, Phillips M, Pearson JC, Christiansen KJ, Coombs GW, et al. Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis. 2012;31:2421–2428. doi: 10.1007/s10096-012-1585-y. [DOI] [PubMed] [Google Scholar]
- 9.Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious disease consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine. 2009;88:263–267. doi: 10.1097/MD.0b013e3181b8fccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus bacteraemia. Clin Infect Dis. 2012;56:527–535. doi: 10.1093/cid/cis889. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Chronic Disease Prevention and Health Promotion DoPH, CDC (2013) The state of aging and health in America 2013
- 12.Lyytikäinen O, Ruotsalainen E, Järvinen A, Valtonen V, Ruutu P. Trends and outcome of nosocomial and community-associated bloodstream infections due to Staphylococcus aureus in Finland, 1995–2001. Eur J Clin Microbiol Infect Dis. 2005;24:399–404. doi: 10.1007/s10096-005-1345-3. [DOI] [PubMed] [Google Scholar]
- 13.Benfield T, Espersen F, Frimodt-Møller N, Jensen AG, Larsen AR, Pallesen LV, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–263. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 14.Big C, Malani PN. Staphylococcus aureus bloodstream infections in older adults: clinical outcomes and risk factors for in-hospital mortality. J Am Geriatr Soc. 2010;58:300–305. doi: 10.1111/j.1532-5415.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 15.Bader MS. Staphylococcus aureus bacteremia in older adults: predictors of 7-day mortality and infection with a methicillin-resistant strain. Infect Control Hosp Epidemiol. 2006;27:1219–1225. doi: 10.1086/507924. [DOI] [PubMed] [Google Scholar]
- 16.Tacconelli E, Pop-Vicas AE, D’Agata EM. Increased mortality among elderly patients with meticillin-resistant Staphylococcus aureus bacteraemia. J Hosp Infect. 2006;64:251–256. doi: 10.1016/j.jhin.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.McClelland RS, Fowler VG, Jr, Sanders LL, Gottlieb G, Kong LK, Sexton DJ, et al. Staphylococcus aureus bacteremia among elderly vs younger adult patients: comparison of clinical features and mortality. Arch Intern Med. 1999;14:1244–1247. doi: 10.1001/archinte.159.11.1244. [DOI] [PubMed] [Google Scholar]
- 18.Kullar R, Rybak MJ, Kaye KS. Comparative epidemiology of bacteremia due to methicillin-resistant Staphylococcus aureus between older and younger adults: a propensity score analysis. Infect Control Hosp Epidemiol. 2013;34:400–406. doi: 10.1086/669868. [DOI] [PubMed] [Google Scholar]
- 19.Yahav D, Schlesinger A, Shaked H. Clinical presentation, management and outcomes of Staphylococcus aureus bacteremia (SAB) in older adults. Aging Clin Exp Res. 2017;29:127–133. doi: 10.1007/s40520-016-0543-4. [DOI] [PubMed] [Google Scholar]
- 20.McCabe WR, Jackson GG. Gram-negative bacteremia: I. Etiology and ecology. Arch Intern Med. 1962;110:847–855. doi: 10.1001/archinte.1962.03620240029006. [DOI] [Google Scholar]
- 21.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 22.Ruotsalainen E, Järvinen A, Koivula I, Kauma H, Rintala E, Lumio J, et al. Levofloxacin does not decrease mortality in Staphylococcus aureus bacteraemia when added to the standard treatment: a prospective and randomized clinical trial of 381 patients. J Intern Med. 2006;259:179–190. doi: 10.1111/j.1365-2796.2005.01598.x. [DOI] [PubMed] [Google Scholar]
- 23.Vogel M, Schmitz RP, Hagel S, Pletz MW, Gagelmann N, Scherag A, et al. Infectious disease consultation for Staphylococcus aureus bacteremia—a systematic review and meta-analysis. J Infect. 2016;72:19–28. doi: 10.1016/j.jinf.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Kang CI, Song JH, Ko KS, Chung DR, Peck KR, Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group Clinical features and outcome of Staphylococcus aureus infection in elderly versus younger adult patients. Int J Infect Dis. 2011;15:58–62. doi: 10.1016/j.ijid.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Yahav D, Eliakim-Raz N, Leibovici L, Paul M. Bloodstream infections in older patients. Virulence. 2016;2:341–352. doi: 10.1080/21505594.2015.1132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Park WB, Lee KD. Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus non-eradicable foci. Clin Infect Dis. 2003;37:794–799. doi: 10.1086/377540. [DOI] [PubMed] [Google Scholar]
- 27.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 28.Forsblom E, Ruotsalainen E, Järvinen A. Improved outcome with early rifampicin combination treatment in methicillin-sensitive Staphylococcus aureus bacteraemia with a deep infection focus—a retrospective cohort study. PLoS One. 2015;10:e0122824. doi: 10.1371/journal.pone.0122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 30.Deulofeu F, Cervello B, Capell S, Martí C, Mercadé V. Predictors of mortality in patients with bacteremia: the importance of functional status. J Am Geriatr Soc. 1998;46:14–18. doi: 10.1111/j.1532-5415.1998.tb01007.x. [DOI] [PubMed] [Google Scholar]