Abstract
Objective
The expression level of monocyte chemoattractant protein-1 (MCP-1) is increased in atherosclerotic regions, inducing monocyte migration to the blood vessel wall. Although the serum MCP-1 concentration is higher in patients with than without cardiovascular disease, the precise correlations between the serum MCP-1 concentration and factors associated with smoking and atherosclerosis are unknown.
Methods
The serum MCP-1 concentration was measured using an enzyme-linked immunosorbent assay in 207 consecutive smokers who visited our smoking cessation clinic.
Results
Sex-adjusted analysis of smokers revealed that the MCP-1 concentration was positively correlated with age (β = 0.311), smoking duration (β = 0.342), systolic blood pressure (β = 0.225), and diastolic blood pressure (β = 0.137) but not with the body mass index. Multivariate regression analysis showed that smoking duration and systolic blood pressure were independent determinants of the MCP-1 concentration.
Conclusions
The MCP-1 concentration was positively correlated with blood pressure among smokers. Long-term smokers with high blood pressure may be more susceptible to plaque rupture at atherosclerotic lesion sites.
Keywords: Smoking, MCP-1, obesity, blood pressure, atherosclerosis, inflammation, cytokine
Introduction
Tobacco smoke contains >200 toxins, including nicotine, carbon monoxide (CO), and oxygen free radicals.1 Nicotine acts directly on the vessel walls, activates the sympathetic nervous system, and induces vasoconstriction, whereas CO and oxygen free radicals induce oxidative stress in the vessel walls and cause vascular inflammation and arteriosclerosis. The risk of cardiovascular events, such as cerebral and myocardial infarction, increases with the duration of tobacco use and the number of cigarettes smoked per day.2
Monocyte chemoattractant protein-1 (MCP-1), also known as C-C motif chemokine 2, is an important chemotactic factor for monocytes and macrophages.3 Inflammation of the vessel walls in response to oxidative stress results in synthesis of chemokines, including MCP-1, by endothelial cells, smooth muscle cells, and macrophages. MCP-1 induces monocyte migration to vascular walls and activates monocytes during atherosclerosis development.4–7 Previous studies have shown that MCP-1 increases the risk of cardiovascular disease8 and contributes to the development of coronary artery diseases such as atherosclerosis.9,10 MCP-1 plays a vital role in endothelial dysfunction7 and the instability and subsequent rupture of atheromatous plaques,11 resulting in myocardial12 and cerebral infarction.13,14 Hence, the MCP-1dependent pathway, which is activated during atherosclerosis development, represents an important therapeutic target.15,16
MCP-1 is involved in the pathogenesis of metabolic syndrome and is associated with various metabolic parameters, such as obesity,7,17,18 diabetes,17–19 and essential hypertension.20,21 The quantitative evaluation of MCP-1 is a diagnostic and prognostic marker of atherosclerotic disease.15,22 With regard to inflammation caused by tobacco smoke, in vitro studies have demonstrated that nicotine promotes MCP-1 production by neutrophils and fibroblasts23 and causes inflammation in cancerous tissues.24 Moreover, the MCP-1 concentration in mice is reportedly increased by passive smoke.25 However, the role of MCP-1 in smoking-induced atherosclerosis in humans is unknown. Therefore, this study was performed to determine the correlation between the serum MCP-1 concentration and smoking- and atherosclerosis-related factors.
Materials and methods
Patients
This prospective study included consecutive smokers who consulted the Smoking Cessation Clinic, Health Evaluation Center, National Hospital Organization, Kyoto Medical Center (Kyoto, Japan) from April 2007 to March 2010. During the same period, a retrospective study of nonsmokers (no smoking for >1 year) who consulted the outpatient clinic of the Department of Cardiology, National Hospital Organization, Kyoto Medical Center was also performed. The exclusion criteria in both studies were as follows: concomitant acute coronary syndrome, infection or pyrexia, recent (within the last 3 months) myocardial infarction or stroke, renal transplantation, a serum creatinine concentration of ≥3 mg/dL, hepatic failure [defined as chronic hepatic disease (i.e., cirrhosis)] or biochemical evidence of significant hepatic derangement (e.g., bilirubin concentration of >3 times the upper limit of normal in association with an aspartate aminotransferase/alanine aminotransferase/alkaline phosphatase concentration of >3 times the upper limit of normal), and active inflammatory disease.
Written informed consent was obtained from all participants. No patient was coerced into participating in this study. The study data were anonymized with no personal identifiers. The Ethical Review Board of the National Hospital Organization, Kyoto Medical Center approved the study protocol.
Data collection
The body mass index (BMI) was calculated as the weight in kilograms divided by the height squared in meters. The waist circumference was measured at the level of the umbilicus with the patient in the supine position. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in a sitting position after resting for >5 min using an automatic electronic sphygmomanometer (BP-103iII; Nippon Colin, Komaki, Japan).26 A regular-sized cuff appropriate for the Japanese population (arm length: 17–32 cm) was used as recommended. At each visit, a nurse measured the expiratory CO concentration with an EC50 Micro Smokerlyzer (Bedfont Scientific, Ltd., Kent, UK), which electronically measures the end-tidal CO concentration with a reported precision of >98%.27 At the initial consultation, nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence, a global standard test to assess physical dependence on nicotine.28–30 The score ranges from 0 to 10, with higher scores indicating more severe nicotine dependence. The number of cigarettes smoked per day was determined by questioning the smoker as follows: “On average, in the past month, how many cigarettes did you smoke per day?” The Brinkman index was calculated as the daily number of cigarettes multiplied by the number of smoking years. The Zung Self-Rating Depression Scale, a self-reported questionnaire, was used to assess the severity of depression, with a higher score indicating a more severe depressive state. This questionnaire is a useful tool with which to track changes in depression levels over time in research studies or during the post-treatment clinical course.31,32 At each visit, a member of the study staff reviewed the questionnaires completed by the patients. If any omissions or errors were found, the patients were requested to complete the questionnaire again.33,34
Blood sampling
Blood testing was performed to assess the biochemical and hematological profiles of the patients. Blood samples were collected from the antecubital vein 2 to 3 h after a meal to determine the estimated glomerular filtration rate, hemoglobin A1c (HbA1c) concentration, high-density lipoprotein-cholesterol (HDL-C) concentration, low-density lipoprotein-cholesterol (LDL-C) concentration, and high-sensitivity C-reactive protein concentration. The blood samples were immediately centrifuged at 3,000 rpm for 10 min at 4℃. The plasma HbA1c and serum HDL-C and LDL-C concentrations were measured using an automatic analyzer (LABOSPECT 008; Hitachi High-Technologies Co., Ltd., Tokyo, Japan) with enzyme-based reagents (Kyowa Medex Co., Ltd., Tokyo, Japan).35 The serum MCP-1 concentration was measured using a specific sandwich enzyme-linked immunosorbent assay (Ikagaku Co., Ltd., Kyoto, Japan). The serum MCP-1 concentration was quantified with a sandwich enzyme immunoassay (human MCP-1, Quantikine; R&D Systems GmBH, Wiesbaden, Germany) according to the manufacturer’s protocol.36 The maximum storage time of the aliquots before analysis was 24 months without intermittent thawing. After completion of the study, sequential samples from each patient were run concurrently. The average serum recovery rate was 103%, with a sensitivity of 5.0 pg/mL and intra-assay and interassay variability of 4.8% and 5.8%, respectively. To detect any systematic drift in the MCP-1 protein concentration during storage, a subset of 35 pairs was measured twice: at the time of blood collection and 24 months later.36
Statistical analysis
All statistical analyses were performed by a professional statistician using the Statistical Package for the Social Sciences (SPSS) Statistics 17.0 (SPSS Inc., Chicago, IL, USA). Normality of the data was assessed using the Shapiro–Wilk test. The MCP-1 values were logarithmically transformed for statistical analysis. Correlations between the serum MCP-1 concentration and smoking- and atherosclerosis-related factors were examined according to a sex-adjusted correlation analysis. Factors influencing the serum MCP-1 concentration were analyzed using a sex-adjusted multivariate analysis.
The power of the multiple regression analysis of smokers was calculated using a post-hoc statistical power analysis. At that time, the power was calculated using the sample size, number of independent values, and actual obtained R2 values. For the multiple regression analysis of smokers, the R2 value was 0.206. Three independent variables (sex, SBP, and smoking duration) were assessed using a sample size of 207; therefore, the power was calculated as 0.999, confirming a large value. The type I error probability (α) was 0.05.
Results
Various parameters were evaluated in 207 consecutive smokers (age range, 25–81 years) who visited our smoking cessation clinic. Among the smokers, 77 (37.2%) received antihypertensive agents, 38 (18.4%) received statins, and 43 (20.8%) received medications for diabetes mellitus.
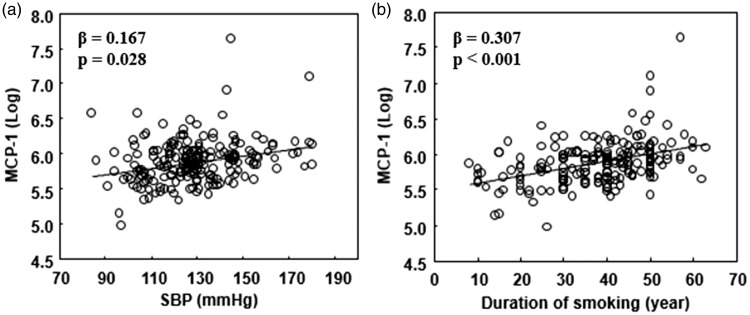
The clinical characteristics of the smokers are shown in Table 1. The results of the clinical correlation analysis of the log-transformed serum MCP-1 concentrations in smokers are shown in Table 2. The log-transformed serum MCP-1 concentrations in smokers showed no correlation with the BMI, metabolic parameters (e.g., HbA1c, HDL-C, and LDL-C), number of cigarettes per day, Brinkman index (pack-years), expiratory CO concentration, Zung Self-Rating Depression Scale score, or Fagerström Test for Nicotine Dependence score. However, the MCP-1 concentration was positively correlated with age (β = 0.311, p < 0.001), SBP (β = 0.225, p = 0.001), DBP (β = 0.137, p = 0.045), estimated glomerular filtration rate (β = −0.244, p = 0.001), and smoking duration (β = 0.342, p < 0.001). In smokers, there was no significant correlation between the waist circumference and MCP-1 concentration. Waist circumference data were excluded from the multivariate analysis because of the significant correlation with the BMI and missing data of many patients. The sex-adjusted multivariate regression analysis revealed that SBP (β = 0.167, p = 0.028) (Figure 1(a)) and smoking duration (β = 0.307, p < 0.001) (Figure 1(b)) were independent determinants of the MCP-1 concentration.
Table 1.
Clinical characteristics of smokers.
| Smokers (n = 207) | |
|---|---|
| Age (years) | 59 ± 13 |
| Male/female | 155/52 |
| BMI (kg/m2) | 22.8 [21.0, 25.2] |
| Waist circumference (cm) | 86 ± 11 |
| SBP (mmHg) | 129 ± 19 |
| DBP (mmHg) | 74 ± 12 |
| eGFR (mL/min/1.73 m2) | 78 [66, 93] |
| HbA1c (%) | 5.9 [5.6, 6.7] |
| HDL-C (mg/dL) | 55 ± 17 |
| LDL-C (mg/dL) | 112 [90, 130] |
| MCP-1 (pg/mL) | 360 [294, 416] |
| hsCRP (mg/dL) | 0.3 [0.1, 1.0] |
| Daily cigarette consumption (n) | 20 [20, 30] |
| Duration of smoking (years) | 38 ± 12 |
| Brinkman index (pack-years) | 880 [600, 1181] |
| CO (ppm) | 16.0 [10.0, 25.8] |
| FTND score | 7.2 ± 1.9 |
| SDS score | 37.3 ± 10.0 |
Data are presented as mean ± standard deviation or median [interquartile range].
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MCP-1, monocyte chemoattractant protein-1; hsCRP, high-sensitivity C-reactive protein; CO, carbon monoxide; FTND, Fagerström Test for Nicotine Dependence; SDS, Zung Self-Rating Depression Scale.
Table 2.
Sex-adjusted analysis on correlation between serum MCP-1 concentration and clinical parameters in smokers.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| β value | p value | β value | p value | |
| Age (years) | 0.311 | <0.001 | – | – |
| BMI (kg/m2) | −0.037 | 0.581 | – | – |
| Waist circumference (cm) | 0.050 | 0.528 | ||
| SBP (mmHg) | 0.225 | 0.001 | 0.167 | 0.028 |
| DBP (mmHg) | 0.137 | 0.045 | – | – |
| HbA1c (%) | −0.005 | 0.940 | – | – |
| eGFR (mL/min/1.73 m2) | −0.244 | 0.001 | – | – |
| HDL-C (mg/dL) | 0.065 | 0.386 | – | – |
| LDL-C (mg/dL) | −0.002 | 0.976 | – | – |
| hsCRP (mg/L) | −0.008 | 0.921 | ||
| Daily tobacco consumption (n) | −0.065 | 0.344 | – | – |
| Duration of smoking (years) | 0.342 | <0.001 | 0.307 | <0.001 |
| Brinkman index (pack-years) | 0.053 | 0.459 | – | – |
| CO (ppm) | −0.131 | 0.056 | – | – |
| FTND score | −0.119 | 0.080 | – | – |
| SDS score | −0.092 | 0.194 | – | – |
β value: correlation coefficient, R2 = 0.206
MCP-1, monocyte chemoattractant protein-1; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; CO, carbon monoxide; FTND, Fagerström Test for Nicotine Dependence; SDS, Zung Self-Rating Depression Scale.
Figure 1.
Correlation of log-transformed serum monocyte chemoattractant protein-1 (MCP-1) concentration with systolic blood pressure (SBP) and smoking duration
Correlation between log-transformed serum MCP-1 concentration and (a) SBP (β = 0.167, p = 0.028) and (b) smoking duration (β = 0.307, p < 0.001).
Various parameters were evaluated in 80 nonsmokers (age range, 26–80 years) who were recruited from the outpatient clinic of the Department of Cardiology. Among nonsmokers, 43 (53.8%) received antihypertensive agents, 22 (27.5%) received statins, and 2 (2.5%) received medications for diabetes mellitus.
The clinical characteristics of the nonsmokers are shown in Supplemental Table 1. In nonsmokers, we performed a sex-adjusted correlation analysis of the log-transformed serum MCP-1 concentration with age, BMI, blood pressure, and metabolic parameters (e.g., HbA1c, HDL-C, and LDL-C) (Supplemental Table 2). The serum MCP-1 concentration in nonsmokers showed a significant positive correlation with age, similar to that observed in smokers. In contrast with the serum MCP-1 concentrations in smokers, those in nonsmokers were not correlated with SBP or DBP. The sex-adjusted multivariate regression analysis revealed that age (β = 0.418, p = 0.001) and BMI (β = 0.285, p = 0.029) were independent determinants of the MCP-1 concentration among nonsmokers.
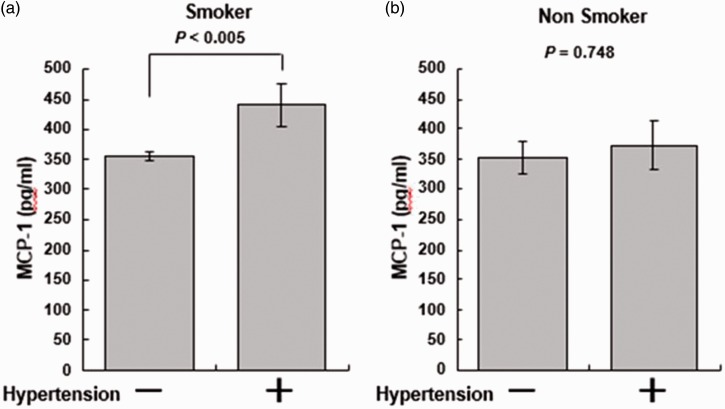
The MCP-1 concentration was compared between hypertensive patients (SBP of ≥140 mmHg or DBP of ≥90 mmHg) and normotensive patients (SBP of <140 mmHg and DBP of <90 mmHg). As shown in Figure 2, the MCP-1 concentrations were significantly higher in hypertensive smokers (n = 59) than in normotensive smokers (n = 148) (p < 0.005) (Figure 2(a)). However, there was no significant difference in the MCP-1 concentrations between hypertensive (n = 11) and normotensive (n = 69) nonsmokers (Figure 2(b)).
Figure 2.
Comparison of serum monocyte chemoattractant protein-1 (MCP-1) concentration between hypertensive and normotensive smokers and nonsmokers
Comparison of serum MCP-1 concentration between hypertensive [systolic blood pressure (SBP) of ≥ 140 mmHg or diastolic blood pressure (DBP) of ≥90 mmHg] and normotensive patients (SBP of <140 mmHg and DBP of <90 mmHg). (a) The MCP-1 concentration was significantly higher in hypertensive smokers (n = 59) than in normotensive smokers (n = 141) (p < 0.005). (b) Among nonsmokers, however, there was no significant difference in the MCP-1 concentration between hypertensive (n = 11) and normotensive (n = 69) patients (p = 0.748).
Discussion
The results of the present study demonstrated that the MCP-1 concentration increased with age in both smokers and nonsmokers. Arterial aging is the main contributor to the increased risk of morbidity from cardiovascular diseases, mainly because of the presence of chronic and low-grade inflammation of the vessels,37 indicating that the MCP-1 concentration increases because of aging-related chronic inflammation.
The serum MCP-1 concentration was significantly and positively correlated with the SBP and DBP among smokers. The multivariate analysis showed that the SBP and smoking duration were determinants of the MCP-1 concentration. These findings imply that an increase in the serum MCP-1 concentration is closely associated with the presence of hypertension and a long-term history of smoking. Consistent with previous reports,21,38 the MCP-1 concentration was significantly higher in hypertensive than normotensive smokers. However, there was no significant difference in the serum MCP-1 concentration between hypertensive and normotensive nonsmokers. The finding that the association between the MCP-1 concentration and blood pressure is dependent on the smoking status raises the possibility that MCP-1 contributes to the decreased vascular elasticity and increased vascular resistance caused by long-term smoking.
Obesity is a state of chronic inflammation that induces the release of fatty acids and inflammatory cytokines. In present study, the MCP-1 concentration was significantly correlated with the BMI among nonsmokers, which is in agreement with previous reports showing that the MCP-1 concentration increases because of obesity.7,17,18 In contrast, the MCP-1 concentration was not associated with the BMI among smokers. In vitro studies have shown that nicotine promotes MCP-1 production23,24 and that the MCP-1 concentration in mice increases due to tobacco smoke.25 Additionally, the MCP-1 concentration increases because of obesity.7,17,18 Therefore, the MCP-1 concentration in smokers may reflect the effects of both body weight and smoking status. Because MCP-1 is a marker closely related to arteriosclerosis, the MCP-1 concentration in smokers can serve as a cardiovascular marker that comprehensively reflects both the body weight and smoking status.
This study had some limitations. With respect to the backgrounds of the patients (207 smokers and 80 nonsmokers), the nonsmokers were relatively older than the smokers. Hence, further studies are warranted to compare the traits between smokers and nonsmokers that impact the MCP-1 concentration. In addition, we investigated the association between the serum MCP-1 concentration and smoking- and atherosclerosis-related factors using a cross-sectional design. Additional studies are required to clarify the cause-and-effect correlation between the MCP-1 concentration and the smoking-induced decreased blood vessel elasticity and increased vascular resistance.
Conclusions
The serum MCP-1 concentration was positively correlated with the smoking duration and blood pressure in smokers. These findings suggest that MCP-1 contributes to the decrease in vascular elasticity and increase in vascular resistance caused by long-term smoking. MCP-1 plays a central role in inflammation of the vessel walls. Hence, long-term smokers with high blood pressure may be more susceptible to plaque rupture at atherosclerotic lesion sites.
Supplementary Material
Acknowledgements
We thank Yuko Iida and Sachiko Terashima for providing technical assistance.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This work was supported in part by a Grant-in-Aid for Clinical Research from the National Hospital Organization. The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 2013; 10: 219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 2.[No authors listed] Relationship of blood pressure, serum cholesterol, smoking habit, relative weight and ECG abnormalities to incidence of major coronary events: final report of the pooling project. The pooling project research group. J Chronic Dis 1978; 31: 201–306. [DOI] [PubMed]
- 3.Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 1998; 187: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis 1999; 147: 213–225. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Kakkar V, Lu X. Impact of MCP-1 in atherosclerosis. Curr Pharm Des 2014; 20: 4580–4588. [DOI] [PubMed] [Google Scholar]
- 6.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29: 313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohman MK, Eitzman DT. Targeting MCP-1 to reduce vascular complications of obesity. Recent Pat Cardiovasc Drug Discov 2009; 4: 164–176. [DOI] [PubMed] [Google Scholar]
- 8.Kang YS, Cha JJ, Hyun YY, et al. Novel C-C chemokine receptor 2 antagonists in metabolic disease: a review of recent developments. Expert Opin Investig Drugs 2009; 20: 745–756. doi: 10.1517/13543784.2011.575359. [DOI] [PubMed] [Google Scholar]
- 9.Bai XY, Li S, Wang M, et al. Association of monocyte chemoattractant protein-1 (MCP-1)-2518A > G polymorphism with susceptibility to coronary artery disease: a meta-analysis. Ann Hum Genet 2015; 79: 173–187. doi: 10.1111/ahg.12105. [DOI] [PubMed] [Google Scholar]
- 10.Kitamoto S, Egashira K. Anti-monocyte chemoattractant protein-1 gene therapy for cardiovascular diseases. Expert Rev Cardiovasc Ther 2003; 1: 393–400. [DOI] [PubMed] [Google Scholar]
- 11.Alekperov ÉZ, Nadzhafov RN. Contemporary concepts of the role of inflammation in atherosclerosis. Kardiologiia 2010; 50: 88–91. [PubMed] [Google Scholar]
- 12.Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 2009; 117: 95–109. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- 13.Bonifačić D, Toplak A, Benjak I, et al. Monocytes and monocyte chemoattractant protein 1 (MCP-1) as early predictors of disease outcome in patients with cerebral ischemic stroke. Wien Klin Wochenschr 2016; 128: 20–27. doi: 10.1007/s00508-015-0878-4. [DOI] [PubMed] [Google Scholar]
- 14.Arakelyan A, Zakharyan R, Hambardzumyan M, et al. Functional genetic polymorphisms of monocyte chemoattractant protein 1 and C-C chemokine receptor type 2 in ischemic stroke. J Interferon Cytokine Res 2014; 34: 100–105. doi: 10.1089/jir.2013.0030. [DOI] [PubMed] [Google Scholar]
- 15.Coll B, Alonso-Villaverde C, Joven J. Monocyte chemoattractant protein-1 and atherosclerosis: is there room for an additional biomarker? Clin Chim Acta 2007; 383: 21–29. [DOI] [PubMed] [Google Scholar]
- 16.Poupel L, Combadière C. Atherosclerosis: on the trail of chemokines. Biol Aujourdhui 2010; 204: 285–293. doi: 10.1051/jbio/2010026. [DOI] [PubMed] [Google Scholar]
- 17.Panee J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2010; 60: 1–12. doi: 10.1016/j.cyto.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sell H, Eckel J. Monocyte chemotactic protein-1 and its role in insulin resistance. Curr Opin Lipidol 2007; 18: 258–262. [DOI] [PubMed] [Google Scholar]
- 19.Dragomir E, Simionescu M. Monocyte chemoattractant protein-1 –a major contributor to the inflammatory process associated with diabetes. Arch Physiol Biochem 1996; 112: 239–244. [DOI] [PubMed] [Google Scholar]
- 20.Martynowicz H, Janus A, Nowacki D, et al. The role of chemokines in hypertension. Adv Clin Exp Med 2014; 23: 319–325. [DOI] [PubMed] [Google Scholar]
- 21.Antonelli A, Fallahi P, Ferrari SM, et al. High serum levels of CXC (CXCL10) and CC (CCL2) chemokines in untreated essential hypertension. Int J Immunopathol Pharmacol 2012; 25: 387–395. [DOI] [PubMed] [Google Scholar]
- 22.Melgarejo E, Medina MA, Sánchez-Jiménez F, et al. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol 2009; 41: 998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Almasri A, Wisithphrom K, Windsor LJ, et al. Nicotine and lipopolysaccharide affect cytokine expression from gingival fibroblasts. J Periodontol 2007; 78: 533–541. [DOI] [PubMed] [Google Scholar]
- 24.Lazar M, Sullivan J, Chipitsyna G, et al. Induction of monocyte chemoattractant protein-1 by nicotine in pancreatic ductal adenocarcinoma cells: role of osteopontin. Surgery 2010; 148: 298–309. doi: 10.1016/j.surg.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu BQ, Heeschen C, Sievers RE, et al. Second hand smoke stimulates tumor angiogenesis and growth. Cancer Cell 2003; 4: 191–196. [DOI] [PubMed] [Google Scholar]
- 26.McManus RJ, Mant J, Hull MR, Hobbs FD. Does changing from mercury to electronic blood pressure measurement influence recorded blood pressure? An observational study. Br J Gen Pract 2003; 53: 953–956. [PMC free article] [PubMed] [Google Scholar]
- 27.Hald J, Overgaard J, Grau C. Evaluation of objective measures of smoking status–a prospective clinical study in a group of head and neck cancer patients treated with radiotherapy. Acta Oncol 2003; 42: 154–159. [DOI] [PubMed] [Google Scholar]
- 28.Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J 1990; 69: 763–765. [PubMed] [Google Scholar]
- 29.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 30.Terry A, Rustin MD. Assessing nicotine dependence. Am Fam Physician 2000; 62: 579–584. [PubMed] [Google Scholar]
- 31.Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965; 12: 63–70. [DOI] [PubMed] [Google Scholar]
- 32.Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry 1965; 13: 508–515. [DOI] [PubMed] [Google Scholar]
- 33.Komiyama M, Shimada S, Wada H, et al. Time-dependent changes of atherosclerotic LDL complexes after smoking cessation. J Atheroscler Thromb 2016; 23: 1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada S, Hasegawa K, Wada H, et al. High blood viscosity is closely associated with cigarette smoking and markedly reduced by smoking cessation. Circ J 2011; 75: 185–189. [DOI] [PubMed] [Google Scholar]
- 35.Komiyama M, Wada H, Ura S, et al. The effects of weight gain after smoking cessation on atherogenic α1-antitrypsin-low-density lipoprotein. Heart Vessels 2015; 30: 734–739. doi: 10.1007/s00380-014-0549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Störk S, Baumann K, von Schacky C, et al. The effect of 17 beta-estradiol on MCP-1 serum levels in postmenopausal women. Cardiovasc Res 2002; 53: 642–649. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Jiang L, Monticone RE, et al. Proinflammation: the key to arterial aging. Trends Endocrinol Metab 2014; 25: 72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng D, Liu T, Su DF, et al. The association between smoking quantity and hypertension mediated by inflammation in Chinese current smokers. J Hypertens 2013; 31: 1798–1805. doi: 10.1097/HJH.0b013e328362c21a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.