Short abstract
Objective
Accumulation of advanced glycation end products (AGEs) occurs during normal aging but markedly accelerates in people with diabetes. AGEs may play a role in various age-related disorders. Several studies have demonstrated that skin autofluorescence (SAF) reflects accumulated tissue levels of AGEs. However, very few studies have investigated SAF in the general population. The purpose of the present study was to more thoroughly evaluate the potential association among SAF, chronological age, and lifestyle habits in the general population.
Methods
A large cross-sectional survey of 10,946 Japanese volunteers aged 20 to 79 years was conducted. Volunteers completed a self-administered questionnaire and underwent SAF measurement on their dominant forearms. The associations of SAF with age and lifestyle habits were analyzed using a multiple stepwise regression analysis.
Results
Age was independently correlated with SAF. Lifestyle habits such as physical activity, nonsmoking, adequate sleep, low mental stress level, eating breakfast, and abstaining from sugary food were each independently associated with lower SAF.
Conclusions
SAF was associated with age and healthy lifestyle habits in this general Japanese population. The present study suggests that SAF measurement is a convenient tool for evaluating habitual lifestyle behaviors and may have potential for preventative health education.
Keywords: Skin autofluorescence, advanced glycation end products, lifestyle, Japanese, health education, general population
Introduction
Advanced glycation end products (AGEs) are formed by nonenzymatic reactions between sugars and amino groups of macromolecules, such as proteins, lipids, and nucleic acids.1–3 Because glycation occurs constantly during normal aging and the rate of degradation of glycated macromolecule derivatives is very slow, AGEs accumulate with increasing age.1–3
The pathogenicity of AGEs involves their cross-linking ability, which alters the functions and tertiary structures of proteins and lipids, as well as their proinflammatory interactions with AGE cellular receptors. Accumulating evidence shows that AGEs play important roles in the development of age-related disorders including diabetic vascular complications, osteoporosis, Alzheimer’s disease, cancer, and nonalcoholic steatohepatitis.3–10
Pentosidine and crossline have structural properties that cause them to emit fluorescent light across a specific range of wavelengths upon excitation by ultraviolet light.11,12 This unique characteristic has been used to develop technology that quantifies accumulated AGEs within the human skin.13 Although several confounding factors exist, such as other fluorophores, skincare cream use, and skin pigmentation, skin autofluorescence (SAF) is a prominent biomarker that may reflect tissue accumulation of AGEs.14–17 Indeed, a positive correlation between SAF and AGE levels in human skin has been found, even with nonfluorescent AGEs.13–17 Moreover, SAF has been shown to predict future cardiovascular events and death among patients with diabetes.16,17 SAF values are also associated with the severity of chronic kidney disease and are correlated with an increased risk of death from cardiovascular disease in patients with chronic kidney disease.15,16 These observations suggest that SAF measurement is a convenient, noninvasive tool with which to evaluate the prevalence and severity of various age- and/or diabetes-related disorders.
The formation and accumulation of AGEs progress under inflammatory and oxidative stress conditions.3 Furthermore, food- or tobacco-derived AGEs are absorbed by the human body and may have causative roles in the pathogenesis of chronic lifestyle-related diseases.18–21 Therefore, it is conceivable that lifestyle behaviors, including dietary patterns, physical activity, and amount of sleep, can affect AGE accumulation and resultant SAF values. However, very few studies have investigated SAF in the general population, with most involving a limited population size or number of lifestyle habits evaluated.22–25 Therefore, we conducted a survey among an exceptionally large number of apparently healthy adult volunteers in Japan to more thoroughly analyze the correlation among age, lifestyle habits, and SAF of the dominant volar forearm.
Methods and study design
Study population
From April to June 2013, public health education and promotion events were held in 10 urban areas in Japan (Sapporo, Sendai, Tokyo, Kanazawa, Nagoya, Osaka, Hiroshima, Fukuoka, Kagoshima, and Okinawa). During these events, participants were recruited for the present study, a questionnaire was conducted, and SAF was measured. From 2015 to 2016, additional monthly meetings that were open to the public were held in Tokyo, Japan, and walk-ins were welcomed. Apparently healthy volunteers were also recruited at these events and asked to complete a self-administered questionnaire before undergoing SAF measurement. Written informed consent was obtained from all participants. This study was approved by the research ethics committee of Kyoritsu Women’s College, Tokyo, Japan.
Questionnaire
The questionnaire comprised 12 multiple-choice questions regarding age, sex, and health-related lifestyle habits such as physical activity, smoking history, alcohol consumption, sleeping time, level of mental stress, and eating behaviors (Table 1). Each question was evaluated using a 5-point scale, where 1 was the unhealthiest behavior and 5 was the healthiest. For example, the question, “Do you eat a lot of vegetables?” was scored by the following answers: 5 = strongly agree, 4 = agree, 3 = undecided, 2 = disagree, and 1 = strongly disagree.
Table 1.
Questionnaire on lifestyle habits
| A. How often do you exercise (30-minute walk or equivalent)? | ||||
| 1. Not at all | 2. No exercise but walk in office or house | 3. Once a week | 4. 2–3 times a week | 5. More than 4 times a week |
| B. Do you smoke? | ||||
| 1. Daily smoking for ≥10 years | 2. Daily smoking for <10 years | 3. Quit smoking within 1 year | 4. Quit smoking >1 year ago | 5. Never have smoked |
| C. Do you drink alcohol? | ||||
| 1. More than 4 times a week | 2. 2–3 times a week | 3. Once a week | 4. Sometimes | 5. Never |
| D. How long do you sleep every day? | ||||
| 1. Less than 4 hours | 2. 4–5 hours | 3. 5–7 hours | 4. 7–8 hours | 5. More than 8 hours |
| E. Do you feel mental stress? | ||||
| 1. Strongly agree | 2. Agree | 3. Undecided | 4. Disagree | 5. Strongly disagree |
| Questions about food habits | ||||
| F. Do you eat a lot of vegetables? | ||||
| 1. Strongly disagree | 2. Disagree | 3. Undecided | 4. Agree | 5. Strongly agree |
| G. Do you eat breakfast every morning? | ||||
| 1. Strongly disagree | 2. Disagree | 3. Undecided | 4. Agree | 5. Strongly agree |
| H. Do you eat moderately? (Do you eat until you are 80% full?) | ||||
| 1. Strongly disagree | 2. Disagree | 3. Undecided | 4. Agree | 5. Strongly agree |
| I. Do you avoid eating oily food? | ||||
| 1. Strongly disagree | 2. Disagree | 3. Undecided | 4. Agree | 5. Strongly agree |
| J. Do you avoid eating processed foods? | ||||
| 1. Strongly disagree | 2. Disagree | 3. Undecided | 4. Agree | 5. Strongly agree |
| K. Do you avoid eating sugary food (cakes and candies)? | ||||
| 1. Strongly disagree | 2. Disagree | 3. Undecided | 4. Agree | 5. Strongly agree |
| L. Do you eat vegetables at the start of meals? | ||||
| 1. Strongly disagree | 2. Disagree | 3. Undecided | 4. Agree | 5. Strongly agree |
SAF measurement
SAF was measured on the dominant volar forearm using the TruAge Scanner (Morinda Inc., American Fork, UT, USA), a consumer version of the AGE Reader mu (Diagnoptics Technologies B.V., Groningen, Netherlands), as previously described.26 Certain types of AGEs autofluoresce when exposed to ultraviolet light.11,12 The AGE Reader illuminates skin with ultraviolet light (excitation range = 300–420 nm) and then detects the resulting fluorescent light (emission range = 420–600 nm), while simultaneously detecting light reflected from the skin in the 300- to 420-nm range. SAF, reported in arbitrary units, is defined as the ratio of the intensity of the emitted fluorescent light to that of the reflected light.17 For comparison with SAF data from earlier studies, the AGE score from the TruAge Scanner was divided by 100 because the AGE score is simply a multiple (100×) of the SAF arbitrary unit.
Statistical analysis
Summary statistics, such as mean and standard deviation, were calculated for SAF by age group. Correlations among SAF, age, and lifestyle habits were examined by linear regression analysis in BellCurve™ for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). Multiple stepwise regression analysis was performed to evaluate the influence of each lifestyle habit, described in Table 1, on the measured SAF values. This stepwise regression analysis was performed with the use of IBM SPSS for Windows, version 21.0.0 (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered significant.
Results
In total, 10,946 apparent healthy Japanese adults (age of 20–70 years) were enrolled in the present study (male, n = 2493; female, n = 8453). The numbers of volunteers, by age strata, were 810 (20–29 years), 1403 (30–39 years), 2522 (40–49 years), 2875 (50–59 years), 2247 (60–69 years), and 1089 (70–79 years). As shown in Table 2, SAF significantly increased with the volunteers’ calendar age (p < 0.001).
Table 2.
Comparisons of skin autofluorescence values of the volar forearm, by age group, of three population studies including the present study
|
Skin autofluorescence |
Number of participants |
|||||
|---|---|---|---|---|---|---|
| Age group (years) | Present study | Netherlands | China | Present study | Netherlands | China |
| 20–29 | 1.61 ± 0.32 | 1.53 ± 0.30 | 1.58 ± 0.23 | 810 | 62 | 129 |
| 30–39 | 1.85 ± 0.35 | 1.73 ± 0.42 | 1.71 ± 0.26 | 1403 | 86 | 120 |
| 40–49 | 2.01 ± 0.36 | 1.81 ± 0.36 | 1.83 ± 0.33 | 2522 | 72 | 152 |
| 50–59 | 2.10 ± 0.38 | 2.09 ± 0.36 | 1.95 ± 0.39 | 2875 | 64 | 96 |
| 60–69 | 2.20 ± 0.40 | 2.46 ± 0.57 | 1.97 ± 0.38 | 2247 | 45 | 44 |
| 70–79 | 2.31 ± 0.45 | 2.73 ± 0.55 | 2.14 ± 0.51 | 1089 | 27 | 39 |
Data are presented as mean ± standard deviation.
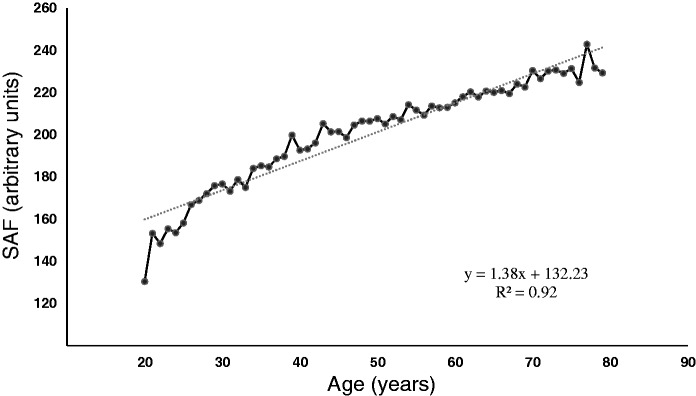
The stepwise regression analysis showed that eight independent lifestyle factors were significantly associated with SAF. Physical activity (p < 0.001), nonsmoking status (p < 0.001), adequate sleep (p < 0.005), lower mental stress (p < 0.001), eating breakfast (p < 0.001), and avoiding sugary food (p < 0.005) were correlated with lower SAF levels, whereas eating moderately and eating vegetables first were associated with higher SAF values (Table 3). Among all factors, calendar age had the strongest association (R2 = 0.92 and y = 1.38x + 132.23, where x = calendar age and y = average SAF) (Figure 1).
Table 3.
Multivariate analyses of associations with skin autofluorescence
|
Multivariate |
|||
|---|---|---|---|
| Parameters | β* | F | p-value |
| Age | 0.46 | 2297.5 | 0.000 |
| Physical activity | −0.057 | 39.9 | 0.000 |
| No smoking | −0.085 | 85.8 | 0.000 |
| Adequate sleep | −0.030 | 11.4 | 0.001 |
| Free of mental stress | −0.040 | 20.8 | 0.000 |
| Eating vegetables | −0.014 | 2.0 | 0.160 |
| Eating breakfast | −0.034 | 12.8 | 0.000 |
| Eating moderately | 0.033 | 10.6 | 0.001 |
| Avoiding oily food | 0.021 | 3.8 | 0.052 |
| Avoiding processed foods | −0.018 | 2.6 | 0.107 |
| Avoiding sugary food | −0.035 | 12.1 | 0.001 |
| Eating vegetables first | 0.035 | 11.6 | 0.001 |
*β is the regression coefficient.
R2 = 0.20
Figure 1.
Regression analysis of average skin autofluorescence and age, where x = age (years) and y = average SAF (arbitrary units). SAF, skin autofluorescence.
Discussion
AGE accumulation is part of the normal aging process.1–3 Indeed, several previous studies have demonstrated that calendar age is associated with increased AGE levels in humans.2,27–30 A comparison of eye lens samples from children younger than 4 years and adults aged 25 to 89 years revealed age-related increases in the AGE content of both water-soluble and alkaline-extracted fractions of lens proteins.28 Gas chromatography–mass spectrometry analysis revealed that levels of a nonfluorescent AGE, carboxymethyl lysine (CML), and a fluorescent AGE, pentosidine, increased with age in the skin collagen of both patients with type 1 diabetes and individuals without diabetes.29 Furthermore, while fetal and juvenile tissue samples contained no detectable CML-modified proteins, CML was present in adult tissues, where higher concentrations occurred with increased calendar age.27 Age-related increases in CML, pentosidine, and carboxyethyl lysine were also observed in human cartilage and skin collagen samples.30
In this study, we found that SAF increased with calendar age in apparently healthy Japanese adults. The present results extend previous findings from studies in the Netherlands and China that had relatively small numbers of subjects.22,23 However, the age-specific mean SAF values were moderately different among those studies (Table 2). Among individuals aged <60 years, the mean SAF values tended to be higher in our study than among those previously reported. However, among individuals aged 60 to 79 years, the mean SAF values of our subjects ranged between those of China and the Netherlands. Whether these differences are due to demographic and/or ethnic differences among the groups remains unclear. However, Yue et al.23 reported that there was no statically significant difference in SAF between Dutch and Chinese populations in the 20- to 60-year age range. Thus, the middle-aged segment of the Japanese general population (40–59 years old) might have higher SAF values than the middle-aged segment of the Chinese or Dutch populations.
AGEs may reflect cumulative glycemic exposure and are less sensitive to enzymatic degradation and proteolysis.31 Moreover, aging may reduce the expression and activities of antioxidant enzymes, such as glyoxalase, a crucial enzyme for detoxification of methylglyoxal.32 AGEs may also suppress antioxidant enzyme expression and activity.33 Therefore, crosstalk exists between aging and AGEs; physiological aging creates numerous conditions that stimulate the accumulation of AGEs, which then accelerate the aging process in target organs.
In the present study, we also found that SAF values were correlated with unhealthy lifestyle habits. Cigarette smoke is one of the major sources of exogenous AGEs.34 In previous studies, SAF levels were shown to be elevated among cigarette smokers, although in a small number of subjects.22,23,35 Additionally, a smoking history may have an impact on SAF, with ex-smokers having higher levels than those who have never smoked.36,37 Consistent with these previous findings, we found that a longer smoking history was correlated with increases in SAF. Moreover, SAF was recently found to be correlated with pack-years of smoking in a large number of subjects, and smoking cessation resulted in a gradual decrease in SAF values,21 supporting our observations.
Physical inactivity is a modifiable risk factor for obesity and diabetes, both of which can accelerate the formation and accumulation of AGEs.38 Moreover, Japanese adult men with higher SAF had lower muscle strength and power.39 Therefore, physical inactivity may have a pathological role in the elevation of SAF. However, very few studies have been conducted to address the causal link between physical inactivity and increased SAF values. It would be interesting to examine whether regular exercise or fitness may help control the accumulation of AGEs during the normal aging process.
Another interesting finding of the present study was the inverse association between sleeping time and SAF. In a previous study with a small number of Japanese subjects (n = 244), a trend toward increased SAF was observed among those receiving <6 hours of sleep per night, with a more significant relationship occurring in those aged 20 to 29 years.40 Indeed, our larger sample size (n = 10,946) showed that adequate sleeping time was significantly correlated with lower SAF values, regardless of age. Sleep deprivation may evoke oxidative stress and increase the risk of obesity and diabetes,41,42 which may stimulate the formation and accumulation of AGEs. Furthermore, emotional and psychological stress not only disturbs adequate sleep but also activates the sympathetic nervous system, thereby impairing glucose metabolism.43 This may partly explain the link between mental stress and increased SAF in our subjects.
Eating more vegetables may limit oxidative stress and AGE formation,44 whereas high intake of processed foods rich in sugar and fats could augment the accumulation of AGEs.20 Consumption of food-derived AGEs, occurring in high levels in many processed foods, may also have a causative role in some chronic diseases.45 Moreover, skipping breakfast is associated with obesity and diabetes, and men who skipped breakfast had a higher risk of cardiovascular disease than men who did not skip breakfast.46 Consistent with these observations, avoidance of sugary foods and eating breakfast every day were associated with lower SAF values in our subjects, while there were trends toward lower SAF values with avoidance of processed foods and eating more vegetables. Therefore, the present findings suggest that unhealthy lifestyle habits may accelerate the formation and accumulation of AGEs in apparently healthy adults. However, the seemingly healthy lifestyle habits of not eating too much and eating vegetables first were positively rather than inversely associated with SAF. Although the reason for the association between these specific habits and elevated SAF remains unknown, it is possible that these two lifestyle patterns were not adequately evaluated by our questionnaire.
Conclusions
The present study, which employed a cross-sectional survey, revealed associations between SAF and lifestyle habits but was unable to clarify any causal relationships. However, our findings suggest that SAF measurement may be useful for evaluating lifestyle habits in healthy adults and motivating them to make improvements.
Declaration of conflicting interest
FI and BJW are employees of Morinda Worldwide Inc. in Japan and the US, respectively.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol 1990; 45: B105–B111. [DOI] [PubMed] [Google Scholar]
- 2.Corstjens H, Dicanio D, Muizzuddin N, et al. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp Gerontol 2008; 43: 663–667. [DOI] [PubMed] [Google Scholar]
- 3.Yamagishi S. Potential clinical utility of advanced glycation end product cross-link breakers in age- and diabetes-associated disorders. Rejuvenation Res 2012; 15: 564–572. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt AM andStern D.. Atherosclerosis and diabetes: the RAGE connection. Curr Atheroscler Rep 2000; 2: 430–436. [DOI] [PubMed] [Google Scholar]
- 5.Vlassara H andStriker GE.. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinol Metab Clin North Am 2013; 42: 697–719. [DOI] [PubMed] [Google Scholar]
- 6.Yamagishi S andImaizumi T.. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 2005; 11: 2279–2299. [DOI] [PubMed] [Google Scholar]
- 7.Yamagishi S. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr Drug Targets 2011; 12: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi M andYamagishi S.. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer's disease. Curr Pharm Des 2008; 14: 973–978. [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi S Matsui T andFukami K.. Role of receptor for advanced glycation end products (RAGE) and its ligands in cancer risk. Rejuvenation Res 2015; 18: 48–56. [DOI] [PubMed] [Google Scholar]
- 10.Hyogo H andYamagishi S.. Advanced glycation end products (AGEs) and their involvement in liver disease. Curr Pharm Des 2008; 14: 969–972. [DOI] [PubMed] [Google Scholar]
- 11.Monnier VM, Vishwanath V, Frank KE, et al. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med 1986; 314: 403–408. [DOI] [PubMed] [Google Scholar]
- 12.Obayashi H, Nakano K, Shigeta H, et al. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun 1996; 226: 37–41. [DOI] [PubMed] [Google Scholar]
- 13.Meerwaldt R, Graaff R, Oomen PHN, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004; 47: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 14.Mulder DJ, Water TV, Lutgers HL, et al. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: an overview of current clinical studies, evidence, and limitations. Diabetes Technol Ther 2006; 8: 523–535. [DOI] [PubMed] [Google Scholar]
- 15.Smit AJ andGerrits EG.. Skin autofluorescence as a measure of advanced glycation endproduct deposition: a novel risk marker in chronic kidney disease. Curr Opin Nephrol Hypertens 2010; 19: 527–533. [DOI] [PubMed] [Google Scholar]
- 16.Bos DC de Ranitz-Greven WL andde Valk HW.. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther 2011; 13: 773–779. [DOI] [PubMed] [Google Scholar]
- 16.Sell DR, Lapolla A, Odetti P, et al. Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes 1992; 41: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi S Fukami K andMatsui T.. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: a novel marker of vascular complications in high-risk patients for cardiovascular disease. Int J Cardiol 2015; 185: 263–268. [DOI] [PubMed] [Google Scholar]
- 18.Uribarri J, Cai W, Ramdas M, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care 2011; 34: 1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai W, Uribarri J, Zhu L, et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A 2014; 111: 4940–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi S andMatsui T.. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition 2016; 32: 157–165. [DOI] [PubMed] [Google Scholar]
- 21.Van Waateringe RP, Mook-Kanamori MJ, Slagter SN, et al. The association between various smoking behaviors, cotinine biomarkers and skin autofluorescence, a marker for advanced glycation end product accumulation. Plos One 2017; 12: e0179330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koetsier M, Lutgers HL, de Jonge C, et al. Reference values of skin autofluorescence. Diabetes Technol Ther 2010; 12: 399–403. [DOI] [PubMed] [Google Scholar]
- 23.Yue X, Hu H, Koetsier M, et al. Reference values for the Chinese population of skin autofluorescence as a marker of advanced glycation end products accumulated in tissue. Diabetic Med 2011; 28: 818–823. [DOI] [PubMed] [Google Scholar]
- 24.Van Waateringe RP, Slagter SN, van der Klauw MM, et al. Lifestyle and clinical determinants of skin autofluorescence in a population-based cohort study. Eur J Clin Invest 2016; 46: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellow NJ Coughlan MT andReid CM.. Association between habitual dietary and lifestyle behaviours and skin autofluorescence (SAF), a marker of tissue accumulation of advanced glycation endproducts (AGEs), in healthy adults. Eur J Nutr 2017. DOI: 0.1007/s00394-017-1495-y. [DOI] [PubMed] [Google Scholar]
- 26.West BJ, Uwaya A, Isami F, et al. Antiglycation activity of iridoids and their food sources. Int J Food Sci 2014. ; 2014: 276950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schleicher ED Wagner E andNerlich AG.. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest 1997; 99: 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki N, Ueno N, Chakrabarti B, et al. Immunochemical evidence for the presence of advanced glycation end products in human lens proteins and its positive correlation with aging. J Biol Chem 1992; 267: 10211–10214. [PubMed] [Google Scholar]
- 29.Dyer DG, Dunn JA, Thorpe SR, et al. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest 1993; 91: 2463–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000; 275: 39027–39031. [DOI] [PubMed] [Google Scholar]
- 31.Tsakiri EN, Iliaki KK, Hohn A, et al. Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radic Biol Med 2013; 65: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 32.Mailankot M, Padmanabha S, Pasupuleti N, et al. Glyoxalase I activity and immunoreactivity in the aging human lens. Biogerontol 2009; 10: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ookawara T, Kawamura N, Kitagawa Y, et al. Site-specific and random fragmentation of Cu, Zn-superoxide dismutase by glycation reaction. Implication of reactive oxygen species. J Biol Chem 1992; 267: 18505–18510. [PubMed] [Google Scholar]
- 34.Cerami C, Founds H, Nicholl I, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A 1997; 94: 13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monami M, Lamanna C, Gori F, et al. Skin autofluorescence in type 2 diabetes: beyond blood glucose. Diabetes Res Clin Prac 2008; 79: 56–60. [DOI] [PubMed] [Google Scholar]
- 36.Gopal P, Reynaert NL, Scheijen JL, et al. Plasma advanced glycation end-products and skin autofluorescence are increased in COPD. Eur Respir J 2014; 43: 430–438. [DOI] [PubMed] [Google Scholar]
- 37.Hoonhorst SJ, Lo Tam Loi AT, Hartman JE, et al. Advanced glycation end products in the skin are enhanced in COPD. Metabolism 2014; 63: 1149–1156. [DOI] [PubMed] [Google Scholar]
- 38.He CT, Lee CH, Hsieh CH, et al. Soluble form of receptor for advanced glycation end products is associated with obesity and metabolic syndrome in adolescents. Int J Endocrinol 2014; 2014: 657607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momma H, Niu K, Kobayashi Y, et al. Skin advanced glycation end product accumulation and muscle strength among adult men. Eur J Appl Physiol 2011; 111: 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomoto K, Yagi M, Arita S, Ogura M, et al. Skin accumulation of advanced glycation end products and lifestyle behaviors in Japanese. Anti Aging Med 2012; 9: 165–173. [Google Scholar]
- 41.Knutson KL. Impact of sleep and sleep loss on glucose homeostasis and appetite regulation. Sleep Med Clin 2007; 2: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villafuerte G, Miguel-Puga A, Rodriguez EM, et al. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Long 2015; 2015: 234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCurley JL, Mills PJ, Roesch SC, et al. Chronic stress, inflammation, and glucose regulation in U.S. Hispanics from the HCHS/SOL Sociocultural Ancillary Study. Psychophysiology 2015; 52: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamagishi SI, Matsui T, Ishibashi Y, et al. Phytochemicals against advanced glycation end products (AGEs) and the receptor system. Curr Pharm Des 2017; 23: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 45.Uribarri J, del Castillo MD, de la Maza MP, et al. Dietary advanced glycation end products and their role in health and disease. Adv Nutr 2015; 6: 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cahill LE, Chiuve SE, Mekary RA, et al. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 2013; 128: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]