Short abstract
An uncommon side effect of cyclosporine A (CsA) use is posterior reversible encephalopathy syndrome (PRES). PRES usually develops because of disturbed capacity of posterior cerebral blood flow to autoregulate an acute rise in blood pressure. We present the case of a 10-year-old girl who was previously diagnosed in our department with focal segmental glomerulosclerosis. She was treated with CsA and developed seizures, progressive loss of consciousness, and visual disturbance on the 7th day of treatment. Brain magnetic resonance imaging showed degeneration of white matter with diffuse demyelination in the parietal and posterior occipital lobes, consistent with the diagnosis of PRES. Cases of PRES reported in children are usually secondary to immunosuppressive therapy in oncological and haematological diseases. Our case is the fifth reported case of focal segmental glomerulosclerosis in children treated with CsA and complicated by PRES. Rapid recognition of PRES and stopping neurotoxic therapy early are essential for a good prognosis.
Keywords: Focal segmental glomerulosclerosis, posterior reversible encephalopathy syndrome, cyclosporine A, hypertension, seizure, immunosuppressive therapy, children, end-stage renal disease
Introduction
Focal segmental glomerulosclerosis (FSGS) is a form of glomerulonephritis that develops in different kidney injuries, evolving with nephrotic-range proteinuria. Fixed and persistent proteinuria over several months may indicate underlying FSGS.1,2 Because most children with nephrotic syndrome (NS) do not routinely have a renal biopsy performed, rigorous estimation of the incidence of FSGS in children is hindered. Determining minimal change nephrotic syndrome is usually based on steroid responsiveness. Most frequently, the diagnosis of FSGS is underestimated because 15%–20% of these patients have an initial good response to steroids.2 In 2003, studies from North America and the United Kingdom reported an incidence of NS of 2–4 new cases/100,000 children per year and biopsy-confirmed FSGS encompassed 15%–20% of the total.3
Children with renal disease, hypertensive encephalopathies, or those receiving immunosuppressive treatment are at particular risk of developing posterior reversible encephalopathy syndrome (PRES). PRES is a neurological condition that is characterised by seizures, altered mental status, headaches, and visual impairment.4 PRES has a reported incidence of 4%–9% of children with renal conditions.5,6 However, this incidence might be underestimated because some patients may develop PRES without seizures.7,8
We report a rare case of FSGS in a child who was complicated by seizures, altered conscious level, and impaired vision, and was treated with cyclosporine A (CsA).
Case report
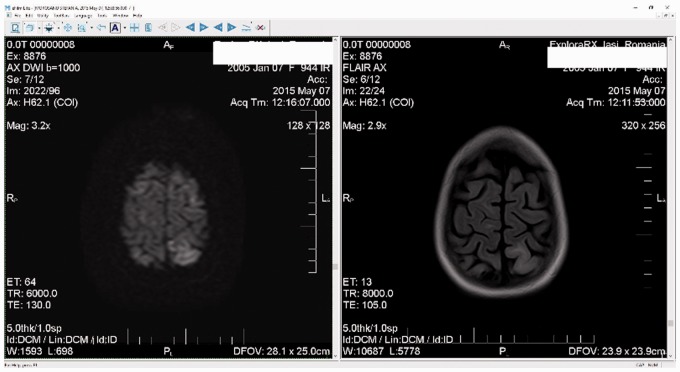
We present the case of a 10-year-old girl who was initially admitted for generalised oedema and malignant hypertension. She had hypoalbuminaemia, hypercholesterolaemia, hypertriglyceridaemia, and nephrotic-range proteinuria. Complement fractions, anti-nuclear antibodies, anti-double-stranded DNA, and liver enzymes were within normal limits. Her symptoms did not disappear after 4 weeks of corticoid therapy (60 mg/m2/day) and severe oedema was maintained. She was prescribed clonidine 5 mcg/kg, enalapril 0.5 mg/kg, telmisartan 1 mg/kg, and spironolactone 2 mg/kg, with good control of blood pressure. A kidney biopsy showed FSGS. The patient was started on methylprednisolone pulse therapy (30 mg/kg, 3 days), in association with CsA 5 mg/kg/day, according to current guidelines.9 After just 1 week of therapy, the child was admitted to the paediatric intensive care unit because of generalised seizures, and respiratory distress. Her blood pressure was 120/65 mmHg, heart rate was 90 beats/min, respiratory rate was 36/min, and SaO2 was 88%. Blood pressure remained stable and was within the normal range during the whole period of hospital stay. Serum CsA levels were normal (125 ng/mL). The patient’s symptoms progressively worsened, with development of loss of consciousness, focal seizures, intermittent bilateral amaurosis, drooling, and severe respiratory insufficiency requiring mechanical ventilation. A blood culture and uroculture showed negative results. The cerebrospinal fluid was normal as follows: osmolarity at 37°C was 281 mOsm/L, pH was 7.30, protein level was 0.11g/L, Cl level was 6.41 mgNaCl/L, glucose level was 50 mg/dL, cellularity was 1 cell/mm2, and culture was negative. A brain computed tomography scan was normal. Brain magnetic resonance imaging (MRI) showed degeneration of white matter with diffuse demyelination in the parietal and posterior occipital lobes (Figure 1). The rapid progressive course of the disease and MRI features suggested posterior encephalopathy.
Figure 1.
Diffusion-weighted magnetic resonance imaging (left panel) and fluid attenuated inversion recovery (right panel) show cortical hyperdensity in the parietal lobe, indicating white matter damage
Acute toxicity of CsA was considered and thus immunosuppressive therapy was stopped. The evolution of disease was complicated with severe bone marrow aplasia and pneumonia, which required blood transfusions, platelet concentrate, and antibiotic therapy. Furthermore, she continued to develop right-sided focal tonic–clonic seizures. We concomitantly observed continuous deterioration of kidney function, which required continuous veno-venous haemofiltration. Intravenous immunoglobulins (0.5 g/kg/day) were administered for 5 days in association with continuous veno-venous haemofiltration and antihypertensive therapy (nifedipine and clonidine). The patient was on mechanical ventilation for 1 week. She had no neurologic sequelae at 6 months of follow-up. However, she still had renal impairment (stage 2 of chronic kidney disease). During these 6 months, we continued FSGS treatment with monthly intravenous pulses of methylprednisolone. Our patient stopped receiving medical care for the following 18 months when she was in end-stage renal disease and a chronic haemodialysis program was implemented. She is currently having regular haemodialysis sessions.
The patient’s guardian provided informed consent. The case report was approved by the local hospital ethics committee (registration number: 11247/8.05.2017).
Discussion
The use of immunosuppressive therapy may result in development of PRES. This situation has been frequently reported in haemato-oncological diseases, such as acute lymphocytic leukaemia, aplastic anaemia, thalassemia, acute myeloblastic leukaemia, non-lymphoid lymphoma, autoimmune lymphoproliferative syndrome, lymphohistiocytosis, and Evans syndrome. Additionally, immunosuppressive therapy resulting in development of PRES can be found in Henoch–Schonlein purpura, systemic lupus erythematosus, Guillain–Barre syndrome, and preeclampsia.10,11
Our systematic search of all PubMed case reports of PRES in children showed that our case was the fifth reported case of FSGS in children who were treated with CsA and complicated by seizures, altered conscious level, and impaired vision. The other case reports were by Saeed et al.,12 Gera et al.,13 Tenta et al.,14 and Sakai et al.15
The diagnosis of FSGS is exclusively bioptic because there are no distinctive clinical features that differentiate it from other types of chronic glomerulopathy. FSGS in children is often coupled with steroid resistance and hypertension, and there is also a risk of progression towards end-stage renal disease.16,17 According to North American Pediatric Renal Transplant Cooperative Study data, nearly 60% of such children with a diagnosis of FSGS progress to dialysis or transplantation within 24 months of entry into the registry (patients with FSGS account for 14.2% of dialysis patients and for 11.5% of transplant patients).18
Treating the underlying condition is the main trigger of management of secondary forms of FSGS. Treatment of primary FSGS includes conservative and immunosuppression regimens aimed at controlling proteinuria and preserving kidney function. Renal survival has been directly associated with proteinuria control in long-term cohort studies in patients with primary FSGS.1 The current treatment strategies of primary FSGS involve one or more of the following: steroid therapy (Mendoza protocol), cyclophosphamide treatment (rare cases), calcineurin inhibitors (CsA and tacrolimus), mycophenolate mofetil, mizobirine, renin–angiotensin system blockade, galactose, rituximab, a synthetic adrenocorticotropin analogue, abatacept (cytotoxic T-lymphocyte-associated antigen 4–immunoglobulin fusion protein), adalimumab, and fresolimumab.19,20
CsA reduces the relapse rate by 80% in patients with steroid-dependent NS.21 Long-term use of CsA in children with steroid-resistant NS positively affects progression of chronic kidney disease by reducing proteinuria.22 The use of a cyclosporine dosage of 4–6 mg/kg/day to maintain a trough level of 60–80 ng/mL in patients with steroid-dependent NS results in a significantly higher rate of remission. Therefore, the Canadian Society of Nephrology recently recommended using a calcineurin inhibitor, either CsA or tacrolimus, following standard therapy with steroids for children with steroid-resistant NS.23
Side effects of CsA include hypertrichosis, gum hypertrophy, hypertension, and nephrotoxicity. A rare side effect of CsA, with an incidence of 40/100,000 people, is PRES. PRES is a clinical-radiological syndrome, which was described for the first time in 1996 by Hinchey et al.20 PRES has been observed in many clinical settings, such as organ or bone marrow transplantation, preeclampsia, haemolytic uremic syndrome, chronic glomerulosclerosis, and drug toxicity. This syndrome has been reported in children and adults.4,7 PRES is most frequently attributed to malignant hypertension or to neurotoxicity induced by immunosuppressive therapy, such as calcineurin inhibitors (tacrolimus or CsA). Immunosuppressant blood levels do not appear to correlate with severe neurotoxicity or PRES, but discontinuation of immunosuppressants usually results in clinical improvement.24 In our case, serum CsA levels were normal. PRES occurs secondary to a disturbed capacity of posterior cerebral blood flow for autoregulation following an acute rise in blood pressure. Vasogenic cerebral oedema is considered the major pathophysiological event in PRES. There are two main theories regarding the genesis of this vasogenic oedema. One theory is hyperperfusion due to autoregulatory failure of the cerebral vasculature1 and the other is hypoperfusion due to vasoconstriction of the cerebral arteries.20,24,25 However, PRES can develop even in the absence of hypertension. In such cases, PRES might occur as a result of a systemic inflammatory state causing endothelial dysfunction.8,25
Symptoms of PRES include back pain, seizures, headaches, temporary blindness or visual disturbances, altered mental status, and hemiparesis. The clinical picture of PRES is highly variable and may be complicated by cerebral haemorrhage, blindness, coma, and even death. Local injuries are visible through MRI, which shows high-density signals in the white matter, especially in the occipital or temporal area. All lesions are usually reversible after interruption of immunosuppressive therapy.26 Oliveira et al.27 reported remission of PRES after 2 to 6 months. In our case, a full recovery was attained after 6 months.
The precipitating factors of PRES should be the first target of treatment.10,28 Early diagnosis, rapid and efficient communication with stakeholders, as well as consideration of moral values when life is at stake29 are paramount for achieving a good prognosis. Rapid recognition of PRES and stopping neurotoxic therapy early are essential for a good prognosis. PRES must be considered in all paediatric patients with kidney disease with immunosuppressive treatment (e.g., CsA) if they develop an unexpected episode of neurological signs, even if MRI results are not limited to white matter in the occipital region.
Declaration of Conflicting Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Gipson DS, Gibson K, Gipson PE, et al. Therapeutic approach to FSGS in children. Pediatr Nephrol 2007; 22: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg R Middleton J andVehaskari VM.. Focal segmental glomerulosclerosis–epidemiology aspects in children and adults. Pediatr Nephrol 2007; 22: 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filler G, Young E, Greier P, et al. Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis 2003; 42: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 4.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome, N Engl J Med 1996; 334: 494–500. [DOI] [PubMed] [Google Scholar]
- 5.Onder AM, Lopez R, Teomete U, et al. Posterior reversible encephalopathy syndrome in the pediatric renal population. Pediatr Nephrol 2007; 22: 1921–1929. [DOI] [PubMed] [Google Scholar]
- 6.Ishikura K, Hamasaki Y, Sakai T, et al. Posterior reversible encephalopathy syndrome in children with kidney diseases. Pediatr Nephrol 2012; 27: 375–384. [DOI] [PubMed] [Google Scholar]
- 7.Ishikura K, Ikeda M, Hamasaki Y, et al. Posterior reversible encephalopathy syndrome in children: its high prevalence and more extensive imaging findings. Am J Kidney Dis 2006; 48: 231–238. [DOI] [PubMed] [Google Scholar]
- 8.Gavrilovici C, Miron I, Voroneanu L, et al. Posterior reversible encephalopathy syndrome in children with kidney disease. Int Urol Nephrol 2017; 49: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 9.KDIGO Clinical Practice Guideline for Glomerulonephritis, Vol 2, supplement 2, 2012, available at http://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf
- 10.Endo A, Fuchigami T, Hasegawa M, et al. Posterior reversible encephalopathy syndrome in childhood: report of four cases and review of the literature. Pediatr Emerg Care 2012; 28: 153–157. [DOI] [PubMed] [Google Scholar]
- 11.Arzanian MT, Shamsian BS, Karimzadeh P, et al. Posterior reversible encephalopathy syndrome in pediatric hematologic-oncologic disease: literature review and case presentation. Iran J Child Neurol. 2014; 8: 1–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Saeed B, Abou-Zor N, Amer Z, et al. Cyclosporin-A induced posterior reversible encephalopathy syndrome. Saudi J Kidney Dis Transpl 2008; 19: 439–442. [PubMed] [Google Scholar]
- 13.Gera DN, Patil SB, Iyer A, et al. Posterior reversible encephalopathy syndrome in children with kidney disease, Indian J Nephrol 2014; 24: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenta M, Uchida HA, Nunoue T, et al. Successful treatment by mycophenolate mofetil in a patient with focal segmental glomerulosclerosis associated with posterior reversible encephalopathy syndrome, CEN Case Rep 2015; 4: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai N, Kawasaki Y, Imaizumi T, et al. Two patients with focal segmental glomerulosclerosis complicated by cyclosporine-induced reversible posterior leukoencephalopathy syndrome. Clin Nephrol. 2010; 73: 482–486. [DOI] [PubMed] [Google Scholar]
- 16.Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nature reviews Nephrology 2015; 11: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrilovici C, Goldsmith DJ, Reid C, et al. What is the role of ambulatory BP monitoring in pediatric nephrology? Journal of nephrology 2004; 17: 642–652. [PubMed] [Google Scholar]
- 18.North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) 2014. Annual report. Available at https://web.emmes.com/study/ped/annlrept/annualrept2014.pdf
- 19.Han KH andKim SH.. Recent Advances in Treatments of Primary Focal Segmental Glomerulosclerosis in Children. BioMed Res Int 2016; 2016: 3053706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemper MJ, Lehnhardt A, Zawischa A, et al. Is rituximab effective in childhood nephrotic syndrome? Yes and no. Pediatr Nephrol 2014; 29: 1305–1311. [DOI] [PubMed] [Google Scholar]
- 21.Niaudet P andHabib R.. Cyclosporine in the treatment of idiopathic nephrosis. J Am Soc Nephrol 1994; 5: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 22.Cattran DC, Appel GB., Hebert LA, et al. , A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int 1999; 56: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 23.Samuel S., Bitzan M., Zappitelli M, et al. , Canadian society of nephrology commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis: management of nephrotic syndrome in children. Am J Kidney Dis 2014; 63: 354–362. [DOI] [PubMed] [Google Scholar]
- 24.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008; 29: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz RB. Hyperperfusion encephalopathies: hypertensive encephalopathy and related conditions. Neurologist 2002; 8: 22–34. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhary M, Kabbani AA, Tobey D, et al. Posterior reversible encephalopathy syndrome in a woman with focal segmental glomerulosclerosis. Neuropsychiatr Dis Treat 2015; 11: 1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira RA, Fechine LM, Neto FC, et al. Posterior reversible encephalopathy syndrome (PRES) induced by cyclosporine use in a patient with collapsing focal glomerulosclerosis. Int Urol Nephrol 2008; 40: 1095–1098. [DOI] [PubMed] [Google Scholar]
- 28.Dima-Cozma C, Mitu F, Szalontay A, et al. Socioeconomic status and psychological factors in patients with essential hypertension. Revista de Cercetare si Interventie Sociala 2014; 44: 147–159. [Google Scholar]
- 29.Gavrilovici C andOprea L.. Clinical ethics, research ethics and community ethics-the moral triad of nowadays society. Revista Romana De Bioetica 2013; 11: 3–5. [Google Scholar]