Abstract
The studies of 3-deoxy-D-manno-octulosonic acid (KDO) have been hindered due to its limited availability. Herein, an efficient enzymatic system for the facile synthesis of KDO from easy-to-get starting materials is described. In this one-pot three-enzyme (OPME) system, D-ribulose 5- phosphate, which was prepared from D-xylose, was employed as starting materials. The reaction process involves the isomerization of D-ribulose 5-phosphate to D-arabinose 5-phosphate catalyzed by D-arabinose 5-phosphate isomerase (KdsD), the aldol condensation of D-arabinose 5-phosphate and phosphoenolpyruvate (PEP) catalyzed by KDO 8-phosphate synthetase (KdsA), and the hydrolysis of KDO-8-phosphate catalyzed by KDO 8-phosphate phosphatase (KdsC). By using this OPME system, 72% isolated yield was obtained. The obtained KDO was further transferred to Lipid A by KDO transferase from E.coli (WaaA).
Keywords: KDO, enzymatic synthesis, one-pot multienzyme, biocatalysis, LPS
Graphical abstract
Lipopolysaccharides (LPS), also known as endotoxins, are large molecules that anchored in the outer membrane of Gram-negative bacteria by lipid A, to which a nonrepeating core oligosaccharide and a distal polysaccharide termed as O-antigen are attached (Figure 1).1 Nonrepeating core oligosaccharide part contains 3-deoxy-D-manno-octulosonic acid (KDO) and heptose and is highly conserved in different bacteria.2 KDO is the only sugar that found in all known core structures, although in some cases a derivative, D-glycero-D-talo-2-octulosonic acid (KO), is also present.2 KDO was also found in capsular polysaccharides of many bacteria. For example, the repeating unit of Neisseria meningitides serogroup E capsule consists of alternating D-galactosamine and KDO residues.3 Escherichia coli K12 capsule contains rhamnose and KDO residues.4 Besides, KDO was found in plant and green algae.5–8 Concerning the importance of KDO in kinds of biological processes, enzymes that involved in KDO biosynthetic pathway are exciting targets for the development of new classes of antibiotics.9,10 Core polysaccharides of LPS are also the potential vaccines against bacterial infection. Many KDO-containing polysaccharides have been synthesized and evaluated in recent years.11–14 The fact that immunizations with many of these polysaccharides lead to antibody responses provides an impetus to explore further KDO-containing polysaccharides as a vaccine candidate.14 Nevertheless, such studies have been hampered by the lack of efficient and convenient preparation methods for KDO preparation.
Figure 1.
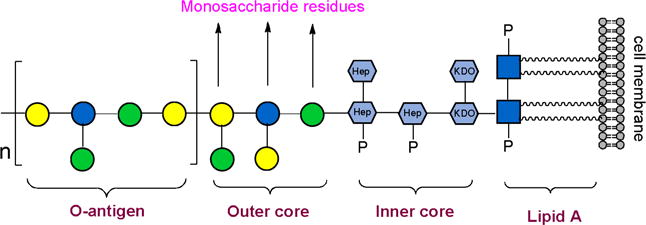
The structure of E.coli LPS
Chemical methods for KDO synthesis have been developed over the past decades,15–21 but the tedious protection/de-protection steps can be complicated and suffer from low yield. Alternatively, enzymatic syntheses employing KDO aldolase,22 sialic acid aldolase,23,24 KDO phosphate synthetase25 proceed regio- and stereoselectively without protection. KDO aldolase and sialic acid aldolase could condense arabinose and pyruvate into KDO directly, but both enzymes suffer from low specific activity,22,23 making these processes impractical for the scalable synthesis of KDO. In contrast with both aldolases, KDO 8-phosphate synthetase showed significantly higher specific activity,25–27 and more than 120 mg of protein could be obtained from one liter of LB culture medium by using pET protein expression system (data in this work). KDO 8-phosphate synthetase catalyzes the aldol condensation of D-arabinose 5-phosphate and phosphoenolpyruvate (PEP), resulting in KDO 8-phosphate, which can be hydrolyzed to afford KDO by phosphatase.25 The only block of this process for large scale synthesis is the low accessibility of D-arabinose 5-phosphate. Commercially available D-arabinose 5-phosphate is extremely expensive ($643/25mg, Sigma-Aldrich) for preparative scale synthesis. Moreover, D-arabinose 5-phosphate has been difficult to prepare in quantity because there is a lack of kinase that could efficiently phosphorylate D-arabinose at C-5 position directly. Bednarski and co-workers used hexokinase and ATP-regeneration system to produce D-arabinose 5-phosphate for KDO synthesis.25 Nevertheless, the low specific activity of hexokinase towards D-arabinose requires a large amount of hexokinase. To avoid using expensive starting materials, Pohl and co-workers have developed a biological “living factory”, by which KDO was produced from glucose through cell fermentation.28 Although hundreds milligram of KDO could be produced in one liter of medium, the purification of the final product from fermentation broth can be complicated. Therefore, an efficient and convenient method to readily provide KDO in considerable amounts is highly attractive in enabling the studies of KDO.
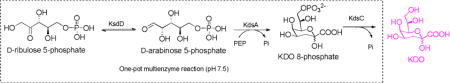
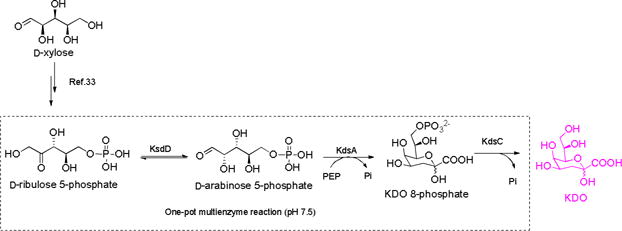
Herein, an efficient enzymatic strategy for the facile synthesis of KDO from easy-to-get starting materials is described (Scheme 1). In the first stage, D-ribulose 5-phosphate was prepared from D-xylose in multi-gram scale. In the second stage, D-ribulose 5-phosphate was incubated with D-arabinose 5-phosphate isomerase (KdsD), KDO 8-phosphate synthetase (KdsA), and KDO 8-phosphate phosphatase (KdsC) in one-pot fashion to produce KDO. The obtained KDO was further transferred into lipid A by KDO transferase from E.coli (WaaA) (Scheme 3).
Scheme 1.
One-pot multienzyme system for the production of KDO
Scheme 3.
One-pot multienzyme system for the synthesis of Re-LPS.
D-arabinose 5-phosphate is a rare sugar phosphate, and there is a lack of kinase that could efficiently phosphorylate D-arabinose directly. Therefore, D-arabinose 5-phosphate has been difficult to prepare in quantity, and the commercially available product is extremely expensive. Meanwhile, many synthetic methods have been explored for the synthesis of D-ribulose 5-phosphate, which is a key intermediate in pentose phosphate pathway(PPP) and widely exists in bacteria, plants, and animals.29 The reported methods for the synthesis of D-ribulose 5-phosphate relies on the isomerization of D-ribose 5-phosphate,30 the phosphorylation of D-ribulose,31 and the oxidization of D-gluconate 6-phosphate.32 Although scalable product could be produced by using these methods, these processes still suffer from expensive starting materials, low yields, or a complicated purification step. As a consequence, commercially available D-ribulose 5-phosphate is also extremely expensive ($1245/25mg, Sigma-Aldrich). Recently, we have developed an efficient and convenient platform for the facile synthesis of phosphorylated ketopentoses,33 in which the synthesis of D-ribulose 5-phosphate was also included. In this strategy, D-ribulose was prepared from D-xylose by a one-pot two-enzyme system in first reaction stage,34 and then D-ribulose was phosphorylated by using L-ribulose kinase at C-5 position. The product was purified by using silver nitrate precipitation.33 Having got a considerable amount of D-ribulose 5-phosphate in hand in this work (multi-gram), we try to use a sequential one-pot three-enzyme (OP3E) system containing KdsD, KdsA, and KdsC to synthesize KDO (Scheme 1).
The requirement of several enzyme-catalyzed reactions being carried in one-pot is that the enzymes must explicitly recognize their individual substrate. Otherwise, the cross-reactions will result in unpredictable by-products and increase the purification difficulties. KDO 8-phosphate synthetase could specifically recognize D-arabinose 5-phosphate but not D-ribulose 5-phosphate,27 making our design (Scheme 1) reasonable. KdsC is highly active to hydrolyze the phosphate group of KDO 8-phosphate, while only trace activity towards D-arabinose 5-phosphate and PEP was observed (thousands of times lower than KDO 8-phosphate),35 indicates its potential applications in OPME reaction. However, its substrate specificity towards D-ribulose 5-phosphate is unknown. To test the substrate specificity of KdsC towards D-ribulose 5-phosphate, D-ribulose 5-phosphate was incubated with KdsC in excess amount for three hours, while D-ribulose 5-phosphate was incubated with alkaline phosphatase as a control. No observable D-ribulose was found on TLC (Figure S1), indicating D-ribulsoe 5-phosphate can’t serve as the substrate of KdsC.
To test the practicability of the designed OPME reaction for the production of KDO, analytical scale reaction was carried in a 50 ul system containing D-ribulose 5-phosphate KdsD, KdsA, and KdsC. The reactions were monitored by TLC while employing authentic KDO as a control. After observing the formation of KDO on TLC (Figure S2), preparative scale synthesis was carried in 300 ml system (gram scale). To efficiently convert D-ribulose 5-phosphate, excess PEP (2.5 equiv) was used. For the convenience of the final purification, no buffer was used. The reaction pH was held near 7.5 using sodium hydroxide as the reaction was ongoing. Once reaction no longer moves forward, KDO was purified by using DEAE column (HCO3− form). After desalting by Bio-Gel P-2 column, the product was isolated in 72% yield concerning D-ribulose 5-phosphate. The product was confirmed by NMR and HRMS analysis. 1H-NMR of the obtained KDO is well accord with the authentic standard (Figure 2). A single peak (237.0561, M-H) on high resolution mass spectrum was observed as well (Figure 2).
Figure 2.
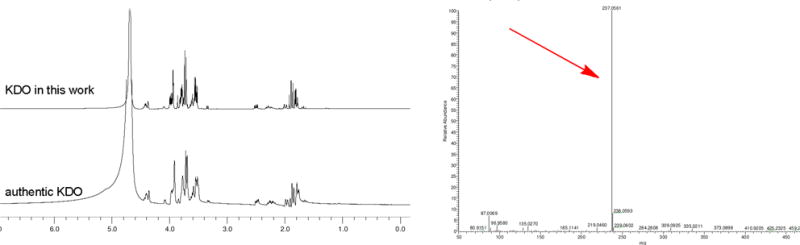
NMR and HRMS analysis of the obtained KDO
To further confirm the structure of the obtained KDO, the product was converted to the known pentaacetate methyl ester of KDO 3 (Scheme 2). 3 was characterized by NMR and HRMS (see Supporting Information). The proton and carbon NMR spectra of the 3 were good accordance with the previously reported data.8,22
Scheme 2.
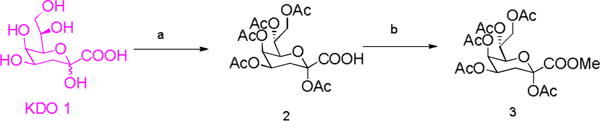
Synthesis of the pentaacetate methyl ester of KDO. (a) Ac2O, DMAP, pyridine, rt; (b) TMSCHN2, DCM/MeOH
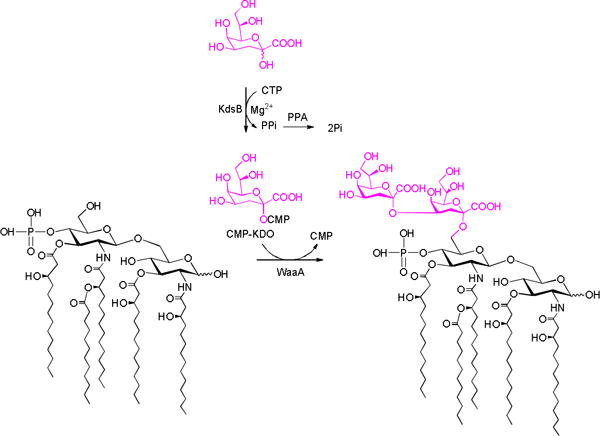
Having got a considerable amount of KDO in hand, we further try to install it on lipid A to synthesize Re-type LPS (lipid A linked with KDO residues)36 by using KDO transferase. The synthesis of Re-type LPS is the key step to synthesize lipopolysaccharide (lipid A linked with polysaccharide) to develop vaccine candidate against Gram-negative bacteria infection. Lipid A can serve as an adjuvant to enhance the immunogenicity of polysaccharide portion.37,38 Although many efforts have been made to install polysaccharide on lipid A, only Re-type LPS has been synthesized successfully by using chemical strategy to the best of our knowledge.39 Nevertheless, the process undergoing multi protection/deprotection steps can be complicated and suffer from very low yield. The synthesis of more complex lipopolysaccharides is still challenged. Compared to chemical method, enzymatic synthesis of oligosaccharides has distinct advantages in regio- and stereo-selectivity. In this work, we tried to develop an enzymatic system to prepare Re-LPS for further synthesis of complex lipopolysaccharides (Scheme 3).
KDO transferase from E.coli (WaaA) can transfer two KDO residues onto lipid A.40,41 Before its incorporation into LPS or CPS, KDO is activated to CMP-KDO, which serves as the substrate for KDO transferase, by CMP-KDO synthase.42–44 However, CMP-KDO is very unstable under physiological conditions. It has been reported that the half-life-time of CMP-KDO is only 34 min at 25°C.45 Therefore, a one-pot reaction system was used to transfer KDO on lipid A (Scheme 3),41 in which KDO, CTP, CMP-KDO synthesase from E.coli (KdsB), inorganic pyrophosphatase (PPA) from Pasteurella multocida and WaaA were included. KdsB catalyzes the formation of CMP-KDO from KDO and CTP. PPA was added to improve the whole reaction by hydrolyzing the by-product of PPi. The produced CMP-KDO could serve as the substrate of WaaA. Although we successfully observed the formation of the product on the high resolution mass spectrum, a large-scale synthesis to obtain enough products for NMR analysis and vaccine evaluation was not achieved. The difficulties line in the extremely poor solubility of lipid A in water. Kinds of detergents have been tested to improve the reaction, but a practical scale synthesis is still unsuccessful. Tylor and co-workers recently found that heptose transferases can recognize lipid A without intact lipid tails,46,47 which solubility is better than the normal lipid A. Inspired by this result, a circuitous strategy is re-designed to try to synthesize Re-LPS in large scale in our lab.
In summary, a practical system for the facile synthesis of KDO in large scale is described. We demonstrate herein that KDO could be efficiently and conveniently prepared from D-ribulose 5-phosphate by using a one-pot multienzyme (OPME) system. The advantages of this strategy are that all the materials used in this strategy are easy-to-get and all enzymes involved in the synthetic process are highly active. Moreover, an attempt for the installation of KDO to lipid A is also made. Although two KDO residues can be easily transferred to lipid A by a single KDO transferase, a practical reaction system that could produce enough products is still necessary. We anticipate this work will accelerate an understanding of both biological roles and synthetic applications of KDO.
Supplementary Material
Acknowledgments
This work was financially supported National Institute of Health (R01 GM085267 and R01 AI083754).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at
References and notes
- 1.Brade H, Brade L, Schade U, Zahringer U, Holst O, Kuhn HM, Rozalski A, Rohrscheidt E, Rietschel ET. Progress in clinical and biological research. 1988;272:17. [PubMed] [Google Scholar]
- 2.Raetz CR, Whitfield C. Annu Rev Biochem. 2002;71:635. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, Frasch CE, Stephens DS, Feavers I, Frosch M, Parkhill J, Vogel U, Quail MA, Bentley SD, Maiden MC. Emerg Infect Dis. 2013;19:566. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitfield C. Annu Rev Biochem. 2006;75:39. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 5.Li YT, Wang LX, Pavlova NV, Li SC, Lee YC. J Biol Chem. 1997;272:26419. doi: 10.1074/jbc.272.42.26419. [DOI] [PubMed] [Google Scholar]
- 6.Droge W, Lehmann V, Luderitz O, Westphal O. Eur J Biochem. 1970;14:175. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt H, Hansen G, Singh S, Hanuszkiewicz A, Lindner B, Fukase K, Woodard RW, Holst O, Hilgenfeld R, Mamat U, Mesters JR. Proc Natl Acad Sci U S A. 2012;109:6253. doi: 10.1073/pnas.1119894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camci-Unal G, Mizanur RM, Chai Y, Pohl NL. Org Biomol Chem. 2012;10:5856. doi: 10.1039/c2ob25168j. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla L, Polissi A, Airoldi C, Gabrielli L, Merlo S, Nicotra F. Curr Med Chem. 2011;18:830. doi: 10.2174/092986711794927676. [DOI] [PubMed] [Google Scholar]
- 10.Adachi H, Kondo KI, Kojima F, Umezawa Y, Ishino K, Hotta K, Nishimura Y. Nat Prod Res. 2006;20:361. doi: 10.1080/14756360500183699. [DOI] [PubMed] [Google Scholar]
- 11.Boltje TJ, Zhong W, Park J, Wolfert MA, Chen WX, Boons GJ. J Am Chem Soc. 2012;134:14255. doi: 10.1021/ja306274v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Martin CE, Seeberger PH. Chem Sci. 2012;3:896. [Google Scholar]
- 13.Yang Y, Oishi S, Martin CE, Seeberger PH. J Am Chem Soc. 2013;135:6262. doi: 10.1021/ja401164s. [DOI] [PubMed] [Google Scholar]
- 14.Broecker F, Aretz J, Yang Y, Hanske J, Guo XQ, Reinhardt A, Wahlbrink A, Rademacher C, Anish C, Seeberger PH. ACS chemical biology. 2014;9:867. doi: 10.1021/cb400925k. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Dong J, Xu F, Liu A, Wang L, Zhang Q, Chai Y. Org Lett. 2015;17:2388. doi: 10.1021/acs.orglett.5b00901. [DOI] [PubMed] [Google Scholar]
- 16.Li LS, Wu YL. Curr Org Chem. 2003;7:447. [Google Scholar]
- 17.Hekking KFW, van Delft FL, Rutjes FPJT. Tetrahedron. 2003;59:6751. [Google Scholar]
- 18.Kuboki A, Tajimi T, Tokuda Y, Kato DI, Sugai T, Ohira S. Tetrahedron Lett. 2004;45:4545. [Google Scholar]
- 19.Hartmann K, Kim BG, Linker T. Synlett. 2004:2728. [Google Scholar]
- 20.Kikelj V, Plantier-Royon R, Portella C. Synthesis-Stutt gart. 2006:1200. [Google Scholar]
- 21.Hekking KFW, Moelands MAH, van Delft FL, Rutjes FPJT. J Org Chem. 2006;71:6444. doi: 10.1021/jo060913x. [DOI] [PubMed] [Google Scholar]
- 22.Sugai T, Shen GJ, Ichikawa Y, Wong CH. Journal of the American Chemical Society. 1993;115:413. [Google Scholar]
- 23.Wada M, Hsu CC, Franke D, Mitchell M, Heine A, Wilson I, Wong CH. Bioorg Med Chem. 2003;11:2091. doi: 10.1016/s0968-0896(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CC, Hong Z, Wada M, Franke D, Wong CH. Proc Natl Acad Sci U S A. 2005;102:9122. doi: 10.1073/pnas.0504033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bednarski MD, Crans DC, DiCosimo R, Simon ES, Stein PD, Whitesides GM, Schneider MJ. Tetrahedron letters. 1988;29:427. [Google Scholar]
- 26.Strohmaier H, Remler P, Renner W, Hogenauer G. J Bacteriol. 1995;177:4488. doi: 10.1128/jb.177.15.4488-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray PH. J Bacteriol. 1980;141:635. doi: 10.1128/jb.141.2.635-644.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camci-Unal G, Mizanur RM, Chai YH, Pohl NLB. Org Biomol Chem. 2012;10:5856. doi: 10.1039/c2ob25168j. [DOI] [PubMed] [Google Scholar]
- 29.Kruger NJ, von Schaewen A. Curr Opin Plant Biol. 2003;6:236. doi: 10.1016/s1369-5266(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 30.Pontremoli S, Mangiarotti G. J Biol Chem. 1962;237:643. [PubMed] [Google Scholar]
- 31.Imker HJ, Fedorov AA, Fedorov EV, Almo SC, Gerlt JA. Biochemistry. 2007;46:4077. doi: 10.1021/bi7000483. [DOI] [PubMed] [Google Scholar]
- 32.Pontremoli S, De Flora A, Grazi E, Mangiarottig. Bonsignore A, Horecker BL. J Biol Chem. 1961;236:2975. [PubMed] [Google Scholar]
- 33.Wen L, Huang K, Liu Y, Wang PG. ACS Catalysis. 2016 [Google Scholar]
- 34.Wen L, Huang K, Wei M, Meisner J, Liu Y, Garner K, Zang L, Wang X, Li X, Fang J, Zhang H, Wang PG. Angew Chem Int Ed Engl. 2015 doi: 10.1002/anie.201505714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Woodard RW. J Biol Chem. 2003;278:18117. doi: 10.1074/jbc.M301983200. [DOI] [PubMed] [Google Scholar]
- 36.Zähringer U, Lindner B, Seydel U, Rietschel ET, Naoki H, Unger F, Imoto M, Kusumoto S, Shiba T. Tetrahedron letters. 1985;26:6321. [Google Scholar]
- 37.Carter D, Reed Sg. Current Opinion in Hiv and Aids. 2010;5:409. doi: 10.1097/COH.0b013e32833d2cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou ZF, Liao GC, Mandal SS, Suryawanshi S, Guo ZW. Chemical Science. 2015;6:7112. doi: 10.1039/c5sc01402f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshizaki H, Fukuda N, Sato K, Oikawa M, Fukase K, Suda Y, Kusumoto S. Angew Chem Int Ed. 2001;40:1475. doi: 10.1002/1521-3773(20010417)40:8<1475::AID-ANIE1475>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 40.Brabetz W, Muller-Loennies S, Brade H. J Biol Chem. 2000;275:34954. doi: 10.1074/jbc.M005204200. [DOI] [PubMed] [Google Scholar]
- 41.Belunis CJ, Raetz CR. J Biol Chem. 1992;267:9988. [PubMed] [Google Scholar]
- 42.Heath EC, Mayer RM, Edstrom RD, Beaudreau CA. Ann N Y Acad Sci. 1966;133:315. doi: 10.1111/j.1749-6632.1966.tb52374.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghalambor MA, Levine EM, Heath EC. J Biol Chem. 1966;241:3207. [PubMed] [Google Scholar]
- 44.Ghalambor MA, Heath EC. J Biol Chem. 1966;241:3216. [PubMed] [Google Scholar]
- 45.Schmidt H, Mesters JR, Wu J, Woodard RW, Hilgenfeld R, Mamat U. PLoS One. 2011;6:e23231. doi: 10.1371/journal.pone.0023231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czyzyk DJ, Liu C, Taylor EA. Biochemistry. 2011;50:10570. doi: 10.1021/bi201581b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mudapaka J, Taylor EA. Febs Letters. 2015;589:1423. doi: 10.1016/j.febslet.2015.04.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


