Summary
Antiplatelet agents (APAs) are proven to reduce risk of major cardiovascular events in patients with cardiovascular disease and normal kidney function. With recent post hoc analyses of large trials questioning the safety and efficacy of APAs in CKD, major gaps exist in our understanding of platelet aggregability and the effects of APAs on thrombosis and bleeding in CKD. Clinical practice guidelines are ambiguous about use of such agents in CKD patients, because patients with moderate to advanced CKD were systematically excluded from clinical trials of APAs. CKD patients experience excessive rates of cardiovascular thrombotic events, yet paradoxically are at higher risk for major bleeding while receiving APAs. Furthermore, observational studies suggest that CKD patients may exhibit poor response to APAs. High residual platelet aggregability, as determined by inhibition of platelet aggregation, is associated with increased risk for cardiovascular events. In addition, metabolism of certain APAs may be altered in CKD patients. It is, therefore, imperative to explore the mechanisms responsible for poor response to APAs in CKD patients in order to use these drugs more safely and effectively. This review identifies the knowledge gaps and future trials needed to address those issues with the use of APAs in CKD patients.
Introduction
CKD affects 10%–16% of adults worldwide. Compared with the general population, individuals with CKD are at increased risk of cardiovascular events and death from such events (1). Furthermore, in-hospital mortality after acute myocardial infarction (MI) and other related adverse events increase with worsening kidney function (1). CKD patients experience higher rates of coronary in-stent thrombosis (2) and, paradoxically, more major bleeding events on antiplatelet therapy, resulting in four-fold higher mortality at 1 year compared with patients with normal kidney function (3). Increased oxidative stress (4), inflammation (4), and platelet dysfunction (5), as well as altered pharmacodynamic properties of antiplatelet agents (6) and accelerated plaque progression and rupture (7) are proposed mechanisms for the observed increase in the composite outcome of stroke, MI, fatal coronary heart disease, and death in CKD patients, which is double the risk from diabetes mellitus alone (8).
Antiplatelet agents (APAs) are proven to reduce major cardiovascular events in patients with coronary artery disease (CAD). However, limited data exist regarding APA use in CKD patients, because they were systematically excluded from large randomized trials. Aggressive risk factor modifications are often delayed and CKD patients are less likely to receive cardioprotective medications and early cardiovascular interventions due to concerns regarding safety (9,10). This review summarizes current guidelines and evidence for APA use in CKD patients and discusses gaps and limitations in our current understanding given the lack of adequately powered randomized controlled trials (RCTs).
Platelet Dysfunction in CKD
Kidney failure may cause two opposite hemostatic complications: a bleeding diathesis (11) and a thrombotic predisposition (3). It is thought that platelet-platelet and platelet-vessel wall interactions are abnormal in CKD patients (5). Platelet defects range from diminished responsiveness to platelet agonists like ADP, abnormal platelet adherence to foreign surfaces, reduced platelet procoagulant activity, decreased thromboxane and cyclic AMP (cAMP) production, and decreased platelet membrane glycoprotein Ib (GPIb) expression (5). Most of these findings were observed in dialysis patients and are based on older methods of measuring platelet function. It is postulated that urea, other nitrogenous compounds, phenols, middle molecules, and fibrinogen fragments decrease platelet aggregability by causing configurational changes in GPIIb/IIIa receptors on the platelet plasma membrane (12,13). Endothelial dysfunction in CKD patients with resultant abnormal prostacyclin and thromboxane production may reduce aspirin’s ability to inhibit cycloxygenase-1 (COX-1) (5,14).
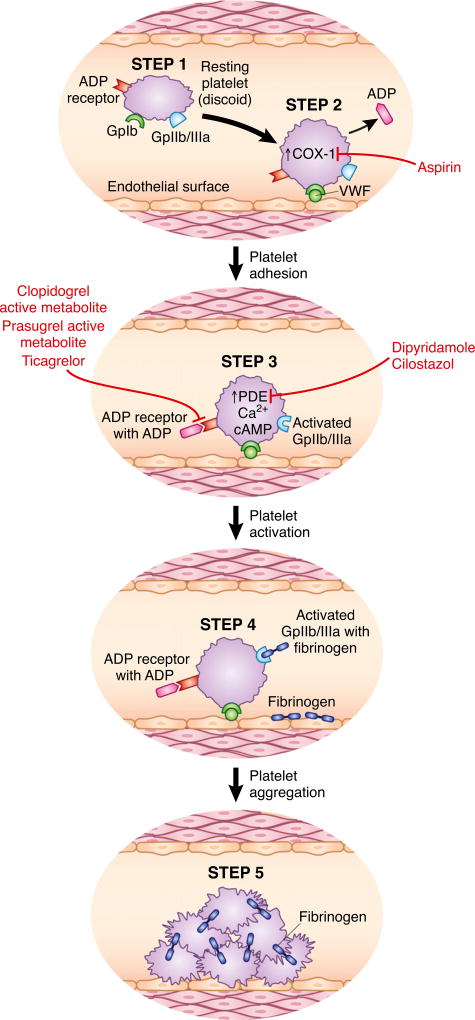
vWf, a large multimeric glycoprotein secreted from endothelium, plays an important role in hemostasis by facilitating platelet adhesion and aggregation. vWf antigen levels, as a result of endothelial cell activation, are elevated in CKD patients (4,14). It is not yet clear whether elevations in vWf antigen levels are causal for increased thrombosis or mirror endothelial dysfunction in CKD. However, studies associating increased antigen levels to cardiovascular events are fraught with small sample sizes, as well as heterogeneous populations or methodologies that limit their interpretation (15). It is believed that although vWf antigen levels may be high, vWf activity may be impaired in CKD due to decreased renal clearance of middle molecules that interfere with vWf binding to platelets. Furthermore, desmopressin (1-desamino-D-arginine vasopressin), which is commonly used to reduce bleeding risk in uremic patients, is known to induce release of coagulator factor VIII, vWf, and tissue plasminogen activator possibly via extrarenal V2 receptors (16). Although desmopressin is used to shorten or normalize bleeding time temporarily in uremic patients, vWf levels are already increased in these patients with CKD. Hence, there remains poor understanding of these mechanisms (17). Figure 1 shows platelet activation and aggregation in normal individuals and various steps where platelet dysfunction occurs in CKD.
Figure 1. Steps in platelet activation and aggregation, and sites of action of APAs.
(1) Resting platelet (discoid in shape) with GPIb (vWf receptor), GPIIb/IIIa (fibrinogen receptor), thrombin receptor, ADP receptor (P2Y12), and thromboxane receptor. (2) A stimulus such as endothelial injury causes thrombin release and vWf interaction with GPIb. Resting platelets change their shape from discoid to spherical. Thrombin binds to its receptor, resulting in increase of COX-1 activity, thromboxane, and ADP release from platelet granules. (3) ADP and thromboxane bind to their receptors and increase intracellular calcium (Ca2+) and cAMP. Intracellular cAMP levels are maintained by phosphodiesterase enzyme (PDE). (4) Platelets are activated and GPIIb/IIIa undergoes a conformational change to allow fibrinogen binding. (5) Platelet aggregation occurs with cross-linking of platelet along with fibrinogen. Uremia alters all of these steps in that it reduces expression of GPIb, limits intracellular calcium and cAMP increase, and hinders GPIIb/IIIa activation. Sites of action of aspirin, clopidogrel, prasugrel, ticagrelor, dipyridamole, and cilostazol are also shown.
Available APAs
Table 1 lists different antiplatelet agents, their mechanism of action, and response to various agonists (collagen, AA, and ADP) used in platelet function testing. Figure 1 also illustrates different sites of actions of individual APAs. Aspirin is the most commonly used APA in CKD patients. It inhibits COX-1 and decreases platelet thromboxane production. The antiplatelet effect of dipyridamole is mediated via phosphodiesterase inhibition (PDEI) and depends on stimulation of platelet cAMP by circulating prostacyclin (18). Dipyridamole is a weak antiplatelet agent and its effects are potentiated by concomitant use of low-dose aspirin. Compared with low-dose aspirin (75–100 mg/d), which selectively inhibits platelet COX-1 and potentiates the antiplatelet effect of dipyridamole, high-dose aspirin (>1000 mg/d) prevents prostacyclin formation, nullifying the effects of dipyridamole (19). Thus, the antiplatelet effect of an aspirin/dipyridamole combination depends critically on doses used (19).
Table 1.
Antiplatelet agents, mechanism of action and response to agonists used in platelet function testing (19,64–67)
| Drug (Type) | Action | Mean Platelet Inhibition (Time Required) |
Response to Collagen/AA |
Response to ADP |
Dosing in CKD and ESRD (68) |
|---|---|---|---|---|---|
| Aspirin (NSAIDs) | Inhibits COX-1 | <30 min | Inhibits | None | Use with caution eGFR <10 ml/min per 1.73 m2 |
| Clopidogrel (Thienopyridine) | Inhibits P2Y12 receptor | <1 h with loading dose of 300 mg; 2–3 d for daily dose of 75 mg | No | Inhibits | No dose adjustment required |
| Prasugrel (Thienopyridine) | Inhibits P2Y12 receptor | 2–4 h | No | Inhibits | No dose adjustment required; no data in ESRD available |
| Ticagrelor (ATP analog) | Inhibits P2Y12 receptor | 2–4 h | No | Inhibits | No dose adjustment required; no data in ESRD available |
| Dipyridamole (PDE inhibitor) | Increases platelet cAMP | Variable | Variable | Inhibits in whole blood not in platelet-rich plasma | No dose adjustment required |
| Cilostazol (PDE inhibitor) | Increases platelet cAMP | Variable | Inhibits | Inhibits | No dose adjustment required; caution advised for creatinine clearance <25 ml/min |
NSAIDs, nonsteroidal anti-inflammatory agents; COX-1, cycloxygenase-1; eGFR, estimated GFR; PDE, phosphodiesterase; cAMP, cyclic AMP.
Cilostazol is a newer PDEI that inhibits platelet aggregation induced by collagen, ADP, and AA and improves endothelial cell function (20). Cilostazol is a potent APA. It may be especially pertinent in CKD patients, in whom endothelial cell dysfunction results in malfunction of the antithrombogenic activity of the vascular endothelium. Although platelets are not usually activated while circulating through normal endothelium, they are when endothelial cells are activated by oxidative stress.
Clopidogrel, a P2Y12 receptor inhibitor, is a prodrug and inhibits the P2Y12 receptor selectively and irreversibly, utilizing the reactive thiol group of its active metabolite and forming a disulfide bridge between ≥1 cysteine residues of the P2Y12 receptor with resultant irreversible blockade (21). Activation of the P2Y12 receptor, a G protein–coupled purinergic receptor that binds ADP, leads to activation of the GPIIb/IIIa receptor, granule release, amplification of platelet aggregation, and stabilization of the platelet aggregate. P2Y12 receptor blockade acts early in the cascade of platelet aggregation and prevents platelet degranulation and inhibits activation of GPIIb/IIIa receptor blocking fibrinogen binding and platelet cross-linking. Prasugrel and ticagrelor are the newest additions to this group (22,23). Unlike clopidogrel and prasugrel, ticagrelor is not a prodrug and does not require metabolism to an active metabolite (24). Ticagrelor induces hyperuricemia and should be used with caution in patients with gout (24). Maintenance aspirin doses >100 mg/d reduce the efficacy of ticagrelor and are not recommended (24).
Safety and Efficacy of Antiplatelet Therapy in CKD
Safety
CKD patients are not only at increased risk for bleeding complications, but also paradoxically at increased risk of thrombosis due to the underlying complex hemostatic disorder seen with progressive kidney failure. In addition, increased risk of drug-drug interactions with dual antiplatelet therapy, changes in renal and nonrenal drug clearance, and underlying qualitative platelet defects with altered reactivity and aggregability from uremia may be other factors that increase risk in CKD patients.
To examine concerns regarding safety of low-dose aspirin in secondary prevention of future cardiovascular events in CKD patients, two studies reported no increased risk of major bleeding. The First United Kingdom Heart and Renal Protection (UK-HARP) trial, a RCT of 242 predialysis CKD patients, 73 chronic hemodialysis or peritoneal dialysis patients, and 133 previous kidney transplant recipients compared 100 mg/d versus no aspirin in secondary prevention of cardiovascular events without undue toxicity. There was no increased risk of major bleeding, defined as fatal or requiring hospitalization (relative risk [RR], 0.66; 95% confidence interval [95 CI%], 0.19–2.31) on 100 mg/d aspirin in CKD patients, however, there was a three-fold increase in risk of minor bleeding, defined as epistaxis, ecchymosis, or bruising (RR, 2.8; 95% CI, 1.5–5.3) (25). Subsequently, the Dialysis Outcomes and Practice Patterns Study (DOPPS) showed no increased risk of gastrointestinal bleeding (RR, 1.01; 95% CI, 0.88–1.17) in those who were taking 100 mg/d of aspirin versus no aspirin (26). Studies that have addressed the safety and efficacy of aspirin in patients with CKD stages 3–5 are shown in Table 2 (25–28).
Table 2.
Safety and efficacy of aspirin in CKD
| Study | Study Type | Participants | N | Efficacy | Safety |
|---|---|---|---|---|---|
| UK-HARP (25) | RCT | Predialysis CKD; chronic dialysis; renal transplant | 448 | Not analyzed in this study | No evidence of major bleeding; 3-fold risk of minor bleeding |
| DOPPS (26) | Nested case control study of DOPPS 1–2 | Randomly selected chronic HD patients | 28,320 | No benefit on all-cause mortality; reduced risk of MI and stroke | No increase in GI bleeds |
| Sciahbasi et al. (27) | Retrospective analysis | Patients admitted to community hospital with ACS | 595 | Patients on chronic low-dose aspirin and statin are less likely to get admitted with STEMI | Not analyzed |
| Palmer et al. (28) | Meta-analysis | 9 trials on ACS/PCI; 31 trials on stable or no CAD | 21,670 | Little or no effect on all-cause mortality; uncertain effects on cardiovascular mortality | Increase in major and minor bleeding; uncertain risk of hemorrhagic stroke |
UK-HARP, First United Kingdom Heart and Renal Protection study; RCT, randomized controlled trial; DOPPS, Dialysis Outcomes and Practice Patterns Study; HD, hemodialysis; MI, myocardial infarction;; GI, gastrointestinal; ACS, acute coronary syndrome; STEMI, ST elevation myocardial infarction; PCI, percutaneous coronary intervention; CAD, coronary artery disease.
The majority of safety data for clopidogrel comes from post hoc analyses of RCTs comparing clopidogrel versus placebo for secondary prevention of future cardiovascular events. Post hoc analysis of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial was performed on a subgroup of 2009 patients with diabetic nephropathy (defined as patients with a random spot urine albumin/creatinine ratio >30 mg/g of creatinine with no data on serum creatinine concentration available). The authors reported that clopidogrel increased cardiovascular and overall mortality compared with placebo (29). The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial evaluated patients with acute coronary syndrome (ACS), excluding ST segment elevation MI and ESRD patients (30). A subgroup analysis of patients with an estimated GFR <60 ml/min per 1.73 m2 (calculated by the Modified Diet in Renal Disease study equation) showed no benefit with respect to reduction of all-cause mortality (RR, 0.95; 95% CI, 0.78–1.16) or cardiovascular death (RR, 0.95; 95% CI, 0.77–1.17) with addition of clopidogrel to standard therapy for secondary prevention of ACS. Risk of major and minor bleeding was increased in CKD patients taking clopidogrel (RR, 1.37; 95% CI, 0.89–2.12; and RR, 1.50; 95% CI, 1.21–1.86, respectively).
A recent meta-analysis by Palmer et al. evaluated safety of aspirin and clopidogrel in nine trials (all post hoc subgroup analyses for CKD) involving 9969 patients with ACS undergoing percutaneous coronary intervention (PCI) and 31 trials involving 11,701 patients with stable or no cardiovascular disease (28). They concluded that although the evidence was low quality, there was an increase in major and minor bleeding episodes with APA use in CKD patients (RR, 1.40; 95% CI, 1.07–1.86; RR, 1.47; 95% CI, 1.25–1.72, respectively). Although the relative risk of hemorrhagic stroke was increased, it was not statistically significant. Therefore, although individual studies reported no increased risk of major bleeding, Palmer’s meta-analysis does report increased risk of bleeding with aspirin in secondary prevention.
In conclusion, low-dose aspirin monotherapy should be used cautiously for secondary prevention of future cardiovascular events in CKD patients. Furthermore, there is a lack of data for low-dose aspirin use in primary prevention. Clopidogrel is more commonly used as dual APA therapy with aspirin in secondary prevention and monotherapy for primary prevention in those allergic to aspirin. Initial data from the two post hoc analyses above raise serious safety concerns with the use of dual APA therapy (aspirin and clopidogrel) in CKD patients.
Evidence for Efficacy
The efficacy of aspirin in CKD patients presenting with ACS is well established (31). However, data on reduction of overall mortality in CKD patients are limited. In a cross-sectional analysis of the DOPPS cohort (26), benefit of aspirin versus no aspirin on cardiovascular morbidity and mortality was evaluated in chronic hemodialysis patients. Although there was no effect on all-cause mortality, there was a reduction in MI risk (RR, 1.21; 95% CI, 1.06–1.38) and stroke (RR, 0.82; 95% CI, 0.69–0.98). In a retrospective analysis of consecutive patients admitted to a community hospital for ACS, 404 patients had normal kidney function and 191 had CKD defined as eGFR ≤60 ml/min per 1.73 m2 on admission (27). Prior aspirin or statin use was associated with reduced odds of admission with ST elevation MI in all patients and those with CKD stages 3–5 (RR, 0.5; 95% CI, 0.2–1.0) as shown in Table 2.
There is controversy regarding the efficacy of clopidogrel in CKD patients. A post hoc analysis of a subgroup of 331 patients with creatinine clearances <60 ml/min in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial showed that clopidogrel versus placebo did not decrease the composite outcome of death, MI, and stroke in CKD patients compared with those with normal kidney function (32). Unfortunately, the trial excluded patients with a serum creatinine concentration >3 mg/dl. Furthermore, there are limited data regarding pharmacodynamics, duration of therapy, added benefit of higher loading, and maintenance dose of clopidogrel in CKD patients.
Newer APAs have been approved by the US Food and Drug Administration (FDA). The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) showed prasugrel to be more efficacious than clopidogrel in those with reduced creatinine clearance (20% risk reduction in patients with creatinine clearance ≥60 ml/min and 14% risk reduction in patients with creatinine clearance <60 ml/min) (22). In fact, a sub-analysis of TRITON-TIMI showed that the reduction in risk of coronary stent thrombosis with prasugrel was independent of creatinine clearance (33–34). Furthermore, the greatest absolute benefit was observed in those patient groups that were at highest risk for coronary in-stent thrombosis. These included patients with CKD, diabetes mellitus, longer stents, and stents located at bifurcation points. The Platelet Inhibition and Patient Outcomes (PLATO) trial showed that ticagrelor was superior to clopidogrel in all patients irrespective of their creatinine clearance, with relative risk of cardiovascular events for patients with creatinine clearance ≥60 ml/min and <60 ml/min reported as 0.90 (95% CI, 0.79–1.02) and 0.77 (95% CI, 0.65–0.90), respectively (23), as shown in Table 3. Newer agents provide clinicians more drug options to choose from when deciding the right APA for prevention of future cardiovascular events. However, data on these drugs are not currently available beyond 12 months of follow-up.
Table 3.
Safety and efficacy of P2Y12 receptor antagonists in CKD
| Study | Intervention | Participants |
N (CKD) |
Efficacy | Safety |
|---|---|---|---|---|---|
| CURE (30) | Clopidogrel versus placebo | ACS patients (no STEMI) | 3262 | No reduction in death and cardiovascular death | Increased risk of minor bleeding |
| CREDO (32) | Clopidogrel versus placebo for 1 yr (post hoc analysis) | Elective PCI patients | 331 | No difference in death, MI, or stroke between clopidogrel and placebo arm | No difference in bleeding based on CKD |
| CHARISMA (29) | Clopidogrel versus placebo for 28 mo (post hoc analysis) | Elective PCI patients | 2009 | Increased all-cause and cardiovascular mortality | No difference in bleeding |
| TRITON-TIMI (22) | Prasugrel versus clopidogrel | ACS patients with scheduled PCI | 1490 | Reduced ischemic events (no effect on mortality) | Increased risk of major bleeding |
| PLATO (23) | Ticagrelor versus clopidogrel | ACS patients | 3237 | Fewer ischemic end points and less mortality | No significant increase in major bleeding; more nonprocedure-related bleeding |
| Palmer et al. (28) | Anti-platelet agents | ACS/PCI or cardiovascular disease patients | 21,670 | Little or no effect on all-cause mortality; uncertain effects on cardiovascular mortality | Increase in major and minor bleeding; uncertain risk of hemorrhagic stroke |
CURE, Clopidogrel in Unstable Angina to Prevent Recurrent Events; ACS, acute coronary syndrome; STEMI, ST segment elevation myocardial infarction; CREDO, Clopidogrel for the Reduction of Events During Observation; PCI, percutaneous coronary intervention; MI, myocardial infarction; CHARISMA, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; TRITON-TIMI, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction); PLATO, Platelet Inhibition and Patient Outcomes.
For secondary prevention of stroke (especially an ischemic stroke while taking aspirin), there is no evidence that increasing aspirin dose provides additional benefit in patients with CKD (35). Although alternative antiplatelet agents are often considered for noncardioembolic patients, no single agent or combination has been studied in patients that have had an event while receiving aspirin. There are multiple factors that influence thrombosis in vivo, and it may be naive to expect a drug that influences one parameter to work in all patients. A combination of drugs that affect the multifactorial pathogenesis of arterial thrombosis, such as aspirin-clopidogrel (36), clopidogrelcilostazol (37), aspirin-dipyridamole (38), is more efficacious than monotherapy alone. However, there are insufficient data available to make evidence-based recommendations about choices between antiplatelet options other than aspirin for secondary stroke prevention in CKD patients (35).
The meta-analysis by Palmer et al. reported that although evidence was of low quality, APA had no effect on all-cause mortality (RR, 0.87; 95% CI, 0.61–1.24) and cardiovascular mortality (RR, 0.91; 95% CI, 0.60–1.36), but did reduce events rates for MI (RR, 0.66; 95% CI, 0.51–0.87) (28). There was a reduction in the relative risk for stroke but this was not statistically significant (RR, 0.66; 95% CI, 0.16–2.78).
Overall, aspirin is beneficial in reducing mortality in CKD patients presenting with ACS. However, data are limited to determine the efficacy of aspirin in primary or secondary prevention of future cardiovascular events in CKD patients with stable CAD. The efficacy of clopidogrel may be limited in CKD patients given current data. However, there are data to support no added benefit of dual APAs (aspirin and clopidogrel) in secondary prevention. Prasugrel and ticagrelor are new APAs and appear superior to clopidogrel in CKD patients on the basis on the RCTs published recently. However, data on long-term safety and efficacy of these agents are not currently available.
Potential Mechanisms for Reduced Efficacy of APAs in CKD Patients
There are several potential mechanisms that may explain why some CKD patients have poor response to APAs. Residual platelet aggregability, cytochrome P450 monoxygenase system (CYP) polymorphisms, altered nonrenal drug metabolism from uremia, and increased vWf antigen and impaired vWf activity are the most important mechanistic pathways that require further discussion.
Residual platelet aggregability is defined as poor inhibition of platelet aggregation in response to various agonists (AA, collagen, and ADP) used in analyzing platelet function. Several observational studies in non-CKD patients indicate that residual platelet aggregability while receiving treatment with APAs is associated with an increase in adverse cardiovascular outcomes (39–41). Although marked variability exists in the platelet-inhibitory effects of aspirin (42) and clopidogrel (43) in asymptomatic individuals and those with overt CAD, high residual platelet aggregability while taking aspirin and clopidogrel predicts risk for cardiovascular death, nonfatal MI, recurrent ischemic events, and coronary in-stent thrombosis. Consequently, targeting high residual platelet aggregability as a novel modifiable cardiovascular risk factor is an emerging strategy that utilizes more potent and targeted APAs in an attempt to improve cardiovascular outcomes.
This observation was recently extended to the CKD population. In a cross-sectional analysis of 306 diabetic patients with CAD, platelet aggregation with ADP and collagen was assessed in light transmittance aggregometry (using platelet-rich plasma). Markers of platelet activation (P-selectin expression and GPIIb/IIIa activation) were assessed by flow cytometry in patients stratified by CKD stages (44). The authors noted higher residual platelet aggregability in patients with CKD versus those with normal kidney function on dual APA therapy (aspirin and clopidogrel). This finding was shown in another retrospective analysis of 1567 patients with CKD stages 3–5 with symptomatic CAD who underwent PCI compared with non-CKD controls. Higher residual platelet aggregability while taking clopidogrel was associated with higher cardiovascular events within 1 year of PCI. Furthermore, a higher proportion of patients with CKD stages 3–5 exhibited higher residual platelet aggregability while taking clopidogrel (45) compared with those with normal kidney function. This finding is important because higher residual platelet aggregability in CKD patients is associated with worse outcomes after PCI (46).
To evaluate whether residual platelet aggregability in CKD patients is modifiable, a controlled study was conducted in 74 chronic hemodialysis patients versus 50 non-CKD controls undergoing PCI to compare effects of standard therapy (75 mg of clopidogrel daily), higher-dose therapy (150 mg of clopidogrel daily), and dual therapy (75 mg of clopidogrel daily and 100 mg of cilostazol twice daily) (37). This study showed that compared with non-CKD patients, CKD patients have higher residual platelet aggregability, with no added benefit of increased clopidogrel dose on platelet inhibition; however, there was improvement in residual platelet aggregability with the addition of cilostazol.
In analyzing other potential mechanisms accounting for poor efficacy of APAs in CKD patients, it is pertinent to understand the complex metabolism of clopidogrel that may be altered in uremia (46). It is first metabolized to 2-oxo-clopidogrel by cytochrome monoxygenase system enzymes CYP1A2, CYP2B6, and CYP2C19 (47). The 2-oxo-clopidogrel is then hydrolyzed to its active metabolite by CYP2B6, CYP2C9, CYP2C19, and CYP3A4. This has potential pharmacokinetic importance for two reasons. First, CYP2C19 plays an important role in both steps, and several common polymorphisms were identified that either increase or reduce its activity. Polymorphisms that reduce activity can occur in up to 30% of patients and vary depending on race. In one study, carriers of one reduced function CYP2C19 allele had a 32.4% reduced exposure to the active clopidogrel metabolite and a 53% increase in risk of the combined endpoint of death, MI, or stroke (48). Risk of coronary in-stent thrombosis was increased three-fold in carriers of the reduced function allele. Second, CYP3A4 plays an important role in the formation of the active metabolite and its activity can be either increased or decreased by a variety of drugs, creating the potential for drug-drug interactions.
Poor clopidogrel responsiveness in CKD patients was shown using ADP-induced platelet aggregation (45). As GFR declined, there was a progressive increase in the percentage of patients that were poor responders from 20% in patients with CKD stage 2 to 38% in those with CKD stages 4 and 5 (P value for trend <0.001). This may result from the effects of uremia on nonrenal metabolism and clearance of clopidogrel. In clinical practice, medication dosages are commonly adjusted for renally excreted drugs. However, the potential pharmacokinetic effects of uremia on drug transport and nonrenal metabolism must also be considered (49). For example, uremia reduces expression and activity of a variety of CYP450s, including CYP2C19 and CYP3A4, that are involved in clopidogrel metabolism (50). In addition, kidney failure reduces expression of the organic anion transporter responsible for drug transport into enterocytes and hepatocytes (50). However, the exact mechanism of downregulation of such transporters is not known.
Recently, vWf antigen and activity levels were shown to be associated with cardiovascular events. Elevated vWf antigen levels in CKD can possibly explain high cardiovascular thrombotic events (15). However, it is postulated that impaired vWf activity (due to decreased expression of GPIb on platelet surface in CKD) may explain the increased bleeding tendency of patients with kidney disease (5). Future research is needed as newer APAs are discovered and this pathway is explored.
Current Recommendations
The National Kidney Foundation (NKF) task force has evaluated strategies for preventing and treating cardiovascular diseases in patients with CKD (51). Unfortunately, these recommendations have not been updated recently. With limited evidence to support the efficacy of aspirin in patients with CKD, the NKF recommendation for the usual aspirin dose in patients with CKD and CAD was based on extrapolation of the benefits of aspirin in the general population in patients with pre-existing cardiovascular disease (51). Furthermore, the 2009 Kidney Disease Improving Global Outcomes practice guidelines for secondary prevention of cardiovascular events in kidney transplant recipients with diabetes or cardiovascular disease suggest use of low-dose aspirin based on very poor quality of evidence (52). The American Heart Association (AHA) Task Force and American College of Cardiology (ACC) and US Preventive Task Force make no specific recommendations for APA use in CKD patients, because they acknowledge the lack of sufficient data (53,54). A recent study reported that >75% of published CAD trials have excluded CKD patients (55). Evidence is “level C” at best (35,56–58). This is especially concerning given that APAs are the third most commonly prescribed medications that lead to adverse drug events and hospitalizations (55).
The AHA/ACC guidelines encourage the performance of appropriately designed clinical trials to identify the optimal clopidogrel loading dose, realizing that there is considerable interpatient variability in clopidogrel response, with a wide range of inhibition of platelet aggregation after a given dose. However, techniques for monitoring for poor clopidogrel response and the appropriate dosing strategy when this is detected remain to be established. The AHA does not mandate routine platelet function testing to guide APA therapy because it is expensive and not routinely covered by insurance. However, it endorses platelet function testing for those coronary interventions that are high risk, such as in CKD patients, in order to maximize efficacy and safety of APAs. Because there is a lack of controlled studies to prove that routine platelet function testing will improve hard clinical outcomes, no specific recommendations can currently be made.
Overall, selection of an APA should be individualized on the basis of patient risk factor profiles, tolerance, and other clinical characteristics. Because CKD patients are at higher risk for not only thrombotic but also bleeding events, there is an urgent need for future trials to guide APA therapy in CKD patients.
Current Limitations
A major limitation of our current understanding and interpretation of the available evidence results from the heterogeneity of studies that were not powered to adequately address APA safety and efficacy in CKD patients. In addition, the majority of concerns raised result from subgroup or post hoc analyses. Furthermore, if APA therapy is efficacious in some CKD patients but not others, then how to identify such subgroups is an important question that remains to be answered. Clinicians also face the dilemma of choosing the correct type, dose duration, and combination of APAs in order to minimize side effects while maximizing cardiovascular and survival benefits. Major gaps exist in our knowledge of the pharmacodynamics of platelet aggregation and inhibition by APAs in the CKD population. Moreover, studies lacked data on proven adherence to APA medications, were underpowered, and used nonstandardized cutoffs to measure residual platelet aggregability, limiting the interpretation of these results (59,60). Finally, many studies did not control for sex, body mass index, and diabetes mellitus, factors that are known to affect platelet aggregability (61–63).
Another major limitation of previous studies exploring platelet aggregability in patients with CKD is the large methodological heterogeneity in assessing platelet function. There are numerous in vitro or ex vivo laboratory assays available for platelet functional assessment, but whole blood platelet aggregation (WBPA) measured by ex vivo impedance aggregometry via Chrono-log or Multi-plate aggregometers appear to be better tests for assessing platelet function compared with the traditional light transmittance method using platelet-rich plasma. WBPA to assess platelet function appears superior to light transmittance aggregation using platelet-rich plasma. WBPA is more sensitive and faster, evaluates platelets in a near physiologic milieu in the presence of red blood cells and white blood cells that are known to affect platelet function, and does not require centrifugation that results in platelet injury (64).
In an effort to improve cardiovascular outcomes for CKD patients, clinicians face uncertainty in how to best use APAs. As nephrologists, we face the conundrum of having to balance bleeding risk in patients with advanced CKD and the need for antiplatelet drugs in order to reduce the burden of thrombotic cardiovascular events in this high-risk population. High residual platelet reactivity on APAs is a novel modifiable risk factor predicting future cardiovascular events in patients with CAD. Limited data exist regarding platelet aggregability in predialysis CKD patients to guide current clinical practice. The ACC/AHA recommends conducting RCTs for testing the hypothesis that routine platelet function testing should be used to tailor APAs with special focus on high-risk patients such as those with CKD. Large prospective randomized trials with homogenous CKD populations are required to determine the significance of elevated levels of platelet (P-selectin, platelet factor-4, and vasodilator-stimulated phosphoprotein) and endothelial (vWf antigen and activity, E-selectin) markers in predicting hard clinical outcomes. With recent trials exploring newer APAs, there is uncertainty among clinicians in how to use generic drugs like aspirin and clopidogrel versus newer expensive drugs with unknown long-term safety profiles and higher costs.
That being said, our approach is based on the following principles until further evidence is available to guide management in CKD patients. First, low-dose aspirin monotherapy should be used for primary and secondary prevention. Caution is advised with respect to higher aspirin doses given limited data on added benefits and concern for increased adverse events. Second, dual therapy with aspirin and clopidogrel should be considered for secondary prevention in patients undergoing PCIs and other high-risk events. The optimal duration of dual therapy is unclear even in those without CKD. To date, there is no added benefit of administering higher loading or maintenance doses of clopidogrel. Third, platelet function testing should be considered in those patients that experience thrombotic events despite dual therapy to guide treatment. Newer agents should be contemplated in patients who demonstrate higher residual platelet aggregability on standard treatment. However, there remains an urgent need for more studies to assess the platelet and endothelial functional profile, pharmacodynamics and pharmacokinetics of APAs, and long-term safety and efficacy of such therapies in CKD in an attempt to safely reduce cardiovascular events in this high-risk population.
Acknowledgments
This work is supported in part by an AHA Clinical Research Program grant (12CRP11830004) awarded to N.J. Support for S.S.H. is provided by a Veterans Affairs MERIT grant (CX000217-01) and by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK085512).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHA; the Department of Veterans Affairs; the National Institute of Diabetes, Digestive and Kidney Diseases; or the National Institutes of Health.
Disclosures
S.B. is a consultant/advisor to Medtronic and Covidien, has received grants from Gilead, and has ownership in MDcare Global and HygieaTel.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Machecourt J, Danchin N, Lablanche JM, Fauvel JM, Bonnet JL, Marliere S, Foote A, Quesada JL, Eltchaninoff H, Vanzetto G EVASTENT Investigators. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and non-diabetic patients: The EVASTENT Matched-Cohort Registry. J Am Coll Cardiol. 2007;50:501–508. doi: 10.1016/j.jacc.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 3.Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113–1119. doi: 10.1016/s0735-1097(02)01745-x. [DOI] [PubMed] [Google Scholar]
- 4.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 5.Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579–589. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 6.Leblond F, Guévin C, Demers C, Pellerin I, Gascon-Barré M, Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001;12:326–332. doi: 10.1681/ASN.V122326. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: Results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 8.Debella YT, Giduma HD, Light RP, Agarwal R. Chronic kidney disease as a coronary disease equivalent—a comparison with diabetes over a decade. Clin J Am Soc Nephrol. 2011;6:1385–1392. doi: 10.2215/CJN.10271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson PN, Rumsfeld JS, Liang L, Hernandez AF, Peterson ED, Fonarow GC, Masoudi FA American Heart Association Get With The Guidelines-Heart Failure Program. Treatment and risk in heart failure: Gaps in evidence or quality? Circ Cardiovasc Qual Outcomes. 2010;3:309–315. doi: 10.1161/CIRCOUTCOMES.109.879478. [DOI] [PubMed] [Google Scholar]
- 10.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD Acute Coronary Treatment and Intervention Outcomes Network registry. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105–110. doi: 10.2215/CJN.01810407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozek-Langenecker SA, Masaki T, Mohammad H, Green W, Mohammad SF, Cheung AK. Fibrinogen fragments and platelet dysfunction in uremia. Kidney Int. 1999;56:299–305. doi: 10.1046/j.1523-1755.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 13.Sloand JA, Sloand EM. Studies on platelet membrane glycoproteins and platelet function during hemodialysis. J Am Soc Nephrol. 1997;8:799–803. doi: 10.1681/ASN.V85799. [DOI] [PubMed] [Google Scholar]
- 14.Landray MJ, Wheeler DC, Lip GYH, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: The chronic renal impairment in Birmingham (CRIB) study. Am J Kid Dis. 2004;43:244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: Focus on acute coronary syndromes. Circulation. 2008;117:1449–1459. doi: 10.1161/CIRCULATIONAHA.107.722827. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP) J Thromb Haemost. 2003;1:682–689. doi: 10.1046/j.1538-7836.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 17.Sica DA, Gehr TW. Desmopressin: Safety considerations in patients with chronic renal disease. Drug Saf. 2006;29:553–556. doi: 10.2165/00002018-200629070-00001. [DOI] [PubMed] [Google Scholar]
- 18.Moncada S, Korbut R. Dipyridamole and other phosphodiesterase inhibitors act as antithrombotic agents by potentiating endogenous prostacyclin. Lancet. 1978;1:1286–1289. doi: 10.1016/s0140-6736(78)91269-2. [DOI] [PubMed] [Google Scholar]
- 19.FitzGerald GA. Dipyridamole. N Engl J Med. 1987;316:1247–1257. doi: 10.1056/NEJM198705143162005. [DOI] [PubMed] [Google Scholar]
- 20.Goto S. Cilostazol: Potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl. 2005;6:3–11. doi: 10.1016/j.atherosclerosissup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Mullangi R, Srinivas NR. Clopidogrel: Review of bioanalytical methods, pharmacokinetics/pharmacodynamics, and update on recent trends in drug-drug interaction studies. Biomed Chromatogr. 2009;23:26–41. doi: 10.1002/bmc.1128. [DOI] [PubMed] [Google Scholar]
- 22.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITONTIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 23.James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, Harrington RA, Horrow J, Katus H, Keltai M, Lewis BS, Parikh K, Storey RF, Szummer K, Wojdyla D, Wallentin L. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122:1056–1067. doi: 10.1161/CIRCULATIONAHA.109.933796. [DOI] [PubMed] [Google Scholar]
- 24.Gaglia MA, Jr, Waksman R. Overview of the 2010 Food and Drug Administration Cardiovascular and Renal Drugs Advisory Committee meeting regarding ticagrelor. Circulation. 2011;123:451–456. doi: 10.1161/CIRCULATIONAHA.110.985325. [DOI] [PubMed] [Google Scholar]
- 25.Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A, Cairns HS, Collins R, Foley RN, Frighi V, Kourellias K, Ratcliffe PJ, Rogerson M, Scoble JE, Tomson CR, Warwick G, Wheeler DC. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: Biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis. 2005;45:473–484. doi: 10.1053/j.ajkd.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Ethier J, Bragg-Gresham JL, Piera L, Akizawa T, Asano Y, Mason N, Gillespie BW, Young EW. Aspirin prescription and outcomes in hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2007;50:602–611. doi: 10.1053/j.ajkd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Sciahbasi A, Arcieri R, Quarto M, Pendenza G, Lanzillo C, Summaria F, Romagnoli E, Commisso C, Penco M, Lioy E. Impact of chronic aspirin and statin therapy on presentation of patients with acute myocardial infarction and impaired renal function. Prev Cardiol. 2010;13:18–22. doi: 10.1111/j.1751-7141.2009.00050.x. [DOI] [PubMed] [Google Scholar]
- 28.Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, Copetti M, Graziano G, Tognoni G, Jardine M, Webster A, Nicolucci A, Zoungas S, Strippoli GF. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: A systematic review and meta-analysis. Ann Intern Med. 2012;156:445–459. doi: 10.7326/0003-4819-156-6-201203200-00007. [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta A, Steinhubl SR, Bhatt DL, Berger PB, Shao M, MakK-H, Fox KAA, Montalescot G, Weber MA, Haffner SM, Dimas AP, Steg PG, Topol EJ CHARISMA Investigators. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial) Am J Cardiol. 2009;103:1359–1363. doi: 10.1016/j.amjcard.2009.01.342. [DOI] [PubMed] [Google Scholar]
- 30.Keltai M, Tonelli M, Mann JF, Sitkei E, Lewis BS, Hawken S, Mehta SR, Yusuf S CURE Trial Investigators. Renal function and outcomes in acute coronary syndrome: Impact of clopidogrel. Eur J Cardiovasc Prev Rehabil. 2007;14:312–318. doi: 10.1097/01.hjr.0000220582.19516.a6. [DOI] [PubMed] [Google Scholar]
- 31.Basra SS, Tsai P, Lakkis NM. Safety and efficacy of antiplatelet and antithrombotic therapy in acute coronary syndrome patients with chronic kidney disease. J Am Coll Cardiol. 2011;58:2263–2269. doi: 10.1016/j.jacc.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 32.Best PJM, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, Califf RM, Topol EJ CREDO Investigators. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: Results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008;155:687–693. doi: 10.1016/j.ahj.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Wiviott SD, Trenk D, Frelinger AL, O’Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: The Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116:2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 34.Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EM TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626–1636. doi: 10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 35.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T American Heart Association/American Stroke Association Council on Stroke Council on Cardiovascular Radiology and Intervention American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: Co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–e449. [PubMed] [Google Scholar]
- 36.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 37.Woo JS, Kim W, Lee SR, Jung KH, Kim WS, Lew JH, Lee TW, Lim CK. Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: Results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease (PIANO-2 CKD) randomized study. Am Heart J. 2011;162:1018–1025. doi: 10.1016/j.ahj.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): Randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 39.Aradi D, Komócsi A, Vorobcsuk A, Rideg O, Tokés-Füzesi M, Magyarlaki T, Horváth IG, Serebruany VL. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: Systematic review and meta-analysis. Am Heart J. 2010;160:543–551. doi: 10.1016/j.ahj.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, Moschi G, Gori AM, Abbate R, Antoniucci D. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;49:2312–2317. doi: 10.1016/j.jacc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 41.Frere C, Cuisset T, Quilici J, Camoin L, Carvajal J, Morange PE, Lambert M, Juhan-Vague I, Bonnet JL, Alessi MC. ADP-induced platelet aggregation and platelet reactivity index VASP are good predictive markers for clinical outcomes in non-ST elevation acute coronary syndrome. Thromb Haemost. 2007;98:838–843. [PubMed] [Google Scholar]
- 42.Gurbel PA, Bliden KP, DiChiara J, Newcomer J, Weng W, Neerchal NK, Gesheff T, Chaganti SK, Etherington A, Tantry US. Evaluation of dose-related effects of aspirin on platelet function: Results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115:3156–3164. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: A review of the evidence. J Am Coll Cardiol. 2005;45:1157–1164. doi: 10.1016/j.jacc.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabaté M, Ferreiro JL, Ueno M, Jimenez-Quevedo P, Alfonso F, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Htun P, Fateh-Moghadam S, Bischofs C, Banya W, Müller K, Bigalke B, Stellos K, May AE, Flather M, Gawaz M, Geisler T. Low responsiveness to clopidogrel increases risk among CKD patients undergoing coronary intervention. J Am Soc Nephrol. 2011;22:627–633. doi: 10.1681/ASN.2010020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, Caillard S, Campia U, Moulin B, Gachet C, Ohlmann P. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011;57:399–408. doi: 10.1016/j.jacc.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 47.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 48.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 49.Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, Pichette V, Himmelfarb J. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol. 2009;20:2269–2276. doi: 10.1681/ASN.2009010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dreisbach AW. The influence of chronic renal failure on drug metabolism and transport. Clin Pharmacol Ther. 2009;86:553–556. doi: 10.1038/clpt.2009.163. [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT., Jr Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 52.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EM Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 53.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Jacobs AK. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:2022–2060. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- 54.Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: An update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]
- 55.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 56.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr 2011 WRITING GROUP MEMBERSACCF/AHA TASK FORCE MEMBERS. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 57.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA American Heart Association Science Advisory and Coordinating Committee. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 58.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, 3rd, Morrison DA, O’Neil WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology/American Heart Association Task Force on Practice Guidelines ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–e286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 59.Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, Deneer VH, Harmsze AM, van der Heyden JA, Rensing BJ, Suttorp MJ, Hackeng CM, ten Berg JM. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754–762. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 60.Serebruany V, Cherala G, Williams C, Surigin S, Booze C, Kuliczkowski W, Atar D. Association of platelet responsiveness with clopidogrel metabolism: Role of compliance in the assessment of “resistance”. Am Heart J. 2009;158:925–932. doi: 10.1016/j.ahj.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Becker DM, Segal J, Vaidya D, Yanek LR, Herrera-Galeano JE, Bray PF, Moy TF, Becker LC, Faraday N. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. 2006;295:1420–1427. doi: 10.1001/jama.295.12.1420. [DOI] [PubMed] [Google Scholar]
- 62.Bordeaux BC, Qayyum R, Yanek LR, Vaidya D, Becker LC, Faraday N, Becker DM. Effect of obesity on platelet reactivity and response to low-dose aspirin. Prev Cardiol. 2010;13:56–62. doi: 10.1111/j.1751-7141.2009.00058.x. [DOI] [PubMed] [Google Scholar]
- 63.DiChiara J, Bliden KP, Tantry US, Hamed MS, Antonino MJ, Suarez TA, Bailon O, Singla A, Gurbel PA. The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: An analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes. 2007;56:3014–3019. doi: 10.2337/db07-0707. [DOI] [PubMed] [Google Scholar]
- 64.Dyszkiewicz-Korpanty AM, Frenkel EP, Sarode R. Approach to the assessment of platelet function: Comparison between optical-based platelet-rich plasma and impedance-based whole blood platelet aggregation methods. Clin Appl Thromb Hemost. 2005;11:25–35. doi: 10.1177/107602960501100103. [DOI] [PubMed] [Google Scholar]
- 65.Sun B, Le SN, Lin S, Fong M, Guertin M, Liu Y, Tandon NN, Yoshitake M, Kambayashi J. New mechanism of action for cilostazol: Interplay between adenosine and cilostazol in inhibiting platelet activation. J Cardiovasc Pharmacol. 2002;40:577–585. doi: 10.1097/00005344-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Bhatt DL. Antiplatelet therapy: Ticagrelor in ACS-what does PLATO teach us? Nat Rev Cardiol. 2009;6:737–738. doi: 10.1038/nrcardio.2009.192. [DOI] [PubMed] [Google Scholar]
- 67.Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126–142. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
- 68.Micromedex 2.0 (Healthcare Series). Version 5.1. Greenwood Village, CO: Thomson Reuters Healthcare; [Google Scholar]