Abstract
Dopamine D2 receptors (D2Rs) in the ventral tegmental area (VTA) and the nucleus accumbens (NAc) are associated with vulnerability to addiction; however, whether D2Rs in these two brain regions play differential roles in regulation of drug intake is unknown. Here, we compared the effect of decreased mRNA level of Drd2 in each region on cocaine self-administration in a dose-response function. Drd2 mRNA levels in rat VTA or NAc were knocked down by bilateral microinjection of lentivirus coding shRNAs against rat Drd2. Drd2 knockdown was persistent and stable between 20 and 90 days after lentiviral infection. Animals were trained to self-administer cocaine 20 days after Drd2 shRNA treatment. Compared to scrambled shRNA treated rats, Drd2 knockdown in the VTA increased cocaine self-administration at all tested doses (0.02-0.56 mg/kg/infusion) producing an upward shift (both the ascending and descending limb) in the dose-response curve of cocaine self-administration. In contrast, intra-NAc knockdown increased cocaine self-administration only on the ascending limb of the dose-response curve (0.02-0.07 mg/kg/infusion). These data suggest that D2Rs in the VTA, not in the NAc, regulate high-dose cocaine intake. The present study not only demonstrates that low levels of D2Rs in either region increase low doses of cocaine intake, but also reveals for the first time their dissociable roles in limiting high doses of cocaine self-administration.
Keywords: Dopamine D2 autoreceptors, Drd2 knockdown, cocaine self-administration, ventral tegmental area, nucleus accumbens
Introduction
Dopamine plays an important role in drug addiction. The rewarding and reinforcing effects of drugs of abuse are mediated in part by the mesolimbic dopamine system, which includes dopamine neuron projections from the midbrain ventral tegmental area (VTA) to the nucleus accumbens (NAc) and the prefrontal cortex [1]. Dopamine signaling in the mesolimbic dopaminergic system is controlled by dopamine D1 receptors as well as dopamine D2 receptors (D2Rs) located on midbrain dopaminergic neurons (autoreceptors) and striatal medium spiny neurons (postsynaptic D2Rs). Although low availability of striatal D2Rs has long been associated with vulnerability to drug addiction [2, 3], new evidence also indicates that the levels of midbrain D2Rs are inversely correlated with impulsivity, enhanced drug reward and dopamine release [4, 5]. To date, it remains to be determined whether these two populations of D2Rs distinctly regulate drug intake. This is important because these two anatomical and neuronal segregated populations of D2Rs may regulate different aspects of addiction behavior. This study provides new insight into brain region- and D2R subtype-specific regulation of high and low doses of cocaine intake which has not been explored previously.
Dopamine D2 autoreceptors are located on the soma and dendrites of the midbrain dopamine neurons as well as on their axon terminals in various projection areas [6]. Somatodendritic autoreceptors modulate the firing rate of dopaminergic neurons, dopamine synthesis and release [7]. Activation of autoreceptors attenuates neuron excitability and diminishes dopamine release through a negative feedback mechanism [8]. Chronic exposure to psychostimulants increases dopamine neuron excitability and dopamine release which parallels reduced D2 autoreceptor function [9, 10]. Thus, altered expression and/or function of D2 autoreceptors would disrupt dopamine neuron activity and eventually influence drug-taking behavior. In rodents, vulnerability to cocaine self-administration is associated with enhanced dopamine neuron excitability resulting from reduced D2 autoreceptor function in the VTA [10–12]. In human, decreased availability of D2Rs in the midbrain parallels enhanced dopamine release in striatum and is correlated to greater impulsivity and craving for amphetamine [4]. Moreover, reduced D2R levels in mice and rats enhance dopamine release, cocaine-induced locomotor activity and conditioned place preference, and increase incentive motivation for cocaine under the progressive-ratio schedule and the salience of cocaine-paired cues [5, 13, 14]. However, it is puzzling that reduced D2R levels in the VTA of rodents do not affect fixed-ratio responding to high doses of cocaine treatment in recent reports [13, 14]. It is possible that low levels of D2 autoreceptors may cause a left-ward shift in cocaine sensitivity to low, but not high, doses of cocaine. Thus, the current study examined the effect of D2 autoreceptor knockdown in the VTA on a dose-response function of cocaine self-administration.
Striatal D2Rs are mostly located on specific subclasses of GABAergic medium spiny neurons and are coupled to Gαi/o proteins to inhibit cAMP production. Prolonged activation of striatal D2Rs in combination with D1-like receptors induced by psychostimulants leads to profound changes in the activity of many protein kinases and transcription factors that contribute to neuroplasticity [15, 16]. Repeated psychostimulant exposure persistently reduces striatal D2R levels in humans [17] and monkeys [18, 19]. Moreover, low levels of striatal D2Rs are associated with more pleasant experience to methylphenidate administration in non-drug abusers [3, 20]. It is generally considered that low levels of striatal D2Rs are inversely associated with vulnerability to drug-taking behavior in human and various animals models of drug addiction [21] . Given the known important roles of D2Rs in both the VTA and the NAc, the second goal of the present study was to examine whether these two populations of receptors distinctly regulate cocaine intake. Lentiviral gene delivery approach was utilized to knock down Drd2 mRNA levels via shRNAs in rat VTA or NAc. The knockdown effects of Drd2 mRNA in these two regions were directly compared in a dose-response function of cocaine self-administration. We found that D2Rs in these two regions have differential roles in regulation of high and low doses of cocaine intake.
Materials and Methods
Animals
Male Sprague Dawley rats (325-350 g; Charles River, Wilmington, MA) were housed in a temperature-controlled vivarium on a 12-hour reversed light/dark cycle (lights on at 6:00 PM). Rats were group-housed upon arrival and housed individually after stereotaxic surgery and catheterization. Water was available ad libitum while food access was restricted to maintain consistent body weight during the experiment. Experimental sessions were conducted during the dark phase of the light/dark cycle. Health of the rats was monitored daily by the experimenters and weekly by institutional veterinarians. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Wake Forest School of Medicine.
Production of Drd2 shRNA-containing lentiviral vectors
Four siRNA sequences targeting the rat Drd2 (accession number: NM_012547.1) were selected using Dharmacon siDESIGN software. The sequences targeting both the short and long form of rat Drd2 were: a) ccaccaactacttgatagtca, b) catcgtcactctgctggtcta, c) cttcggactcaacaatacaga, and d) caacctgaagacaccactcaa. Hairpins were designed and engineered into the shRNA expression vector psi-LVRU6GP (GeneCopoeia, Rockville, MD) using BamHI and EcoRI. The shRNA expression is driven by the U6 promoter. The scrambled siRNA sequence is GCCTATCACCGTCATAATA. Lentivirus was produced as described previously [22]. Briefly, HEK293 cells were co-transfected with the shRNA vectors, pMDLg/pRRE, pRSV-Rev and pMD2G (Addgene, Cambridge, MA) using lipofectamine 2000 (Invitrogen). Lentivirus-containing culture supernatants were collected 48 and 72 hrs after transfection, filtered through 0.45 μm cellulose acetate membrane filters and concentrated by ultracentrifugation at 100,000 × g for 1.5 hrs at 4°C. Pellets containing the lentiviral particles were suspended in the phosphate-buffered saline (PBS, pH 7.2) and stored in aliquots at −80°C. The titer of the lentivirus was 2.5×108 TU/ml determined by p24 ELISA kit (Sigma Aldrich, St. Louis, MO). The control lentivirus was generated from the lentiviral vector containing the scrambled shRNA. The specificity and efficiency of each Drd2 shRNA were validated and quantitated by the quantitative polymerase chain reaction (qPCR) in PC12 cells expressing rat Drd2 and dopamine D3 receptor (Drd3) gene which shares high sequence homology with Drd2. Because all four shRNAs knocked down the mRNA level of Drd2 by more than 50% in PC12 cells compared to the scrambled shRNA treatment, a mixture of these four shRNA vectors was used for lentiviral infection of rat brain.
Stereotaxic surgery, lentiviral microinjection and Intravenous catheter implantation
Animals were anesthetized by pentobarbital (40 mg/kg; i.p.). The lentivirus coding for Drd2 shRNAs was injected bilaterally into the VTA (1.5 μl, AP −5.2, lateral ±−0.6, ventral −8.1) or the NAc core-shell borderline region (2.0 μl, AP 1.8, lateral ±−1.6, ventral −7.4) according to the coordinates (Paxinos & Watson, 1997). Injections were carried out using a microinfusion pump (0.2 μL/min) and the injection cannulae remained in place 10 min following the injection to allow for diffusion and prevent retraction of the virus along the cannula track. Control animals were injected with scrambled shRNA. Then animals were implanted with a single intravenous catheter into the right jugular vein as described previously [23]. To maintain the patency of catheters, programed infusions (0.2 ml delivered over 6.2 s) of heparinized saline (1.7 U/ml) were administered at hourly intervals in the home cage when experiments were not conducted. Patency of catheters was evaluated at regular intervals by delivering an intravenous infusion of methohexital (10 mg/kg). If the catheter was patent, loss of consciousness would occur within 2 to 3 sec [24]. We previously showed that daily infusions of heparinized saline maintained the patent catheters for as long as 3-5 months [24, 25]. Self-administration was initiated 20 days after the catheter implantation.
Cocaine Self-Administration
Self-administration was performed during the dark phase of the light/dark cycle in operant chambers as previously described [26]. Briefly, animals were transferred from the home cages to operant conditioning chambers (24.5 × 23.5 × 21 cm) that were enclosed in sound-attenuating chambers and contained a retractable lever positioned 2.5 cm above the floor, an exhaust fan, a tone source (2.9 kHz, 45 dB), a house light, a red stimulus light directly above the retractable lever and a 20-ml syringe pump attached to the outside. Extraneous noise was masked by an exhaust fan. A counterbalanced arm was mounted to the rear corner of the operant chamber onto which the single channel swivel at the end of the rat’s leash was attached. A motor-driven 20-ml syringe pump was attached outside of the sound-attenuating chamber and polyethylene tubing was fed from the drug syringe into the operant chamber through a small hole in the outer chamber.
The animals were trained to self-administer cocaine (0.14 mg/kg/infusion). Sessions were performed daily over the course of 8-10 days. Responding was engendered under a fixed ratio 1 (FR1): time-out (20 s) schedule of 2 hrs or 35 infusions daily. Before each daily session, a 5-min blackout was followed by a priming infusion of a dose to be administered. After the priming infusion, the lever was extended and the cue light above the lever was illuminated. Upon completion of the response requirement, a 20 s time-out was initiated where a drug infusion was delivered (200 μl in 6 s), the lever was retracted, the lever lights extinguished, a tone was generated, and the house light illuminated. When the responding was stabilized, the ratio was increased to FR2 with an inter-response time contingency (IRT<10 sec). Response times on the lever had to be 10 sec or less or the FR was reset. Once acquired, the schedule for self-administration was switched to FR2: time-out 20 s schedule of three 1-hr components. The dose-response curves for cocaine self-administration were obtained in the within-session procedure using a decreasing order of dose presentation described previously [25, 27]. The cocaine dose ranges were 0.02, 0.04, 0.07, 0.14, 0.28 and 0.56 mg/kg/infusion (200 μl delivered in 6 s). The daily session was comprised of a 3-hr dose-set which included 3 adjacent cocaine doses in a descending order with one dose being accessible for an hour. The cocaine dose (mg/kg/infusion) sets were: 0.56-0.28-0.14, 0.28-0.14-0.07, 0.14-0.07-0.04 and 0.07-0.04-0.02. The order of dose presentation for each dose-set was randomized across sessions. The 3-hr segments of the daily session were separated by 10-min time-out periods followed by a priming infusion of the dose to be administered in the succeeding component. The initial dose-set used for cocaine was 0.14, 0.28 and 0.56 mg/kg/infusion. Each dose-set was repeated at least twice before moving to the next dose-set. Eighteen hours following the last self-administration session, rats were sacrificed by rapid decapitation. Each brain was removed from the skull, immediately immersed in isopentane packed in dry ice and stored at −80°C. Brains were later removed and prepared for sectioning (20 μm thickness) in a cryostat at −20°.
Quantitative Polymerase Chain Reaction (qPCR)
The VTA and the NAc were dissected from brain slices containing the respective regions according to the coordinates (Paxinos & Watson, 1997) for determination of the Drd2 mRNA levels. Total RNA was extracted using TRIZOL in a combination with linear acrylamide (Ambion, Forster City, CA). Contaminating genomic DNA was removed by DNase I digestion using DNA-free RNA kit (ZYMO Research, Irvine, CA). Quality and concentrations of RNA were examined and measured using NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific). Total RNA (20 ng) was reverse transcribed into single stranded cDNA using a high-capacity cDNA reverse transcription kit containing random primers (Applied Biosystems, Foster City, CA). The mRNA levels of Drd2 in the VTA and the NAc at various time intervals following lentiviral injections were determined by qPCR. The primers for Drd2 amplification were designed using qPCR primer design software from Integrated DNA Technology as following: 5′-CTGCTCTTCGGACTCAACAATA-3′ (forward primer) and 5′-GATGAAGGGCACGTAGAATGA-3′ (reverse primer). The qPCR was performed using All-in-One qPCR SYBR Green Master Mix (GeneCopoeia Inc., Rockville MD) in a 96-well format on an ABI PRISM 7500 Fast real-time PCR System (Applied Biosystems, Forester City, CA) as described previously [28]. The relative change in the target gene expression was analyzed using 2-ΔΔCT method as described [29]. Samples containing no cDNA template and no reverse transcriptase were run as negative controls for contamination and amplification of genomic DNA, respectively. All samples were run in triplicate. For each gene, qPCR reactions for the control and cocaine self-administration groups were run concurrently on the same 96-well plate. The mRNA levels of Drd2 were normalized to the housekeeping gene β-actin (ACTB) and calculated as relative to the control. The primers for ACTB are: 5′-ACAGGATGCAGAAGGAGATTAC-3′ (forward primer) and 5′-ACAGTGAGGCCAGGATAGA-3′ (reverse primer). We also examined the specificity of Drd2 knockdown by determining the mRNA levels of Drd3, which share a high sequence homology with Drd2. The primers for quantitating Drd3 mRNA levels are: 5′-GGTCATTGTGCTTGGAGCCTTCAT-3′ (forward primer) and 5′-GGCTTTGCGGAACTCCACATTGAA-3′ (reverse primer).
Statistics
Graph Pad Prism (version 6, La Jolla, CA, USA) was used for statistical analyses. A one-way ANOVA was used to examine the duration of Drd2 knockdown effect in the VTA. A two-way ANOVA with repeated measures was conducted to examine the effect of Drd2 knockdown in each region on the dose-response function of cocaine self-administration. Multiple comparisons were examined using a posthoc Bonferroni analysis. Student t-test was used to assess the group difference in Drd2 mRNA levels between scrambled shRNA and Drd2 shRNA-treated animals. Statistical significance was set at p<0.05.
Results
Reduced mRNA Levels of Drd2 in the VTA and the NAc by Drd2shRNAs and Cocaine
The specificity and efficiency of Drd2 were confirmed in rat PC12 cells that express endogenous Drd2 and Drd3 gene. PC12 cells were infected with lentivirus coding Drd2 Drd2 shRNA for 3 days and then qPCR was performed to determine the mRNA levels of Drd2 and Drd3 (N=5). Each Drd2 shRNA reduced Drd2 mRNA level more than 50% (posthoc Bonferroni test, p<0.01, Fig. 1a) and had no effect on Drd3 mRNA level (Fig. 1b) when compared to the treatment with scrambled shRNA or no treatment. Thus, a mixture of all four Drd2 shRNAs was used for lentiviral infection in rat brain.
Fig. 1. Validation of Drd2 knockdown in rat PC12 cells.
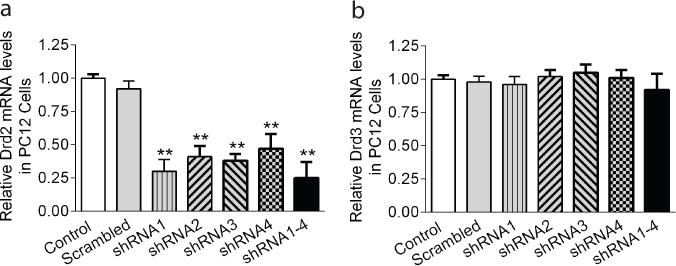
Rat PC12 cells plated in 24-well plates were infected by lentivirus containing Drd2 shRNA or scrambled shRNA for 3 days and then harvested for qPCR analysis. (a) The scrambled shRNA treatment had no effect on Drd2 mRNA level when compared to the control (no treatment). A one-way ANOVA revealed a significant main effect of treatment (F5,24=41.74, p<0.010). Bonferroni posthoc analysis indicated a significant reduction of Drd2 mRNA level in cells treated with one or the mixture of the four Drd2 shRNAs when compared to scrambled shRNA treatment (**p<0.01). (b) Drd2 shRNAs did not alter Drd3 mRNA levels (n=5).
Lentivurs was microinjected into rat VTA or NAc. To examine the efficiency and the duration of Drd2 knockdown in vivo, Drd2 mRNA levels in rat VTA were assessed 20 and 90 days after injection of lentivirus coding shRNAs against Drd2 or scrambled shRNA in the VTA. Because the Drd2 mRNA levels from the 20- (100.00 ± 9.98, N=5) and 90-day (112.26 ± 11.17, N=5) control groups (scrambled shRNA injection) did not differ (p=0.11 by student t-test), they were combined as one control group (N=10). The Drd2 levels after 20- and 90- days of Drd2 shRNA treatment were expressed as relative to the combined control. A one-way ANOVA revealed a significant main effect of group on Drd2 mRNA levels (F2,18=15.58, p<0.01). A posthoc Bonferroni test indicated that there was 30.2% ± 5.85% reduction of the Drd2 mRNA level 20 days after Drd2 shRNA treatment (N=5) when compared to scrambled shRNA treatment (Figs. 2a, N=10, p<0.05). The mRNA level of Drd2 at 90 days post-treatment with Drd2 shRNAs (N=6) was significantly lower compared to the scrambled shRNA-treated controls (p<0.01) but did not differ from that at 20 days post-treatment, suggesting that the reduced Drd2 mRNA level in the VTA by Drd2 shRNA treatment persisted at least 90 days after infection. The stability of Drd2 knockdown within 90 days provides feasibility to study the knockdown effect on cocaine self-administration which requires long duration. Drd2 knockdown efficiency was also confirmed in the animals treated with Drd2 shRNAs in the NAc. Microinjection of Drd2 shRNAs in the NAc (N=6) resulted in 25.75% ± 0.96 reduction in Drd2 mRNA level in this region 20 days post-infection when compared to their corresponding controls (N=6) (t=4,312, p<0.01; Fig. 2a). It is important to point out that intra-VTA Drd2 knockdown did not alter Drd2 mRNA level in the NAc when compared to the controls treated with scrambled shRNA 20 days after neuron infection (100.00 ± 8.32 and 104.32 ± 9.75 for control and knockdown animals, respectively). Similarly, intra-NAc Drd2 knockdown did not change Drd2 mRNA level in the VTA when compared to the controls (100.00 ±10.47 and 98.49 ± 11.28 for control and knockdown animals, respectively).
Fig. 2. Drd2 mRNA levels in scrambled shRNA and Drd2 shRNA treated animals.
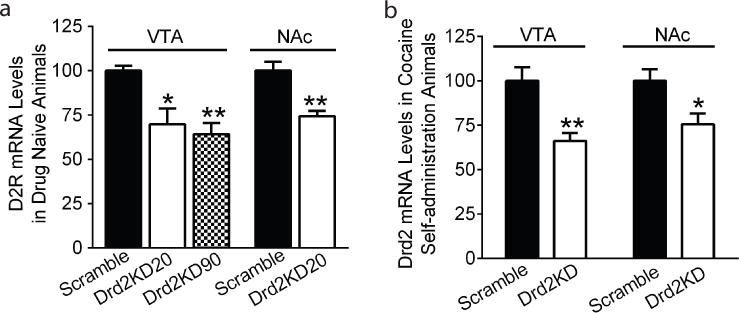
Lentivirus containing scrambled shRNA or shRNAs against rat Drd2 was bilaterally injected into the VTA or the NAc. (a) The Drd2 mRNA levels were examined 20- (Drd2KD20) and 90-days (Drd2KD90) post-treatment of Drd2shRNAs in the VTA by quantitative PCR and normalized by β-actin gene. Drd2 shRNAs significantly reduced the mRNA levels 20 days after infection and remained stable even after 90 days. (b) Cocaine self-administration itself reduced Drd2 mRNA levels in the scrambled control (Scramble) and knockdown (Drd2KD) animals in the VTA. (c) Drd2 shRNA in the NAc reduced the mRNA level and cocaine self-administration reduced Drd2 mRNA levels in both scrambled control and knockdown animals in the NAc. Data were presented as the mean Drd2 mRNA levels ± SEM (*p<0.05, **p<0.01 vs control).
We also assessed Drd2 mRNA levels in the scrambled shRNA- and Drd2 shRNA-treated animals once they completed cocaine self-administration. Animals treated with Drd2 shRNAs in the VTA (N=7) maintained significantly lower levels of Drd2 mRNA levels in the VTA when compared to their corresponding control animals (N=9) after cocaine self-administration (t=3.25, p<0.01, Fig. 2b). Similarly, animals treated with Drd2 shRNAs in the NAc (N=6) showed reduced Drd2 mRNA levels in the NAc when compared with their corresponding controls (N=6) after cocaine exposure (t=2.74, p<0.05, Fig. 2b). These data suggest that reduced Drd2 level induced by Drd2 shRNAs was sustained during cocaine self-administration. Due to the lack of saline controls, whether cocaine self-administration further reduced Drd2 mRNA levels in these Drd2 knockdown animals could not be determined.
Drd2 Knockdown in the VTA and the NAc Differentially Regulated High- and Low-dose Cocaine Self-administration
All animals treated with scrambled or Drd2 shRNAs acquired cocaine self-administration (35 infusions in 2 hrs) by day 5 and remained stable for at least 3 consecutive days. Cocaine dose-response curves were then generated. The number of cocaine infusions between the two control groups treated with scrambled shRNAs in the VTA (N=9) and the NAc (N=6) did not differ (Fig. 3a) as revealed by one-way ANOVA (F1,78=1,379, p=0.24). There was also no significant interaction effect (F5,78=0.45, p=0.81). Thus, data from these two groups were combined. The average number of cocaine infusions per hr was a bell-shaped function of cocaine doses in all groups of animals (Figure 3b). A two-way ANOVA with repeated measurement showed that there were significant main effects of group (F2,150=39.86, p<0.01), cocaine dose (F5,150=44.29, p<0.01) and interaction (F10,150=3.94, p<0.01). Although Drd2 knockdown in both the VTA (N=7) and the NAc (N=6) increased the overall number of cocaine infusions, these two groups showed different responses to high and low doses of cocaine when compared to control animals. Intra-VTA Drd2 knockdown produced greater responses to cocaine across all tested doses as reflected by an upward shift (both the ascending and descending limb) in the cocaine dose-response function (Fig. 3b). The increased cocaine responses from intra-VTA Drd2 knockdown animals were even more pronounced when the total amount of cocaine intake was plotted across the cocaine doses on the semi-log scale (Fig. 3c). A two-way ANOVA indicated a significant main effect of group (F2,150=65.0, p<0.01) and a significant interaction effect (F10,150=5,186, p<0.01). A posthoc Bonferroni test showed a significant increase in cocaine intake across all doses of cocaine. In contrast, Drd2 knockdown in the NAc resulted in a vertical shift only in the ascending limb of the cocaine dose-response curve. Compared to control animals, intra-NAc Drd2 knockdown animals self-administered more infusions of cocaine at the low end of the cocaine dose range (0.02, 0.04 and 0.07 mg/kg) and did not differ from control animals at the high end of the cocaine dose range (Fig. 3b). This observation was also reflected in the cocaine dose-intake curve, showing a greater cocaine intake at the low end of the cocaine dose range without changing the maximal response revealed by posthoc Bonferroni test (Fig. 3c).
Fig. 3. The differential effects of Drd2 knockdown in the VTA and the NAc on cocaine self-administration.
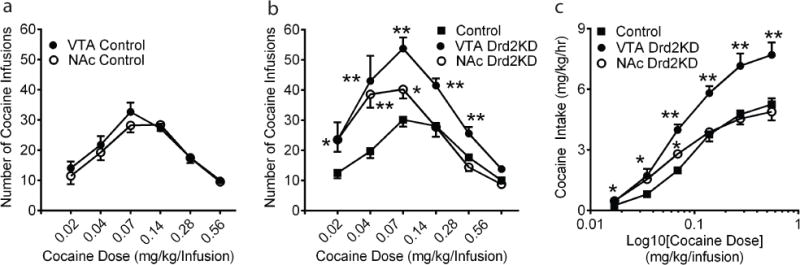
(a) The number of cocaine infusions did not differ between scrambled shRNA injection in the VTA and the NAc. Cocaine dose-infusion (b) and dose-intake (c) curves were generated for Drd2 knockdown in the VTA and in the NAc. Data were represented as the mean number of cocaine infusion/hr or the total cocaine intake (mg/kg/hr) ± SEM (*p<0.05, **p<0.01 vs. control). Fig. 3c is in the semi-logarithmic scale.
Discussion
The present study utilized a lentiviral gene knockdown approach to determine whether Drd2 mRNA levels in the VTA and the NAc differentially regulate the amount of cocaine intake. We found that reduced mRNA levels of Drd2 in both brain regions increase cocaine self-administration but in a distinct manner. D2Rs in the VTA appear to be responsible for regulating cocaine intake at relatively higher doses compared to those in the NAc.
A significant finding of the current study is that a reduced mRNA level of D2Rs in the VTA is associated with increased cocaine intake, especially at low doses, in a FR schedule of reinforcement. Our finding is consistent with the general notion that there is an inverse relationship between the level and/or function of D2Rs in the midbrain and addiction vulnerability based on a few recent reports. For example, midbrain level of D2Rs is inversely correlated with impulsivity/novelty-seeking in humans [4, 30] and rodents [31]. Moreover, conditional midbrain D2R knockout mice show enhanced progressive ratio responding for cocaine [13]. However, it is intriguing that reduced midbrain D2R levels in rodents were reported to have no effect on the number of cocaine infusions under the fixed ratio schedule in two recent studies. Specifically, midbrain D2R knockout mice did not show increased cocaine self-administration for a single unit cocaine dose (1 mg/kg/infusion for 2 hrs) under the FR1 schedule when compared to their counterpart wildtype mice [13]. Additionally, D2R knockdown in rat VTA via adeno-associated virus (AAV) failed to alter cocaine self-administration for a unit cocaine dose of 0.25 mg/infusion (approximately 0.84 mg/kg/infusion) under the FR1 reinforcement schedule for 2 hrs [14]. A major discrepancy between the previous reports and the current study was the variation in the experimental design including cocaine dosage (a unit dose vs. a range of cocaine doses). We found that lentiviral knockdown of D2Rs in the VTA resulted in more cocaine intake at all tested doses (e.g., 0.04-0.56 mg/kg/infusion for 1 hr) with one exception (0.56 mg/kg/infusion) when compared to the control treatment. The dose-response curve revealed that Drd2 knockdown in the VTA increased cocaine self-administration using doses lower than those in the references cited above (0.84 and 1 mg/kg/infusion). Thus, the dosage of cocaine is likely a significant source contributing to the discrepancy. Additionally, variation in other experimental procedures may also explain the discrepancy such as the reinforcement schedule (FR1 in 2 hrs vs. FR2 in 1 hr), the degree of Drd2 gene manipulation (knockout vs. knockdown) and the type of virus used for knockdown (adeno-associated virus vs. lentivirus). Although low striatal D2R level has long been associated with enhanced addiction vulnerability in humans and monkeys [21], the present study provided the first evidence of a direct causal effect of reduced Drd2 mRNA level in the NAc on cocaine intake in a FR schedule of reinforcement. Our finding is in agreement with recent evidence indicating that striatal Drd2 activation is critical in regulation of cocaine’s effects. For example, chemicogenetic inhibition of D2R-containing medium spiny neurons in mouse NAc enhances motivation to obtain cocaine whereas neuron activation attenuates cocaine self-administration [32]. Accordingly, overexpression of D2Rs in mouse striatal GABAergic neurons abolishes cocaine-induced hyperlocomotor activity [33]. Collectively, our data suggests that reduced Drd2 levels in both the VTA and the NAc contribute to enhanced cocaine intake, but particularly at relatively low doses. The increased cocaine intake in intra-VTA and intra-NAc Drd2 knockdown animals may suggest increased cocaine sensitivity which has been shown in Drd2 knockout mice [5]. It is important to point it out that increases in the number of cocaine infusion on the descending limb may indicate that animals are attempting to overcome diminished reinforcing effects by taking more drugs; however, this is unlikely to be the case on the ascending limb of the dose-response curve. Previous studies from our group and others indicate that competitive and noncompetitive antagonists will shift the dose-effect curve in such a manner on the descending limb for example, but generally lead to reduced or extinction of responding on the ascending portion of the curve [34, 35]. Future experiments using progressive ratio schedule of reinforcement and in vivo microdialysis would specifically address whether Drd2 knockdown in these two regions produce more or less sensitivity to cocaine reinforcement.
The second notable finding of the present study is that different parts of the dose-response curve for cocaine self-administration are susceptible to manipulation of Drd2 mRNA levels in the VTA versus the NAc. Because Drd2 knockdown in the VTA did not change the Drd2 mRNA level in the NAc and vice versa, the differential effects in cocaine intake from these two groups of animals are due to a reduction of Drd2 mRNA level in either the VTA or the NAc. In the dose-intake curve, there was an upward shift in cocaine intake (both ascending and descending limbs) across all doses of cocaine in the intra-VTA Drd2 knockdown rats when compared to their corresponding controls, indicating the increased cocaine responses in these animals. However, Drd2 knockdown in the NAc resulted in a vertical shift of the dose-intake curve showing an increase in cocaine intake for the low end of the cocaine dose range (the ascending limb of the dose-intake curve) but not for the high end (the descending limb). Thus, it appears that D2R knockdown in the NAc leads to enhanced cocaine intake. It is believed that the ascending limb of the cocaine dose-response curve is more indicative of reinforcing effects while the descending limb results from a combination of rate-decreasing effects at higher doses and pharmacokinetics [36]. Our data suggest that Drd2 in the VTA and the NAc mediate cocaine reinforcement with dissociable roles. D2Rs have been known for their role in regulation of cocaine intake at high doses although it is unclear which D2R subpopulation primarily mediates this effect. For example, the D2R antagonist eticlopride increased self-administration of high doses of cocaine (1 and 3.2 mg/kg/infusion) on the descending limb of the cocaine dose-effect curve in monkeys, rats and mice [37, 38]. Moreover, whole-body D2R knockout mice self-administered more cocaine at higher dosage than their counterpart control mice [37]. The present study indicates for the first time that D2Rs (autoreceptors) in the VTA, not D2Rs (postsynaptic D2Rs) in the NAc, plays a primary role in the regulation of high-dose cocaine intake. Although there is variation in the degree of Drd2 mRNA knockdown in the VTA and the NAc in the current study; the significant interaction (brain region × cocaine dose) suggests that the difference in Drd2 mRNA levels could not explain the differential effects of Drd2 knockdown in these two regions on low- and high-dose cocaine intake. These dissociable roles of Drd2 mRNA levels in the VTA and the NAc may be clinically relevant. It has been shown that extensive exposure to psychostimulants in human results in dysregulation of brain function in regions (e.g. NAc) that receive dopaminergic projections from the midbrain (e.g. VTA) [39, 40]. Furthermore, the availability and/or function of D2Rs in both the midbrain and the striatum are sensitive to chronic psychostimulant treatment and associated with drug reward [4, 19, 28]. Importantly, we previously found that D2Rs in the midbrain are more susceptible to changes by chronic amphetamine self-administration than D2Rs in the striatum [28]. Thus, it is possible that functional changes of D2Rs in the midbrain may precede D2Rs in the striatum during chronic exposure to cocaine. Reduced D2 receptor function in the midbrain would result in abnormal dopamine signaling which eventually leads to altered striatal D2R function. We did not observe changes in the Drd2 mRNA levels in the NAc after Drd2 shRNA was given in the VTA, which is consistent with the report on no change in striatal D2R expression when Drd2 was knocked out in mouse midbrain dopamine neurons [5]. Thus, future experiments are necessary to determine whether there are functional changes of D2Rs in the striatum when the level of D2Rs in the VTA is altered.
It is worth noting the limitation of lentiviral application in vivo to knock down Drd2 gene. Because lentivirus integrates within the genome, the lentivirus-based vector can modulate gene expression up to 6-9 months [41] and thus, this technique is useful for studies that require lengthy experimental procedures such as drug self-administration in the present study. However, the major disadvantage of lentiviral approach is the restricted, small infection area. The lentiviral particles are relatively large (>100 nm in diameter) when compared to adeno-associated virus (AAV, ~10 nm). The large size of lentiviral particles restricts their passive diffusion through the extracellular space in the brain; therefore, the infection area by lentivirus is more concentrated and much smaller compared to that from the AAV infection. Although the limited spreading of lentivirus produces more region-specific effect compared to AAV, it makes impossible to examine the effect of lentivirus on the expression and function of targeted proteins given the amount of tissues required for these assays. The second limitation of this approach is that D2R knockdown in the VTA and the NAc is not neuron-specific. Neurons in the rat VTA are heterogeneous with more than 55% being dopaminergic, 35% GABAergic and most of the remaining VTA neurons glutamatergic and cholinergic [42–44]. Although the vast majority of VTA D2Rs is located in dopaminergic neurons (D2 autoreceptors), D2Rs are also present in non-dopaminergic neurons in the VTA [45]. Similarly, neurons in the NAc are also heterogeneous with GABA, glutamate, dopamine and acetylcholine neurons [46]. Thus, future studies require applications that can selectively knock down D2Rs in dopaminergic and GABAergic neurons in the VTA and the NAc, respectively, to dissect the contributions of D2 autoreceptors from postsynaptic D2R to cocaine self-administration.
In conclusion, the present study indicates that reduced Drd2 mRNA levels in both the VTA and the NAc are a risk factor for increased cocaine intake at lower doses. However, D2Rs in the VTA, not the NAc, are responsible for regulating high-dose cocaine intake. Several Drd2 polymorphisms have been identified in humans and some of them are associated with the expression of D2Rs [47]. This study provides a strong rationale for future screening of vulnerable human subpopulation to addiction by genotyping Drd2 polymorphisms or mutations that lead to reduced Drd2 mRNA level or D2R function in the VTA and the NAc. Additionally, this study may help improve our understanding of differential pharmacological responses to D2R-based ligands for addiction treatment among human addicts with varying availability of D2Rs in the brain and provide additional justification for personalized medications to treat addiction. It is worth noting that the expression levels or function of D2Rs in the VTA and the NAc have also been associated with food reward [5, 48]. Whether the differential roles of Drd2 in the VTA and the NAc in regulating high and low doses of cocaine are applicable to other non-stimulants including food warrants further investigation.
Highlights.
Knockdown of Drd2 in the VTA increases cocaine self-administration at both low and high doses
Knockdown of Drd2 in the NAc increases self-administration of low doses of cocaine only
Drd2 in the VTA, not the NAc, is involved in mechanisms limiting high doses of cocaine intake in rats
Acknowledgments
This research was supported by National Institute of Drug Abuse grant P50DA006634 and R01DA042862.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard review of psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 4.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nature neuroscience. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 7.Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87:273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe AL, Varela E, Bettinger L, Beckstead MJ. Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology (Berl) 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- 11.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCall NM, Kotecki L, Dominguez-Lopez S, Marron Fernandez de Velasco E, Carlblom N, Sharpe AL, Beckstead MJ, Wickman K. Selective Ablation of GIRK Channels in Dopamine Neurons Alters Behavioral Effects of Cocaine in Mice. Neuropsychopharmacology. 2017;42:707–715. doi: 10.1038/npp.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holroyd KB, Adrover MF, Fuino RL, Bock R, Kaplan AR, Gremel CM, Rubinstein M, Alvarez VA. Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug-paired cues. Neuropsychopharmacology. 2015;40:1495–1509. doi: 10.1038/npp.2014.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong JW, Roelofs TJ, Mol FM, Hillen AE, Meijboom KE, Luijendijk MC, van der Eerden HA, Garner KM, Vanderschuren LJ, Adan RA. Reducing Ventral Tegmental Dopamine D2 Receptor Expression Selectively Boosts Incentive Motivation. Neuropsychopharmacology. 2015;40:2085–2095. doi: 10.1038/npp.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baik JH. Dopamine signaling in reward-related behaviors. Frontiers in neural circuits. 2013;7:152. doi: 10.3389/fncir.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with DeltaFosB as a key mediator. J Neurosci. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998;30:88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature neuroscience. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 21.Gould RW, Duke AN, Nader MA. PET studies in nonhuman primate models of cocaine abuse: translational research related to vulnerability and neuroadaptations. Neuropharmacology. 2014;84:138–151. doi: 10.1016/j.neuropharm.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. Journal of virology. 2003;77:8957–8961. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- 24.Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- 26.Pattison LP, McIntosh S, Sexton T, Childers SR, Hemby SE. Changes in dopamine transporter binding in nucleus accumbens following chronic self-administration cocaine: heroin combinations. Synapse. 2014;68:437–444. doi: 10.1002/syn.21755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh S, Sexton T, Pattison LP, Childers SR, Hemby SE. Increased Sensitivity to Cocaine Self-Administration in HIV-1 Transgenic Rats is Associated with Changes in Striatal Dopamine Transporter Binding. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2015;10:493–505. doi: 10.1007/s11481-015-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, Howlett AC, Jones SR, Chen R. Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacology. 2014;39:1833–1842. doi: 10.1038/npp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tournier BB, Steimer T, Millet P, Moulin-Sallanon M, Vallet P, Ibanez V, Ginovart N. Innately low D2 receptor availability is associated with high novelty-seeking and enhanced behavioural sensitization to amphetamine. Int J Neuropsychopharmacol. 2013;16:1819–1834. doi: 10.1017/S1461145713000205. [DOI] [PubMed] [Google Scholar]
- 32.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature neuroscience. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Martin TJ, DeMontis MG, Kim SA, Sizemore GM, Dworkin SI, Smith JE. Effects of beta-funaltrexamine on dose-effect curves for heroin self-administration in rats: comparison with alteration of [3H]DAMGO binding to rat brain sections. Drug and alcohol dependence. 1998;52:135–147. doi: 10.1016/s0376-8716(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 36.Woods J, Winger GD, France CP. Reinforcing and discriminative stimulus effects of cocaine: analysis of pharmacological mechanisms. In: Fisher RAS, Uhlenhuth EH, editors. Cocaine: clinical and biobehavioral aspects. Oxford: Oxford UP; 1987. pp. 21–65. [Google Scholar]
- 37.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods JH, Herling S, Winger G. Chlorpromazine- and haloperidol-induced changes in some behavioral effects of cocaien and amphetamine. In: Deniker R-TCP, Villeneuve A, editors. Proceedings of the 10th Congress, Collegium Internationale Neuro-Psychopharmacologicum. New York: Pergamon; 1978. pp. 1485–1502. [Google Scholar]
- 39.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- 41.Osten P, Dittgen T, Licznerski P. Lentivirus-Based Genetic Manipulations in Neurons In Vivo. In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton (FL): 2006. [Google Scholar]
- 42.Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? The Journal of physiology. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salgado S, Kaplitt MG. The Nucleus Accumbens: A Comprehensive Review. Stereotactic and functional neurosurgery. 2015;93:75–93. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- 47.Gluskin BS, Mickey BJ. Genetic variation and dopamine D2 receptor availability: a systematic review and meta-analysis of human in vivo molecular imaging studies. Translational psychiatry. 2016;6:e747. doi: 10.1038/tp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]


