Abstract
Background
Use of tobacco is the leading preventable cause of death in the United States. Racial/ethnic minorities and individuals of low socioeconomic status disproportionately experience tobacco-related disease and illness. Unique challenges and circumstances exist at each point in the cancer care continuum that may contribute to the greater cancer burden experienced by these groups.
Methods
We reviewed tobacco-related disparities from cancer prevention to cancer survivorship. We also describe research that seeks to reduce tobacco-related disparities.
Results
Racial/ethnic minorities and low-income individuals experience unique social and environmental contextual challenges such as greater environmental cues to smoke and greater levels of perceived stress and social discrimination. Clinical practice guidelines support the effectiveness of pharmacotherapy and behavioral counseling for racial and ethnic minorities, yet smoking cessation rates are lower in this group when compared with non-Hispanic whites. Superior efficacy for culturally adapted interventions has not yet been established.
Conclusions
To reduce health disparities in this population, a comprehensive strategy is needed with efforts directed at each point along the cancer care continuum. Strategies are needed to reduce the impact of contextual factors such as targeted tobacco marketing and social discrimination on smoking initiation and maintenance. Future efforts should focus on increasing the use of evidence-based cessation treatment methods and studying its effectiveness in these populations. Attention must also be focused on improving treatment outcomes by reducing smoking in diverse racial and ethnic patient populations.
Graphical abstract

Introduction
In the United States, tobacco smoking represents the leading preventable cause of death.1,2 Nearly one-third of all cancer-related deaths are attributable to tobacco use.1–3 The health burden of tobacco use is not equally distributed: Racial and ethnic minorities who smoke and have lower levels of education, income, and occupational status disproportionately experience tobacco-related disease burden and illness.4 Examples of tobacco-related disparities include exposure to tobacco smoke, current patterns of use and cessation, subsequent health consequences among specific population groups, capacity and infrastructure, and access to resources.4 Attention to tobacco-related health disparities has been growing, and the number of priority populations has extended beyond racial and ethnic minorities to include individuals of low socioeconomic status as well as sexual minorities (lesbian, gay, bisexual, and transgender).5 In addition, those in the tobacco-related health disparities field have moved beyond descriptive studies and have begun to develop and test interventions to help reduce smoking among these vulnerable populations.6
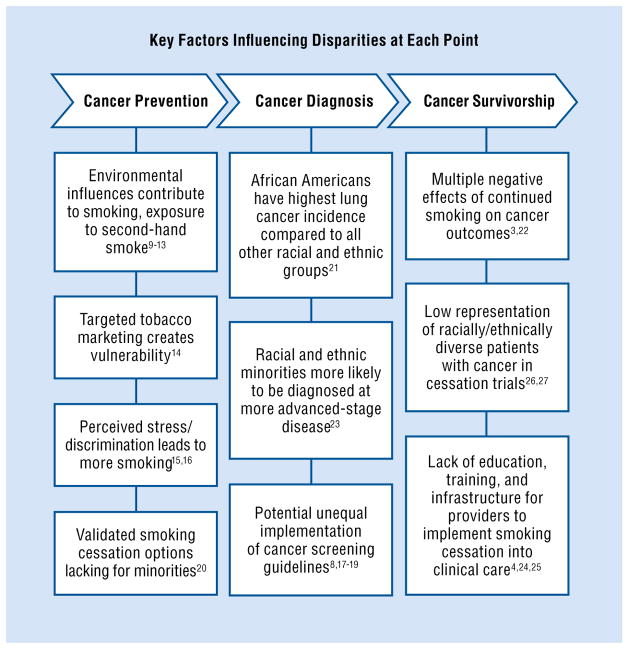
Although tobacco use contributes to the presence and exacerbation of health disparities with respect to health outcomes such as stroke, diabetes, and coronary heart disease, this paper specifically focuses on the association between tobacco use and cancer.7 Unique challenges and circumstances also exist at different points along the cancer care continuum that contribute to the larger cancer burden experienced by ethnic/racial minorities and underserved populations (Fig).3,4,8–27
Fig.
Tobacco-related health disparities exist across the cancer care continuum.
Cancer Prevention
Evidence suggests that cigarette smoking is associated with increased risks of developing oropharyngeal, laryngeal, esophageal, tracheal, bronchial, lung, stomach, liver, pancreatic, kidney, ureteral, cervical, bladder, and colorectal cancers as well as acute myeloid leukemia.1,2 Thus, decreasing rates of tobacco initiation, as well as increasing smoking cessation rates, are important steps for cancer prevention. To further our understanding of tobacco initiation and maintenance among racial/ethnic minorities and individuals of lower socioeconomic status, it is important to consider environmental and social contextual influences.9
Environmental Influences
Tobacco-control policies, such as smoking restrictions, can be effective at reducing tobacco initiation, use, and related health care costs.2 Despite progress on reducing the amount of exposure to second-hand smoke via smoke-free restrictions in public places, disparities in work-place policies can create or exacerbate tobacco-related disparities in racial/ethnic minority and under-served populations.28 For example, those who smoke and are employed in blue-collar or service occupations are more likely to work in environments that permit smoking.29 In addition to the potential health outcomes of increased exposure to second-hand smoke, less-restrictive policies may serve to maintain smoking behavior. Compared with workplaces that do not have smoking restrictions, workplaces that prohibit smoking result in fewer cigarettes smoked, greater cessation attempts, and more quitting successes among employees.30 Research also suggests that lack of self-imposed smoking restrictions in the home contributes to greater maintenance of smoking behavior, whereas the adoption of smoke-free restrictions results in greater cessation ability.31 Individuals who smoke, have a lower socioeconomic status, and are African American are less likely than non-Hispanic whites to impose smoking bans in the home and automobile; thus, environmental exposure in these settings is an additional barrier to consider.10–13 Disproportionate exposure to tobacco smoke is also a public health issue among residents of multiunit housing properties who may be exposed to second-hand smoke due to shared air between units and poor ventiliation.32 Residents of subsidized housing also experience high levels of stress, which can impact smoking behavior.33
Targeted Marketing
Another key factor that influences the initiation and maintenance of tobacco smoking among low-income and racial/ethnic minority populations is targeted tobacco marketing.2 The tobacco industry uses targeted marketing and promotions to reach ethnic/racial minorities, sexual minorities, and low-income communities.2,14 Examples of these industry efforts include a greater presence of tobacco billboards in specific neighborhoods and the targeted marketing of menthol cigarettes.14 In a systematic review, the presence and quantity of tobacco marketing at tobacco retailers were examined by neighborhood characteristics such as race, ethnicity, and socioeconomic status.14 The results suggested that lower-income neighborhoods are exposed to more tobacco marketing, and the amount of marketing of menthol products is higher in both urban neighborhoods and communities with larger numbers of African American residents.14 Among neighborhoods with a greater proportion of Hispanic residents, no observed increase in tobacco marketing was seen (possibly due to cultural reasons).2,14
Perceived Stress and Social Discrimination
Racial/ethnic minorities and low-income individuals report higher levels of perceived stress, which is defined by the degree to which individuals perceive circumstances in their life as stressful.15,34 Elevated levels of stress may can be attributed to several factors such as lower wages, more daily hassles, and stressful life events.15,35 Social discrimination is also a commonly perceived stressor among African Americans and Latinos.34 Perceived levels of stress and social discrimination have been associated with cancer burden and tobacco smoking.2 The prevalence of tobacco smoking is higher among individuals who perceive greater rates of discrimination.16 For example, compared with nonminority individuals, lesbian, gay, bisexual, and transgender individuals are more likely to experience stressors such as discrimination and stigma and have a higher smoking prevalence rate (between 24% and 45%).36 In addition, an inverse relationship has been observed between perceived levels of stress and rates of smoking cessation.37
A primary mechanism through which stress may influence smoking behavior is via negative-affect management.34 Although tobacco smoking may not actually reduce stress (except for nicotine withdrawal-related stress), if those who smoke believe that smoking reduces stress, then that belief will motivate smoking — ie, smoking is often used as a coping strategy for dealing with stress, and, as stress increases, so does smoking.38 The relationship between negative affect and smoking has been well documented.39,40 The greater perceived level of stress among racial/ethnic minorities and low-income populations, as well as the robust relationship between stress and smoking, remains an important factor to assess and consider in the development of targeted interventions for underserved persons who smoke.
Secondary Prevention
To work toward eliminating tobacco-related cancer disparities, we must add to our understanding of social and environmental factors that influence tobacco use among underserved populations so that primary prevention strategies to prevent smoking initiation may be implemented. Equally important are secondary prevention strategies that seek to develop effective smoking cessation interventions to help those who have started smoking. Several evidence-based treatments exist for treating tobacco dependence. Clinical practice guidelines from the US Public Health Service summarize these treatments and describe a brief intervention model called the 5 As8,41: ask about tobacco use, advise to quit smoking, assess interest in quitting smoking, assist in quitting smoking via counseling and pharmacotherapy, and arrange follow-up. These recommendations apply to a broad population of persons who smoke and include those from all racial and ethnic groups, individuals with low socioeconomic status, those with limited formal education, those who are hospitalized, and those who identify as lesbian, gay, bisexual, or transgender.41 Despite the applicability of these guidelines for diverse populations, the unequal implementation of these guidelines has been documented.8,17–19 Among those who smoke, Hispanics and those with low socioeconomic status are less likely to receive advice and assistance from their health care professional about smoking cessation compared with non-Hispanic whites and those with higher socioeconomic status, respectively.17–19 Access to evidence-based smoking cessation methods is also lacking for individuals with limited or no English proficiency.42 The absence of readily available, Spanish-language, evidence-based materials has been cited as a key barrier to the ability of primary care physicians to assist with smoking cessation.43
Targeted Smoking Cessation
The US Public Health Service guidelines for evidence-based smoking cessation interventions apply to all racial and ethnic minorities.8 However, important differences exist between racial/ethnic minorities and whites that warrant research on the effectiveness of smoking cessation interventions.44 The smoking prevalence rate for non-Hispanic whites (18.2%) is comparable with that seen in African Americans (17.5%) but is lower for Hispanics (11.2%).45 However, smoking prevalence varies by cultural subgroup; for example, the smoking prevalence among individuals of Puerto Rican and Cuban descent exceeds 30%.46 Moreover, racial and ethnic minorities are disproportionately represented in lower-income categories, where smoking prevalence is highest.45 Several notable differences in smoking behavior patterns also exist among different groups. Although smoking prevalences among African Americans and non-Hispanic whites are comparable, African Americans start smoking at a later age, are more likely to smoke menthol cigarettes, and smoke fewer cigarettes per day on average.3,47,48 Despite lower daily rates of smoking among Hispanics and African Americans compared with non-Hispanic whites, racial and ethnic minorities who smoke have greater difficulty quitting smoking.49
Cultural factors and acculturation must be taken into account, because those who are more acculturated are more likely to smoke and have lower odds of sustaining cessation.46 Limited interventional research has also been conducted with American Indians and Alaska Natives, who have the highest rates of tobacco use — including both ceremonial and commercial tobacco use.5,45
Benowitz et al50,51 also reports that differences may exist in the genetics and physiology (eg, how fast nicotine is metabolized) among racial and ethnic groups that warrant the evaluation of specific smoking cessation interventions among these groups, rather than assuming equivalent rates of efficacy across groups. This concept is relevant given that most clinical trials for smoking cessation medications include few minorities who smoke.50
Narrative and meta-analytic reviews of smoking cessation interventions have been conducted for racial and ethnic minorities who smoke.44,52–54 One comprehensive and narrative review included 64 studies involving African Americans, Latinos, American Indians, Asians, and Pacific Islanders who smoked.44 Pharmacotherapy, behavioral interventions, and community interventions for smoking cessation were examined. The authors concluded that existing data support the use of pharmacotherapy treatments for smoking cessation among African Americans, Latinos, and Hispanics who smoke.44 Specifically, nicotine patches, nicotine nasal sprays, and bupropion were effective in African Americans who smoke, whereas the nicotine patch alone was effective for Hispanics who smoke.44 Nicotine gum has not demonstrated efficacy in 3 studies with African Americans who smoke, and trials have not been conducted with nicotine lozenges, inhalers, or combination nicotine-replacement therapy.44
Regardless of the outcomes of efficacy trials, real-world effectiveness and use of pharmacotherapy must be considered. Qualitative research conducted by Fu et al55 with minorities who smoke (African Americans, American Indians, and Southeast Asians) revealed that knowledge is lacking about how pharmacotherapy works as well as the skepticism regarding the effectiveness of smoking cessation. These beliefs may explain the low utilization of pharmacotherapy among racial and ethnic minorities who smoke.56 Further research on the beliefs and barriers of racial and ethnic differences regarding pharmacotherapy may aid in the development of strategies to increase use of cessation methods.
Tested modalities for behavioral interventions have included both group and individual counseling.46,54 In a randomized trial, Webb et al57 found that African Americans who smoked and received group-based cognitive behavioral therapy to help them quit smoking had higher cessation rates than African Americans who smoked and were involved in a general health education group that controlled for contact and intensity. In addition to support for group therapy, researchers concluded that individual counseling (in-person and on the telephone) was effective for aiding smoking cessation among racial and ethnic minorities.44
Some research has focused on the development of culturally adapted smoking cessation interventions that take into account unique cultural characteristics.44 However, little research has been conducted to evaluate the effectiveness of culturally adapted or culturally specific interventions compared with generic interventions. Select studies conducted with African Americans who smoke have been mixed.20,58–62 One study found evidence of a greater level of interest and attention for culturally specific materials, yet readiness to quit and cessation attempts were greater for the standard materials.58 By contrast, a study conducted with African Americans who smoke found greater rates of smoking abstinence at a 12-month follow-up period among participants who received culturally tailored materials than those who received standard materials.59 Other studies have also demonstrated the benefits of culturally tailored interventions for Hispanics who smoke.60,61 Overall, researchers have reported that study participants are generally accepting of interventional materials that include information about cultural adaptation, yet additional research is needed to determine whether such cultural tailoring is superior to standard materials to improve smoking cessation rates among minorities.20,58,62
Cancer Diagnosis
Higher rates of cancer morbidity and mortality are evident among racial/ethnic minorities and those with low socioeconomic status.21 Several pathways exist in which the relationship between tobacco and cancer health disparities can present. A higher risk of tobacco-related cancers among persons who smoke and have low incomes could, in part, be a function of higher smoking prevalence rates. Although the overall prevalence of smoking has declined to 16.8%, low-income individuals who smoke (ie, household income below the poverty level) have a smoking prevalence rate of 30.4%.63 Other pathways are less clear. Referred to as the “smoker’s paradox,” African Americans smoke fewer cigarettes per day and are less likely to smoke daily, yet they have higher rates of lung, esophageal, and oral cancers than non-Hispanic whites who smoke.64
Disparities in Lung Cancer
Lung cancer is the leading cause of cancer-related deaths in the United States, and 80% of these deaths are attributable to cigarette smoking.2 African Americans have the highest lung cancer incidence and mortality rates compared with all other racial and ethnic groups.3,21 Risk factors for poor cancer outcomes have been attributed to several factors such as treatment access and lower socioeconomic status, as well as smoking-related factors.22 The disproportionate burden in lung cancer risk among African Americans cannot be explained by a greater number of cigarettes smoked.64 However, a distinguishing smoking behavior among African Americans who smoke, which may aid in explaining disparate disease outcomes, is that African Americans are more likely to smoke menthol cigarettes than whites.3 As part of the 2009 Family Smoking Prevention and Tobacco Control Act, the Tobacco Products Scientific Advisory Committee of the US Food and Drug Administration was tasked with submitting a report on the impact of menthol.65 Findings from that report suggest that little evidence exists to demonstrate that menthol contributes to greater disease risk; however, menthol cigarettes may have more addictive qualities than nonmenthol cigarettes, which could contribute to low rates of smoking cessation.3,65 Menthol also has cooling and anesthetic effects that reduce the harshness of cigarette smoke.65 Use of menthol cigarettes is also associated with increased initiation and progression to cigarette smoking and difficulty quitting smoking.65 Based on these findings, the Tobacco Products Scientific Advisory Committee concluded that no longer selling menthol cigarettes would be of a public health benefit.65 However, more research is needed to further examine the potential role that menthol plays in the unequal disease burden experienced by African Americans.3 Specifically, as noted by Alexander et al,64 research is needed on examining additional pathways such as the role of taste sensitivity and the effects of menthol on cellular mechanisms and subsequent disease processes.
Role of Beliefs About Lung Cancer
Research suggests that beliefs about lung cancer may differ by race/ethnicity. Compared with whites, African Americans have lower perceived notions of risk for lung cancer and are more likely to doubt the relationship between lung cancer and behavior.66,67 African Americans also have demonstrated greater skepticism regarding surgical treatment for lung cancer.68,69 Moreover, the belief that exposure to air during surgery can cause the tumor to spread and fatalistic beliefs (eg, “If I have lung cancer, I believe it was meant to be”) may serve as barriers to accepting surgical intervention among racial and ethnic minorities.68,69 These findings underscore the need to understand perceptions of lung cancer by race/ethnicity to identify potential barriers to smoking cessation and lung cancer treatment.
Challenges in Screening
Based on evidence from the National Lung Cancer Screening Trial, low-dose computed tomography for lung cancer screening provides an opportunity to reduce the rate of lung cancer mortality.70 A concern raised by Holford et al71 is that the 30 pack-years’ eligibility criteria required by the US Preventive Services Task Force and Centers for Medicare & Medicaid Services may result in fewer screenings for African American men who smoke and have fewer pack years. African Americans have also been found to be more reluctant to get screened for lung cancer due to fear of the disease.66 Thus, it is possible that unequal implementation of newer screening modalities and heightened fears of disease may broaden cancer disparities.
Head and Neck Cancers
Tobacco use is a major risk factor for the development of head and neck cancers: 75% of all head and neck cancers are attributable to tobacco and alcohol use.72 Molina et al23 examined survival rates of head and neck cancers based on race/ethnicity and socioeconomic status. Their findings suggested that, compared with non-Hispanic whites, African Americans were diagnosed with head and neck cancers at a younger age and at more advanced-disease stages.23 In addition, the average survival time for African American patients was significantly lower than whites (21 vs 40 months).23 Poor survival outcomes in African Americans were observed despite the lack of difference in the frequency of smoking. Living in an area of high poverty was found to be an independent predictor of worse outcomes.73 Poor cancer outcomes among patients from impoverished communities has also been attributed to lack of access to health care, decreased likelihood of insurance coverage, and advanced disease at presentation.73 Among Hispanics and Latinos, incidence and mortality rates of head and neck cancers are lower than both African Americans and non-Hispanic whites.74
Cancer Survivorship
Once an individual who smokes has been diagnosed with cancer, he or she may mistakenly believe that it is too late to quit smoking; however, a growing body of research suggests that patients with cancer who continue to smoke are at risk for several adverse health outcomes.1 The 2014 report of the US Surgeon General concluded that sufficient evidence has accumulated to infer a causal relationship between individuals with cancer who smoke cigarettes and cancer survivors for both all-cause mortality and cancer-specific mortality.1 Evidence also suggests that continued smoking results in an increased risk for second primary cancers known to be caused by cigarette smoking, and, although this evidence is insufficient to infer a causal relationship, an association does exist between cigarette smoking and risk of cancer recurrence, poor response to treatment, and increased rates of cancer treatment-related toxicity.1 Because racial and ethnic minorities are more likely to be diagnosed with late-stage tobacco-related cancers, it is possible that they could be particularly vulnerable to the adverse effects of continued smoking. Park et al22 reported that smoking cessation represents a modifiable risk factor that could result in better treatment outcomes in African Americans with lung cancer by improving rates of treatment efficacy and quality of life. Given the strong associations between continued smoking and cancer outcomes, organizations such as the American Society of Clinical Oncology and American Association for Cancer Research have called for smoking cessation assistance to be provided to cancer survivors.75 The National Comprehensive Cancer Network also established practice guidelines for smoking cessation that call for all patients with cancer to receive evidence-based smoking cessation interventions.76
Implementation of the 5As model for a brief cessation intervention has been suboptimal in the oncology setting.8,24,25,77 Although the “ask” and “advise” steps are often implemented, the remaining 3 steps are not routinely completed.8,24 A review of members of the American Society of Clinical Oncology reported that 44% of its members surveyed said they discuss medication options for smoking cessation, and 39% said they provided smoking cessation treatment.24 These findings coincide with patient reports of low levels of cessation assistance provided by oncology specialists.77 Because racial and ethnic minorities who smoke are generally less likely to receive assistance from their physicians in quitting smoking, it is plausible that this assistance may also not occur in the oncology setting.18,19 Perceptions of inadequate training in tobacco cessation interventions and low rates of self-efficacy in helping patients to quit have been cited barriers to cessation support by health care professionals.24 These findings suggest a need for improved education and training of health care professionals to better convey the rationale for smoking cessation and to develop strategies to implement smoking cessation into clinical care. In the absence of health care professional training, and taking into consideration the constraint of time, an alternative ask–advise–refer model may be more feasible to implement.25 This model involves asking about tobacco use, advising the patient to quit, and then referring the patient to existing, evidence-based resources (eg, national tobacco quit line [800-QUIT-NOW]).
Despite the importance of quitting smoking for individuals with cancer, few clinical trials of smoking cessation have been conducted in this population. In a systematic review and meta-analysis, 10 randomized controlled trials and 3 prospective cohort studies were evaluated.26 Studies included a range of nonpharmacological (eg, counseling, self-help) and pharmacological (eg, nicotine-replacement therapy) interventions.26 Overall, findings indicated that tobacco cessation interventions in the oncology setting were not significantly different from usual care, suggesting the need for additional research to enhance smoking cessation among patients with cancer.26 Although a single smoking cessation trial demonstrated feasibility of recruiting African American patients, most smoking cessation trials conducted in the oncology setting have had low representation of racially and ethnically diverse patients.26,27 To our knowledge, cessation interventions are not available and have not been tested in Spanish-speaking patients. Thus, as new interventions are developed and tested in the oncology setting, the inclusion of racial and ethnic minorities is imperative to extend the generalizability of findings and reduce tobacco-related cancer disparities.
Conclusions
Racial and ethnic minorities experience shared social and environmental contextual factors that impact the initiation and maintenance of smoking, and the potential for tobacco-related health disparities exist at each point of the oncology care continuum. Thus, a comprehensive strategy for reducing health disparities must call for future research efforts at each of these points.
Health disparities exist in the incidence and mortality of the 2 most strongly associated tobacco-related cancers, namely lung and head and neck cancers.2,3,21,23,72–74 Future research is needed to elucidate the causes of these disparities. As new cancer screening tools become available, attention must be paid to prevent the widening of health disparities due to unequal implementation.
Overall rates of smoking cessation tend to be lower among racial and ethnic minorities.46,49 Culturally adapted interventions are more acceptable44,46; however, research has not yet supported their effectiveness when compared with standard materials.55,61 Future research efforts should be directed at increasing the use of treatment and greater rates of efficacy in these vulnerable populations. Individuals with cancer must also be provided with cessation assistance and interventions aimed at helping them quit smoking to improve cancer outcomes.75,76 Additional education and training are needed for oncologists so they can begin to understand the impact of continued smoking for patients as well as to increase their comfort level for providing smoking cessation treatment to their patients who need it.25 Due to the disproportionate disease burden experienced by African Americans and other underserved populations who smoke, future trials on smoking cessation interventions must include diverse racial and ethnic patient populations and those cessation tools should be validated for use in all vulnerable patient populations.3,26,27
Acknowledgments
Dr Brandon receives grants and research support from Pfizer and is a consultant for Voxiva and the Australian General Solicitor. The other authors report no significant relationships with the companies/organizations whose products or services may be referenced in this article.
References
- 1.US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years Of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; [Accessed August 19, 2016]. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures for African Americans 2016–2018. Atlanta, GA: American Cancer Society; [Accessed August 19, 2016]. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-047403.pdf. [Google Scholar]
- 4.Fagan P, King G, Lawrence D, et al. Eliminating tobacco-related health disparities: directions for future research. Am J Public Health. 2004;94(2):211–217. doi: 10.2105/ajph.94.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Surgeon General. Tobacco use among U.S. racial/ethnic minority groups--African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. Executive summary. MMWR Recomm Rep. 1998;47(RR-18):v–xv. 1–16. [PubMed] [Google Scholar]
- 6.Okuyemi KS, Reitzel LR, Fagan P. Interventions to reduce tobaccorelated health disparities. Nicotine Tob Res. 2015;17(8):887–891. doi: 10.1093/ntr/ntv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrett BE, Dube SR, Winder C, et al. Cigarette smoking — United States, 2006–2008 and 2009–2010. MMWR Suppl. 2013;62(3):81–84. [PubMed] [Google Scholar]
- 8.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline: Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; Public Health Service; 2008. [Accessed August 19, 2016]. http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. [Google Scholar]
- 9.Garrett BE, Dube SR, Babb S, et al. Addressing the social determinants of health to reduce tobacco-related disparities. Nicotine Tob Res. 2015;17(8):892–897. doi: 10.1093/ntr/ntu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honjo K, Tsutsumi A, Kawachi I, et al. What accounts for the relationship between social class and smoking cessation? Results of a path analysis. Soc Sci Med. 2006;62(2):317–328. doi: 10.1016/j.socscimed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Muilenburg Legge J, Latham T, Annang L, et al. The home smoking environment: influence on behaviors and attitudes in a racially diverse adolescent population. Health Educ Behav. 2009;36(4):777–793. doi: 10.1177/1090198109339461. [DOI] [PubMed] [Google Scholar]
- 12.Norman GJ, Ribisl KM, Howard-Pitney B, et al. The relationship between home smoking bans and exposure to state tobacco control efforts and smoking behaviors. Am J Health Promot. 2000;15(2):81–88. doi: 10.4278/0890-1171-15.2.81. [DOI] [PubMed] [Google Scholar]
- 13.Mills AL, White MM, Pierce JP, et al. Home smoking bans among U.S. households with children and smokers. Opportunities for intervention. Am J Prev Med. 2011;41(6):559–565. doi: 10.1016/j.amepre.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Lee JG, Henriksen L, Rose SW, et al. A systematic review of neighborhood disparities in point-of-sale tobacco marketing. Am J Public Health. 2015;105(9):e8–e18. doi: 10.2105/AJPH.2015.302777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb Hooper M, Kolar SK. Distress, race/ethnicity and smoking cessation in treatment-seekers: implications for disparity elimination. Addiction. 2015;110(9):1495–1504. doi: 10.1111/add.12990. [DOI] [PubMed] [Google Scholar]
- 16.Purnell JQ, Peppone LJ, Alcaraz K, et al. Perceived discrimination, psychological distress, and current smoking status: results from the Behavioral Risk Factor Surveillance System Reactions to Race module, 2004–2008. Am J Public Health. 2012;102(5):844–851. doi: 10.2105/AJPH.2012.300694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruger J, Shaw L, Kahende J, et al. Health care providers’ advice to quit smoking, National Health Interview Survey, 2000, 2005, and 2010. Prev Chronic Dis. 2012;9:E130. doi: 10.5888/pcd9.110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Quintero C, Crum RM, Neumark YD. Racial/ethnic disparities in report of physician-provided smoking cessation advice: analysis of the 2000 National Health Interview Survey. Am J Public Health. 2006;96(12):2235–2239. doi: 10.2105/AJPH.2005.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenfeld N, Schappert SM, Lin SX. Racial and ethnic differences in delivery of tobacco-cessation services. Am J Prev Med. 2009;36(1):21–28. doi: 10.1016/j.amepre.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Simmons VN, Quinn G, Litvin EB, et al. Transcreation of validated smoking relapse-prevention booklets for use with Hispanic populations. J Health Care Poor Underserved. 2011;22(3):886–893. doi: 10.1353/hpu.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howlader N, Noone AM, Krapcho M, et al., editors. [Accessed August 3, 2016];SEER cancer statistics review, 1975–2012. http://seer.cancer.gov/csr/1975_2012. Based on November 2014 SEER data submission, posted April 2015.
- 22.Park ER, Japuntich SJ, Traeger L, et al. Disparities between blacks and whites in tobacco and lung cancer treatment. Oncologist. 2011;16(10):1428–1434. doi: 10.1634/theoncologist.2011-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113(10):2797–2806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- 24.Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: a survey of American Society of Clinical Oncology members. J Oncol Pract. 2013;9(5):258–262. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder SA. What to do with a patient who smokes. JAMA. 2005;294(4):482–487. doi: 10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- 26.Nayan S, Gupta MK, Strychowsky JE, et al. Smoking cessation interventions and cessation rates in the oncology population: an updated systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2013;149(2):200–211. doi: 10.1177/0194599813490886. [DOI] [PubMed] [Google Scholar]
- 27.Martinez E, Tatum KL, Weber DM, et al. Issues related to implementing a smoking cessation clinical trial for cancer patients. Cancer Causes Control. 2009;20(1):97–104. doi: 10.1007/s10552-008-9222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moolchan ET, Fagan P, Fernander AF, et al. Addressing tobacco-related health disparities. Addiction. 2007;102(suppl 2):30–42. doi: 10.1111/j.1360-0443.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- 29.Aakko E, Schafer E, Gyarmathy VA, et al. Smoking policies in manufacturing and assembly workplaces, Wisconsin, 1999. WMJ. 2001;100(3):67–69. [PubMed] [Google Scholar]
- 30.Glasgow RE, Cummings KM, Hyland A Community Intervention Trial for Smoking Cessation. Relationship of worksite smoking policy to changes in employee tobacco use: findings from COMMIT. Tob Control. 1997;6(suppl 2):S44–S48. doi: 10.1136/tc.6.suppl_2.s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizacani BA, Martin DP, Stark MJ, et al. A prospective study of household smoking bans and subsequent cessation related behaviour: the role of stage of change. Tob Control. 2004;13(1):23–28. doi: 10.1136/tc.2003.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraev TA, Adamkiewicz G, Hammond SK, et al. Indoor concentrations of nicotine in low-income, multi-unit housing: associations with smoking behaviours and housing characteristics. Tob Control. 2009;18(6):438–444. doi: 10.1136/tc.2009.029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews JO, Mueller M, Newman SD, et al. The association of individual and neighborhood social cohesion, stressors, and crime on smoking status among African-American women in southeastern US subsidized housing neighborhoods. J Urban Health. 2014;91(6):1158–1174. doi: 10.1007/s11524-014-9911-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendzor DE, Businelle MS, Reitzel LR, et al. The influence of discrimination on smoking cessation among Latinos. Drug Alcohol Depend. 2014;136:143–148. doi: 10.1016/j.drugalcdep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skinner ML, Shirtcliff EA, Haggerty KP, et al. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011;23(4):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grady ES, Humfleet GL, Delucchi KL, et al. Smoking cessation outcomes among sexual and gender minority and nonminority smokers in extended smoking treatments. Nicotine Tob Res. 2014;16(9):1207–1215. doi: 10.1093/ntr/ntu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg CJ, Thomas JL, Guo H, et al. Predictors of smoking reduction among Blacks. Nicotine Tob Res. 2010;12(4):423–431. doi: 10.1093/ntr/ntq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landrine H, Klonoff EA. Racial segregation and cigarette smoking among blacks: findings at the individual level. Journal Health Psychol. 2000;5(2):211–219. doi: 10.1177/135910530000500211. [DOI] [PubMed] [Google Scholar]
- 39.Brandon TH. Negative affect as motivation to smoke. Curr Dir Psychol Sci. 1994;3(2):33–37. [Google Scholar]
- 40.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 41.Fiore MC, Jaén CR. A clinical blueprint to accelerate the elimination of tobacco use. JAMA. 2008;299(17):2083–2085. doi: 10.1001/jama.299.17.2083. [DOI] [PubMed] [Google Scholar]
- 42.Wetter DW, Mazas C, Daza P, et al. Reaching and treating Spanish-speaking smokers through the National Cancer Institute’s Cancer Information Service. A randomized controlled trial. Cancer. 2007;109(2 suppl):406–413. doi: 10.1002/cncr.22360. [DOI] [PubMed] [Google Scholar]
- 43.Blumenthal DS. Barriers to the provision of smoking cessation services reported by clinicians in underserved communities. J Am Board Fam Med. 2007;20(3):272–279. doi: 10.3122/jabfm.2007.03.060115. [DOI] [PubMed] [Google Scholar]
- 44.Cox LS, Okuyemi K, Choi WS, et al. A review of tobacco use treatments in U.S. ethnic minority populations. Am J Health Promot. 2011;25(5 suppl):S11–S30. doi: 10.4278/ajhp.100610-LIT-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 46.Merzel CR, Isasi CR, Strizich G, et al. Smoking cessation among U.S. Hispanic/Latino adults: findings from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Prev Med. 2015;81:412–419. doi: 10.1016/j.ypmed.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 48.Trinidad DR, Gilpin EA, Lee L, et al. Do the majority of Asian-American and African-American smokers start as adults? Am J Prev Med. 2004;26(2):156–158. doi: 10.1016/j.amepre.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Irvin Vidrine J, Reitzel LR, Wetter DW. The role of tobacco in cancer health disparities. Curr Oncol Rep. 2009;11(6):475–481. doi: 10.1007/s11912-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benowitz NL. Smoking cessation trials targeted to racial and economic minority groups. JAMA. 2002;288(4):497–499. doi: 10.1001/jama.288.4.497. [DOI] [PubMed] [Google Scholar]
- 51.Benowitz NL, Perez-Stable EJ, Herrera B, et al. Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94(2):108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence D, Graber JE, Mills SL, et al. Smoking cessation interventions in U.S. racial/ethnic minority populations: an assessment of the literature. Prev Med. 2003;36(2):204–216. doi: 10.1016/s0091-7435(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 53.Robles GI, Singh-Franco D, Ghin HL. A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations. Clin Ther. 2008;30(5):800–812. doi: 10.1016/j.clinthera.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Webb MS. Treating tobacco dependence among African Americans: a meta-analytic review. Health Psychol. 2008;27(3 suppl):S271–S282. doi: 10.1037/0278-6133.27.3(suppl.).s271. [DOI] [PubMed] [Google Scholar]
- 55.Fu SS, Burgess D, van Ryn M, et al. Views on smoking cessation methods in ethnic minority communities: a qualitative investigation. Prev Med. 2007;44(3):235–240. doi: 10.1016/j.ypmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Levinson AH, Perez-Stable EJ, Espinoza P, et al. Latinos report less use of pharmaceutical aids when trying to quit smoking. Am J Prev Med. 2004;26(2):105–111. doi: 10.1016/j.amepre.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Webb MS, de Ybarra DR, Baker EA, et al. Cognitive-behavioral therapy to promote smoking cessation among African American smokers: a randomized clinical trial. J Consult Clin Psychol. 2010;78(1):24–33. doi: 10.1037/a0017669. [DOI] [PubMed] [Google Scholar]
- 58.Webb MS. Culturally specific interventions for African American smokers: an efficacy experiment. J Natl Med Assoc. 2009;101(9):927–935. doi: 10.1016/s0027-9684(15)31041-5. [DOI] [PubMed] [Google Scholar]
- 59.Orleans CT, Boyd NR, Bingler R, et al. A self-help intervention for African American smokers: tailoring cancer information service counseling for a special population. Prev Med. 1998;27(5 pt 2):S61–S70. doi: 10.1006/pmed.1998.0400. [DOI] [PubMed] [Google Scholar]
- 60.Nevid JS, Javier RA. Preliminary investigation of a culturally specific smoking cessation intervention for Hispanic smokers. Am J Health Promot. 1997;11(3):198–207. doi: 10.4278/0890-1171-11.3.198. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez Esquivel D, Webb Hooper M, Baker EA, et al. Culturally specific versus standard smoking cessation messages targeting Hispanics: an experiment. Psychol Addict Behav. 2015;29(2):283–289. doi: 10.1037/adb0000044. [DOI] [PubMed] [Google Scholar]
- 62.Simmons VN, Cruz LM, Brandon TH, et al. Translation and adaptation of smoking relapse-prevention materials for pregnant and postpartum Hispanic women. J Health Commun. 2011;16(1):90–107. doi: 10.1080/10810730.2010.529492. [DOI] [PubMed] [Google Scholar]
- 63.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 64.Alexander LA, Trinidad DR, Sakuma KL, et al. Why we must continue to investigate menthol’s role in the African American smoking paradox. Nicotine Ton Res. 2016;18(suppl 1):S91–S101. doi: 10.1093/ntr/ntv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tobacco Products Scientific Advisory Committee. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. Bethesda, MD: US Food and Drug Administration; 2011. [Accessed August 3, 2016]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/UCM269697.pdf. [Google Scholar]
- 66.Lathan CS, Okechukwu C, Drake BF, et al. Racial differences in the perception of lung cancer: the 2005 Health Information National Trends Survey. Cancer. 2010;116(8):1981–1986. doi: 10.1002/cncr.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park ER, Ostroff JS, Rakowski W, et al. Risk perceptions among participants undergoing lung cancer screening: baseline results from the National Lung Screening Trial. Ann Behav Med. 2009;37(3):268–279. doi: 10.1007/s12160-009-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George M, Margolis ML. Race and lung cancer surgery--a qualitative analysis of relevant beliefs and management preferences. Oncol Nurs Forum. 2010;37(6):740–748. doi: 10.1188/10.ONF.740-748. [DOI] [PubMed] [Google Scholar]
- 69.Jonnalagadda S, Bergamo C, Lin JJ, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer. 2012;77(3):526–531. doi: 10.1016/j.lungcan.2012.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holford TR, Levy DT, Meza R. Comparison of smoking history patterns among African American and white cohorts in the United States born 1890 to 1990. Nicotine Tob Res. 2016;18(suppl 1):S16–S29. doi: 10.1093/ntr/ntv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–3287. [PubMed] [Google Scholar]
- 73.Conway DI, Petticrew M, Marlborough, et al. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int J Cancer. 2008;122(12):2811–2819. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- 74.Fagan P, Moolchan ET, Lawrence D, et al. Identifying health disparities across the tobacco continuum. Addiction. 2007;102(suppl 2):5–29. doi: 10.1111/j.1360-0443.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 75.Toll BA, Brandon TH, Gritz ER, et al. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19(8):1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Comprehensive Cancer Network. [Accessed August 4, 2016];NCCN Clinical Practice Guidelines in Oncology: Smoking Cessation. v.2.2015. https://www.nccn.org/professionals/physician_gls/pdf/smoking.pdf.
- 77.Simmons VN, Litvin EB, Unrod M, et al. Oncology healthcare providers’ implementation of the 5A’s model of brief intervention for smoking cessation: patients’ perceptions. Patient Educ Couns. 2012;86(3):414–419. doi: 10.1016/j.pec.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]