Abstract
Chemotherapy associated cardiomyopathy is a well known cardiotoxicity of contemporary cancer treatment and a cause of increasing concern for both cardiologists and oncologists. As cancer outcomes improve, cardiovascular disease has become a leading cause of morbidity and mortality among cancer survivors. Asymptomatic or symptomatic left ventricular systolic dysfunction in the setting of cardiotoxic chemotherapy is an important entity to recognize. Early diagnosis of cardiac injury through the use of novel blood based biomarkers or noninvasive imaging modalities may allow for the initiation of cardioprotective medications or modification of chemotherapy regimen in order to minimize or prevent further damage. Several clinical trials are currently underway to determine the efficacy of cardioprotective medications for the prevention of chemotherapy associated cardiomyopathy. Implementing a strategy that includes both early detection and prevention of cardiotoxicity will likely have a significant impact on the overall prognosis of cancer survivors. Continued coordination of care between cardiologists and oncologists remains critical to maximizing the oncologic benefit of cancer therapy while minimizing any early or late cardiovascular effects.
Keywords: cardiotoxicity, chemotherapy, congestive heart failure
Introduction
The landscape of cancer care has evolved over the past 20 years with the development of more aggressive cancer screening programs, improvements in diagnostic testing, and more effective treatment options. As a result, cancer death rates have declined 20% from 1991 (215.1 per 100,000 population) to 2009 (173.1 per 100,000 population) and the population of cancer survivors is projected to increase to nearly 18 million by 2022.(1) What has become clear, however, is that the benefit of many successful anticancer therapies is attenuated by adverse cardiotoxic effects. As cancer survivorship increases in the new era of improved chemotherapeutics, competing cardiac causes of morbidity and mortality will have a significant impact on long-term patient outcomes. This is an area of growing concern for both oncologists and cardiologists and has led to the development of a new field of cardio-oncology which focuses on the treatment and prevention of cardiovascular disease among cancer patients.
Chemotherapy associated cardiomyopathy is a well known cardiotoxicity and is the primary focus of this review. A list of chemotherapeutic agents associated with cardiomyopathy is summarized in Table 1. Anthracyclines are among the oldest chemotherapeutic agents, and their cardiotoxic effects have been studied for over 30 years.(2–4) Several other classes of chemotherapeutic agents have also been identified to cause significant cardiac toxicity, including alkylating agents, tyrosine kinase inhibitors, antimicrotubule agents, and monoclonal antibody-based targeted therapies.
Table 1.
Chemotherapeutic agents associated with cardiomyopathy
| Chemotherapeutic agent | Incidence (%)(84) | Proposed mechanism of action | Comments |
|---|---|---|---|
|
| |||
| Anthracyclines | |||
| Doxorubicin | 3–26 | Free radical formation and increased oxidative stress, leading to apoptosis and cell death; potentially mediated by topoisomerase-II β(70) | Acute cardiotoxicity, a rare complication, occurs immediately after infusion (<1%). Chronic cardiotoxicity can first be detected many years after exposure but not uncommonly occurs within the first year of treatment. Risk is dose dependent and increases with cumulative dosing > 400mg/m2. |
| Liposomal | 4–13 (62, 85, 86) | ||
| Epirubicin | 0.9–3.3 | ||
| Idarubicin | 5–18 | ||
|
| |||
| Alkylating agents | |||
| Cyclophosphamide | 7–28 | Increase in free oxygen radicals; direct endothelial injury (87) | Acute cardiac toxicity is associated with high dose conditioning regimens (120–180mg/kg) commonly used for bone marrow transplantation. |
| Ifosfamide | 17 | ||
|
| |||
| Monoclonal antibodies | |||
| Trastuzumab | 2–28 | Inhibition of ERBB2 signaling, activation of mitochondrial apoptotic pathway; impaired cardiac repair pathways | Associated risk factors include anthracycline exposure, age, and baseline LVEF.(88) |
| Bevacizumab (Avastin®) | 1.7–3 | Anti-angiogenesis | CHF reported among patients with metastatic breast cancer treated with prior anthracycline.(89) |
|
| |||
| Tyrosine kinase inhibitors | |||
| Abl Kinase Inhibitors | |||
| Imatinib | 0.5–1.7 | ABL kinase inhibition and mitochondrial dysfunction | Most commonly seen in elderly patients with underlying cardiac risk factors (e.g. diabetes, hypertension, coronary artery disease, and arrhythmia)(91) |
| Dasatanib | 2–4 | ||
| Multi-kinase Inhibitors | |||
| Sunitinib | 15–20 (90) | Off-target kinase inhibition | Cardiomyopathy may be exacerbated by sunitinib induced hypertension(92) |
| ERBB2 inhibitors | |||
| Lapatinib | 1.5–2.2 | Inhibition of ERBB2 and EGFR | Lower incidence of cardiomyopathy and heart failure compared to trastuzumab(93) |
|
| |||
| Antimicrotubules | |||
| Docetaxel (Taxotere) | 2.3–8 | Increased microtubule density leading to contractile dysfunction(94) | Potentiates the cardiotoxicity of anthracyclines when given concurrently |
| Paclitaxel | --- | Histamine release; induction of myocyte damage by affecting subcellular organelles(87) | |
|
| |||
| Proteasome inhibitors | |||
| Bortezomib | 2–5 | Interference with the ubiquitin proteasome system, resulting in accumulation of toxic proteins within cardiomyocytes | --- |
| Carfilzomib | 4(95) | ||
Attempts to develop improved strategies for the diagnosis of cardiotoxicity beyond measurement of left ventricular ejection fraction (LVEF) have been a major focus of recent investigation. Biomarkers and non-invasive imaging modalities [i.e. tissue Doppler imaging, speckle tracking strain echocardiography, and cardiac magnetic resonance imaging (MRI)] have been proposed for the early detection of cardiotoxicity. Small clinical trials have shown modest success with the use of standard heart failure pharmacotherapy, including beta blockers and angiotensin converting enzyme inhibitors (ACE-I), to prevent left ventricular (LV) dysfunction associated with cancer therapy. However there remains no clear consensus on the appropriate use of these therapies in the cancer setting. We will review the current evidence relating to the early detection, treatment, and prevention of cancer therapy associated cardiomyopathy.
Clinical Criteria for Chemotherapy Associated Cardiomyopathy
The term “cardiotoxicity” broadly refers to any cardiovascular side effect related to cancer therapy (i.e. heart failure, cardiomyopathy, arrhythmias, ischemia, valvular disease, pericardial disease, hypertension, or thrombosis). For the purposes of this review, however, cardiotoxicity will be used to refer to LV dysfunction that develops as a result of chemotherapy induced myocardial injury. Anthracycline induced cardiomyopathy was first described in the 1970s and was defined in early trials by the presence of clinical signs and symptoms of heart failure believed to be secondary to anthracycline exposure.(5) The diagnosis can be confirmed by endomyocardial biopsy which shows several characteristic findings including myofibrillar dropout, distortion and disruption of Z-lines, mitochondrial disruption, and intramyocyte vacuolization.(6, 7) Although it is considered to be the most sensitive and specific test for anthracycline induced cardiomyopathy, use of endomyocardial biopsy is limited in clinical practice due to its invasive nature.
More recently, inconsistencies in the literature on the definition and criteria for cardiotoxicity pose a major challenge to the field of cardio-oncology, especially in the context of newer targeted therapies (e.g. trastuzumab) that are associated with adverse cardiac effects. In 2002 a Cardiac Review and Evaluation Committee (CREC) was formed in order to obtain independent and unbiased estimates of trastuzumab associated cardiac dysfunction, and the following criteria for cardiotoxicity were proposed:(8) (1) cardiomyopathy characterized by a decrease in cardiac LVEF (global or septal predominance), (2) symptoms of congestive heart failure (CHF), (3) associated signs of CHF (i.e. S3 gallop, tachycardia, or both), or (4) a decline in LVEF of at least 5% to < 55% with signs/symptoms of CHF, or decline of 10% to below 55% without symptoms. Despite this effort, significant heterogeneity exists in the criteria for cardiotoxicity in subsequent clinical trials (Table 2), leading to significant variability in the reported incidence of chemotherapy associated cardiac dysfunction.
Table 2.
Chemotherapy associated cardiomyopathy data from adjuvant trastuzumab clinical trials
| Trial name | Imaging modality for LVEF determination |
Frequency of monitoring |
Criteria for withholding trastuzumab |
Cardiac event rates |
|---|---|---|---|---|
| NSABP B-31 (96) | MUGA | Baseline, after AC, and at 6, 9, 18 months | LVEF decrease of ≥ 16%, or decrease of 10–15% below the LLN (defined by each institution) | Discontinuation of trastuzumab in 14% of patients due to asymptomatic decrease in LVEF; NYHA class III or IV heart failure or death from cardiac causes occurred in 1.3% in control vs. 4% in trastuzumab arm after 7 year follow-up.(88) |
| HERA(97) | Echo or MUGA | Baseline, and at 3, 6, 12, 18, 24, 30, 36, and 60 months after randomization | Symptomatic heart failure with LVEF < 45%, or LVEF decrease of ≥ 10% to < 50% | During median follow-up of 3.6 years, NYHA class III or IV heart failure occurred in 0% of control vs. 0.8% of trastuzumab group; Significant decrease in LVEF occurred in 2.9% of control vs. 9.8% of trastuzumab group (98) |
| N-9831(99, 100) | Echo or MUGA | Baseline, after AC, 6, 9, and 18 months | LVEF decrease of ≥ 16%, or decrease of 10–15% below the LLN (defined by each institution) | NYHA class III or IV heart failure or death from cardiac causes at 3y: 0.3% in control vs. 3.3% in concurrent trastuzumab-paclitaxel group.(101) |
| FinHer (102) | Echo or MUGA | Before chemotherapy, after FEC, and 12 and 36 months after chemotherapy | None | No patients receiving trastuzumab developed heart failure or a decline in LVEF > 10% to < 50%. |
| BCIRG 006(103) | Echo or MUGA | Seven time points throughout study period | LVEF decrease of ≥ 16%, or decrease of 10–15% below the LLN (defined by each institution), or decrease of < 10% to ≥ 6% below the LLN | NYHA class III or IV heart failure occurred in 0% of AC-T, 0.4% of TCH, and 2% AC-T and trastuzumab group; >10% decrease in LVEF occurred in 11.2% AC-T, 9.4% of TCH, and 18.6% of AC-T and trastuzumab group.* |
Abbreviations: NSABP = National Surgical Adjuvant Breast and Bowel Project; HERA = Herceptin Adjuvant; FinHeR = Finland Herceptin; BCIRG =Breast Cancer International Research Group; MUGA = Multi Gated Acquisition Scan; AC = doxorubicin and cyclophosphamide; FEC = 5=fluorouracil, epirubicin, cyclophosphamide; LVEF = left ventricular ejection fraction; LLN = lower limit of normal; NYHA = New York Heart Association; T = docetaxel; TCH = docetaxel, carboplatin, and trastuzumab
Imaging for Early Detection of Cardiotoxicity
Radionuclide Ventriculography and Echocardiography
Measurement of LVEF is the most commonly used method to evaluate for cardiotoxicity, and a baseline LVEF is routinely obtained prior to the initiation of cardiotoxic chemotherapy. Repeat serial LVEF assessments are recommended in the setting of certain cardiotoxic agents, such as trastuzumab,(9–11) and can also be performed as needed if signs or symptoms of CHF develop. Radionuclide ventriculography, or multiple gated acquisition scan (MUGA), has been validated as an accurate and reproducible method for LVEF estimation,(12) but exposes patients to approximately 6–7mSv of ionizing radiation per examination. Echocardiography is often preferred because it is a readily accessible and safe technology that does not involve the use of ionizing radiation. Although 2-dimensional echocardiography can be limited by significant variability and poor agreement with reference methods, this has significantly improved with the use of ultrasound contrast agents. In a study of 110 patients by Malm et al, LVEF by unenhanced echocardiography and cardiac MRI differed by ≥ 10% in 23 patients (26%) versus 0 with contrast echo.(13) 3-dimensional echocardiography offers additional incremental benefit over 2D techniques for determination of LVEF.(14). Moreover, among cancer patients undergoing serial monitoring of LVEF, noncontrast 3D echocardiography is feasible, accurate, and reproducible. (15, 16) Although some studies suggest that diastolic dysfunction may be an early sign of cardiotoxicity, the utility of diastolic function assessment during cancer treatment remains uncertain.(17, 18)
Although 2D echocardiography is routinely used for surveillance of LVEF, this modality can be limited by suboptimal image quality as well as significant inter- and intra-observer variability. A change in LVEF of ~10% is the minimum that can be recognized with 95% confidence,(15) but this degree of change is commonly used as the threshold to define cardiotoxicity. In addition, LVEF abnormalities likely represent a late manifestation of cardiotoxicity and may indicate the presence of irreversible myocardial damage. A prior study by Ewer et al showed that biopsy proven abnormalities due to anthracycline cardiotoxicity correlated poorly with LVEF, suggesting that LVEF is an insensitive marker for cardiotoxicity.(19) More sensitive and specific noninvasive markers of LV dysfunction would be useful for identifying patients at increased risk for treatment associated LV dysfunction, thereby allowing oncologists and cardiologists to tailor the treatment regimen for optimal efficacy while minimizing cardiac toxicity.
Myocardial Strain Imaging
Tissue Doppler and speckle tracking strain imaging have emerged as two quantitative techniques for estimating global and regional myocardial mechanical function and have the potential to detect early signs of LV dysfunction.(20) The first description of strain was derived from tissue Doppler imaging (TDI) for assessment of regional myocardial function and was validated in an ischemia model.(21, 22) However, this technique is both user and angle dependent and is unable to differentiate translational motion or tethering effects from myocardial contractility. Speckle tracking echocardiography is an angle independent technique that utilizes an image processing algorithm for analyzing motion of “speckles” or “fingerprints” within a 2-dimensional echo image, and has replaced TDI strain as the preferred method for quantitative assessment of cardiac deformation (Figure 1).(23, 24)
Figure 1.
Two-dimensional myocardial strain measurement
Example of assessment of longitudinal myocardial strain in the apical 4-chamber view: (A) normal longitudinal strain in a healthy patient; (B) abnormal longitudinal strain in a Hodgkin lymphoma survivor previously treated with anthracycline chemotherapy and mediastinal radiotherapy. The colored lines represent measurements of regional myocardial deformation. The white dotted line represents the global average of all segments in each view.
Several studies have evaluated the utility of strain imaging for the detection of chemotherapy associated cardiotoxicity. Fallah-Rad et al(25) evaluated 42 patients with breast cancer overexpressing human epidermal growth factor receptor 2 (HER2) receiving trastuzumab in the adjuvant setting after anthracycline therapy. Within 3 months, peak global longitudinal and radial strain detected pre-clinical changes in LV systolic function prior to a decrease in LVEF observed several months later. A more recent prospective multicenter study by Sawaya et al demonstrated that global longitudinal strain < 19% was predictive of subsequent cardiotoxicity as defined by CREC criteria and present in all patients who later developed symptoms of heart failure.(26) Negishi et al also showed that a ≥ 11% relative reduction in global longitudinal strain was predictive of subsequent trastuzumab associated cardiotoxicity.(27) Abnormalities in strain parameters can also be seen several years after a cardiotoxic exposure. This was reported in a study among 75 asymptomatic breast cancer survivors who received anthracycline with or without adjuvant trastuzumab in which global longitudinal strain was significantly decreased in the chemotherapy group up to 6 years after therapy compared to controls.(28)
Although these novel echocardiographic markers of subclinical LV dysfunction may allow for earlier detection of patients at increased risk for developing cardiotoxicity, the clinical significance of these changes remains unclear. Further studies are required to determine which patients would benefit most from this additional testing, when the testing should occur, and whether changes in these early echocardiographic markers are of sufficient clinical relevance to warrant an alteration in the oncologic treatment plan or intervention with cardioprotective medication.
Cardiac MRI
Cardiac MRI provides accurate measurements of LV dimensions and is considered the gold standard to which other imaging modalities are compared for LVEF determination. Unlike echocardiography, cardiac MRI does not rely on geometric assumptions for calculating volumes and is not hindered by poor acoustic windows. As a result, it has been shown to have superior intra- and inter-observer reproducibility and accuracy compared with echocardiography.(29) The use of echocardiography and cardiac MRI for evaluation of LV structure and function was compared in 114 adult survivors of childhood cancer by Armstrong et al.(30) Compared with cardiac MRI, 2D and 3D echocardiography were less sensitive (25% and 53%, respectively) for the detection of LVEF < 50%. However, using a higher LVEF cutoff of <60% by echocardiography increased the sensitivity to 75% for detecting a LVEF < 50% by cardiac MRI. These results suggest that the prevalence of cardiotoxicity may be underestimated by 2D echocardiography compared to more sensitive volumetric measures of LVEF such as cardiac MRI.
Beyond cardiac function and remodeling, cardiac MRI can directly assess myocardial tissue characteristics that are potentially useful for the identification of cardiotoxicity during or after cancer therapy. Several studies have shown the presence of myocardial fibrosis through the detection of late gadolinium enhancement (LGE) during and soon after completion of cancer therapy,(25, 31) although the prevalence of LGE appears to be low (<10%) during long term follow-up.(32, 33) New tissue characterization methods, such as T1 mapping, enable quantification of extracellular volumes, and preliminary studies have shown this to be elevated among patients with anthracycline associated cardiotoxicity.(34, 35) Additional studies are needed to determine the role that cardiac MRI will play in the surveillance and diagnostic algorithm for cardiotoxicity. Evaluation of chemotherapy associated cardiomyopathy and quantification of LV function are both approved indications for cardiac MRI based on the 2006 ACC/AHA appropriate use guidelines,(36) however the key disadvantages of cardiac MRI are high cost and limited availability of cardiac MRI scanners and trained personnel.
Biomarkers for Prediction of Cardiotoxicity
Cardiac biomarkers may serve a role as an alternative diagnostic tool for the detection of chemotherapy associated cardiotoxicity. A biomarker strategy would allow for early intervention with cardioprotective medications or alteration in the cancer treatment regimen to minimize the risk of cardiac dysfunction. Several biomarkers have been proposed, including troponin, natriuretic peptide, and C-reactive protein (CRP).
Troponin
Cardiac troponin T and I (TnT and TnI), long known for the important role they play in the diagnosis of acute coronary syndromes, are sensitive and specific markers for myocardial injury. Multiple studies have investigated the role of troponin as a promising biomarker for the diagnosis of chemotherapy associated cardiomyopathy (Table 3). In one study of 204 patients receiving high dose chemotherapy (HDC), TnI was elevated in 32% of patients and occurred >50% of the time soon after the end of drug administration. LVEF was also significantly reduced among patients with positive TnI.(37) A follow-up study to investigate the time course of TnI elevation and its impact on clinical outcome showed that patients with negative TnI (<0.08ng/ml), immediately and one month after chemotherapy, showed no reduction in LVEF and a very low incidence of cardiac events.(38) In contrast, patients with elevated TnI had a higher incidence of adverse cardiac events consisting mostly of heart failure and asymptomatic LV dysfunction. An elevated troponin may also identify those who are less likely to recover despite maximal heart failure therapy, whereas a negative troponin may suggest that any incident LV dysfunction will be transient.(39) This information could help clinicians to risk stratify patients and minimize unnecessary interruption of cancer treatment.
Table 3.
Utility of troponin as a biomarker for predicting chemotherapy associated cardiomyopathy
| Author | Criteria for biomarker positivity |
Patient population | n | Frequency of monitoring | Outcome |
|---|---|---|---|---|---|
| Broeyer et al (104) | TnT ≥ 0.01ng/ml | Recipients of doxorubicin chemotherapy | 26 | Before, at completion, and 24h after chemotherapy administration | TnT below limit of detection in most cases |
| Cardinale et al (37) | TnI > 0.4ng/ml | Recipients of HDC | 204 | Before, immediately after, and 12, 24, 36, 72h after each cycle | LVEF < 50% observed in 19/65 (29%) TnI+ and 0/139 TnI− patients (p < 0.001) |
| Cardinale et al (38) | TnI ≥ 0.08ng/ml | Recipients of HDC | 703 | Before, immediately after, 12, 24, 36, 72h after each cycle (early TnI), and 1 month after (late TnI) last administration of HDC | Higher cardiac event rate in patients with TnI positivity |
| Cardinale et al (39) | TnI ≥ 0.08ng/ml | Early, advanced, and metastatic Her2+ breast cancer patients treated with receiving trastuzumab | 251 | Before and soon after each trastuzumab treatment | Trastuzumab induced cardiotoxicity was more frequent in patients with elevated TnI (62% v. 5%, p<0.001); LVEF recovery occurred less frequently in patients with elevated TnI (35% v. 100%, p<0.001) |
| Fallah-Rad et al(25) | TnT ≥ 0.01ng/ml | HER2+ breast cancer patients receiving adjuvant trastuzumab | 42 | Before initiation of anthracycline, before initiation of trastuzumab, and 3, 6, 9, and 12 months after initiation of trastuzumab | TnT remained within normal limits for both the normal cohort and those who developed trastuzumab mediated cardiomyopathy |
| Sawaya et al(105) | TnI > 0.015µg/L | HER2+ breast cancer treated with anthracyclines and trastuzumab | 43 | Before chemotherapy, after 3 and 6 months of treatment | Elevated TnI at 3 months is an independent predictor of later cardiotoxicity (p<0.02) |
| Sawaya et al(26) | hsTnI > 30pg/ml | HER2+ breast cancer patients treated with adjuvant anthracyclines, taxanes, and trastuzumab | 81 | Before chemotherapy, 3, 6, 9, 12, and 15 months | Elevated hsTnI at the completion of anthracycline therapy is predictive of subsequent cardiotoxicity |
Abbreviations: TnT, troponin-T; TnI, troponin-I; HDC, high-dose chemotherapy; LVEF, left ventricular ejection fraction; hsTnI, high sensitivity troponin-I
More sensitive troponin assays have recently been developed, which allow for detection of troponin release at an earlier stage of myocyte stress. Several studies have demonstrated better diagnostic accuracy of these newer assays in the early diagnosis of acute coronary syndrome,(40–42) but their role in the detection of cardiotoxicity is still unclear. Sawaya et al evaluated the utility of ultrasensitive TnI for predicting subsequent cardiotoxicity among 81 patients with HER2 (+) breast cancer and found that ultrasensitive TnI > 30pg/ml, when combined with global longitudinal strain < 19%, was associated with subsequent decline in LVEF and symptomatic CHF.(26)
Natriuretic peptide
Natriuretic peptides have been studied extensively for their diagnostic and prognostic role in cardiovascular disease. Both atrial and brain natriuretic peptides (ANP, BNP) are important for salt and water handling and are produced by the heart in response to high ventricular filling pressure, as is typically seen with heart failure. Several studies have looked at the value of both atrial and brain natriuretic peptide levels for monitoring and/or prediction of chemotherapy induced cardiotoxicity, however the results have been inconclusive. An early study by Suzuki et al suggested the possible role of BNP in the assessment of cardiac function after anthracycline administration for hematologic malignancies.(43) However, other studies performed in patients of varying ages and different malignancies have failed to show an association between BNP and risk of cardiotoxicity.(25, 44) Daugard et al studied 107 patients receiving anthracycline for a variety of cancer diagnoses including breast cancer, sarcoma, and lymphoma, and concluded that neither baseline levels nor a change in ANP or BNP were predictive of a change in LVEF.(45) More recently, in a homogenous group of 81 women with HER-2 positive breast cancer treated with anthracyclines followed by taxanes and trastuzumab, Sawaya et al found that an elevated N-terminal pro-B type natriuretic peptide (NT-proBNP) was not predictive of subsequent LVEF decline or symptomatic heart failure.(26) At present, there is insufficient data to recommend the routine measurement of natriuretic peptides in the assessment of cardiotoxicity in clinical practice.
C-reactive protein and other novel biomarkers
Few studies have evaluated the association between CRP and cardiotoxicity, and this may be due to the confounding effect of concurrent infectious or inflammatory processes that affect CRP levels and often occur with malignancy. A recent single center clinical trial demonstrated that high sensitivity-CRP had a high sensitivity (92.9%) and negative predictive value (94.1%) for predicting trastuzumab-induced cardiotoxicity.(46) Another study by Ky et al investigated the association of multiple conventional and novel biomarkers with cardiotoxicity, including growth differentiation factor-15 (GDF-15), myeloperoxidase (MPO), placental growth factor (PIGF), soluble fms-like tyrosine kinase receptor-1 (sFlt-1), and galectin-3.(47) Among 78 breast cancer patients treated with doxorubicin and trastuzumab, changes in TnI and MPO but not CRP were associated with subsequent cardiac dysfunction. Additional studies are needed to validate the utility of candidate biomarkers before application in clinical practice.
Management of Cardiotoxicity
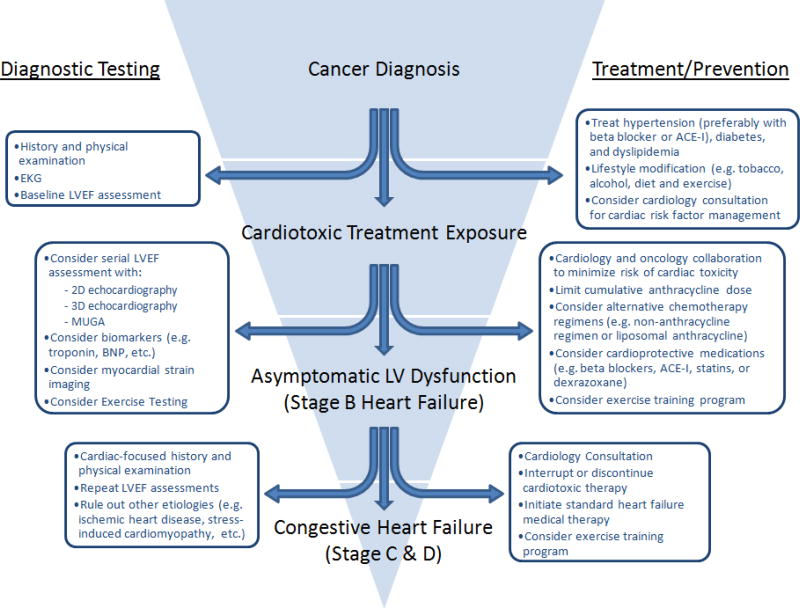
In 2005, the ACC/AHA introduced a new classification system of heart failure that emphasized the preventable nature of heart failure, and this was accompanied by recommendations to treat cardiovascular risk factors in order to prevent or delay the onset of heart failure.(48) Based on this new classification system, patients with chemotherapy associated cardiomyopathy and asymptomatic LV dysfunction are classified with stage B heart failure. According to the 2013 ACC/AHA Guideline for the Management of Heart Failure, patients with stage B heart failure should be treated with ACE-Is (Class I, Level of Evidence A) and beta blockers (Class I, Level of Evidence C).(49) The use of therapies such as implantable cardioverter-defibrillators or cardiac resynchronization therapy for more advanced stages of heart failure should take into consideration the patient’s overall prognosis and quality of life. A proposed diagnostic and treatment algorithm for patients exposed to cardiotoxic therapy is shown in Figure 2.
Figure 2.
Proposed diagnostic, preventive, and treatment strategies for patients at risk for chemotherapy associated cardiomyopathy
Evidence supporting the use of contemporary heart failure therapies is largely based on studies in patients with ischemic or nonischemic dilated cardiomyopathies, and limited data exist regarding the treatment of patients with chemotherapy associated cardiomyopathy.(50–52) Cardinale et al evaluated the response of anthracycline induced cardiomyopathy to modern heart failure therapy and included 201 patients with a LVEF ≤ 45%.(53) Enalapril and, when possible, carvedilol were initiated at the time of detection of LVEF impairment and up-titrated to the maximal tolerated dose, and LVEF was followed serially by echocardiography. A total of 85 patients (42%) normalized their LVEF, 26 patients (13%) showed an increase in LVEF > 10% but below 50%, and 90 patients (45%) showed < 10% increase in LVEF. A short time to initiation of heart failure therapy was an important predictor of LVEF recovery. This was one of the first prospective studies to show the efficacy of ACE-Is and beta-blockers for the treatment of anthracycline mediated cardiomyopathy, suggesting that early treatment may be important to increase the likelihood of LVEF recovery. Several questions remain unanswered, including which specific medication to use, how much, and for what duration? Additional studies are needed to address these gaps in knowledge and better inform the optimal heart failure management of chemotherapy associated cardiomyopathy.
Strategies for Prevention of Cardiotoxicity
Current management strategies have relied on early detection of myocardial injury through serial monitoring of LVEF or cardiac biomarker testing during treatment, followed by temporary or permanent discontinuation of further cardiotoxic exposures. A major goal of cardio-oncology is to prevent the development of cardiotoxicity, either through modification of the cardiotoxic exposure or initiation of cardioprotective medications. Here we will review some of the preventive strategies that have been proposed.
Chemotherapy modification
Anthracycline cardiotoxicity is related to cumulative dose,(54) and cumulative doxorubicin doses should be limited to 450–500mg/m2 in adults. However, given that the sensitivity to cardiotoxic effects of anthracycline can vary by patient, routine surveillance of cardiac function is critical for the prevention of cardiotoxicity, even at lower anthracycline dose ranges. Prolonged infusion schedules have been shown to lower the incidence of cardiotoxicity when compared to bolus therapy.(55) In a Cochrane Database Review of 6 randomized controlled trials in which different anthracycline dosage schedules were used in cancer patients, the rate of heart failure was significantly lower with a long infusion (≥ 6 hours) as compared to a shorter infusion (RR = 0.27; 95% confidence interval 0.09 to 0.81).(56) This strategy has not been shown to adversely affect the cancer response rate or overall survival.
Liposomal preparations of anthracyclines, first used in the early 1990s for the treatment of AIDS-associated Kaposi’s sarcoma, are associated with a lower incidence of cardiotoxicity compared with standard anthracycline preparations.(57) Liposomal preparations of anthracyclines were found to be effective in a variety of malignancies including breast cancer, ovarian cancer, and multiple myeloma, while associated with less cardiac toxicity.(58–62) Less severe cardiac changes were seen on endomyocardial biopsy among patients receiving pegylated liposomal doxorubicin compared to patients receiving non-liposomal doxorubicin.(63) Liposomal anthracycline preparations are currently in use for the treatment of ovarian cancer and multiple myeloma.
Dexrazoxane
Dexrazoxane is an EDTA-like chelator that binds to iron and reduces the formation of superhydroxide radicals that can cause oxidative damage of cardiac tissue. The efficacy of dexrazoxane was recently addressed in a Cochrane database review which included 10 randomized clinical trials of 1619 patients.(64) The majority of patients included in these studies were adults with advanced breast cancer treated with either doxorubicin or epirubicin, and treatment with dexrazoxane significantly reduced the incidence of heart failure (RR 0.29, 95% CI 0.20 to 0.41, P < 0.00001). Although there have been some concerns that dexrazoxane may compromise tumor response to chemotherapy,(65) this meta-analysis showed no significant difference in tumor response rate, progression free survival, overall survival, adverse effects, or secondary malignant disease with dexrazoxane treatment.
The American Society of Clinical Oncology (ASCO) published guidelines in 2008 for the use of dexrazoxane in patients with breast cancer and other malignancies, and recommended the following:(66) (1) Dexrazoxane should be considered for patients with metastatic breast cancer or other malignancies who have received more than 300mg/m2 of doxorubicin in the metastatic setting and who may benefit from continued doxorubicin therapy; (2) Dexrazoxane can be considered for patients with non-breast malignancies who have received ≥ 300 mg/m2 of doxorubicin-based therapy. Caution should be exercised in settings where doxorubicin-based therapy has been shown to improve survival; (3) The use of dexrazoxane in the adjuvant setting is not recommended outside of a clinical trial; (4) There is insufficient evidence to support routine use of dexrazoxane among patients with cardiac risk factors or underlying structural heart disease. Despite the current ASCO guidelines, dexrazoxane is not routinely used in clinical practice due to the continued concern for its interference with conventional cancer treatment. Several clinical trials are currently underway to evaluate the efficacy of dexrazoxane in other cancer patient populations.
Prophylaxis with cardioprotective medications
One of the first clinical trials to investigate the role of cardioprotective medical therapy in preventing cardiotoxicity was performed by Kalay et al.(67) In this small study, 50 patients with planned anthracycline treatment (doxorubicin or epirubicin) were randomized to carvedilol 12.5 mg once daily versus placebo. LV systolic and diastolic function was evaluated by echocardiography before and after exposure to anthracycline treatment. At 6 month follow-up, patients in the control group had a significantly lower LVEF and larger LV systolic and diastolic dimensions compared to the carvedilol group. A retrospective study by Seicean et al also showed that beta-blocker use was associated with a lower incidence of heart failure among patients with breast cancer receiving anthracycline and trastuzumab therapy.(68) One of the proposed mechanisms for the protective effect of carvedilol is its ability to reduce free oxygen radicals, which have been implicated in the pathogenesis of anthracycline mediated toxicity.(69) More recently, a study by Zhang et al showed that topoisomerase-II beta (Top2β) plays an important role in the pathogenesis of doxorubicin-induced cardiotoxicity through the mediation of structural and functional changes in mitochondria of cardiomyocytes as well as generation of reactive oxygen species.(70)
The role of angiotensin antagonists for the prevention of cardiotoxicity was investigated in a randomized trial by Cardinale et al.(71) Among patients with an elevated troponin I (>0.07ng/mL) after high dose chemotherapy, early treatment with enalapril 20mg daily started 1 month following chemotherapy and continued for 1 year prevented the development of cardiotoxicity (defined as an absolute decrease > 10% in LVEF to below 50%). Although the mechanism by which enalapril prevents cardiotoxicity remains unclear, it is postulated that ACE-Is block cardiac-associated renin-angiotensin system activity, reduce left ventricular remodeling, and decrease oxidative stress. This was the first study to implement a prophylactic cardioprotective strategy among patients at high risk of cardiotoxicity using a biomarker directed approach. The Prevention of Left Ventricular Dysfunction During Chemotherapy (OVERCOME) study recently evaluated the effects of combined enalapril and carvedilol in patients with hematologic malignancies treated with intensive chemotherapy and found that LVEF did not change in the enalapril and carvedilol group but significantly decreased in those treated with placebo (p=0.04).(72) These results show that the combination of enalapril and carvedilol may be effective in preventing LV dysfunction during intensive chemotherapy and could have important clinical implications.
Statins, well known for the protective effects in patients treated for coronary artery disease, have also been investigated for their potential to attenuate cardiotoxicity. Using an animal model, Riad et al showed that mice pretreated with fluvastatin showed improved LV function when compared to untreated mice after exposure to doxorubicin.(73) Observational data from Seicean et al also showed that statin therapy appears to be associated with a reduced risk for heart failure and cardiac related mortality among breast cancer patients treated with anthracycline,(74) but prospective clinical trials are needed to further evaluate any association between statin therapy and risk of cardiotoxicity.
Several ongoing clinical trials are currently underway to further investigate the efficacy of prophylactic cardioprotective medications among patients treated with cardiotoxic chemotherapy. The Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE 101-Breast) is a randomized trial among HER2 (+) early breast cancer patients to determine if perindopril or bisoprolol therapy can prevent trastuzumab-associated LV remodeling as measured by LV volume indices using cardiac MRI.(75) A similar trial sponsored by the National Cancer Institute is studying the effect of lisinopril and carvedilol on trastuzumab-induced cardiotoxicity as measured by LVEF (NCT01009918).
Exercise training
Aerobic exercise training has been proposed as a nonpharmacologic therapy that may attenuate the deleterious effects of heart failure.(76, 77) It has been shown to correct endothelial dysfunction by both improving nitric oxide (NO) formation and endothelium-dependent vasodilation of the skeletal muscle vasculature,(78) improve cardiac and skeletal muscle energy metabolism and function,(79) and improve diastolic filling and increase stroke volume.(80) All of these adaptations lead to an improvement in systolic and diastolic function with augmentation of cardiac output and increase in maximal oxygen uptake (VO2max), resulting in improved exercise tolerance and decreased fatigability in heart failure.(81) Several animal studies have investigated the effects of aerobic exercise training prior to and during doxorubicin therapy and shown that exercise prevents doxorubicin-induced impairments in LV function.(82, 83) Exercise training represents a promising strategy for prevention and/or treatment of chemotherapy associated cardiomyopathy, however additional studies are required to better understand the mechanism of this benefit and to inform future recommendations for exercise training among cancer patients.
Conclusion
Given the potential interaction between cancer therapy and the cardiovascular system, cardiologists and oncologists must collaborate in order to ensure the best long-term clinical outcome for cancer patients. Newer targeted therapies are changing the landscape of cancer care, and the impact of cardiotoxicity on overall morbidity and mortality will increase as cancer outcomes improve. Future diagnostic strategies will likely incorporate the use of novel imaging techniques (i.e. speckle tracking strain or cardiac MRI) and biomarker testing in order to identify patients with early or subclinical signs of cardiotoxicity. Translational studies are needed to better understand the mechanism in which cardiotoxic agents cause myocardial injury, and this may help inform the design of future trials investigating the use of cardioprotective medications for the prevention of chemotherapy associated cardiomyopathy. A continued interdisciplinary cardio-oncology approach is critical to maintain a balance between the oncologic benefit of cancer treatment and its associated cardiac toxicities.
Abbreviations
- ACE-I
angiotensin converting enzyme inhibitor
- ASCO
American Society of Clinical Oncology
- CHF
congestive heart failure
- CREC
Cardiac Review and Evaluation Committee
- HDC
high dose chemotherapy
- HER2
human epidermal growth factor 2
- LVEF
left ventricular ejection fraction
- MUGA
multi gated acquisition scan
- TDI
tissue Doppler imaging
Footnotes
Disclosures:
The authors have no disclosures.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Praga C, Beretta G, Vigo PL, Lenaz GR, Pollini C, Bonadonna G, et al. Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treat Rep. 1979;63(5):827–34. [PubMed] [Google Scholar]
- 3.Von Hoff DD, Rozencweig M, Layard M, Slavik M, Muggia FM. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977;62(2):200–8. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]
- 4.Rinehart JJ, Lewis RP, Balcerzak SP. Adriamycin cardiotoxicity in man. Ann Intern Med. 1974;81(4):475–8. doi: 10.7326/0003-4819-81-4-475. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710–7. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann Intern Med. 1978;88(2):168–75. doi: 10.7326/0003-4819-88-2-168. [DOI] [PubMed] [Google Scholar]
- 7.Meinardi MT, van der Graaf WT, van Veldhuisen DJ, Gietema JA, de Vries EG, Sleijfer DT. Detection of anthracycline-induced cardiotoxicity. Cancer Treat Rev. 1999;25(4):237–47. doi: 10.1053/ctrv.1999.0128. [DOI] [PubMed] [Google Scholar]
- 8.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 9.Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100(5):684–92. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herceptin Prescribing Information. Genentech, Inc.: Oct 29, 2010. [Google Scholar]
- 11.Fox KF. The evaluation of left ventricular function for patients being considered for, or receiving Trastuzumab (Herceptin) therapy. Br J Cancer. 2006;95(10):1454. doi: 10.1038/sj.bjc.6603340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burow RD, Strauss HW, Singleton R, Pond M, Rehn T, Bailey IK, et al. Analysis of left ventricular function from multiple gated acquisition cardiac blood pool imaging. Comparison to contrast angiography. Circulation. 1977;56(6):1024–8. doi: 10.1161/01.cir.56.6.1024. [DOI] [PubMed] [Google Scholar]
- 13.Malm S, Frigstad S, Sagberg E, Larsson H, Skjaerpe T. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: a comparison with magnetic resonance imaging. J Am Coll Cardiol. 2004;44(5):1030–5. doi: 10.1016/j.jacc.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 14.Gopal AS, Shen Z, Sapin PM, Keller AM, Schnellbaecher MJ, Leibowitz DW, et al. Assessment of cardiac function by three-dimensional echocardiography compared with conventional noninvasive methods. Circulation. 1995;92(4):842–53. doi: 10.1161/01.cir.92.4.842. [DOI] [PubMed] [Google Scholar]
- 15.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61(1):77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 16.Walker J, Bhullar N, Fallah-Rad N, Lytwyn M, Golian M, Fang T, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(21):3429–36. doi: 10.1200/JCO.2009.26.7294. [DOI] [PubMed] [Google Scholar]
- 17.Bu'Lock FA, Mott MG, Oakhill A, Martin RP. Left ventricular diastolic function after anthracycline chemotherapy in childhood: relation with systolic function, symptoms, and pathophysiology. Br Heart J. 1995;73(4):340–50. doi: 10.1136/hrt.73.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radulescu D, Pripon S, Radulescu LI, Duncea C. Left ventricular diastolic performance in breast cancer survivors treated with anthracyclines. Acta Cardiol. 2008;63(1):27–32. doi: 10.2143/AC.63.1.2025328. [DOI] [PubMed] [Google Scholar]
- 19.Ewer MS, Ali MK, Mackay B, Wallace S, Valdivieso M, Legha SS, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J Clin Oncol. 1984;2(2):112–7. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 20.Gorcsan J, 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58(14):1401–13. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Derumeaux G, Ovize M, Loufoua J, Andre-Fouet X, Minaire Y, Cribier A, et al. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation. 1998;97(19):1970–7. doi: 10.1161/01.cir.97.19.1970. [DOI] [PubMed] [Google Scholar]
- 22.Edvardsen T, Urheim S, Skulstad H, Steine K, Ihlen H, Smiseth OA. Quantification of left ventricular systolic function by tissue Doppler echocardiography: added value of measuring pre- and postejection velocities in ischemic myocardium. Circulation. 2002;105(17):2071–7. doi: 10.1161/01.cir.0000014614.63980.ba. [DOI] [PubMed] [Google Scholar]
- 23.Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20(3):234–43. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23(4):351–69. doi: 10.1016/j.echo.2010.02.015. quiz 453-5. [DOI] [PubMed] [Google Scholar]
- 25.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57(22):2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 26.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circulation Cardiovascular imaging. 2012;5(5):596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26(5):493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010;96(9):701–7. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 29.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? European heart journal. 2000;21(16):1387–96. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(23):2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunning MA, Kutty S, Rome ET, Li L, Padiyath A, Loberiza F, et al. Cardiac Magnetic Resonance Imaging for the Assessment of the Myocardium After Doxorubicin-based Chemotherapy. Am J Clin Oncol. 2013 doi: 10.1097/COC.0b013e31829e19be. [DOI] [PubMed] [Google Scholar]
- 32.Neilan TG, Coelho-Filho OR, Pena-Herrera D, Shah RV, Jerosch-Herold M, Francis SA, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110(11):1679–86. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawley C, Wainwright C, Segelov E, Lynch J, Beith J, McCrohon J. Pilot study evaluating the role of cardiac magnetic resonance imaging in monitoring adjuvant trastuzumab therapy for breast cancer. Asia Pac J Clin Oncol. 2012;8(1):95–100. doi: 10.1111/j.1743-7563.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 34.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111(5):717–22. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48(7):1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36(2):517–22. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 38.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–54. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 39.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–6. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 40.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. The New England journal of medicine. 2009;361(9):868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 41.Weber M, Bazzino O, Navarro Estrada JL, de Miguel R, Salzberg S, Fuselli JJ, et al. Improved diagnostic and prognostic performance of a new high-sensitive troponin T assay in patients with acute coronary syndrome. American heart journal. 2011;162(1):81–8. doi: 10.1016/j.ahj.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. The New England journal of medicine. 2009;361(9):858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Hayashi D, Yamazaki T, Mizuno T, Kanda Y, Komuro I, et al. Elevated B-type natriuretic peptide levels after anthracycline administration. American heart journal. 1998;136(2):362–3. doi: 10.1053/hj.1998.v136.89908. [DOI] [PubMed] [Google Scholar]
- 44.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clinical research in cardiology : official journal of the German Cardiac Society. 2008;97(5):318–26. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 45.Daugaard G, Lassen U, Bie P, Pedersen EB, Jensen KT, Abildgaard U, et al. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. European journal of heart failure. 2005;7(1):87–93. doi: 10.1016/j.ejheart.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Onitilo AA, Engel JM, Stankowski RV, Liang H, Berg RL, Doi SA. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast cancer research and treatment. 2012;134(1):291–8. doi: 10.1007/s10549-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 47.Ky B, Putt M, Sawaya H, French B, Januzzi JL, Sebag IA, et al. Early Increases in Multiple Biomarkers Predict Subsequent Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt SA American College of C, American Heart Association Task Force on Practice G. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 50.Jensen BV, Nielsen SL, Skovsgaard T. Treatment with angiotensin-converting-enzyme inhibitor for epirubicin-induced dilated cardiomyopathy. Lancet. 1996;347(8997):297–9. doi: 10.1016/s0140-6736(96)90469-9. [DOI] [PubMed] [Google Scholar]
- 51.Noori A, Lindenfeld J, Wolfel E, Ferguson D, Bristow MR, Lowes BD. Beta-blockade in adriamycin-induced cardiomyopathy. Journal of cardiac failure. 2000;6(2):115–9. [PubMed] [Google Scholar]
- 52.Tallaj JA, Franco V, Rayburn BK, Pinderski L, Benza RL, Pamboukian S, et al. Response of doxorubicin-induced cardiomyopathy to the current management strategy of heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(12):2196–201. doi: 10.1016/j.healun.2004.12.108. [DOI] [PubMed] [Google Scholar]
- 53.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213–20. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 54.Bristow MR, Mason JW, Billingham ME, Daniels JR. Dose-effect and structure-function relationships in doxorubicin cardiomyopathy. American heart journal. 1981;102(4):709–18. doi: 10.1016/0002-8703(81)90096-x. [DOI] [PubMed] [Google Scholar]
- 55.Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982;96(2):133–9. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 56.van Dalen EC, van der Pal HJ, Caron HN, Kremer LC. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane database of systematic reviews. 2006(4):CD005008. doi: 10.1002/14651858.CD005008.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Young AM, Dhillon T, Bower M. Cardiotoxicity after liposomal anthracyclines. The lancet oncology. 2004;5(11):654. doi: 10.1016/S1470-2045(04)01605-5. [DOI] [PubMed] [Google Scholar]
- 58.Jones RL, Berry GJ, Rubens RD, Miles DW. Clinical and pathological absence of cardiotoxicity after liposomal doxorubicin. The lancet oncology. 2004;5(9):575–7. doi: 10.1016/S1470-2045(04)01570-0. [DOI] [PubMed] [Google Scholar]
- 59.Harris KA, Harney E, Small EJ. Liposomal doxorubicin for the treatment of hormone-refractory prostate cancer. Clinical prostate cancer. 2002;1(1):37–41. doi: 10.3816/cgc.2002.n.005. [DOI] [PubMed] [Google Scholar]
- 60.Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94(1):25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 61.O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15(3):440–9. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 62.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(5):1444–54. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 63.Berry G, Billingham M, Alderman E, Richardson P, Torti F, Lum B, et al. The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi's sarcoma patients treated with pegylated liposomal doxorubicin. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1998;9(7):711–6. doi: 10.1023/a:1008216430806. [DOI] [PubMed] [Google Scholar]
- 64.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane database of systematic reviews. 2011;(6):CD003917. doi: 10.1002/14651858.CD003917.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15(4):1318–32. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 66.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(1):127–45. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 67.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48(11):2258–62. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 68.Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circ Heart Fail. 2013;6(3):420–6. doi: 10.1161/CIRCHEARTFAILURE.112.000055. [DOI] [PubMed] [Google Scholar]
- 69.Kametani R, Miura T, Harada N, Shibuya M, Wang R, Tan H, et al. Carvedilol inhibits mitochondrial oxygen consumption and superoxide production during calcium overload in isolated heart mitochondria. Circulation journal : official journal of the Japanese Circulation Society. 2006;70(3):321–6. doi: 10.1253/circj.70.321. [DOI] [PubMed] [Google Scholar]
- 70.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 71.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–81. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 72.Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J Am Coll Cardiol. 2013;61(23):2355–62. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 73.Riad A, Bien S, Westermann D, Becher PM, Loya K, Landmesser U, et al. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009;69(2):695–9. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- 74.Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60(23):2384–90. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 75.Pituskin E, Haykowsky M, Mackey JR, Thompson RB, Ezekowitz J, Koshman S, et al. Rationale and design of the Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE 101--Breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC Cancer. 2011;11:318. doi: 10.1186/1471-2407-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328(7433):189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98(24):2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 79.Ventura-Clapier R, Mettauer B, Bigard X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovascular research. 2007;73(1):10–8. doi: 10.1016/j.cardiores.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation. 2011;124(5):642–50. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 82.Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. Journal of cardiovascular pharmacology. 2006;47(2):182–9. doi: 10.1097/01.fjc.0000199682.43448.2d. [DOI] [PubMed] [Google Scholar]
- 83.Hydock DS, Lien CY, Schneider CM, Hayward R. Effects of voluntary wheel running on cardiac function and myosin heavy chain in chemically gonadectomized rats. Am J Physiol Heart Circ Physiol. 2007;293(6):H3254–64. doi: 10.1152/ajpheart.00801.2007. [DOI] [PubMed] [Google Scholar]
- 84.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 85.Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94(1):25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 86.van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010;(5):CD005006. doi: 10.1002/14651858.CD005006.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer treatment reviews. 2004;30(2):181–91. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(31):3792–9. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29(6):632–8. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 90.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19(9):1613–8. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 91.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110(4):1233–7. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 92.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83(6):679–86. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 94.Shimoyama M, Murata Y, Sumi KI, Hamazoe R, Komuro I. Docetaxel induced cardiotoxicity. Heart. 2001;86(2):219. doi: 10.1136/heart.86.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–25. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan-Chiu E, Yothers G, Romond E, Geyer CE, Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23(31):7811–9. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 97.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 98.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422–8. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 99.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 100.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(1):14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 101.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(8):1231–8. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. The New England journal of medicine. 2006;354(8):809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 103.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Broeyer FJ, Osanto S, Ritsema van Eck HJ, van Steijn AQ, Ballieux BE, Schoemaker RC, et al. Evaluation of biomarkers for cardiotoxicity of anthracyclin-based chemotherapy. Journal of cancer research and clinical oncology. 2008;134(9):961–8. doi: 10.1007/s00432-008-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. The American journal of cardiology. 2011;107(9):1375–80. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]