Abstract
Sterility in hybrid animals is widely known to be due to a cytological mechanism of aberrant homologous chromosome pairing during meiosis in hybrid germ cells. In this study, the gametes of four marine fish species belonging to the Sciaenid family were artificially fertilized, and germ cell development was examined at the cellular and molecular levels. One of the intergeneric hybrids had gonads that were testis-like in structure, small in size, and lacked germ cells. Specification of primordial germ cells (PGCs) and their migration toward genital ridges occurred normally in hybrid embryos, but these PGCs did not proliferate in the hybrid gonads. By germ cell transplantation assay, we showed that the gonadal microenvironment in hybrid recipients produced functional donor-derived gametes, suggesting that the germ cell-less phenotype was caused by cell autonomous proliferative defects of hybrid PGCs. This is the first evidence of mitotic arrest of germ cells causing hybrid sterility in animals.
Keywords: gametogenesis, germline, hybridization, mitosis, primordial germ cells, sexual reproduction, sterility, genetics of sex
HYBRID sterility is one of the postzygotic reproductive isolation mechanisms between species or subspecies that is thought to result from the acquisition of genetic incompatibilities, and it plays an important role in maintaining speciation (Mayr 1963; Mallet 2007). The nature and complexity of hybrid incompatibilities, probably caused by the combination of the diverged alleles and incorrect epistatic interactions between genes, remain poorly understood. Focusing on the cytological studies of disturbed gametogenesis in hybrid animals of various taxa (Chandley et al. 1975; Sawamura et al. 2004; Bhattacharyya et al. 2013; Islam et al. 2013), failures of synapsis between homologous chromosomes during meiosis are often reported in the hybrids resulting from crossing karyotypically identical species of house mice (Flachs et al. 2014). More recently, failure in pairing between homologous chromosomes followed by meiotic silencing of unsynapsed chromatin has been proposed to be the cause of apoptosis of gametocytes and sterility in mammals (Torgasheva and Borodin 2016).
As fertilization is mainly external in fish, a number of cases of hybrid sterility have been reported in fish (Chevassus 1983; Bartley et al. 2001; Rahman et al. 2012; Piva et al. 2017). Morphological and histological studies of the gonads of sterile hybrid fish have indicated that sexual maturation is affected in several ways that depend on the combination of parental species. Some hybrid fish possess gonads that are normal in size and structure, but they produce morphologically and/or karyotypically abnormal gametes or fertilizable but unviable gametes (Hooe et al. 1994; Shimizu et al. 1997). In experimental model freshwater fish, Wong et al. (2011) reported that a hybrid fish, produced by in vitro fertilization of zebrafish (Danio rerio) eggs with pearl danio (D. albolineatus) sperm, had gonads with reduced size that mostly consisted of spermatogonia or oogonia, and neither female nor male hybrids were able to develop functional gonads. Testes with small size and spermatogenic disruption were also found in interspecific hybrid medaka (Hamaguchi and Sakaizumi 1992; Shimizu et al. 1997). Aberrant chromosome synapsis caused by a difference in the meiotic germ cell karyotype and chromosome structure of the parental species of interspecific hybrids is widely believed to be a key mechanism of hybrid sterility in fish, as well as in other vertebrates. Vestigial and thread-like gonads in adult fish have been reported in hybrids resulting from systematically distinct species (Kitamura et al. 1991; Sugama et al. 1992; Murata et al. 1997; Gorshkov et al. 2002), and may suggest the presence of unrevealed mechanisms governing hybrid sterility.
Although the characteristic features of abnormal gonads of hybrids—such as meiotic arrest, abnormal sex ratio, and reduced fecundity—have been known for centuries, there have been few studies of early gonadal development of sterile hybrid animals, including of the differentiation and proliferation of mitotic germ cells [i.e., primordial germ cells (PGCs), gonocytes, and gonial cells] and sex differentiation. Although gonads at least form in sterile hybrid animals (Bullini 1994), no studies have been conducted to determine whether cell fate decisions and early migration of hybrid PGCs, as well as the formation of genital ridges, occur normally. It is also unknown whether the behavior of hybrid mitotic germ cells affects the gonadal development of hybrid animals. The more important question is whether the meiotic arrest observed in hybrid germ cells is the only barrier for maintaining speciation in animals.
In the present paper, we produced hybrids with four marine fishes belonging to three genera of family Sciaenidae, which is commonly known as drums and croakers, and which was estimated to have diverged ∼15–18 MYA (Lo et al. 2015). We studied viability, fertility, and gonadal development from larval to sexual maturation stages of the hybrid offspring, with a focus on the characteristics of early gonadal development, i.e., the differentiation and proliferation of PGCs, formation of genital ridges, sex differentiation, and gonadal structure of adult hybrids at the cellular and molecular levels. Furthermore, to evaluate whether gonadal somatic cells of sterile hybrids retain the function to produce viable gametes, a germ cell transplantation technique using normal (nonhybrid) spermatogonial cells as donor cells was utilized.
Materials and Methods
Ethics
All experiments were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals of Tokyo University of Marine Science and Technology.
Broodstock, artificial insemination, and larvae rearing
Four species of sciaenids [blue drum (BD, Nibea mitsukurii), yellow drum (YD, N. albiflora), white croaker (WC, Pennahia argentata), and mulloway, also known as kob or Japanese meager (Mu, Argyrosomus japonicus)] were captured from the wild and maintained at Tateyama Station (Banda), Field Science Center of Tokyo University of Marine Science and Technology (Figure 1A). Eggs of BD and sperm of BD, YD, WC, and Mu were separately collected by applying gentle pressure to the abdomen, and crosses were made in the laboratory. Fertilized eggs were reared in 100-liter seed production tanks with seawater at 24–25°. Fertilization and hatching rates at 2 and 24 hr postfertilization (hpf) were determined as described previously (Takeuchi et al. 2016). To confirm successful hybridization, species-specific sequences of BD, YD, WC, and Mu were detected by PCR analysis of the genomic DNA extracted from newly hatched larvae obtained from each cross (see below).
Figure 1.
Interspecific hybridization among Sciaenidae fishes (A). (B and C) Fertilization and hatching rate at 24 hr postfertilization (B), and survival rate at 10 dph (C). All experimental hybridizations were replicated at least three times and an average of 23,000 eggs (range = 4500–44,000 eggs) of BD were used in each cross. Data are mean ± SEM. Different letters indicate statistically significant differences (P < 0.05). F1 offspring obtained by cross between BD eggs and BD sperm, YD sperm, WC sperm, and Mu sperm are represented by BD, BD-YD, BD-WC, and BD-Mu, respectively. (D) Species-specific PCR amplification of vasa genomic DNA in BD-YD, BD-WC, and BD-Mu larvae. Lanes 1–4, genomic DNA templates obtained from hybrid larvae. Lanes BD, YD, WC, Mu show genomic DNA templates obtained from parents. (E) Survival rate and TL of BD (control) and BD-YD and BD-WC hybrids at 10, 30, and 60 dph. Each experimental cross was repeated four times. TL was determined of a randomly selected sample of an average of 30 individuals (n = 10–41) at each age. Data are shown as mean ± SEM. No statistically significant differences were detected at any age (P > 0.05). BD, blue drum; dph, days posthatching; Mu, mulloway; TL, total length; WC, white croaker; YD, yellow drum.
Total length (TL) and number of larvae in the 100-liter larval rearing tanks were counted at 10, 30, and 60 days posthatch (dph), and the growth and survival rates of hybrid larvae were compared between groups. These assays and experiments were repeated four and five times, respectively, using different batches of fertilized eggs in hybrids and BD.
Detection of parental genomic DNA in hybrids
DNA was extracted using the Gentra Puregene cell kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. To generate PCR primer sets for the detecting of genomic DNA from BD, YD, WC, and Mu, the vasa gene sequence, including the introns and partial coding regions, was amplified using the primer sets in Supplemental Material, Table S1. Amplified genomic DNA fragments were cloned using a TOPO TA cloning kit for sequencing (Invitrogen, Carlsbad, CA) and sequenced. Based on the sequence of vasa (BD, YD, WC, and Mu), common and species-specific primer sets were generated (Table S1), and amplification by thermal cycling was carried out with an initial denaturation for at 94° for 5 min, followed by 30 cycles of 94° for 30 sec, 62° for 30 sec, and 72° for 30 sec, and a final extension at 72° for 5 min. The genomic DNA was amplified using Takara Ex Taq (Takara Bio, Shiga, Japan). PCR products were electrophoresed on 2–3% agarose gels.
Chromosome preparations from embryos and adults
Chromosome slides from hybrid embryos in the gastrulae stage were prepared. A total of 200–300 eggs were collected and dechorionated in 0.005% colchicine for 1 hr, followed by hypotonic treatment in 0.075 M/liter KCl solution for 30 min to obtain a cell suspension. The cells were then fixed in Carnoy’s solution (methanol:acetic acid, 3:1, v/v). Then, liquid samples were dropped on cooled clean glass slides, air-dried, and stained with 15% Giemsa solution diluted with phosphate buffer (pH 6.8).
For the four sciaenid species, juvenile fish at the age of 4–6 months old were injected with 0.1% phytohemagglutinin at a dose of 0.5 ml/100 g of body weight for 12 hr and 0.05% colchicine at a dose of 0.5 ml/100 g of body weight for 3 hr. After this period, fish were anesthetized by tricaine methanesulfonate (MS222), and kidney tissues were collected and placed in hypotonic 0.075 M/liter KCl solution for 30 min. Thereafter, metaphase spreads were obtained as described above. Chromosomes were observed, selected, and photographed under an Olympus uplight microscope BX51 (Olympus).
RT-PCR
Total RNA was extracted from the gonads of BD, WC, and BD-WC hybrid fish using ISOGEN (Nippon Gene). Purified total RNA (1 μg) was reverse transcribed with SUPERSCRIPT III (Invitrogen) using an oligo d(T) primer, as described in the manufacturer’s protocol. Gene expression profiles based on RT-PCR for germ cell markers (vasa, dnd, and dmc1), gonadal somatic cell markers (gsdf, dmrt1, cyp11b, cyp19a1, and foxl2), and internal control [β-actin (actb)] were determined according to the procedure described by Higuchi et al. (2011) using the PCR primers listed in Table S1. Primer sets for each gene were designed using the highly conserved regions of homologs from BD and WC, which were previously obtained and deposited in the National Center for Biotechnology Information GenBank (H. Yoshikawa and Y. Takeuchi, unpublished data).
Thermal cycling was carried out with the following conditions: after initial denaturation for 5 min at 94°; 35 cycles of 30 sec at 94°, 30 sec at 62°, and 30 sec at 72°; and a final extension at 72° for 5 min. The cDNA was amplified using Takara Ex Taq (Takara Bio). PCR products were electrophoresed on 1% agarose gels.
Gonadal anatomy, histology, in situ hybridization, and immunocytochemistry
To evaluate the gonadal maturation of hybrids, gonads were surgically isolated from hybrids aged 6 and 17 months, and the gonadosomatic index (GSI: total gonad weight/total body weight × 100) was calculated.
Histological observation by hematoxylin and eosin (HE) staining and in situ hybridization (ISH) with antisense probes for vasa, gsdf, and cyp11b on the sections of genital ridges, gonads, or whole mounted genital ridges was conducted according to Yazawa et al. (2010). Briefly, the gonads and whole bodies of larvae were fixed with Bouin’s solution and cut into 5 μm-thick sections using standard paraffin embedding methods. The genital ridges of juveniles were removed by fine forceps and mounted on adhesive-coated glass slides (Matsunami Glass Ind., Osaka, Japan) and fixed with Tissue-Tek Ufix (Sakura Fineteck Japan Co., Tokyo, Japan). The cDNA fragments of the conserved 372-bp vasa sequence between BD (nucleotides 1424–1795 bp, GQ404692) and WC (nucleotides 1385–1756 bp, LC317111), the conserved 442- or 445-bp gsdf sequence between BD (nucleotides 26–467 bp, LC317116) and WC (nucleotides 26–470 bp, LC317117), and the conserved 476-bp cyp11b sequence between BD (nucleotides 1–432 bp, LC317120) and WC (nucleotides 1–432 bp, LC317121) were amplified using the primer sets in Table S1, and then used as templates for synthesis of digoxigenin-labeled antisense RNA probes (Roche).
To investigate early gonadal development and proliferation of PGCs in BD-WC hybrid and controls (BD and WC), larvae and juveniles were sampled at 3, 6, 10, 20, 30, 60, and 100 dph. The number of PGCs was counted by observation of serial histological sections from 3 to 20 dph. Excised genital ridges from juveniles at 30 dph were placed on glass slides, fixed with Tissue-Tek Ufix, and hybridized with in situ cRNA antisense probes for BD vasa, and the numbers of vasa-expressing cells in genital ridges were counted.
The proliferation of PGCs in the genital ridges of BD-WC hybrids, BD, and WC at 30 dph was analyzed by double immunostaining with anti-phospho-histone H3 (pH 3) (Ser 10) (Millipore, Bedford, MA) and anti-Vasa. Vasa polyclonal antibody was produced by immunization of a rabbit using a recombinant protein cocktail derived from nucleotides 592 to 608 (VPGDAGFNSSKRNFASS) and 613–630 (GHHGGSFQDNGATSQPA) of BD Vasa (ACV32355). The number of pH 3-positive cells among all Vasa-positive cells (range = 2–50 cells) found in the genital ridges of BD-WC hybrids and those among 30 randomly selected PGCs in the gonads of BD and WC hybrids were reported as a percentage of pH 3-positive PGCs. Apoptotic PGCs in the genital ridges of each fish were detected by double immunostaining of anti-cleaved caspase-3 (Cell Signaling) and anti-Vasa. Gonadal tissue sections and excised gonads were fixed with 4% paraformaldehyde/PBS and Tissue-Tek Ufix, respectively. Samples were treated with Histo VT One solution (Nacalai) for antigen retrieval, blocked with 4% Block-Ace (DS Pharma Biomedical) in PBS, and then incubated with primary antibody. The primary antibodies—anti-Vasa, anti-pH 3 (Ser 10) (Millipore), and anti-cleaved caspase-3—were diluted to 1:1000 in Can Get Signal Immunostain (Toyobo). Secondary antibodies—goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 555 (Invitrogen)—were used according to the manufacturer’s instructions. After DAPI counterstaining, fluorescence images were observed under a confocal microscope (FV1000; Olympus).
Intraperitoneal transplantation of testicular cells into recipient larvae
A transgenic lineage of BD, pHSC-GFP, that carries an enhanced green fluorescent protein (GFP) gene driven by a rainbow trout heat-shock-cognate (HSC) 71 promoter/enhancer, was previously established (Yamamoto et al. 2011). Although expression of the transgene was silenced, pHSC-GFP transgenics maintained a high copy number of transgenes integrated into the host genome (Yamamoto et al. 2011) and provided gynotypable donor germ cells for germ cell transplantation experiments (Yoshikawa et al. 2017). In this study, donor testicular cells were prepared from transgenic BD possessing the pHSC-GFP transgene homozygotically (gfp/gfp). Testicular cells from 3-month-old pHSC-GFP males were dissociated and labeled with PKH26 (Sigma [Sigma Chemical], St. Louis, MO) according to the procedure published by Yoshikawa et al. (2017). Approximately 10,000 cells were transplanted into the peritoneal cavity of 12 dph BD-WC hybrid larvae (n = 418) and BD larvae (n = 100, as control), using the method described in Takeuchi et al. (2009).
To evaluate the incorporation of transplanted testicular cells in recipient genital ridges at 26 dph (i.e., 14 days after transplantation), the digestive organs and the heads of transplanted recipients were dissected out, the remaining body was fixed with Tissue-Tek Ufix containing 28.6 mM DAPI for 5 min on ice, and gonads were further removed by fine forceps. Then, as described in Takeuchi et al. (2009), a germ cell-specific nuclear morphology of PKH26-labeled cells incorporated into the recipient gonads was observed with a confocal microscope (FV1000; Olympus). The following parameters were analyzed under a fluorescent microscope (BX-51): (1) incorporation rates (number of individuals with PKH26-labeled germ cells in the genital ridges/number of observed individuals × 100) and (2) the number of PKH26-labeled germ cells in the gonads. Survival rates of transplanted larvae were also calculated at 26 dph. The expression of BD vasa was analyzed by ISH on excised genital ridges of 30-dph transplanted recipient BD-WC hybrids, as described in Yazawa et al. (2010). Transplantation experiments were repeated three times, and at least eight recipients were used for fluorescence observation for each replicate.
Hybrid recipients transplanted with testicular cells were reared until 6 months, and then semen was collected by applying gentle pressure to the abdomen. DNA was extracted from the semen and subjected to PCR with gfp-specific primers, as previously described (Yoshikawa et al. 2017). After evaluation of the concentration and activity of sperm, as described in Yoshikawa et al. (2017), semen from gfp-positive recipients was used to fertilize eggs obtained from wild-type BD females, and then hatching larvae were analyzed by DNA analysis using gfp-specific primers to determine the production of offspring from spermatozoa derived from donor spermatogonia. Gonadal maturation of transplanted BD-WC hybrid recipients and the production of donor-derived gametes were evaluated by histological observation, GSI, and DNA analysis using gfp-specific primers.
Statistical analysis
Data are presented as mean ± SEM. One-way ANOVA followed by Tukey’s test was used to determine significant differences among group means (GraphPad Prism 5; Graph-Pad Software, San Diego, CA). Two group means were analyzed by Student’s t-test. Regarding sex ratios, the χ2 test was used to test significant difference from a hypothesis of a 1:1 sex ratio. For all statistical tests, differences were considered to be statistically significant when calculated P-values were < 0.05. Means with different letters (A/a) indicate significant difference between the groups.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Fishes are available on request. Table S1 lists primers.
Results
Viability and fertility of F1 hybrid sciaenids generated from BD eggs
To produce hybrid embryos, an average of 23,000 eggs (range, 4500–44,000 eggs) collected from BD females were inseminated with semen from YD, WC, Mu, and BD (control) fish (Figure 1A). As shown in Figure 1B, fertilization and hatching rates for triplicate hybrid crosses (BD-YD hybrid, BD-WC hybrid, and BD-Mu hybrid) and the control cross (BD) ranged from 29 to 56% and 10–26%, respectively, and no significant differences were observed among the groups. However, all BD-Mu hybrids died by 10 dph in all three trials (Figure 1C). Although no significant differences were observed in survival rates between BD-YD hybrids (32.7 ± 5.4%), BD-WC hybrids (15.0 ± 7.5%), and control BD larvae (32.6 ± 7.2%) at 10 dph (P > 0.05), BD-WC hybrids showed the lowest survival rates (Figure 1C). A similar trend was observed in the survival rates of juveniles at 30 and 60 dph (P > 0.05, Figure 1E).
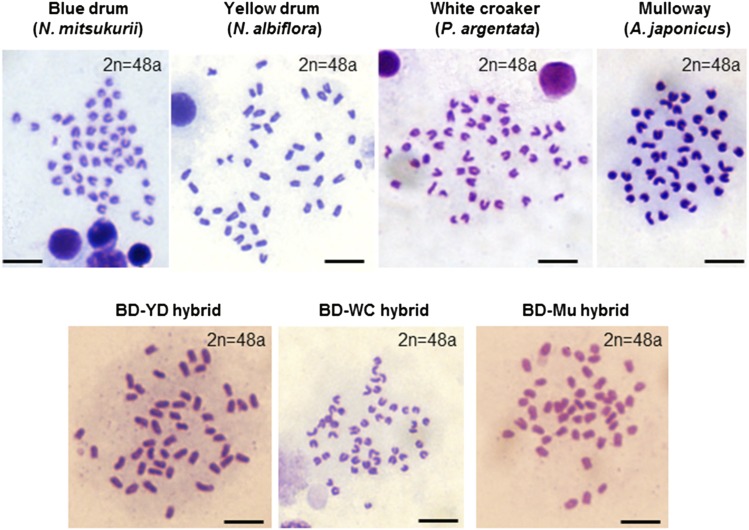
PCR analysis with species-specific primers designed against the vasa genes, including intron regions from individual larvae (n = 30) from each cross, confirmed that all larvae possessed both dam (BD)- and sire (YD, WC, or Mu)-specific sequences, indicating the production of interspecific hybridization in every cross in this study (Figure 1D). In addition, the four sciaenid species exhibited identical karyotypes (2n = 48a), and no abnormalities in chromosome number were observed in hybrid larvae (2n = 48a) (Figure 2).
Figure 2.
Metaphase plates prepared from adult kidneys of individuals of each species and gastrula embryos of each hybrid. “a” of 2n = 48a indicates acrocentric chromosome. Bar, 5 μm. BD, blue drum; Mu, mulloway; WC, white croaker; YD, yellow drum.
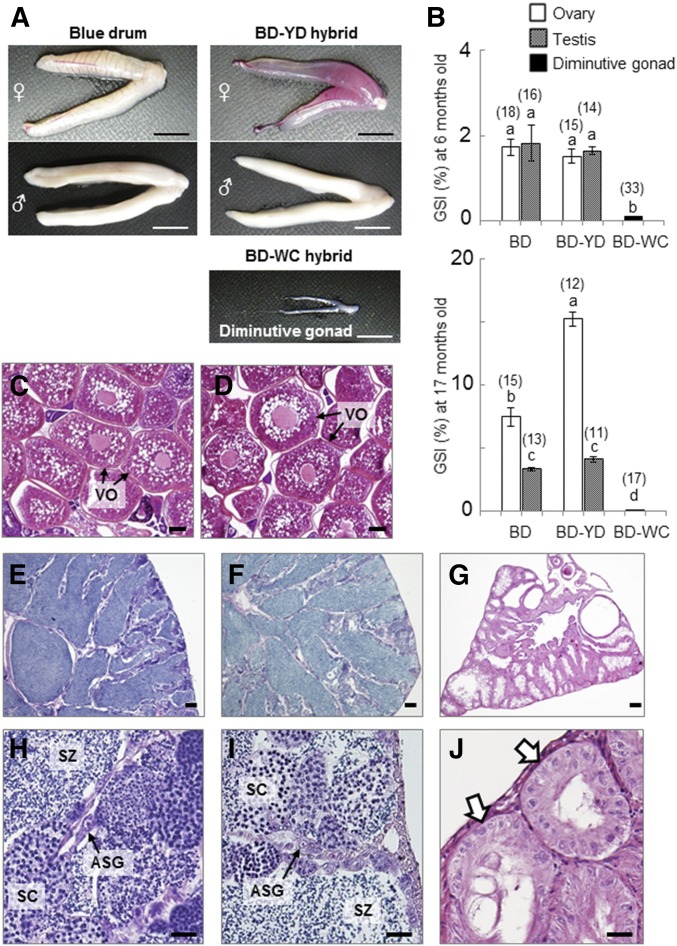
Evaluation of gonadal maturation of BD-YD and BD-WC hybrids at the first (6 months) and second (17 months) spawning seasons by gonadal anatomy and histology showed differences between the two hybrids. For the BD-YD hybrids, the external morphologies of ovaries, testes, and GSI at both spawning seasons were not significantly different from those of the control BD (Figure 3A, top right and Figure 3B) (P > 0.05). Sex ratios of both BD-YD hybrids and BD fish were nearly 50:50 (15 females and 14 males in BD-YD hybrids, and 18 females and 16 males in BD at 6 months). Full-grown vitellogenic oocytes and free spermatozoa were observed in the histological sections of ovaries and testes of both BD fishes (Figure 3, C, E, and H, respectively) and the BD-YD hybrids (Figure 3, D, F, and I, respectively). In addition, eggs and sperm from BD-YD hybrid females (n = 3) and males (n = 21) were used for artificial insemination with sperm and eggs of BD fish, respectively. The fertilization and hatching rates of BD-YD gametes were equivalent to the controls using BD gametes and the F2-hatchlings showed normal external morphology (data not shown).
Figure 3.
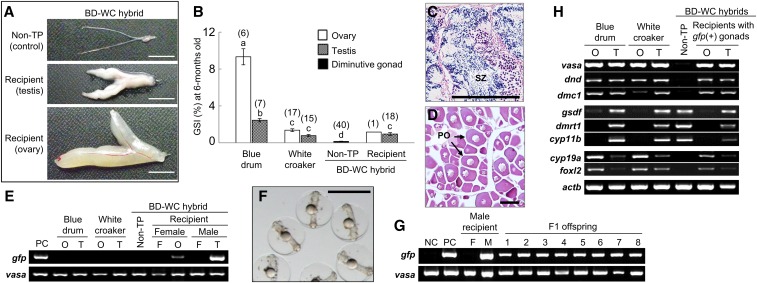
Gonadal development of BD fish and hybrids. (A) Extrarenal morphologies of gonads collected from BD, BD-YD, and BD-WC hybrids at 6 months. (B) GSI at 6 and 17 months. Numbers of weighed gonads in each group are indicated above the bars. Data are shown as mean ± SEM. Different letters indicate statistically significant differences (P < 0.05). (C–G) Histological sections of gonads of each fish at 6 months. Ovaries of BD (C) and BD-YD hybrid (D). Testes of BD (E and H) and BD-YD hybrid (F and I). Diminutive gonad of BD-WC hybrid (G and J). Arrows indicates lobule-like structure of germ cell-less gonads of BD-WC hybrid. Bars, 10 mm (A), 100 μm (C–G), and 20 μm (H–J). ASG, type-A spermatogonia; BD, blue drum; GSI, gonadosomatic index; Mu, mulloway; SC, spermatocyte; SZ, spermatozoa; VO, vittelogenic oocyte; WC, white croaker; YD, yellow drum.
In contrast, all of the BD-WC hybrids had diminutive gonads (Figure 2A, bottom right). GSI of these diminutive gonads at 6 and 17 months was 0.05 ± 0.01 (n = 33) and 0.08 ± 0.01 (n = 17), respectively, which is significantly lower than that of BD and BD-YD hybrids (P < 0.01) (Figure 3B). Neither spermiation nor ovulation was observed in BD-WC hybrids over two consecutive spawning seasons. Surprisingly, histological analysis revealed that all diminutive gonads had lobule-like structures, which is a typical morphological characteristic of teleost testes, but lobules were cavitated and no spermatogenic cells, including spermatogonia, were present (Figure 3, G and J).
Confirmation of the germ cell-less phenotype and testis-like gene expression pattern in gonads of BD-WC hybrids
Gene expression profiling of testes of BD and the diminutive gonads of BD-WC hybrid individuals at 4 months, by ISH using cRNA probes and RT-PCR using primers designed against the conserved regions between BD and WC, revealed differences in the localization of cell types and differences in the expression of germ cell markers. In ISH with vasa, a germ cell marker, probes on serial sections showed vasa mRNA to be localized in both type-A (ASG) and type-B (BSG) spermatogonia cells in the testes of BD fish (Figure 4A, top left), but no vasa-positive cells were observed in the gonads of the BD-WC hybrids (Figure 4A, top right). In contrast, expression of gsdf was confirmed in somatic cells surrounding ASG and BSG in BD testes (Figure 4A, middle left) and cells located inside of lobule-like structures in BD-WC hybrid gonads (Figure 4A, middle right). Distribution patterns and numbers of cyp11b-expressing cells, a molecular marker for Leydig cells, in the interstitial tissues adjacent to the lobules of diminutive gonads of BD-WC hybrids were similar to those of BD testes (Figure 4A, bottom left and right).
Figure 4.
Expression of germ cell- and gonadal somatic cell-specific genes in the testes of BD and WC fish and the diminutive gonads of BD-WC hybrids. (A) In situ hybridization images of vasa, gsdf, and cyp11b, and HE staining of its neighboring histological section. Arrowheads indicate positive signals obtained by gsdf and cyp11b antisense probes and the corresponding cells in HE staining. Red broken lines in the gsdf image of BD testis show the cyst structure surrounding spermatogonia. Bar, 10 μm. (B and C) RT-PCR results for germ cell markers vasa, dnd, and dmc1 (B), and testicular somatic cell markers gsdf, dmrt1, and cyp11b and ovarian somatic cell markers cyp19a and foxl2 (C), in O and T of BD and WC fish and diminutive gonads of four individual BD-WC hybrids (lanes 1–4). β-actin (actb) is used as an internal control. ASG, type-A spermatogonia; BD, blue drum; BSG, type-B spermatogonia; HE, hematoxylin and eosin; O, ovaries; T, testes; WC, white croaker.
RT-PCR analysis confirmed no expression of germ cell markers (vasa, dnd, and dmc1) in gonads of 4-month-old BD-WC hybrids (Figure 4B). Testis-specific expression of dmrt1 and cyp11b, and higher expression levels of gsdf in testes than ovaries, were observed in both BD and WC fish (Figure 4C). In BD-WC hybrid gonads (Figure 4C), expression of supporting somatic cell markers in testes—gsdf, dmrt1, and cyp11b—was detected, but those in ovaries, i.e., cyp19a and foxl2, were not detected. In addition to histological analysis and the cellular distribution patterns of germ cell and gonadal somatic cell markers, germ cell-less and testis-like gene expression patterns were confirmed in the diminutive gonads of BD-WC hybrids.
Early development of PGCs and genital ridges of BD-WC hybrids
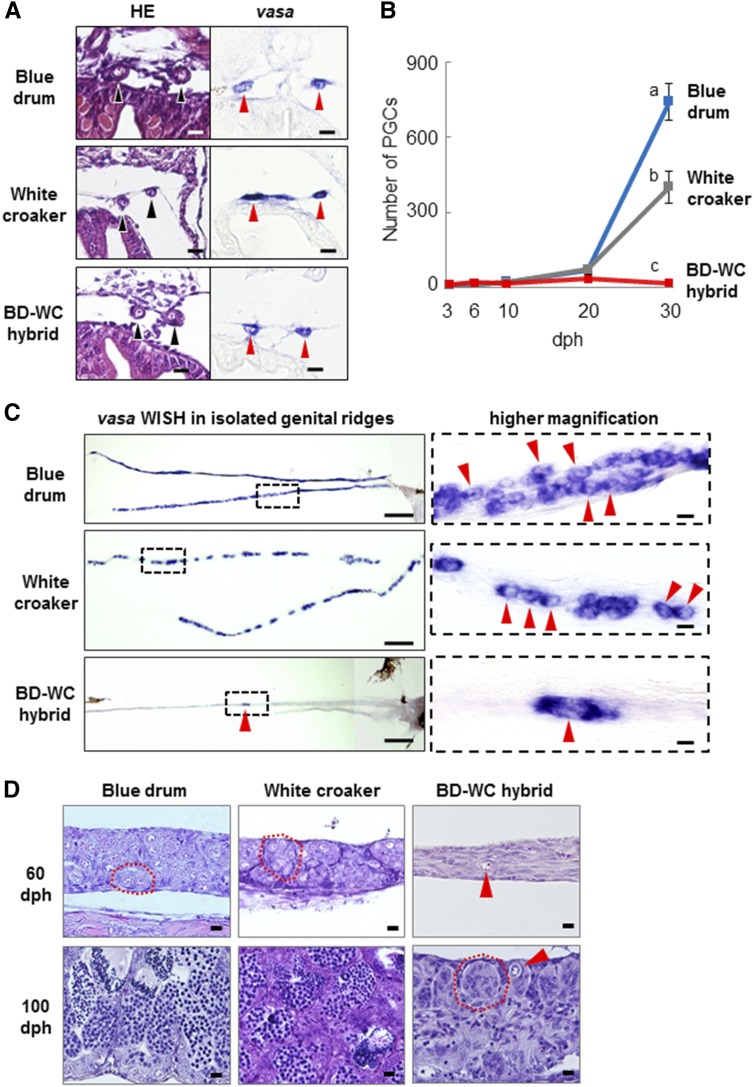
The development of PGCs and genital ridges in BD-WC hybrid larvae and juveniles was investigated during the period from 3 to 100 dph and compared to that of control larvae (BD and WC). PGSs of the hybrid larvae at 10 dph were located bilaterally on the dorsal side of the body cavity (Figure 5A), where genital ridges form by 17 dph [as shown in figure 2 of Takeuchi et al. (2009)]. In addition, PGCs of 10-dph BD-WC hybrid larvae normally expressed vasa mRNA (Figure 5A, bottom right).
Figure 5.
Early gonadal development of BD, WC, and BD-WC hybrid fish. (A) Transverse sections for each larva at 10 dph. Sections were stained with HE (left) and hybridized with vasa-antisense probe (right). Arrowheads indicate PGCs settled bilaterally on the dorsal part of the body cavity in the position where the genital ridges will be formed after a few days. (B) Number of PGCs per individual at 3, 6, 10, 20, and 30 dph. Average number of individuals (n = 5–15) at each age was used for measurement of PGCs. Data are shown as mean ± SEM. Significant reduction of PGC numbers in BD-WC hybrids compared to BD and WC controls was found only at 30 dph (P < 0.05). (C) Distribution of endogenous PGCs in the gonad at 30 dph. Whole genital ridges isolated on the slide glass were hybridized with vasa antisense probe. Right panels are higher magnification views of the areas enclosed in dashed boxes in the left panels. Arrowheads indicate PGCs. (D) Sagittal sections of newly differentiated testis of BD, WC, and BD-WC fish at 60 and 100 dph. Arrowheads indicate isolated germ cells in BD-WC gonads. Red dashed lines indicate lobule structures in the testes. Bars, 10 μm in (A) and right panels of (C and D), 200 μm in left panels of (C). BD, blue drum; dph, days posthatching; HE, HE, hematoxylin and eosin; PGCs, primordial germ cells; WC, white croaker.
For the period from 3 to 20 dph, the numbers of PGCs per individual in the hybrids and control larvae (BD and WC) were counted using whole-body serial sections. As shown in Figure 5B, no significant differences were observed in the number of PGCs in hybrid larvae and those of controls at each developmental stage to 20 dph (n = 5 in each sample). For example, the number of PGCs at 10 dph was 15.2 ± 4.1 cells (n = 6) in the hybrid, which was not significantly different from that of BD (22.7 ± 3.4 cells, n = 6) and WC (20.3 ± 2.1 cells, n = 6) (P > 0.05) (Figure 5B). At 30 dph, the number of PGCs remained steady in the BD-WC hybrid (18 ± 3 cells, n = 15), while the numbers significantly increased in BD (740 ± 70 cells, n = 8) and WC (400 ± 65 cells, n = 15) larvae (P < 0.05) (Figure 5B). The distribution of vasa-expressing cells at 30 dph was observed by whole-mount ISH using the vasa probe on the isolated genital ridges. PGCs in controls (BD and WC) showed wide distribution in the whole of the genital ridges (Figure 5C, top and middle), but only a small number of PGCs were localized mostly in one or two parts of the genital ridges of the hybrids (Figure 4C, bottom).
Gonadal sex differentiation of BD is known to occur at ∼50 dph (Takeuchi et al. 2009). In this study, the distribution of germ cells in the gonads of hybrid juveniles was compared to the newly differentiated testes of control juveniles at 60 and 100 dph by histology (Figure 5D). In the control BD and WC testes, increased numbers of spermatogonia and formation of testis-specific lobule structures were observed at 60 dph, and spermatogenesis took place within the lobules at 100 dph. In contrast, germ cells in the hybrid gonads at both 60 and 100 dph were present as single cells (Figure 5D, top and bottom right), and these gonads maintained similar numbers of germ cells (14 ± 6 cells; range, 0–41 cells, n = 9) as those at 30 dph. Lobule structure was first observed in the germ cell-less gonads of hybrids at 100 dph (Figure 5D, bottom right).
Mitotic arrest of PGCs in the genital ridges of BD-WC hybrids
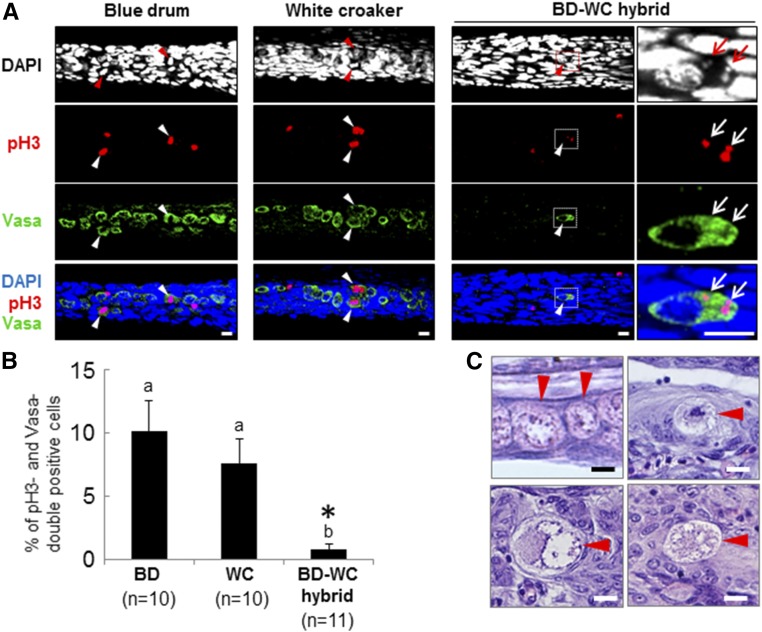
To test the basis of the reduced germ cell numbers in the genital ridges of BD-WC hybrids, cell proliferation and apoptosis of PGCs were examined at 30 dph by whole-mount immunohistochemistry using a mitotic marker, anti-phospho-Histone H3 (Ser10) antibody (anti-pH 3) (Figure 6A), and an apoptosis marker, anti-cleaved caspase-3 antibody. The percentage of pH 3-positive cells among the total number of PGCs was determined by counts following anti-Vasa staining for each larva (Figure 6B). Nuclear staining of pH 3 was observed in 10.1 ± 2.4% and 7.6 ± 1.9% of PGCs in the genital ridges of BD (n = 10) and WC (n = 10) controls, respectively. Nuclear staining of pH 3 was not observed in the PGCs of BD-WC hybrids (n = 11), but 0.8 ± 0.4% of PGCs showed colocalization of pH 3 staining with DAPI in the small extra nuclei within the cytoplasm (Figure 6A, right column), suggesting that lagging or damaged chromosomal fragments were produced in the PGCs of the hybrids. Although cleaved caspase-3-positive PGCs were not observed in the genital ridges of hybrids and controls at 30 dph (data not shown), germ cells with condensed or abnormal nuclei were observed only in the genital ridges of BD-WC hybrids by HE staining at 30 dph (Figure 6C).
Figure 6.
Proliferation and morphological characteristics of PGCs in the gonads of BD, WC, and BD-WC hybrids at 30 dph. (A) Whole-mount immunohistochemistry using anti-pH 3 (pH 3) and anti-Vasa (Vasa) antibodies. Cell nuclei are counterstained with DAPI. Arrowheads in BD and WC samples indicate pH 3-positive PGCs with normal nuclei. An arrowhead in BD-WC hybrids indicates a pH 3-positive PGC with an aberrant nucleus. Right panels of BD-WC hybrids are higher magnification views of the areas enclosed in dashed boxes in the left panels. Arrows indicate small extra nuclei within the cytoplasm of hybrid PGCs. (B) Appearance rates of pH 3-positive PGCs in BD, WC, and BD-WC genital ridges at 30 dph. Data are shown as mean ± SEM. Different letters indicate statistically significant differences (Tukey’s test, P < 0.05). *, PGCs possessing abnormal pH 3 staining, as shown in (A), are included in the data. (C) Normal PGCs of BD (left top) and abnormal PGCs with condensed (right top) or abnormal nuclei (bottom left and right) of the BD-WC hybrids at 30 dph. Arrowheads indicate PGCs. Bars, 10 μm (A), 5 μm (C). BD, blue drum; dph, days posthatching; PGCs, primordial germ cells; WC, white croaker.
Colonization of donor-derived BD spermatogonia in the genital ridges of sterile hybrid recipients
To examine whether the proliferative defect of hybrid PGCs occurred cell autonomously or was caused by incompatibility of gonadal somatic cells supporting gem cell development, the development of normal (nonhybrid) BD germ cells in the gonads of the BD-WC hybrids was studied using intraperitoneal germ cell transplantation. ASG were prepared from immature testes of 3-month-old transgenic BD homozygous for pHSC-GFP (gfp/gfp) (Figure 7A). Dissociated BD testicular cells, labeled with PKH26 (Figure 7B), were transplanted into the peritoneal cavities of the hybrids at 12 dph (TL 4–5 mm) over the course of eight independent experiments. The same donor cells were transplanted into 12-dph BD recipients (as a control). At 14 days after transplantation (26 dph), colonization of BD-WC hybrid recipient genital ridges by PKH26-labeled donor germ cells with large nuclei was observed by confocal microscopy (Figure 7, C and D); such large nuclei are characteristics of ASG, based on HE staining (Figure 7A).
Figure 7.
Colonization of donor BD-derived spermatogonia labeled by PKH26 fluorescent dye in BD-WC hybrid recipient gonads. (A) HE staining of the immature testis of a 3-month-old BD donor consisted of only ASG (arrowheads). (B) Bright-field (left) and fluorescent (right) images of dissociated testicular cells labeled with PKH26. Large cells (arrowheads) possess large and round nuclei, which is a typical morphological characteristic of ASG. (C) Merged image of PKH26 and DAPI staining of a genital ridge of a transplanted BD-WC hybrid recipient at 14 days post-transplantation (26 dph) taken by confocal microscopy. PKH26-positive (red) cells with large and round nuclei are donor-derived germ cells (arrowheads). (D) Higher magnification view of a dashed box in (C). (E) Distribution of PKH26-labeled cells (top) and expression of donor-derived BD vasa visualized by whole-mount ISH (bottom) in the excised genital ridges of the transplanted BD-WC recipient at 18 days post-transplantation (30 dph). PKH26- and vasa-double-positive cells are indicated by asterisks as donor-derived germ cells colonized in the genital ridges of a BD-WC hybrid recipient. (F) View of the entire genital ridge hybridized with the vasa-antisense probe (E). Left is the anterior side of the genital ridge. Bars, 10 μm (A–D) and 100 μm (E and F). ASG, type-A spermatogonia; BD, blue drum; dph, days posthatching; HE, hematoxylin and eosin; ISH, in situ hybridization; WC, white croaker.
The survival rate of transplanted BD-WC hybrid recipients at 26 dph (34.7 ± 6.1%, n = 8) was lower than that of BD recipients (68.1 ± 12.5%, n = 3) (Student’s t-test, P < 0.05). The percentage of BD-WC hybrid recipients with colonized PKH26-labeled germ cells (58.6 ± 5.1%, n = 8) was comparable to that of BD recipients (58.3 ± 11.0%, n = 3). In addition, there were no significant differences in the number of colonized germ cells in the genital ridges of the BD-WC hybrids (9.4 ± 2.6 cells, n = 26) and BD recipients (17.1 ± 7.3 cells, n = 14) at 26 dph (Student’s t-test, P > 0.05).
Whole-mount ISH of isolated recipient genital ridges at 30 dph with the antisense vasa cRNA probe demonstrated vasa expression in PKH26-labeled cells (Figure 7E), proving that they were donor-derived germ cells. Incorporated donor germ cells increased in number and became distributed over the entire genital ridges in BD-WC hybrid recipients at 30 dph (Figure 7F), suggesting that donor germ cells surrounded by gonadal somatic cells of hybrid recipients proliferated after incorporation.
Gonadal development of sterile hybrid recipients following intraperitoneal germ cell transplantation
Gonadal development and donor-derived gametogenesis were evaluated in transplanted hybrid recipients after rearing for 6 months. Among the 125 hybrid recipients, 43 (34.4%) had matured testes (Figure 8A middle) with free spermatozoa (Figure 8C) and 1 (0.8%) had an ovary (Figure 8A bottom) with peri-nucleolus oocytes with cell diameters of 150–200 μm (Figure 8D). The male–female sex ratio (%) of the transplanted BD-WC hybrid recipients possessing developing gonads was 98 (n = 43) to 2 (n = 1), although no significant differences from 50:50 sex ratios were confirmed in the control BD (male = 119, female = 110) and WC (male = 51, female = 52) fish by χ2 tests (P > 0.05). Gonads of the other 81 (64.8%) transplanted BD-WC hybrid recipients showed similar external morphology to nontransplanted BD-WC hybrids (i.e., diminutive gonads, Figure 8A, top).
Figure 8.
Gonadal development, fertility, and gonadal gene expression profile of transplanted BD-WC hybrid recipients at 6 months. (A) External morphology of gonads from BD-WC hybrids (top) and transplanted BD-WC hybrid recipients (middle and bottom). (B) GSI of BD, WC, nontransplanted BD-WC hybrids, and both gfp-positive testes and an ovary of transplanted BD-WC hybrids. Numbers of fish analyzed are indicated above each bar. Data are shown as mean ± SEM. Different letters indicate statistically significant differences (Tukey’s test, P < 0.05). (C and D) HE staining of testis (C) and ovary (D) of transplanted BD-WC hybrid recipients shown in (A). (E) Genomic DNA amplification of gfp gene, a genotype of donor cell, in the O, T, and F of BD, WC, and both nontransplanted and transplanted BD-WC hybrids. Fin of pHSC-GFP transgenic BD was used as PC. Primers designed for conserved vasa sequence among sciaenid species are used as an internal control. (F) F1 offspring obtained from the eggs of wild-type BD female inseminated with sperm of a transplanted BD-WC hybrid recipient male. Bars, 10 mm (A), 100 μm (C and D), and 1 mm (F). (G) Detection of gfp gene in the F, M, and F1 offspring (lanes 1–8) of a transplanted BD-WC hybrid recipient male. DNA from milt of wild-type BD and pHSC-GFP BD are used as NC and PC, respectively. vasa, internal control. (H) RT-PCR results for germ cell markers vasa, dnd, and dmc1; testicular somatic cell markers gsdf, dmrt1, and cyp11b; and ovarian somatic cell markers cyp19a1 and foxl2 in O and T of BD, WC, and Non-TP and transplanted BD-WC hybrid recipients possessing gfp-positive gonads. Actb, internal control. BD, blue drum; F, fin; GSI, gonadosomatic index; HE, hematoxylin and eosin; M, milt; NC, negative control; Non-TP, nontransplanted; O, ovary; PC, positive control; PO, peri-nucleolus oocyte; SZ, spermatozoa; T, testis; WC, white croaker.
Genomic DNA extracted from fin, gonads, and ovaries of the BD-WC hybrid recipients harboring germ cells, nontransplanted BD-WC hybrids, and BD and WC controls was subjected to PCR with gfp primers, together with a set of vasa primers designed against a conserved sequence between BD and WC fish as a positive internal control for the amplification of genomic DNA. The presence of the gfp gene was evident in all recipient gonads harboring germ cells (43 males and 1 female), but not in the fin or other samples (Figure 8E).
The GSI of transplanted hybrid recipients possessing gfp-positive testes (0.95 ± 0.15, n = 18) was significantly higher than that of nontransplanted hybrids (0.15 ± 0.03, n = 40) and significantly lower than for BD (2.45 ± 0.18, n = 7), but not significantly different from WC fish (0.78 ± 0.11, n = 15, P < 0.05) (Figure 8B). One gfp-positive ovary observed in a transplanted hybrid recipient also showed a similar GSI (1.17, n = 1) to WC females (1.37 ± 0.15, n = 17).
Production of donor-derived BD sperm by sterile hybrid recipients
The production of donor-derived sperm by each recipient male at 6 months (n = 43) was investigated by PCR of genomic DNA using gfp-specific primers. All milt collected from each male recipient was positive for the gfp gene (Figure 8G).
Although sperm concentration in hybrid recipient males (1.4 ± 0.5 × 109 cells/ml, n = 3) was ∼60% lower than in control BD males (3.6 ± 0.1 × 109 cells/ml, n = 3) (Student’s t-test, P < 0.05), no significant differences in the duration of sperm moving time was observed between the BD-WC hybrid recipient males (395 ± 18 sec, n = 3) and control BD males (345 ± 23 sec, n = 3). In addition, > 80% of sperm in the microscopic field were found to be motile in both hybrid recipients and BD.
Collected milt samples from hybrid recipients (n = 3) and BD controls (n = 3) were used to fertilize eggs from wild-type (nontransgenic) BD females. Fertilization and hatching rates are summarized in Table 1. F1 embryos developed normally (Figure 8F), and no significant differences were observed between the average fertilization and hatching rates obtained using sperm of BD-WC hybrid recipients (87.5 ± 6.34% and 63.4 ± 16.1%, respectively) and that obtained using sperm of BD controls (66.7 ± 8.8% and 36.1 ± 9.7%, respectively) (Student’s t-test, P > 0.05) (Table 1).
Table 1. Progeny tests and rate of gfp-positive offspring among the F1 generation produced by the BW-WC hybrid recipient male transplanted with spermatogonia of pHSC-GFP transgenic BD fish (gfp/gfp).
| Males | Females | Number of eggs | Fertilization | Hatching (%) | Number of F1 offspring analyzed | gfp+ (%) |
|---|---|---|---|---|---|---|
| Hybrid recipient 1 | Blue drum 1 | 2400 | 75.0 | 33.3 | 32 | 100 |
| Hybrid recipient 2 | Blue drum 2 | 1520 | 92.1 | 88.8 | 32 | 100 |
| Hybrid recipient 3 | Blue drum 3 | 880 | 95.5 | 68.2 | 32 | 100 |
| Blue drum 4 | Blue drum 1 | 8600 | 83.7 | 46.5 | — | — |
| Blue drum 5 | Blue drum 2 | 2000 | 53.8 | 45 0 | — | — |
| Blue drum 6 | Blue drum 3 | 1200 | 62.5 | 16.7 | — | — |
Blue drum 1–6 are wild-type (nontransgenic) fishes.
Genomic DNA analysis using gfp-specific primers showed that all F1 hatchlings from three hybrid recipient males (32 hatchlings per each male) were positive for gfp (Figure 8G and Table 1). This result indicates that the transplanted hybrid recipients only produced functional sperm of donor transgenic BD (gfp/gfp) fish and that the gonadal microenvironment of the germ cell-less BD-WC hybrid recipients was capable of producing functional sperm.
Gene expression profile of the recipient gonads harboring donor-derived germ cells
Gene expression profiles of gfp-positive testes and ovaries of hybrid recipients were investigated using molecular markers for germ cell (vasa, dnd, and dmc1) and gonadal somatic cell markers dominantly expressed in males (gsdf, dmrt1, and cyp11b) and females (cyp19a1 and foxl2) (Figure 8H). Consistent with the results of gonadal histology (Figure 8, C and D), molecular markers for germ cells in mitotic (vasa and dnd) and meiotic (vasa and dmc1) stages were expressed in all gfp-positive gonads but not in the gonads of nontransplanted hybrids. Somatic cell markers for Sertoli cells (gsdf and dmrt) and Leydig cells (cyp11b) were detected in gfp-positive testes. As previously described in Figure 4C, these genes were also expressed in the diminutive gonads of nontransplanted BD-WC hybrids. In contrast, cyp19a1 and foxl2, which were not detected in the gonads of nontransplanted hybrids, were expressed in the gfp-positive ovaries of transplanted hybrid recipients, suggesting that differentiation of granulosa cells occurred under the presence of germ cells in the germ cell-less gonads of the hybrid.
Discussion
Interspecific hybridization between four marine sciaenid species resulted in three hybrids with the following outcomes: fertile (BD-YD hybrid), sterile (BD-WC hybrid), and lethal (BD-Mu hybrid). In sterile BD-WC hybrid larvae, a similar number of PGCs as in BD and WC larvae was observed during the migration period and in newly formed genital ridges, indicating that cell fate specification of PGCs, migration of PGCs to the future gonadal region, and the formation of genital ridges were not affected by interspecific hybridization. However, hybrid PGCs did not proliferate in genital ridges, nor did they show normal morphology or numbers. Notably, an intraperitoneal germ cell transplantation experiment revealed that functional gonads fully supporting gametogenesis of donor-derived BD germ cells could be reconstructed by transplantation of BD germ cells into the gonadal somatic cells of BD-WC hybrids. These results show that the proliferative defects of hybrid PGCs were caused by cell autonomous mechanisms. To our knowledge, this is the first report of hybrid sterility caused by impaired mitotic division of PGCs but not by aberrant chromosome pairing during meiosis.
By intergeneric crossing, BD-WC hybrids lost fertility and BD-Mu hybrids lost viability, while intrageneric crossing resulted in fertile hybrids (BD-YD hybrids). Recent molecular phylogenetic analysis showed an estimated time to the common ancestor of genus Nibea, including BD and YD, of 18.2 MY, and Mu and that of genus Nibea and WC of 15 MY (Lo et al. 2015). The classical isozyme analysis also showed that the genetic distance of two species of Nibea (BD and YD) was more or less equal to that at the subspecies level (Menezes and Taniguchi 1988). Thus, based on experimental data for the three sciaenid hybrid patterns shown in this study, fertility and viability were attributable to the extent of phylogenetic relatedness between the parent species.
In contrast to mouse PGCs, fish PGCs do not proliferate during migration, but they become mitotically active in genital ridges and increase in number up to 10–100 times that prior to sex differentiation (Braat et al. 1999; Molyneaux and Wylie 2004). In this study, PGCs of BD-WC hybrids did not proliferate in the genital ridges and showed abnormal nuclear morphology similar to apoptotic cells. Germ cells may have unique mechanisms that are not present in somatic cells that inhibit mitotic cell divisions when the chromosomes contain the genetic material of a different species. From a cytological point-of-view, further study will be needed to reveal the cell cycle phase of mitotically arrested hybrid PGCs and the molecular mechanisms underlying incompatible mitotic cell division in hybrid PGCs. It would be interesting to examine whether proliferative defects of PGCs in hybrid animals could be observed in other hybrid animals and act as a postzygotic reproductive barrier for maintaining speciation in nature.
At the adult stage, all germ cell-less gonads of BD-WC hybrids showed testis-like lobule structures. In addition, gene expression patterns confirmed by RT-PCR and ISH revealed that these gonads still maintained Sertoli cells and Leydig cells, which play pivotal roles in supporting male germ cell development and controlling the endocrine system related to spermatogenesis, while no expression of ovarian marker genes was detected. In several fish species, it is known that either the quantity or proliferation pattern of PGCs in juvenile fishes influences the sexual differentiation of gonads. In zebrafish, ablation of PGCs by knockdown or knockout studies for the dead end (dnd) gene promotes the masculinization of genetically female fish (Ciruna et al. 2002; Slanchev et al. 2005; Dai et al. 2015; Li et al. 2017). Further, during the embryonic stage of zebrafish, a certain number of PGCs must be present in the genital ridges for the subsequent ovarian development in females to successfully proceed (Tzung et al. 2015). In genetically female medaka Oryzias latipes, high-temperature treatment during larval stages results in elevated cortisol levels, an indicator of stress, retarded PGC proliferation, and the subsequent development of undifferentiated gonads into testes (Selim et al. 2009; Kitano et al. 2012; Nakamura et al. 2012). Thus, it was suggested that mitotic arrest of PGC proliferation in the genital ridges of BD-WC hybrids occurring before the dimorphic sex differentiation stages (20–30 dph) was the major factor behind the appearance of germ cell-less testis-like gonads.
At present, several different approaches to germ cell transplantation techniques, either within the same species (allogenic) or between two different species (xenogenic), have been established in fish (Yoshizaki et al. 2011; Lacerda et al. 2013) and are widely used to study germ cell biology, such as the function of germline stem cells (Yoshizaki et al. 2012; Hayashi et al. 2014). These techniques also have applications in the conservation of endangered species (Lee et al. 2015; Pšenička et al. 2015; Lee and Yoshizaki 2016; Ye et al. 2017), the propagation of valuable fishes with economically useful traits (Morita et al. 2012, 2015; Silva et al. 2016), the cryopreservation of scientific bioresources (Lee et al. 2013, 2016a,b; Seki et al. 2017), and the efficient production of genetically engineered fish (Tonelli et al. 2017). For the purpose of germ cell transplantation experiments, sterilized recipients are useful for obtaining successful colonization of donor germ cells in recipient genital ridges and gonads, and the efficient production of donor-derived gametes without contamination of recipient-derived gametes. Triploidization is a simple approach to produce functionally sterile recipients for germ cell transplantation in various fish species (Okutsu et al. 2007; Yoshikawa et al. 2017). In our previous studies, transplanted triploid BD recipients produced only allogenic donor-derived gametes (Yoshikawa et al. 2017), but they failed to produce xenogenic donor-derived gametes (H. Y. and Y. T., unpublished data). As a characteristic of triploid fish (Piferrer et al. 2009), triploid germ cells continue to underproliferate mitotically and occupy the germ cell niche in the gonads, although they cannot complete meiosis. Thus, competition for the niche between endogenous triploid germ cells and transplanted diploid donor germ cells may be present in the triploid gonads and possibly cause elimination of genetically “less compatible” xenogenic donor cells. Notably, the fertility (i.e., the size of testes and the quantity and quality of sperm) of male BD-WC hybrid recipients transplanted with BD spermatogonia was shown to be recovered in this study, suggesting that the germ cell-less hybrids obtained in this study would have more available niches for donor germ cells. In marine fish, the production of triploids by preventing second polar body exclusion using heat or pressure shocks is technically more difficult than in freshwater fish because of their small egg sizes, unstable egg quality, and high mortality at early stages (Felip et al. 1997; Kaji et al. 1999). Recently, germ cell-less fish have been obtained by dnd-knockdown and -knockout studies in several freshwater fishes, such as zebrafish (Weidinger et al. 2013; Li et al. 2017), loach Misgurnus anguillicaudatus (Fujimoto et al. 2010), goldfish Carassius auratus (Goto et al. 2012), sterlet Acipenser ruthenus (Linhartová et al. 2015), and salmon species (Wargelius et al. 2016; Yoshizaki et al. 2016); however, microinjection experiments of antisense morpholinos or reagents for genome editing to marine fish embryos also require high levels of skill and advanced equipment. Since hybridization can be achieved only by artificial insemination and the usual rearing of the resulting hybrid embryos, hybrid fish showing the germ cell-less phenotype would be suitable recipients for germ cell transplantation, not only in sciaenids but also in other marine fishes.
One of the BD spermatogonia-transplanted BD-WC hybrid recipients possessed an ovary with donor-derived oocytes and expressed ovarian marker genes, suggesting that ovarian differentiation could be induced by the colonization of donor-derived germ cells in sexually undifferentiated genital ridges of this hybrid fish. However, although the major purpose of germ cell transplantation in this study was to test the normality of gonadal somatic cells of hybrid gonads and to reveal the cell autonomous proliferative defects of hybrid PGCs, the occurrence of ovaries harboring donor-derived germ cells in 6-month-old transplanted hybrid recipients was notably low (2%). Skewing of the sex ratio of transplanted recipients would become a potential disadvantage for the use of germ cell-less hybrids as recipients. To overcome this problem, as previously successfully reported in zebrafish (Saito et al. 2008), feminization of transplanted BD-WC hybrid recipients by estradiol treatment should be examined to increase the percentage of recipients harboring ovaries with donor-derived oocytes.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.118.300777/-/DC1.
Acknowledgments
The Japan Society for the Promotion of Science supported this research through the Funding Program for Next Generation World-Leading Researchers (NEXT Program, GS010), initiated by the Council for Science and Technology Policy. This study was also supported, in part, by an Ocean Resource Use Promotion Technology Development Program grant sponsored by the Ministry of Education, Culture, Sports, Science and Technology.
Author contributions: H.Y., D.X., Y.I., T.Y., T.H., and J.W. conducted experiments and analyzed the data. R.Y. and G.Y. contributed to manuscript preparation. Y.T. conceived the study and wrote the manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Communicating editor: B. Draper
Literature Cited
- Bartley D. M., Rana K., Immink A. J., 2001. The use of inter-specific hybrids in aquaculture and fisheries. Rev. Fish Biol. Fish. 10: 325–337. 10.1023/A:1016691725361 [DOI] [Google Scholar]
- Bhattacharyya T., Gregorova S., Mihola O., Anger M., Sebestova J., et al. , 2013. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl. Acad. Sci. USA 110: E468–E477. 10.1073/pnas.1219126110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat A. K., Speksnijder J. E., Zivkovic D., 1999. Germ line development in fishes. Int. J. Dev. Biol. 43: 745–760. [PubMed] [Google Scholar]
- Bullini L., 1994. Origin and evolution of animal hybrid species. Trends Ecol. Evol. 9: 422–426. 10.1016/0169-5347(94)90124-4 [DOI] [PubMed] [Google Scholar]
- Chandley A. C., Short R. V., Allen W. R., 1975. Cytogenetic studies of three equine hybrids. J. Reprod. Fertil. Suppl. 123: 356–370. [PubMed] [Google Scholar]
- Chevassus B., 1983. Hybridization in fish. Aquaculture 33: 245–262. 10.1016/0044-8486(83)90405-2 [DOI] [Google Scholar]
- Ciruna B., Weidinger G., Knaut H., Thisse B., Thisse C., et al. , 2002. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc. Natl. Acad. Sci. USA 99: 14919–14924. 10.1073/pnas.222459999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Jin X., Chen X., He J., Yin Z., 2015. Sufficient numbers of early germ cells are essential for female sex development in Zebrafish. PLoS One 10: e0117824 10.1371/journal.pone.0117824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felip A., Zanuy S., Carrillo M., Martínez G., Ramos J., et al. , 1997. Optimal conditions for the induction of triploidy in the sea bass (Dicentrarchus labrax L.). Aquaculture 152: 287–298. 10.1016/S0044-8486(96)01509-8 [DOI] [Google Scholar]
- Flachs P., Bhattacharyya T., Mihola O., Piálek J., Forejt J., et al. , 2014. Prdm9 incompatibility controls oligospermia and delayed fertility but no selfish transmission in mouse intersubspecific hybrids. PLoS One 9: e95806 10.1371/journal.pone.0095806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nishimura T., Goto-Kazeto R., Kawakami Y., Yamaha E., et al. , 2010. Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc. Natl. Acad. Sci. USA 107: 17211–17216. 10.1073/pnas.1007032107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorshkov S., Gorshkova G., Hadani A., Gordin H., Nibb W., 2002. Chromosome set manipulations and hybridization experiments in gilthead seabream (Sparus aurata). II. Assessment of diploid and triploid hybrids between gilthead seabream and red seabream (Pagrus major). J. Appl. Ichthyology 18: 106–112. 10.1046/j.1439-0426.2002.00334.x [DOI] [Google Scholar]
- Goto R., Saito T., Takeda T., Fujimoto T., Takagi M., et al. , 2012. Germ cells are not the primary factor for sexual fate determination in goldfish. Dev. Biol. 370: 98–109. 10.1016/j.ydbio.2012.07.010 [DOI] [PubMed] [Google Scholar]
- Hamaguchi S., Sakaizumi M., 1992. Sexually differentiated mechanisms of sterility in interspecific hybrids between Oryzias latipes and O. curvinotus. J. Exp. Zool. 263: 323–329. 10.1002/jez.1402630312 [DOI] [PubMed] [Google Scholar]
- Hayashi M., Sato M., Nagasaka Y., Sadaie S., Kobayashi S., et al. , 2014. Enrichment of spermatogonial stem cells using side population in teleost. Biol. Reprod. 91: 1–8. 10.1095/biolreprod.113.114140 [DOI] [PubMed] [Google Scholar]
- Higuchi K., Takeuchi Y., Miwa M., Yamamoto Y., Tsunemoto K., et al. , 2011. Colonization, proliferation and survival of intraperitoneally transplanted yellowtail Seriola quinqueradiata spermatogonia in nibe croaker Nibea mitsukurii recipient. Fish. Sci. 77: 69–77. 10.1007/s12562-010-0314-7 [DOI] [Google Scholar]
- Hooe M. I., Buch D. H., Wahl D. H., 1994. Growth, survival, and recruitment of hybrid crappies stocked in small impoundments. N. Am. J. Fish. Manage. 13: 137–142. [DOI] [Google Scholar]
- Islam F. B., Ishishita S., Uno Y., Mollah M. B. R., Srikulnath K., et al. , 2013. Male hybrid sterility in the mule duck is associated with meiotic arrest in primary spermatocytes. J. Poult. Sci. 50: 311–320. 10.2141/jpsa.0130011 [DOI] [Google Scholar]
- Kaji T., Tanaka M., Tagawa M., 1999. Laboratory study of density-dependent survival after handling in yolk-sac larvae of tunas and a grouper. Fish. Sci. 65: 482–483. 10.2331/fishsci.65.482 [DOI] [Google Scholar]
- Kitamura H., Teong O. Y., Arakawa T., 1991. Gonadal development of artificially induced triploid red sea bream, Pagrus major. Nippon Suisan Gakkaishi 57: 1657–1660. 10.2331/suisan.57.1657 [DOI] [Google Scholar]
- Kitano T., Hayashi Y., Shiraishi E., Kamei Y., 2012. Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature. Mol. Reprod. Dev. 79: 719–726. 10.1002/mrd.22080 [DOI] [PubMed] [Google Scholar]
- Lacerda S. M., Costa G. M., Campos-Junior P. H., Segatelli T. M., Yazawa R., et al. , 2013. Germ cell transplantation as a potential biotechnological approach to fish reproduction. Fish Physiol. Biochem. 39: 3–11. 10.1007/s10695-012-9606-4 [DOI] [PubMed] [Google Scholar]
- Lee S., Yoshizaki G., 2016. Successful cryopreservation of spermatogonia in critically endangered Manchurian trout (Brachymystax lenok). Cryobiology 72: 165–168. 10.1016/j.cryobiol.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Lee S., Iwasaki Y., Shikina S., Yoshizaki G., 2013. Generation of functional eggs and sperm from cryopreserved whole testes. Proc. Natl. Acad. Sci. USA 110: 1640–1645. 10.1073/pnas.1218468110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Seki S., Katayama N., Yoshizaki G., 2015. Production of viable trout offspring derived from frozen whole fish. Sci. Rep. 5: 16045 10.1038/srep16045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Iwasaki Y., Yoshizaki G., 2016a Long-term (5 years) cryopreserved spermatogonia have high capacity to generate functional gametes via interspecies transplantation in salmonids. Cryobiology 73: 286–290. 10.1016/j.cryobiol.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Lee S., Katayama N., Yoshizaki G., 2016b Generation of juvenile rainbow trout derived from cryopreserved whole ovaries by intraperitoneal transplantation of ovarian germ cells. Biochem. Biophys. Res. Commun. 478: 1478–1483. 10.1016/j.bbrc.2016.08.156 [DOI] [PubMed] [Google Scholar]
- Li Q., Fujii W., Naito K., Yoshizaki G., 2017. Application of dead end-knockout zebrafish as recipients of germ cell transplantation. Mol. Reprod. Dev. 84: 1100–1111. 10.1002/mrd.22870 [DOI] [PubMed] [Google Scholar]
- Linhartová Z., Saito T., Kašpar V., Rodina M., Prášková E., et al. , 2015. Sterilization of sterlet Acipenser ruthenus by using knockdown agent, antisense morpholino oligonucleotide, against dead end gene. Theriogenology 84: 1246–1255.e1. 10.1016/j.theriogenology.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Lo P. C., Liu S. H., Chao N. L., Nunoo F. K., Mok H. H., et al. , 2015. A multi-gene dataset reveals a tropical new world origin and early miocene diversification of croakers (Perciformes: Sciaenidae). Mol. Phylogenet. Evol. 88: 132–143. 10.1016/j.ympev.2015.03.025 [DOI] [PubMed] [Google Scholar]
- Mallet J., 2007. Hybrid speciation. Nature 446: 279–283. 10.1038/nature05706 [DOI] [PubMed] [Google Scholar]
- Mayr E., 1963. Animal Species and Evolution. Harvard University Press, Cambridge, MA: 10.4159/harvard.9780674865327 [DOI] [Google Scholar]
- Menezes M. R., Taniguchi N., 1988. Interspecific genetic divergence in Sciaenids from Japan and its adjacent waters. Jpn. J. Ichthyol. 35: 40–46. 10.1007/BF02906682 [DOI] [Google Scholar]
- Molyneaux K., Wylie C., 2004. Primordial germ cell migration. Int. J. Dev. Biol. 48: 537–544. 10.1387/ijdb.041833km [DOI] [PubMed] [Google Scholar]
- Morita T., Morishima K., Miwa M., Kumakura N., Kudo S., et al. , 2012. Production of donor-derived offspring by allogeneic transplantation of spermatogonia in the yellowtail (Seriola quinqueradiata). Biol. Reprod. 86: 1–11. 10.1095/biolreprod.111.097873 [DOI] [PubMed] [Google Scholar]
- Morita T., Morishima K., Miwa M., Kumakura N., Kudo S., et al. , 2015. Functional sperm of the yellowtail (Seriola quinqueradiata) were produced in the small-bodied surrogate, Jack Mackerel (Trachurus japonicus). Mar. Biotechnol. (NY) 17: 644–654. 10.1007/s10126-015-9657-5 [DOI] [PubMed] [Google Scholar]
- Murata O., Kato K., Ishitani Y., Nasu T., Miyashita S., et al. , 1997. Gonadal maturation of Pagrus major × Acanthopagrus schlegeli and Pagrus major × Sparus sarba sea bream hybrids. Suisan Zoshoku 45: 75–80. [Google Scholar]
- Nakamura S., Watakabe I., Nishimura T., Picard J. Y., Toyoda A., et al. , 2012. Hyperproliferation of mitotically active germ cells due to defective anti-Müllerian hormone signaling mediates sex reversal in medaka. Development 139: 2283–2287. 10.1242/dev.076307 [DOI] [PubMed] [Google Scholar]
- Okutsu T., Shikina S., Kanno M., Takeuchi Y., Yoshizaki G., 2007. Production of trout offspring from triploid salmon parents. Science 317: 1517 10.1126/science.1145626 [DOI] [PubMed] [Google Scholar]
- Piferrer F., Beaumont A., Falguière J. C., Flajšhans M., Haffray P., et al. , 2009. Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293: 125–156. 10.1016/j.aquaculture.2009.04.036 [DOI] [Google Scholar]
- Piva L. H., de Siqueira-Silva D. H., Goes C. A. G., Fujimoto T., Saito T., et al. , 2017. Triploid or hybrid tetra: which is the ideal sterile host for surrogate technology? Theriogenology 108: 239–244. 10.1016/j.theriogenology.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Pšenička M., Saito T., Linhartová Z., Gazo I., 2015. Isolation and transplantation of sturgeon early-stage germ cells. Theriogenology 83: 1085–1092. 10.1016/j.theriogenology.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Rahman A. M., Arshad A., Marimuthu K., Ara R., Amin S. M. N., 2012. Inter-specific hybridization and its potential for aquaculture of fin fishes. Asian J. Anim. Vet. Adv. 8: 139–153. [Google Scholar]
- Saito T., Goto-Kazeto R., Arai K., Yamaha E., 2008. Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol. Reprod. 78: 159–166. 10.1095/biolreprod.107.060038 [DOI] [PubMed] [Google Scholar]
- Sawamura K., Roote J., Wu C. I., Yamamoto M.-T., 2004. Genetic complexity underlying hybrid male sterility in Drosophila. Genetics 166: 789–796. 10.1534/genetics.166.2.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Kusano K., Lee S., Iwasaki Y., Yagisawa M., et al. , 2017. Production of the medaka derived from vitrified whole testes by germ cell transplantation. Sci. Rep. 7: 43185 10.1038/srep43185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim K. M., Shinomiya A., Otake H., Hamaguchi S., Sakaizumi M., 2009. Effects of high temperature on sex differentiation and germ cell population in medaka. Aquaculture 289: 340–349. 10.1016/j.aquaculture.2008.12.019 [DOI] [Google Scholar]
- Shimizu Y., Shibata N., Yamashita M., 1997. Spermiogenesis without preceding meiosis in the hybrid medaka between Oryzias latipes and O. curvinotus. J. Exp. Zool. 279: 102–112. [DOI] [Google Scholar]
- Silva M. A., Costa G. M., Lacerda S. M., Brandão-Dias P. F., Kalapothakis E., et al. , 2016. Successful xenogeneic germ cell transplantation from Jundia catfish (Rhamdia quelen) into adult Nile tilapia (Oreochromis niloticus) testes. Gen. Comp. Endocrinol. 230–231: 48–56. 10.1016/j.ygcen.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Slanchev K., Stebler J., de la Cueva-Méndez G., Raz E., 2005. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. USA 102: 4074–4079. 10.1073/pnas.0407475102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama K., Taniguchi N., Seki S., Nabeshima H., 1992. Survival, growth and gonadal development of triploid red sea bream, Pagrus major (Temminick; Schlegel): use of allozyme markers for ploidy and family identification. Aquacult. Fish. Manage. 23: 149–159. [Google Scholar]
- Takeuchi Y., Higuchi K., Yatabe T., Miwa M., Yoshizaki G., 2009. Development of spermatogonial cell transplantation in Nibe croaker, Nibea mitsukurii (Perciformes, Sciaenidae). Biol. Reprod. 81: 1055–1063. 10.1095/biolreprod.109.077701 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Yatabe T., Yoshikawa H., Ino Y., Kabeya N., et al. , 2016. Production of functionally sterile triploid Nibe croaker induced by cold-shock treatment with special emphasis on triploid aptitude as surrogate broodstock. Aquaculture. Available at: http://www.sciencedirect.com/science/article/pii/S0044848616302770. 10.1016/j.aquaculture.2016.05.030 [DOI] [Google Scholar]
- Tonelli F. M., Lacerda S. M., Procópio M. S., Lemos B. L., de França L. R., et al. , 2017. Gene delivery to Nile tilapia cells for transgenesis and the role of PI3K-c2α in angiogenesis. Sci. Rep. 7: 44317 10.1038/srep44317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgasheva A. A., Borodin P. M., 2016. Cytological basis of sterility in male and female hybrids between sibling species of grey voles Microtus arvalis and M. levis. Sci. Rep. 6: 36564 10.1038/srep36564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzung K. W., Goto R., Saju J. M., Sreenivasan R., Saito T., et al. , 2015. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports 4: 61–73. (erratum: Stem Cell Reports 5: 156) 10.1016/j.stemcr.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargelius A., Leininger S., Skaftnesmo K. O., Kleppe L., Andersson E., et al. , 2016. Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci. Rep. 6: 21284 10.1038/srep21284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G., Stebler J., Slanchev K., Dumstrei K., Wise C., et al. , 2013. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr. Biol. 13: 1429–1434. 10.1016/S0960-9822(03)00537-2 [DOI] [PubMed] [Google Scholar]
- Wong T. T., Saito T., Crodian J., Collodi P., 2011. Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol. Reprod. 84: 1190–1197. 10.1095/biolreprod.110.088427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Kabeya N., Takeuchi Y., Higuchi K., Yatabe T., et al. , 2011. Establishment of a stable transgenic strain in a pelagic egg spawning marine teleost, Nibe croaker. Aquaculture 313: 42–49. 10.1016/j.aquaculture.2011.01.042 [DOI] [Google Scholar]
- Yazawa R., Takeuchi Y., Higuchi K., Yatabe T., Kabeya N., et al. , 2010. Chub mackerel gonads support colonization, survival, and proliferation of intraperitoneally transplanted xenogenic germ cells. Biol. Reprod. 82: 896–904. 10.1095/biolreprod.109.081281 [DOI] [PubMed] [Google Scholar]
- Ye H., Li C. J., Yue H.-M., Du H., Yang X. G., et al. , 2017. Establishment of intraperitoneal germ cell transplantation for critically endangered Chinese sturgeon Acipenser sinensis. Theriogenology 94: 37–47. 10.1016/j.theriogenology.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Takeuchi Y., Ino Y., Wang J., Iwata G., et al. , 2017. Efficient production of donor-derived gametes from triploid recipients following intra-peritoneal germ cell transplantation into a marine teleost, Nibe croaker (Nibea mitsukurii). Aquaculture 478: 35–47. 10.1016/j.aquaculture.2016.05.011 [DOI] [Google Scholar]
- Yoshizaki G., Fujinuma K., Iwasaki Y., Okutsu T., Shikina S., et al. , 2011. Spermatogonial transplantation in fish: a novel method for the preservation of genetic resources. Comp. Biochem. Physiol. Part D Genomics Proteomics 6: 55–61. 10.1016/j.cbd.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Yoshizaki G., Ichikawa M., Hayashi M., Iwasaki Y., Miwa M., et al. , 2012. Sexual plasticity of ovarian germ cells in rainbow trout. Development 137: 1227–1230. 10.1242/dev.044982 [DOI] [PubMed] [Google Scholar]
- Yoshizaki G., Takashiba K., Shimamori S., Fujinuma K., Shikina S., et al. , 2016. Production of germ cell-deficient salmonids by dead end gene knockdown, and their use as recipients for germ cell transplantation. Mol. Reprod. Dev. 83: 298–311. 10.1002/mrd.22625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Fishes are available on request. Table S1 lists primers.