Abstract
Exercise improves clinical outcomes in patients diagnosed with heart failure with reduced ejection fraction (HFrEF), in part via beneficial effects on cardiomyocyte Ca2+ cycling during excitation-contraction coupling (ECC). However, limited data exist regarding the effects of exercise training on cardiomyocyte function in patients diagnosed with heart failure with preserved ejection fraction (HFpEF). The purpose of this study was to investigate cardiomyocyte Ca2+ handling and contractile function following chronic low-intensity exercise training in aortic-banded miniature swine and test the hypothesis that low-intensity exercise improves cardiomyocyte function in a large animal model of pressure overload. Animals were divided into control (CON), aortic-banded sedentary (AB), and aortic-banded low-intensity trained (AB-LIT) groups. Left ventricular cardiomyocytes were electrically stimulated (0.5 Hz) to assess Ca2+ homeostasis (fura-2-AM) and unloaded shortening during ECC under conditions of baseline pacing and pacing with adrenergic stimulation using dobutamine (1 μM). Cardiomyocytes in AB animals exhibited depressed Ca2+ transient amplitude and cardiomyocyte shortening vs. CON under both conditions. Exercise training attenuated AB-induced decreases in cardiomyocyte Ca2+ transient amplitude but did not prevent impaired shortening vs. CON. With dobutamine, AB-LIT exhibited both Ca2+ transient and shortening amplitude similar to CON. Adrenergic sensitivity, assessed as the time to maximum inotropic response following dobutamine treatment, was depressed in the AB group but normal in AB-LIT animals. Taken together, our data suggest exercise training is beneficial for cardiomyocyte function via the effects on Ca2+ homeostasis and adrenergic sensitivity in a large animal model of pressure overload-induced heart failure.
NEW & NOTEWORTHY Conventional treatments have failed to improve the prognosis of heart failure with preserved ejection fraction (HFpEF) patients. Our findings show chronic low-intensity exercise training can prevent cardiomyocyte dysfunction and impaired adrenergic responsiveness in a translational large animal model of chronic pressure overload-induced heart failure with relevance to human HFpEF.
Keywords: calcium, excitation-contraction coupling, heart failure
INTRODUCTION
Traditional therapeutic approaches commonly used to treat heart failure with reduced ejection fraction (HFrEF) have largely failed in heart failure with preserved ejection fraction (HFpEF) patients (for reviews, see Refs. 43, 48). Given the difficulties in treating HFpEF and the systemic nature of the disease, utilizing exercise therapeutically for this heart failure subtype may carry added clinical importance (23, 35). Several clinical studies have shown exercise to be a safe way to improve quality of life and cardiorespiratory fitness for both HFrEF and HFpEF patients (7, 10, 12, 16, 26, 39, 42, 44). In HFrEF, improvements to general health status following exercise training may be related to its effects on cardiomyocyte excitation-contraction coupling (ECC) including improvement in speed and amplitude of systolic Ca2+ transients and contraction and enhancement of sarco/endoplasmic reticulum Ca2+ ATPase (SERCA)-mediated diastolic sarcoplasmic reticulum (SR) Ca2+ reuptake and relaxation (25). In contrast, the effects of exercise training on cardiomyocyte function in a setting of HFpEF are largely unknown.
In heart failure, cardiomyocyte Ca2+-handling processes are impaired resulting in aberrant timing and efficacy of ECC, thereby upsetting the inherent stability and plasticity of ventricular contraction and relaxation. Thus, abnormalities in the ability to handle Ca2+ represent a primary underlying factor leading to clinical manifestations of heart failure, including contractile dysfunction and arrhythmia. In contrast with the extensive literature on cardiomyocyte function in HFrEF (1, 33, 57), much less is known regarding the mechanisms disrupting cardiomyocyte ECC in HFpEF as these patients often do not qualify for cardiac transplantation, limiting the ability to study human tissue. Thus the fundamental question is cardiac dysfunction in HFpEF intrinsic or extrinsic to the cardiomyocyte remains controversial.
The goal of this study was to investigate the effects of low-intensity exercise training on cardiomyocyte Ca2+ handling and contractile function in a Yucatan mini-swine model of chronic pressure overload-induced heart failure that exhibits the cardiac features of HFpEF including preserved ejection fraction at rest, diastolic dysfunction, cardiac hypertrophy, diminished left ventricular (LV) contractile reserve, increased LV fibrosis, and lung congestion (13, 14, 21, 34, 40, 41). We hypothesized chronic low-intensity exercise training would attenuate pressure overload-induced impairments to cardiomyocyte ECC and preserve cardiomyocyte functional reserve following adrenergic challenge.
METHODS
Ethical approval, aortic banding, and exercise training.
All animal protocols were in accordance with the Principles for the Utilization and Care of Vertebrate Animals Used in Testing Research and Training and approved by the University of Missouri Animal Care and Use Committee. Male Yucatan miniature swine (29–32 kg; 8 mo old) were assigned into three groups: sedentary control (CON), aortic-banded sedentary (AB), and aortic-banded low-intensity exercise trained (AB-LIT; n = 7 for all groups). Aortic bands were placed around the ascending aorta (proximal to the brachiocephalic artery), and a systolic transstenotic gradient of ~70 mmHg was established (74 ± 2 and 72 ± 1 mmHg for AB and AB-LIT, respectively, P = NS) over a period of ≈6 mo as previously reported (41). Two months after aortic banding, AB-LIT animals started chronic exercise training for 17 wk using low-intensity treadmill training. The exercise training protocol included treadmill running 55 min/day, 3 days/week, for 17 wk with gradually increasing intensity until finally consisting of the following: 1) a 5 min warm up at 1.5 mph; 2) 45 min at 2.5 mph; and 3) a 5 min cool down at 1.5 mph. Dissection of vital tissues and removal of skeletal muscle (deltoid) for analysis of citrate synthase activity (50) occurred at the time of death, 24 h after the last training bout. Animals were fed a standard diet averaging 15–20 g/kg once daily, and water was provided ad libitum.
Transthoracic echocardiography.
Transthoracic echocardiography was performed while animals were under inhaled isoflurane anesthesia (0.5%) in the supine/right lateral position 6 mo postbanding. Short axis two-dimensional and M-mode images were recorded at the mid-papillary level using a 1.5- to 4-MHz transducer on a GE Vividi Ultrasound system. Fractional shortening (FS%) and LV diastolic chamber dimension and wall thickness [LV internal diastolic dimension (LVIDd), LV diastolic wall thickness (LV WTd), interventricular septum diastolic wall thickness (IVS WTd), and relative left ventricular and septal diastolic wall thickness normalized to LVIDd (relative LV + IVS WTd)] were calculated from M-mode recordings as previously reported (20). From an apical four-chamber view, velocity time integral (VTI), a measure directly proportional to stroke volume (SV), was measured at the level of the aortic annulus using pulse wave Doppler. Stroke volume was determined using the equation SV = π(or) × VTI.
Cardiomyocyte Ca2+ and contractile measurements.
Midmyocardial cardiomyocytes were enzymatically isolated from LV wedge preparations of excised hearts as previously described (19, 36). Isolated myocytes were plated on laminin-coated glass coverslips and dye loaded for 20 min at room temperature (22–24°C) with 5 μM fura-2-AM (Thermo/Molecular Probes) in physiological saline solution (PSS) containing the following (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, 10 HEPES, and 1 NaHCO3, pH 7.4 with NaOH. After a 40- to 60-min wash period in PSS, myocytes were placed in a temperature-controlled bath mounted on an inverted fluorescence microscope (IX71; Olympus America, Center Valley, PA). Myocytes were superfused with PSS via an inline solution heater and viewed using a ×40 oil-immersion objective (UApo340: 1.35 NA; Olympus). Action potentials (0.5 Hz) were triggered with electrical field stimulation (S48; Grass Instruments, Warwick, RI) via platinum electrodes placed at the edges of the bath. Epifluorescence microcopy was performed using an IonOptix Ca2+ and Contractility Recording System (IonOptix, Milton, MA) with alternating excitation at 340 and 380 nm, and fluorescence emission was measured from 510 to 565 nm. Background-subtracted fluorescence emission at each wavelength (F340 and F380) was obtained, and the fura-2 ratio (R = F340/F380) was used as an index of intracellular Ca2+ concentration ([Ca2+]i). The fura-2 ratio was acquired simultaneously with sarcomere length (Ls) at 250 Hz. Myocytes were equilibrated to physiological temperature (35–37°C) for 5 min, followed by electrical field stimulation at 0.5 Hz for assessment of Ca2+ transients and sarcomere shortening. In addition to measurements obtained under untreated conditions, a subset of cardiomyocytes were treated with 1 μM dobutamine (±) to assess cardiomyocyte adrenergic functional reserve. With dobutamine, cardiomyocytes experienced a gradual inotropic effect that transitioned into “Ca2+ overload” with incidence of spontaneous Ca2+ transients and contractile activity. Therefore, the action-potential-induced Ca2+ transient and shortening response, which preceded the onset of spontaneous activity, was used for analysis. Action-potential-induced Ca2+ transients and Ls shortening were analyzed offline using Ionwizard 6.3 software (IonOptix) with the following parameters of Ca2+ handling and contractile function assessed: 1) diastolic [Ca2+]i; 2) peak systolic [Ca2+]i; 3) Ca2+ transient amplitude (Δratio), calculated as peak systolic fura-2 ratio – diastolic fura-2 ratio; 4) rate of rise of the Ca2+ transient (ΔR/Δt) in arbitrary units per second; 5) diastolic Ls; 6) systolic Ls; 7) shortening magnitude, calculated as diastolic Ls – systolic Ls; and 8) maximum rate of shortening (ΔL/Δt, in μm/s). For presentation of sarcomere length vs. Ca2+ relationships during ECC, traces of Ca2+ and sarcomere length from each cardiomyocyte within each group were temporally aligned based on the time of electrical field stimulation, and the average of responses of all cells were plotted with Ca2+ on the x-axis and sarcomere length on the y-axis.
Western blotting.
LV tissue was homogenized in lysis buffer (150 mM NaCl, 50 mM Tris-base, pH 8.0, 0.1% Triton-X 100, 2 mM EDTA, and 10 μl/ml HALT protease and phosphatase inhibitor). After centrifugation, supernatants were collected and the protein content was measured according to the method by Bradford (3). Protein (1 μg) was treated with Laemmli sample buffer containing 5% β-mercaptoethanol and heated for 5 min [15 min for phospholamban (PLB)] at 95°C. Protein was loaded into 4–20% polyacrylamide gels (Genscript), separated by SDS-PAGE at 140 V for 50 min, and transferred at 34 V for 60 min to a polyvinylidene difluoride membrane. Membranes were then blocked for 1 h with 5% milk in Tris-buffered saline and 0.1% Tween 20 (TBST). Blots were incubated overnight (25°C) with the primary antibody against SERCA2a (1:1,000; ThermoFisher Scientific), phospholamban (PLB; 1:1,000; ThermoFisher Scientific), sodium-calcium exchanger (NCX; 1:1,000; ThermoFisher Scientific), Na+-K+-ATPase (1:1,000; ThermoFisher Scientific), ryanodine receptor (RyR; 1:1,000; ThermoFisher Scientific), Ser16 phosphorylated PLB (1:1,000; Badrilla), Thr17 phosphorylated PLB (1:1,000; Badrilla), and calsequestrin (CSQ; 1:10,000; ThermoFisher Scientific). The following day, membranes were washed five times for 10 min in TBST and then incubated for 1 h with a horseradish peroxidase-conjugated secondary antibody (anti-mouse polyclonal IgG, 1:1,000; ThermoFisher Scientific; or anti-rabbit IgG, 1:1,000; Cell Signaling). Membranes were again washed five times for 10 min in TBST, visualized via enhanced chemiluminescence (Luminata Forte; Millipore), and quantified by densitometry with Kodak 4000R Imager and Molecular Imagery software.
Statistical analysis.
All data analysis was performed using SigmaPlot version 12.3 (SysStat Software, San Jose, CA). To examine phenotypic changes within populations of cardiomyocytes obtained from the respective animal groups and conditions, the following number of cells (n) from number of animals (N) were sampled: untreated baseline conditions, n = 53/N = 6 CON, n = 37/N = 5 AB, and n = 72/N = 7 AB-LIT; dobutamine, n = 48/N = 6 CON, n = 32/N = 5 AB, and n = 58/N = 7 AB-LIT. The value of n was utilized for subsequent statistical analysis. Group comparisons were made using one-way ANOVA, with Student Newman-Keuls post hoc analysis. Data are reported as individual observations, means of all responses, or means ± SE, with significance denoted at the P < 0.05 and P < 0.10 levels (8, 53).
RESULTS
Aortic-banded animals (AB and AB-LIT) exhibited normal fractional shortening and SV as assessed in vivo using echocardiography (Table 1). Global cardiac hypertrophic remodeling was observed in both the AB and AB-LIT groups and associated with a decrease in LV cardiomyocyte length:width ratio, consistent with concentric cellular remodeling (Table 1). With the use of echocardiography, an increase in relative diastolic wall thickness (predominately in the septum) was observed in parallel with no change in LV internal diastolic dimension in AB animals (vs. CON), confirming our postmortem findings. Changes in relative diastolic wall thickness were attenuated in the AB-LIT group. Decreases in the cardiomyocyte length:width ratio in AB (vs. CON) were due to increased cardiomyocyte width, while the decrease in ratio in AB-LIT (vs. CON) was due to a decrease in cardiomyocyte length. An increase in lung weight in the AB group compared with CON, indicative of pulmonary congestion, was attenuated in AB-LIT animals. No significant differences in citrate synthase activity were found in the deltoid muscle between groups.
Table 1.
Postmortem heart weight and body weight, in vivo echocardiographical measurements (fractional shortening, stroke volume, LVIDd, LV WTd, IVS WTd, and relative LV + IVS WTd), length, width, and length:width ratio of enzymatically isolated cardiomyocytes, lung weight, and deltoid citrate synthase activity of CON, AB, and AB-LIT groups
| CON | AB | AB-LIT | |
|---|---|---|---|
| Post mortem heart weight, g | 207.5 ± 9.8 | 256.3 ± 9.2* | 257.4 ± 7.0* |
| Post mortem body weight, kg | 46.1 ± 2.2 | 47.4 ± 2.4 | 47.7 ± 1.3 |
| In vivo fractional shortening | 46 ± 2 | 47 ± 2 | 47 ± 2 |
| In vivo stroke volume, ml | 67 ± 4 | 65 ± 3 | 62 ± 3 |
| LVIDd, mm | 49 ± 2 | 47 ± 1 | 50 ± 1 |
| LV WTd, mm | 7.4 ± 0.4 | 8.3 ± 0.7 | 8.2 ± 0.4 |
| IVS WTd, mm, | 11.0 ± 0.4 | 12.2 ± 0.2† | 11.8 ± 0.4 |
| Relative LV + IVS WTd | 0.38 ± 0.01 | 0.44 ± 0.02* | 0.40 ± 0.01# |
| Cardiomyocyte length, µm | 214 ± 4 | 207 ± 4 | 191 ± 3*# |
| Cardiomyocyte width, µm | 38 ± 1 | 43 ± 1* | 36 ± 1# |
| Length:width ratio | 6.0 ± 0.2 | 5.4 ± 0.2* | 5.6 ± 0.2* |
| Lung weight, g | 267 ± 14 | 317 ± 21† | 294 ± 10 |
| Deltoid citrate synthase activity, µmol·g wet weight−1·min−1 | 20.4 ± 2.8 | 15.2 ± 2.0 | 21.5 ± 4.3 |
CON, control; AB, aortic-banded; AB-LIT, aortic-banded exercise trained; LVIDd, left ventricular internal diastolic dimension; LV WTd, left ventricular diastolic wall thickness; IVS WTd, interventricular septum diastolic wall thickness; relative LV + IVS WTd, left ventricular and septal diastolic wall thickness normalized to LVIDd calculated as (LV WTd + IVS WTd)/LVIDd.
P < 0.05 vs. CON.
P < 0.05 vs. AB.
P < 0.10 vs. CON.
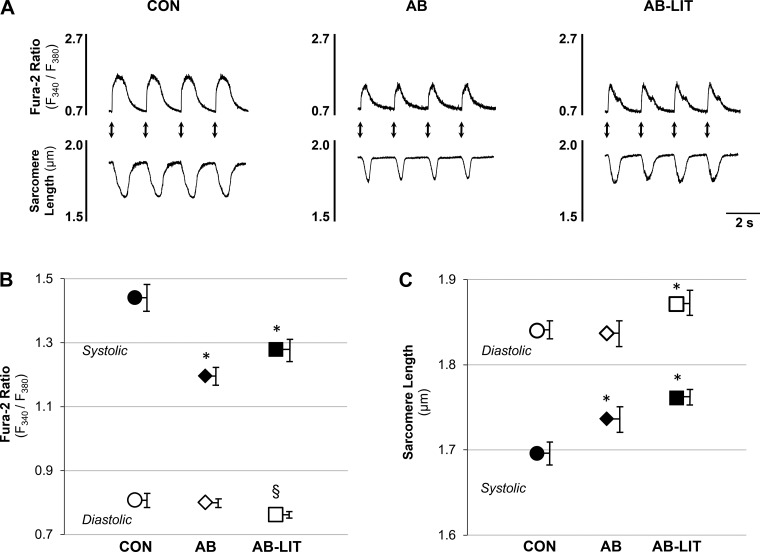
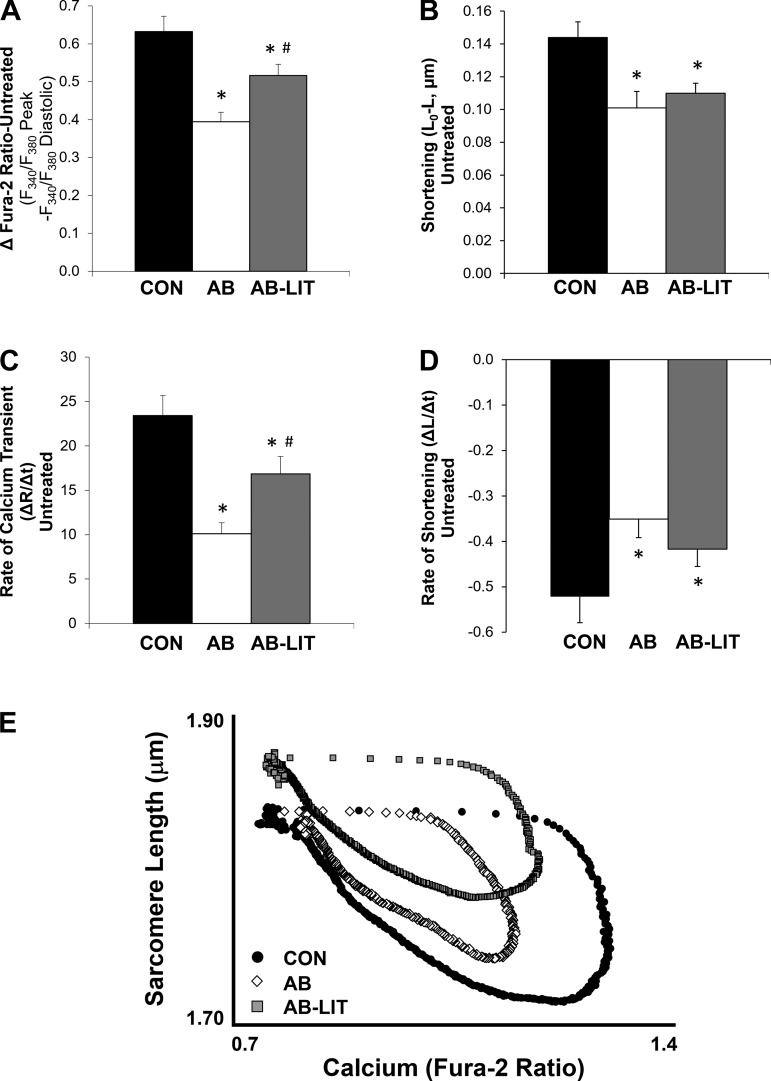
To examine the role of the cardiomyocyte in functional changes observed at the whole organ level, intracellular Ca2+ (fura-2) and sarcomere length were simultaneously monitored in enzymatically isolated LV myocytes in response to electrical field stimulation (Fig. 1A). Compared with CON, cardiomyocytes of AB exhibited similar diastolic Ca2+ and sarcomere length (Fig. 1, B and C). However, systolic Ca2+ was reduced in AB (Fig. 1B) resulting in impaired systolic sarcomere shortening (Fig. 1C), reduced Ca2+ transient amplitude (Fig. 2A), and impaired shortening amplitude (Fig. 2B). Ca2+ transient and shortening rates were also slower in AB vs. CON (Fig. 2, C and D). In AB-LIT animals, a decrease in diastolic Ca2+ was observed compared with both CON and AB (Fig. 1B) along with a longer diastolic sarcomere length (Fig. 1C). Exercise training also attenuated the decrease in Ca2+ transient amplitude and rate (Fig. 2, A and C) observed in the AB group, but did not prevent aortic banding-induced decreases in shortening amplitude or rate (Fig. 2, B and D). Examination of the sarcomere length-Ca2+ relationship during ECC shows an upward and leftward shift during the systolic phase in the AB group, highlighting the impaired amplitude of the Ca2+ transient and associated shortening compared with CON (Fig. 2E). In AB-LIT animals, a longer sarcomere length is evident throughout the ECC cycle in addition to an attenuated leftward shift in the Ca2+ transient.
Fig. 1.
Cardiomyocyte Ca2+ transients and sarcomere shortening during excitation-contraction coupling. A: example traces of action potential-induced Ca2+ transients (fura-2 ratio, top traces) and sarcomere length (bottom traces) in cardiomyocytes of control (CON), aortic-banded (AB), and aortic-banded exercise trained (AB-LIT) groups. Electrical field stimuli are indicated by arrows. B and C: summary data of diastolic (○, ◇, ☐) and systolic (●, ◆, ■) Ca2+ (B) and sarcomere length (C) in CON (○, ●), AB (◇, ◆), and AB-LIT (☐, ■). *P < 0.05 vs. CON. §P < 0.10 vs. CON and AB.
Fig. 2.
Summary data of cardiomyocyte Ca2+ transient and sarcomere shortening amplitude and rate. A and B: summary data of Ca2+ transient (A) and shortening (B) amplitude in cardiomyocytes of CON, AB, and AB-LIT groups. C and D: summary data of maximum Ca2+ transient rate (C) and maximum (most negative) shortening rate (D). E: sarcomere length-Ca2+ loop relationships during excitation-contraction coupling. Loops are averaged traces of all cardiomyocytes within CON (●), AB (◇), and AB-LIT ( ). Traces were aligned for averaging based on electrical stimulation, with error bars omitted for clarity. *P < 0.05 vs. CON. #P < 0.05 vs. AB.
). Traces were aligned for averaging based on electrical stimulation, with error bars omitted for clarity. *P < 0.05 vs. CON. #P < 0.05 vs. AB.
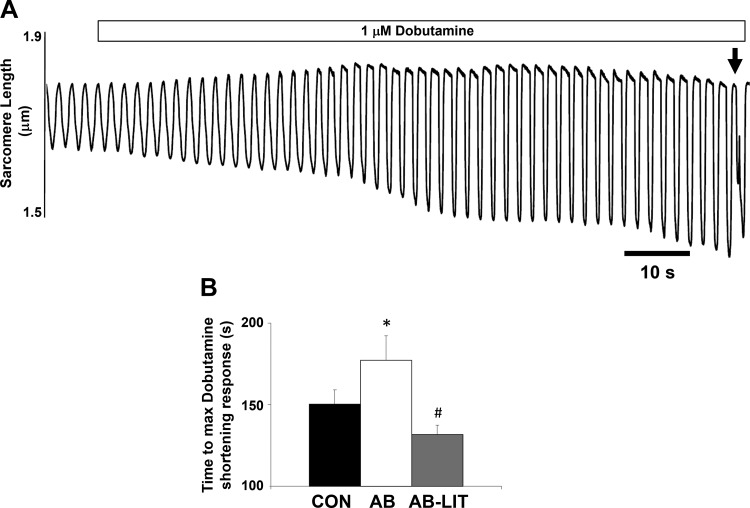
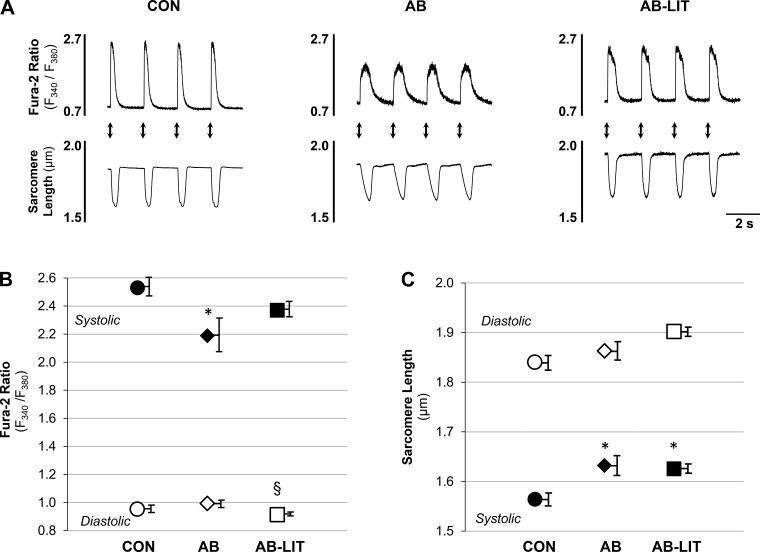
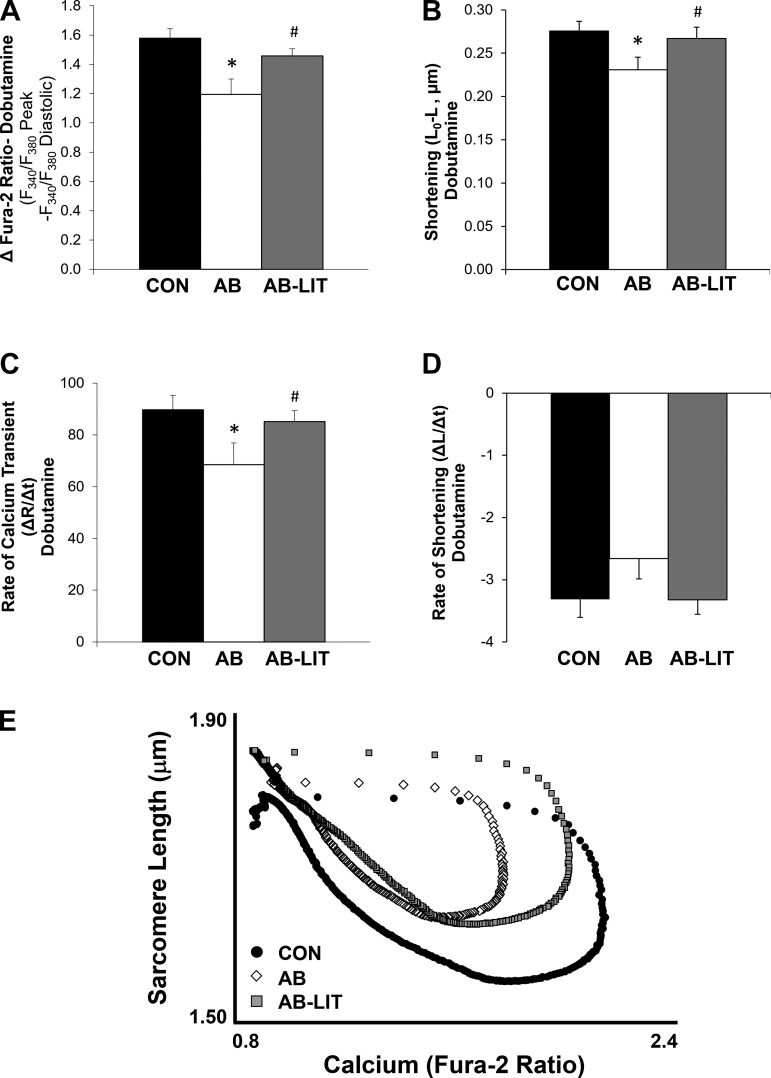
A consistent observation in heart failure is a lack of cardiac functional reserve following stress. We therefore examined cardiomyocyte function following acute application of the adrenergic agonist dobutamine. In all groups, dobutamine application induced the expected increase in Ca2+ transient and shortening amplitude vs. untreated conditions. The maximum inotropic response was defined as the largest action-potential-induced shortening response before the onset of spontaneous contractile activity (Fig. 3A, denoted by arrow). The time to maximum inotropic response was delayed in AB (Fig. 3B) animals compared with CON and AB-LIT. Similar to observations in untreated conditions, cardiomyocytes of AB were similar to CON in diastolic Ca2+ and sarcomere length yet exhibited reduced systolic Ca2+ (Fig. 4B), impaired sarcomere shortening (Fig. 4C), decreased Ca2+ transient amplitude (Fig. 5A), and reduced shortening amplitude (Fig. 5B) vs. CON. The rate of the Ca2+ transient was also slower in AB vs. CON (Fig. 5C). Exercise training completely prevented the AB-induced decreases in cardiomyocyte Ca2+ transient amplitude (Fig. 5A), shortening amplitude (Fig. 5B), and Ca2+ transient rate (Fig. 5C). Sarcomere length-Ca2+ relationship analysis during the ECC cycle again showed an upward and leftward shift in AB during the systolic phase vs. CON, indicative of impaired Ca2+ transients and sarcomere shortening following dobutamine treatment (Fig. 5E). As with untreated conditions, cardiomyocytes of AB-LIT operated at longer sarcomere lengths throughout ECC in the presence of dobutamine. However, with dobutamine, the amplitude of Ca2+ transients and shortening were similar between AB-LIT and CON, resulting in similar shape of the sarcomere length-Ca2+ relationship but at extended sarcomere lengths evident as an upward, but not leftward, shift.
Fig. 3.
Time to maximum inotropic response following dobutamine challenge is prolonged with aortic banding and prevented by exercise training. A: example cardiomyocyte shortening traces (0.5 Hz) before and following addition of dobutamine (1 μM, indicated by bar). Maximum inotropic response was taken as the response before the onset of spontaneous contractile activity (indicated by arrow). B: summary data of time to maximum dobutamine-induced shortening response in cardiomyocytes of CON, AB, and AB-LIT groups. *P < 0.05 vs. CON. #P < 0.05 vs. AB.
Fig. 4.
Cardiomyocyte Ca2+ transients and sarcomere shortening during excitation-contraction coupling following dobutamine challenge. A: example traces of action potential-induced Ca2+ transients (fura-2 ratio, top traces) and sarcomere length (bottom traces) in dobutamine-treated cardiomyocytes of CON, AB, and AB-LIT groups. Electrical field stimuli are indicated by arrows. B and C: summary data of diastolic (○, ◇, ☐) and systolic (●, ◆, ■) Ca2+ (B) and sarcomere length (C) in CON (○, ●), AB (◇, ◆), and AB-LIT (☐, ■) in the presence of dobutamine (1 μM). *P < 0.05 vs. CON. §P < 0.10 vs. CON and AB.
Fig. 5.
Summary data of cardiomyocyte Ca2+ transient and sarcomere shortening amplitude and rate following dobutamine challenge. A and B: summary data of Ca2+ transient (A) and shortening (B) amplitude in dobutamine-treated cardiomyocytes of CON, AB, and AB-LIT groups. C and D: summary data of maximum Ca2+ transient rate (C) and maximum (most negative) shortening rate (D) in the presence of dobutamine (1 μM). E: sarcomere length-Ca2+ loop relationships during excitation-contraction coupling with dobutamine. Loops are averaged traces of all cardiomyocytes within CON (●), AB (◇), and AB-LIT ( ). Traces were aligned for averaging based on electrical stimulation, with error bars omitted for clarity. *P < 0.05 vs. CON. #P < 0.05 vs. AB.
). Traces were aligned for averaging based on electrical stimulation, with error bars omitted for clarity. *P < 0.05 vs. CON. #P < 0.05 vs. AB.
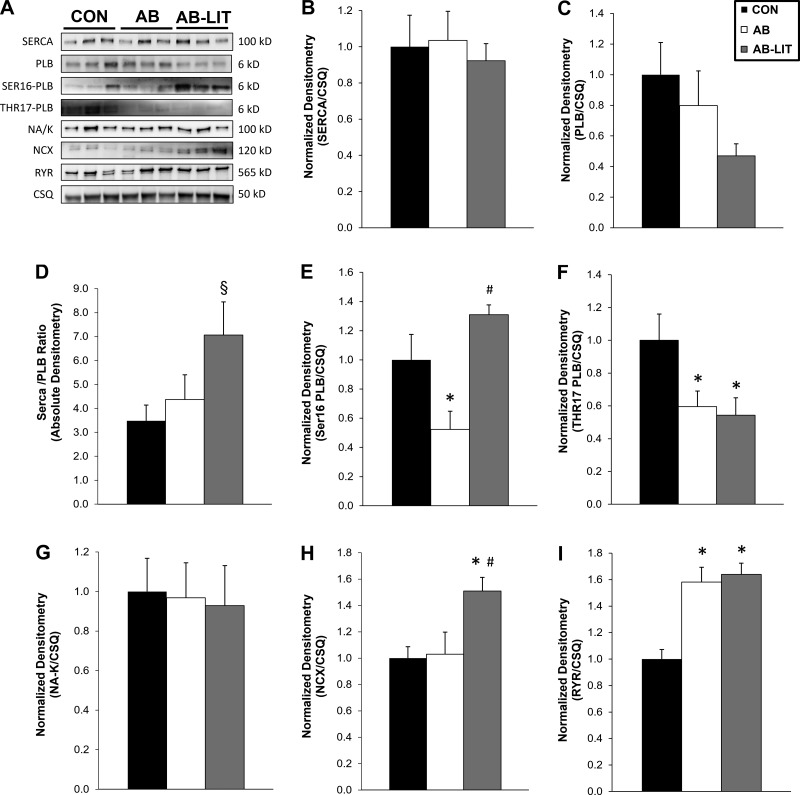
We next examined Ca2+ handling protein levels in whole heart homogenates using Western blotting (Fig. 6). No changes were observed in SERCA levels among groups; however, the SERCA/PLB ratio was increased in AB-LIT (Fig. 6D, vs. CON and AB). Phosphorylation of PLB at the Ser16 site was reduced in AB (Fig. 6E, vs. CON and AB- LIT), while phosphorylation of PLB at the Thr17 site was reduced in both AB and AB-LIT (Fig. 6F, vs. CON). The level of the Na+-K+ ATPase was similar among groups, while protein of a primary Ca2+ extrusion pathway in the cardiomyocyte NCX was increased in AB-LIT (Fig. 6H, vs. CON and AB). RyR levels were increased in AB and AB-LIT vs. CON (Fig. 6I). Taken together, these findings suggest complex regulation of Ca2+ handling protein expression and baseline phosphorylation in response to both pressure overload and exercise training.
Fig. 6.
Protein expression of Ca2+ handling proteins. A: example Western blots of sarco/endoplasmic reticulum Ca2+ ATPase (SERCA; 100 kDa), phospholamban (PLB; 6 kDa), the Ser16 phosphorylation site of PLB (SER16-PLB; 6 kDa), the Thr17 phosphorylation site of PLB (THR17-PLB; 6 kDa), the Na+-K+-ATPase (NA/K; 100 kDa), sodium-calcium exchanger (NCX; 120 kDa), ryanodine receptor (RYR; 565 kDa), and calsequestrin (CSQ; 50 kDa). Summary data of protein expression (relative to CSQ) of SERCA (B), PLB (C), SERCA/PLB ratio (D), SER16-PLB (E), THR17-PLB (F), Na+-K+ ATPase (G), NCX (H), and RYR (I). Data shown are from whole heart homogenates of CON, AB, and AB-LIT groups; n = 5–7 animals per group. *P < 0.05 vs. CON. #P < 0.05 vs. AB. §P < 0.10 vs. CON and AB.
DISCUSSION
The present study in a large animal model with cardiac features of HFpEF reveals chronic low-intensity exercise training attenuates functional impairments in Ca2+ homeostasis induced by pressure overload. In rodent models in the absence of pathological stimuli, exercise training increases expression of SERCA (55), PLB phosphorylation (24), and myofilament sensitivity (11, 55) within cardiomyocytes, all of which augment ECC. Chronic exercise training also increased cardiomyocyte power output in healthy Yucatan mini-swine (22). However, these findings were observed in response to exercise intensities likely intolerable for heart failure patients. This study demonstrates positive adaptations to cardiomyocyte ECC using an exercise stimulus <50% of the absolute intensity typically utilized in comparable physiological swine exercise studies and at a reduced frequency of three exercise bouts per week (vs. 5 sessions per week in healthy animal models) (28).
Beneficial effects of exercise on cardiomyocyte ECC have previously been reported in animal models of heart failure, although they have been predominately observed in rodent models with more relevance to HFrEF. Exercise training initiated following myocardial infarction (MI) in mice (9) and rats (54) prevented MI-associated changes in SR Ca2+-handling protein expression and myofilament dysfunction, with restoration of Ca2+ transient amplitude and contraction during ECC. Mechanistically, we observed an increase in the SERCA/PLB ratio and preservation of normal PLB phosphorylation at Ser16 in the AB-LIT group. Exercise training also induced a significant increase in NCX levels, which favors diastolic Ca2+ extrusion and lower diastolic Ca2+ (9, 56). Theoretically, this effect of training could shift cellular Ca2+ flux toward Ca2+ extrusion and lower SR Ca2+ content, particularly when considered in parallel with the reduction in PLB phosphorylation of Thr17 in the AB-LIT group. Overall, these findings may partially explain how exercise training attenuated, but did not fully prevent, AB-induced decreases to Ca2+ transient amplitude in AB-LIT animals.
Chronic low-intensity exercise training also preserved normal cellular Ca2+ transient and shortening reserve assessed by adrenergic challenge. Evidence of this concept is provided by the maintenance of Ca2+ transient amplitude and rate, as well as shortening amplitude and rate, in AB-LIT cardiomyocytes compared with CON with dobutamine treatment. Although dobutamine exhibits activity at both α- and β-adrenoreceptor subtypes (46), it was utilized for the present study given the rapid and robust inotropic effects on cardiomyocytes via the β1-receptor (2). The time to maximum inotropic response in AB-LIT was also similar to CON, indicative of exercise-induced beneficial effects on β-adrenergic sensitivity (9, 29). Together, our data illustrate the beneficial effects of low-intensity exercise training on cardiomyocyte function in an experimental setting of pressure overload and suggest the preservation of cellular β-adrenergic sensitivity following chronic exercise training may counteract decreases in cardiac reserve highly prevalent in heart failure patients.
Both the AB and AB-LIT groups exhibited global hypertrophic cardiac remodeling and an associated decrease in cardiomyocyte length:width ratio. As expected, cardiomyocytes in the AB group displayed an increase in cell width and no change in cell length compared with CON, consistent with hypertrophic cellular remodeling and the addition of sarcomeres in parallel. However, chronic exercise training caused unexpected structural remodeling in AB-LIT animals characterized by a decrease in length, with no change in width, when compared with CON. This cellular morphological change contrasts the ≈6–12% increase in cardiomyocyte length due to series addition of sarcomeres, along with a commensurate increase in cross-sectional area, traditionally observed following chronic endurance treadmill exercise-training programs in nonpathological animal models (25, 37, 38). An additional intriguing finding was the discordance between global and cellular hypertrophic remodeling in AB-LIT animals, where an increase in heart weight was observed without an associated increase in cardiomyocyte length or width. Cardiomyocyte morphology was obtained from the midmyocardium of the LV free wall, and we cannot exclude that cardiomyocyte hypertrophy in other cardiac regions contributed to an increase in overall cardiac mass. Cardiomyocyte proliferation following the combined pressure overload and exercise stimuli could also account for an increase in heart size. However, endogenous mechanisms of cardiomyocyte renewal in the adult heart are controversial, and most investigations indicate the adult heart has a low capacity for cardiomyocyte proliferation (15). The sarcomere length-Ca2+ relationship analysis also revealed that cardiomyocytes operated at longer sarcomere length throughout the ECC cycle in AB-LIT animals under untreated conditions as well as during adrenergic challenge. Such findings point to sarcomeric ultrastructural remodeling and an extension of cardiomyocyte slack length, which is classically viewed to be dependent on the length of the giant structural protein titin (17, 45). Changes in titin isoform expression and phosphorylation status have been shown with both cardiac disease (30, 31) and following exercise training (6, 18), and it is plausible that titin-based extension of cardiomyocyte slack length in AB-LIT contributes to the longer sarcomere length during ECC. In total, our findings suggest exercise of a low absolute intensity under conditions of chronic pressure overload in this translational swine model promotes alternate cardiomyocyte remodeling processes that differ from previous observations made in healthy animals.
The present investigation adds to previous work from our laboratories, which illustrates cardiomyocyte Ca2+ transients and shortening are impaired in a translational large animal model with cardiac characteristics consistent with HFpEF. The extent by which cardiomyocyte ECC is altered in HFpEF remains controversial, and interpretation of cardiomyocyte data from rodent models with relevance to HFpEF is often complicated by an adaptive hypercontractile compensatory phase (49) that rapidly decompensates to a phenotype more closely resembling HFrEF (32). In this study, data indicate the reduction in Ca2+ transient amplitude and rate in the AB group was not associated with changes in SERCA or PLB protein level. These findings stand in contrast to alterations in cardiomyocyte ECC particularly in end-stage decompensated heart failure where expression of SERCA is decreased (33, 57). Our data indicate decreased PLB phosphorylation at both Ser16 (protein kinase A site) and Thr17 (Ca2+/calmodulin-dependent protein kinase II site) may play a role by impairing SR Ca2+ sequestration via increasing the inhibitory effect of PLB on SERCA. In the absence of changes in SERCA/PLB expression, decreased PLB phosphorylation would lead to greater association of PLB with SERCA, increased inhibition of SERCA-mediated SR Ca2+ uptake, decreased SR Ca2+ content, and a decrease in the Ca2+ transient during ECC.
Decreases in Ser16 phosphorylation of PLB in AB animals, observed in parallel with the delayed time to maximum response after dobutamine exposure, are consistent with the concept of “β-adrenoreceptor desensitization,” which has been observed with advancing age and heart failure (4, 5, 52). When cardiomyocytes were challenged with the adrenergic agonist dobutamine, all groups exhibited an increase in Ca2+ transient amplitude and shortening. However, Ca2+ transient amplitude and myocyte shortening were still decreased in AB vs. CON similar to observations under untreated conditions, indicating that β1-receptor activation was unable to rescue function in the AB group. β1-adrenergic stimulation exerts its cellular effects via the Gs-cAMP-protein kinase A signaling cascade, with resulting phosphorylation of Ca2+ handling proteins including PLB at the Ser16 site. Sustained elevation in Ca2+ cycling following β1-adrenergic stimulation also leads to a Ca2+ dependent increase in PLB phosphorylation at Thr17 via Ca2+/calmodulin-dependent protein kinase II, although this secondary response may be blunted in heart failure due to excessive phosphatase activity (27). Our findings suggest impaired cardiomyocyte Ca2+ transients and shortening in this translational heart failure model are associated with decreased PLB phosphorylation via multiple signaling pathways.
Limitations.
The large animal model of pressure overload-induced heart failure used in this investigation reflects a number of features consistent with HFpEF, including LV hypertrophy, increased lung weight, and normal resting fractional shortening and SV. Taken into consideration with other published studies from our laboratory demonstrating diastolic dysfunction, increased LV fibrosis, and reduced LV contractile reserve in this same model (13, 14, 20, 34, 40, 41), these findings indicate the relevance of this translational model to the pathophysiology of HFpEF. Nevertheless, it is difficult to precisely replicate HFpEF in animal models given the significant aging and sex components associated with the disease that are often accompanied by numerous clinical comorbidities (47, 48). From a practical perspective, aging pigs to true senescence is difficult given their relatively long lifespan (~15 yr) (51). Although this study focused on males, we have also examined aortic-banded ovariectomized female swine as a distinct translationally relevant HFpEF model (40) given the prevalence of HFpEF is twice as high in females (47, 48). Comorbidities common to HFpEF also complicate interpretation of experimental findings, and for this reason we consider a strength of this animal model is the ability to examine mechanisms of cardiac dysfunction independent of confounding disease processes. Systemic hypertension and aortic stenosis also contribute to the development of HFpEF, and while our surgical intervention creates cardiac pressure overload, it does not completely reflect the impact of systemic high blood pressure or decreases in arterial compliance seen in aortic stenosis. Finally, therapeutic exercise regimens are typically not prescribed concurrent with excessive afterload, as patients under the care of physicians are often treated to reduce existing hypertension. Thus, while our experimental exercise modalities do not exactly mimic existing clinical paradigms, our results suggest that exercise under these conditions is not necessarily detrimental and may exert positive cellular benefits on the heart.
In summary, our data provide evidence that chronic low-intensity endurance exercise training is an effective therapeutic alternative that beneficially impacts impaired cardiomyocyte Ca2+ homeostasis, contractile function, and β-adrenergic sensitivity in a large animal model of pressure overload-induced heart failure. Importantly, these results show intrinsic impairments to cardiomyocyte ECC in an experimental setting with relevance to human HFpEF and imply potentially important pathological mechanistic differences compared with HFrEF. Considering the lack of effective pharmacological therapies for HFpEF patients, our findings provide a strong rationale regarding clinical implementation of similar exercise programs for treating this heart failure subtype.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-112998 (to C. A. Emter) and R01-HL-136292 (to T. L. Domeier) and National Institute on Aging Grant K01-AG-041208 (to T. L. Domeier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.H., C.A.E., and T.L.D. conceived and designed research; J.A.H., M.D.L., T.D.O., B.S.F., and C.A.E. performed experiments; J.A.H., A.B.V., M.D.L., K.S.M., C.A.E., and T.L.D. analyzed data; J.A.H., A.B.V., T.D.O., B.S.F., K.S.M., C.A.E., and T.L.D. interpreted results of experiments; J.A.H., A.B.V., M.D.L., C.A.E., and T.L.D. prepared figures; J.A.H., A.B.V., C.A.E., and T.L.D. drafted manuscript; J.A.H., A.B.V., M.D.L., T.D.O., B.S.F., K.S.M., C.A.E., and T.L.D. edited and revised manuscript; J.A.H., A.B.V., M.D.L., T.D.O., B.S.F., K.S.M., C.A.E., and T.L.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jan Ivey, Melissa Cobb, Pamela Thorne, and Daniel G. Dozier for considerable technical contributions, which were essential to the successful completion of the study, and Gore for generous gift of vascular Gore-Tex sleeves used for aortic banding.
REFERENCES
- 1.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21: 380–387, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers, 2001. doi: 10.1007/978-94-010-0658-3 [DOI] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Briston SJ, Caldwell JL, Horn MA, Clarke JD, Richards MA, Greensmith DJ, Graham HK, Hall MC, Eisner DA, Dibb KM, Trafford AW. Impaired β-adrenergic responsiveness accentuates dysfunctional excitation-contraction coupling in an ovine model of tachypacing-induced heart failure. J Physiol 589: 1367–1382, 2011. doi: 10.1113/jphysiol.2010.203984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 6.Chung CS, Hutchinson KR, Methawasin M, Saripalli C, Smith JE III, Hidalgo CG, Luo X, Labeit S, Guo C, Granzier HL. Shortening of the elastic tandem immunoglobulin segment of titin leads to diastolic dysfunction. Circulation 128: 19–28, 2013. doi: 10.1161/CIRCULATIONAHA.112.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 6: 292–300, 2009. doi: 10.1038/nrcardio.2009.8. [DOI] [PubMed] [Google Scholar]
- 8.Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol 287: R247–R249, 2004. doi: 10.1152/ajpregu.00346.2004. [DOI] [PubMed] [Google Scholar]
- 9.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, Dekkers DH, Schoonderwoerd K, Schuurbiers HC, de Crom R, Stienen GJ, Sipido KR, Lamers JM, Duncker DJ. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res 100: 1079–1088, 2007. doi: 10.1161/01.RES.0000262655.16373.37. [DOI] [PubMed] [Google Scholar]
- 10.Dieberg G, Ismail H, Giallauria F, Smart NA. Clinical outcomes and cardiovascular responses to exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. J Appl Physiol (1985) 119: 726–733, 2015. doi: 10.1152/japplphysiol.00904.2014. [DOI] [PubMed] [Google Scholar]
- 11.Diffee GM, Chung E. Altered single cell force-velocity and power properties in exercise-trained rat myocardium. J Appl Physiol (1985) 94: 1941–1948, 2003. doi: 10.1152/japplphysiol.00889.2002. [DOI] [PubMed] [Google Scholar]
- 12.Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 58: 1780–1791, 2011. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Emter CA, Baines CP. Low-intensity aerobic interval training attenuates pathological left ventricular remodeling and mitochondrial dysfunction in aortic-banded miniature swine. Am J Physiol Heart Circ Physiol 299: H1348–H1356, 2010. doi: 10.1152/ajpheart.00578.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emter CA, Tharp DL, Ivey JR, Ganjam VK, Bowles DK. Low-intensity interval exercise training attenuates coronary vascular dysfunction and preserves Ca2+-sensitive K+ current in miniature swine with LV hypertrophy. Am J Physiol Heart Circ Physiol 301: H1687–H1694, 2011. doi: 10.1152/ajpheart.00610.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT, Marbán E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte regeneration: a consensus statement. Circulation 136: 680–686, 2017. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP; HF-ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1451–1459, 2009. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaser ML, Warren CM, Esbona K, Guo W, Duan Y, Parrish AM, Krzesinski PR, Norman HS, Dunning S, Fitzsimons DP, Moss RL. Mutation that dramatically alters rat titin isoform expression and cardiomyocyte passive tension. J Mol Cell Cardiol 44: 983–991, 2008. doi: 10.1016/j.yjmcc.2008.02.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidalgo C, Saripalli C, Granzier HL. Effect of exercise training on post-translational and post-transcriptional regulation of titin stiffness in striated muscle of wild type and IG KO mice. Arch Biochem Biophys 552-553: 100–107, 2014. doi: 10.1016/j.abb.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Hiemstra JA, Gutiérrez-Aguilar M, Marshall KD, McCommis KS, Zgoda PJ, Cruz-Rivera N, Jenkins NT, Krenz M, Domeier TL, Baines CP, Emter CA. A new twist on an old idea part 2: cyclosporine preserves normal mitochondrial but not cardiomyocyte function in mini-swine with compensated heart failure. Physiol Rep 2: e12050, 2014. doi: 10.14814/phy2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiemstra JA, Lee DI, Chakir K, Gutiérrez-Aguilar M, Marshall KD, Zgoda PJ, Cruz Rivera N, Dozier DG, Ferguson BS, Heublein DM, Burnett JC, Scherf C, Ivey JR, Minervini G, McDonald KS, Baines CP, Krenz M, Domeier TL, Emter CA. Saxagliptin and tadalafil differentially alter cyclic guanosine monophosphate (cGMP) signaling and left ventricular function in aortic-banded mini-swine. J Am Heart Assoc 5: e003277, 2016. doi: 10.1161/JAHA.116.003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiemstra JA, Liu S, Ahlman MA, Schuleri KH, Lardo AC, Baines CP, Dellsperger KC, Bluemke DA, Emter CA. A new twist on an old idea: a two-dimensional speckle tracking assessment of cyclosporine as a therapeutic alternative for heart failure with preserved ejection fraction. Physiol Rep 1: e00174, 2013. doi: 10.1002/phy2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinken AC, Korte FS, McDonald KS. Porcine cardiac myocyte power output is increased after chronic exercise training. J Appl Physiol (1985) 101: 40–46, 2006. doi: 10.1152/japplphysiol.00798.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kapiloff MS, Emter CA. The cardiac enigma: current conundrums in heart failure research. F1000Res 5: F1000, 2016. doi: 10.12688/f1000research.7278.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemi OJ, Ellingsen O, Ceci M, Grimaldi S, Smith GL, Condorelli G, Wisløff U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol 43: 354–361, 2007. doi: 10.1016/j.yjmcc.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemi OJ, Wisløff U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol (Oxf) 199: 425–439, 2010. doi: 10.1111/j.1748-1716.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- 26.Keteyian SJ, Piña IL, Hibner BA, Fleg JL. Clinical role of exercise training in the management of patients with chronic heart failure. J Cardiopulm Rehabil Prev 30: 67–76, 2010. doi: 10.1097/HCR.0b013e3181d0c1c1. [DOI] [PubMed] [Google Scholar]
- 27.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res 110: 1646–1660, 2012. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol (1985) 67: 1140–1149, 1989. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- 29.Leosco D, Rengo G, Iaccarino G, Filippelli A, Lymperopoulos A, Zincarelli C, Fortunato F, Golino L, Marchese M, Esposito G, Rapacciuolo A, Rinaldi B, Ferrara N, Koch WJ, Rengo F. Exercise training and beta-blocker treatment ameliorate age-dependent impairment of β-adrenergic receptor signaling and enhance cardiac responsiveness to adrenergic stimulation. Am J Physiol Heart Circ Physiol 293: H1596–H1603, 2007. doi: 10.1152/ajpheart.00308.2007. [DOI] [PubMed] [Google Scholar]
- 30.LeWinter MM, Granzier HL. Titin is a major human disease gene. Circulation 127: 938–944, 2013. doi: 10.1161/CIRCULATIONAHA.112.139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res 77: 637–648, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Ljubojevic S, Radulovic S, Leitinger G, Sedej S, Sacherer M, Holzer M, Winkler C, Pritz E, Mittler T, Schmidt A, Sereinigg M, Wakula P, Zissimopoulos S, Bisping E, Post H, Marsche G, Bossuyt J, Bers DM, Kockskämper J, Pieske B. Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation 130: 244–255, 2014. doi: 10.1161/CIRCULATIONAHA.114.008927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louch WE, Stokke MK, Sjaastad I, Christensen G, Sejersted OM. No rest for the weary: diastolic calcium homeostasis in the normal and failing myocardium. Physiology (Bethesda) 27: 308–323, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Marshall KD, Muller BN, Krenz M, Hanft LM, McDonald KS, Dellsperger KC, Emter CA. Heart failure with preserved ejection fraction: chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. J Appl Physiol (1985) 114: 131–147, 2013. doi: 10.1152/japplphysiol.01059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald KS, Emter CA. Exploring new concepts in the management of heart failure with preserved ejection fraction: is exercise the key for improving treatment? J Appl Physiol (1985) 119: 724–725, 2015. doi: 10.1152/japplphysiol.00570.2015. [DOI] [PubMed] [Google Scholar]
- 36.McDonald KS, Hanft LM, Domeier TL, Emter CA. Length and PKA dependence of force generation and loaded shortening in porcine cardiac myocytes. Biochem Res Int 2012: 371415, 2012. doi: 10.1155/2012/371415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore RL, Musch TI, Yelamarty RV, Scaduto RC Jr, Semanchick AM, Elensky M, Cheung JY. Chronic exercise alters contractility and morphology of isolated rat cardiac myocytes. Am J Physiol Cell Physiol 264: C1180–C1189, 1993. doi: 10.1152/ajpcell.1993.264.5.C1180. [DOI] [PubMed] [Google Scholar]
- 38.Moore RL, Palmer BM. Exercise training and cellular adaptations of normal and diseased hearts. Exerc Sport Sci Rev 27: 285–315, 1999. doi: 10.1249/00003677-199900270-00011. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL, HF-ACTION Investigators; HF-ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1439–1450, 2009. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olver TD, Hiemstra JA, Edwards JC, Ferguson BS, Laughlin MH, Emter CA. The protective role of sex hormones in females and exercise prehabilitation in males on sternotomy-induced cranial hypoperfusion in aortic banded mini-swine. J Appl Physiol (1985) 122: 423–429, 2017. doi: 10.1152/japplphysiol.00817.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olver TD, Klakotskaia D, Ferguson BS, Hiemstra JA, Schachtman TR, Laughlin MH, Emter CA. Carotid artery vascular mechanics serve as biomarkers of cognitive dysfunction in aortic-banded miniature swine that can be treated with an exercise intervention. J Am Heart Assoc 5: e003248, 2016. doi: 10.1161/JAHA.116.003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8: 33–40, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peana D, Domeier TL. Cardiomyocyte Ca2+ homeostasis as a therapeutic target in heart failure with reduced and preserved ejection fraction. Curr Opin Pharmacol 33: 17–26, 2017. doi: 10.1016/j.coph.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, Anker SD, Solal AC, Filippatos GS, Hoes AW, Gielen S, Giannuzzi P, Ponikowski PP. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 13: 347–357, 2011. doi: 10.1093/eurjhf/hfr017. [DOI] [PubMed] [Google Scholar]
- 45.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci USA 104: 3444–3449, 2007. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruffolo RR Jr, Spradlin TA, Pollock GD, Waddell JE, Murphy PJ. Alpha and beta adrenergic effects of the stereoisomers of dobutamine. J Pharmacol Exp Ther 219: 447–452, 1981. [PubMed] [Google Scholar]
- 47.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol 26: 562–568, 2011. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 48.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134: 73–90, 2016. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shorofsky SR, Aggarwal R, Corretti M, Baffa JM, Strum JM, Al-Seikhan BA, Kobayashi YM, Jones LR, Wier WG, Balke CW. Cellular mechanisms of altered contractility in the hypertrophied heart: big hearts, big sparks. Circ Res 84: 424–434, 1999. doi: 10.1161/01.RES.84.4.424. [DOI] [PubMed] [Google Scholar]
- 50.Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 51.Vodicka P, Smetana K Jr, Dvoránková B, Emerick T, Xu YZ, Ourednik J, Ourednik V, Motlík J. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci 1049: 161–171, 2005. doi: 10.1196/annals.1334.015. [DOI] [PubMed] [Google Scholar]
- 52.White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation 90: 1225–1238, 1994. doi: 10.1161/01.CIR.90.3.1225. [DOI] [PubMed] [Google Scholar]
- 53.Williams JL, Hathaway CA, Kloster KL, Layne BH. Low power, type II errors, and other statistical problems in recent cardiovascular research. Am J Physiol Heart Circ Physiol 273: H487–H493, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Wisløff U, Loennechen JP, Currie S, Smith GL, Ellingsen Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res 54: 162–174, 2002. doi: 10.1016/S0008-6363(01)00565-X. [DOI] [PubMed] [Google Scholar]
- 55.Wisløff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 50: 495–508, 2001. doi: 10.1016/S0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- 56.Zhang XQ, Ng YC, Musch TI, Moore RL, Zelis R, Cheung JY. Sprint training attenuates myocyte hypertrophy and improves Ca2+ homeostasis in postinfarction myocytes. J Appl Physiol (1985) 84: 544–552, 1998. doi: 10.1152/jappl.1998.84.2.544. [DOI] [PubMed] [Google Scholar]
- 57.Zima AV, Bovo E, Mazurek SR, Rochira JA, Li W, Terentyev D. Ca handling during excitation-contraction coupling in heart failure. Pflugers Arch 466: 1129–1137, 2014. doi: 10.1007/s00424-014-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]