Abstract
Resistance exercise during the postprandial period lowers venous glucose concentrations in individuals with type 2 diabetes, but the impact of resistance exercise on interstitial glucose concentrations is not well understood. The objective of this study was to compare subcutaneous adipose tissue interstitial glucose and venous blood glucose concentrations during postprandial resistance exercise in patients with type 2 diabetes. Eleven individuals completed two trials in a random order including a no-exercise (NoEx) and a postprandial resistance exercise trial (M-Ex). During the trials, the individuals consumed a meal and either remained sedentary (NoEx) or performed a session of resistance training beginning 45 min after the meal (M-Ex) while interstitial and venous glucose concentrations were simultaneously measured. Venous glucose during exercise was ~11% lower (P = 0.05) during M-Ex (8.0 ± 0.5 mmol/l) compared with NoEx (9.0 ± 0.5 mmol/l) whereas interstitial glucose during M-Ex (10.4 ± 0.7 mmol/l) was not different compared with interstitial glucose during NoEx (10.1 ± 0.7 mmol/l). Bland-Altman plots revealed that the difference (bias) between interstitial and venous glucose during exercise was more than twofold greater during M-Ex (2.36 ± 2.07 mmol/l) compared with NoEx (1.11 ± 1.69 mmol/l). The mean (33.8 ± 6.2 mmol/l) and median (34.7 ± 6.3 mmol/l) absolute relative difference during exercise were 73% and 78% greater compared with the mean (19.5 ± 4.1 mmol/l) and median (19.5 ± 4.1 mmol/l) absolute relative difference during NoEx (P = 0.04). Resistance exercise has unequal effects on glucose concentrations within different bodily compartments as exercise reduced venous glucose concentrations but not adipose tissue interstitial glucose concentrations in the abdominal region in individuals with type 2 diabetes.
NEW & NOTEWORTHY This is the first study to compare subcutaneous adipose tissue interstitial glucose concentrations and venous blood glucose concentrations during postprandial resistance exercise in individuals with type 2 diabetes. We find that resistance exercise effectively reduces systemic venous blood glucose concentrations but not subcutaneous adipose tissue interstitial glucose concentrations in the abdominal region. Resistance exercise has differential effects on glucose concentrations depending on its compartmentalization within the body.
Keywords: weight training, interstitial glucose, blood glucose, diabetes, obesity
INTRODUCTION
Interstitial fluid is an extracellular solution that is found in the spaces between most cells of the body and is spatially different compared with plasma within the blood compartment. Absorption of nutrients (e.g., glucose) into nondigestive tissues, such as adipose or skeletal muscle tissue, is through diffusion across the capillary endothelium lining the blood vessels into the interstitial fluid before reaching the cell membrane. Interstitial glucose and venous glucose concentrations are similar under steady-state conditions in healthy humans (21) and in patients with type 1 diabetes (2). An estimated lag time between equilibration (i.e., a stable gradient) of interstitial fluid and the blood has been reported to be between 0 and 45 min (28), and in one study this lag time has been shown to increase by 8 min when venous blood glucose concentrations change rapidly from euglycemia to hyperglycemia (16).
Exercise rapidly alters venous glucose concentrations in patients with type 2 diabetes, particularly exercise performed in the postprandial period (14, 19, 20). In both nondiabetic and diabetic patients, subcutaneous adipose tissue interstitial glucose concentrations (measured with a continuous glucose monitor) were shown to be higher compared with capillary or venous glucose concentrations during aerobic and/or resistance exercise (11, 15, 18, 22). In another study, the mean difference between subcutaneous adipose tissue interstitial and venous glucose concentrations was greater during low-intensity exercise, whereas the mean difference was lower during high-intensity exercise in patients with type 1 diabetes (10). On the contrary, another study reported subcutaneous adipose tissue interstitial glucose concentrations were not significantly different compared with venous glucose concentrations during aerobic or resistance exercise in patients with type 1 diabetes (32). The mix of findings between studies could possibly be due to the heterogeneity in populations used, different devices used to measure interstitial glucose, different sites of measurement, different exercise modalities prescribed, and/or timing of exercise relative to the last meal.
The impact of the timing of exercise relative to a meal on venous and subcutaneous adipose tissue interstitial glucose concentrations has not been well defined. Although it has been established that both pre- and postdinner resistance exercise lowers postprandial venous glucose concentrations in adults with type 2 diabetes, the magnitude and rate of change are quite different (14). For instance, predinner resistance exercise results in a more gradual decline in postprandial blood glucose concentrations, whereas postdinner resistance exercise results in a rapid decline in blood glucose concentrations at the onset of exercise followed by a rapid increase (rebound) in glucose concentrations shortly after cessation of exercise. Exercise during the postprandial period, when glucose is changing rapidly due to the meal and exercise, may result in greater differences in interstitial and venous glucose concentrations, although this has never been explored in individuals with type 2 diabetes. Therefore, the primary purpose of this study was to compare subcutaneous adipose tissue interstitial glucose concentrations in the abdominal region and venous blood glucose concentrations during sedentary and postprandial resistance exercise conditions in subjects with type 2 diabetes. Additionally, this study sought to determine how distinct phases of the postprandial glucose profile compares between the interstitial and venous compartments. Given that prior research (11, 15, 18, 22) has shown that interstitial glucose concentrations during the fasted state are higher during exercise compared with venous glucose concentrations, we hypothesized that subcutaneous interstitial glucose concentrations during postprandial resistance exercise would be higher compared with venous blood glucose concentrations in individuals with type 2 diabetes.
METHODS
Subjects
Individuals included in this study 1) were men and women (not pregnant or lactating) between the ages of 25 and 68 yr that were physician-diagnosed with type 2 diabetes, 2) were obese, 3) were not using tobacco products, 4) had not had weight-loss surgery, and 5) were not using insulin. This study was approved by the Institutional Review Board at the University of Missouri, and all subjects provided written, informed consent before beginning research activities.
General Study Design
This study was a small part of another study that has been published (14). Initially, the subjects completed two familiarization sessions which were separated by 10-repetition maximum testing (details described later). After these initial testing visits, the subjects completed two different experimental trials in a random order and spaced at least 1 wk apart. The two trials included a no-exercise trial (NoEx) and a postprandial exercise trial (M-Ex, exercise began 45 min after an evening dinner). The day before the NoEx and M-Ex trials a Medtronic iPro (MedTronic MiniMed) continuous glucose monitor was inserted in the periumbilical region of the abdomen of the subject and was used to measure interstitial glucose concentrations (14). During both trials, the glucose monitor was inserted in the same area to limit variability due to differences in site of sensor insertion. After insertion, the subjects were given their prepackaged breakfast and lunch meals for the next day. On the following day, the subjects consumed the prepackaged breakfast and lunch meals on their own. In the evening, the subjects reported to the laboratory for testing during which they consumed a standardized dinner meal with or without a session of whole body resistance exercise. Upon arrival, a venous catheter was inserted into the antecubital vein and frequent venous blood samples were taken to assess venous glucose concentrations with a YSI 2700 SELECT automated glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). The following morning, the participants reported back to the laboratory for a blood draw.
Blood sampling and calibration of the continuous glucose monitor.
Venous blood samples were taken ~5 min before the meal (baseline) and then at 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 150, 180, 210, and 240 min after the meal was completely ingested. These samples were immediately placed on ice and the glucose concentration was measured within 5 and 10 min of taking the blood sample. The continuous glucose monitor (CGM) was calibrated according to manufacturer specifications. Approximately 1 h after inserting the continuous glucose monitor in the evening the subjects took and recorded a capillary blood glucose measurement. The following day, immediately before breakfast and lunch, the subjects took a capillary blood glucose measure and recorded it. These capillary blood glucose values were later used to calibrate the continuous glucose monitor for these time periods. Immediately before dinner and immediately before breakfast the following day, venous blood glucose was taken and measured with the YSI, and these values were used to calibrate the CGM. Pilot testing in our laboratory has shown that venous glucose concentrations are lower compared with capillary blood glucose (data not shown), and since we were comparing venous and interstitial glucose concentrations in this study (and considered the venous compartment to be the reference) we calibrated the continuous glucose monitor with venous glucose values. The continuous glucose monitor values that were determined by calibration with capillary glucose values were not included in this study.
Exercise.
Exercise consisted of a whole body resistance exercise session that targeted all the major muscle groups. The first familiarization session was a general orientation session to teach the subjects how to correctly perform each exercise. The subjects completed 1–2 sets of each exercise which included the leg press, seated calf raises, seated chest flies, seated back flies, back extensions, shoulder raises, leg curls, and abdominal crunches. Subsequently, within a week of this first visit the subjects reported back to the laboratory for 10-repetition maximum testing of each exercise previously listed, except abdominal crunches. Last, after a 1-wk washout the participants reported back to the laboratory for the second familiarization session which included performing three sets of 10 repetitions of each exercise described previously. Three sets of each exercise were completed before moving to the next exercise, and ~1–2 min of rest was allotted between sets. For the first set, the weight used was ~50% of the subjects previously determined 10-repetition maximum. For sets 2 and 3, 100% of the 10-repetition maximum weight was used. The entire exercise session lasted on average ~45–47 min. Following the familiarization and strength testing the subjects completed the NoEx and M-Ex trials. The second familiarization session was identical to the session that was performed during the M-Ex trial.
Diet.
Diet was standardized during the testing day as all subjects were provided breakfast, lunch, and dinner with the macronutrient composition of each meal consisting of 50% carbohydrate, 35% fat, and 15% protein (14). The total caloric load provided to each subject during the testing day was based on their estimated total daily energy expenditure. The total daily energy expenditure of each subject was estimated by measuring the resting energy expenditure with a metabolic cart (ParvoMedics TrueOne 2400, Sandy, UT) and average daily physical activity levels with an armband (BodyMedia armband, BodyMedia, Pittsburgh, PA) of the subjects before testing. The subjects were then provided a diet during the testing day that was equal in caloric content to their estimated total daily energy expenditure, and consumed the same diet during both testing days. The foods included in the breakfast meal were an English muffin, cheddar cheese, one large egg, ham, hash browns, ketchup, and apple or orange juice. The lunch meal contained white bread, ham, mayonnaise, cheddar cheese, a granola bar, and apple or orange juice. The dinner meal consumed in the laboratory during the testing period consisted of spaghetti with meat sauce, garlic bread, caffeine-free soda, and 1.5 g of acetaminophen to assess gastric emptying (an outcome that was a part of the parent study). The subjects took their medications at the same time, dose, and frequency during both conditions.
Calculations and Statistical Methods
Data from the continuous glucose monitor was processed according to manufacturer specifications using Solutions Software for iPro (version 2.0A). The statistical analysis and Bland-Altman plots were computed using GraphPad Prism 6 software (GraphPad Software). The mean absolute relative difference (ARD) and median ARD were calculated using the following formula: ARD = 100 × [(interstitial glucose – venous glucose)/venous glucose], mean ARD = average ARD of all time points for each subject, and median ARD = median ARD of all time points for each subject. Initially, an ARD value was calculated at each of the 19 time points that interstitial and venous glucose concentrations were measured. Subsequently, the mean or median ARD value for all 19 time points was calculated for each subject. Statistically significant differences between interstitial and venous glucose values at each time point during each trial were calculated using a two-way ANOVA with repeated measures. Specific differences were determined with Holm-Sidak post hoc tests. We further explored differences between interstitial and venous glucose concentrations by calculating the change in glucose during the initial 15 min after the meal or the average glucose values during a specifically defined period (i.e., minutes 0–49, 50–100, and 101–240). A one-way repeated-measures ANOVA with Holm-Sidak post hoc tests was then used to determine specific differences in the average change in glucose concentrations or the average glucose concentration during the defined period. A paired samples t-test was used to compare mean and median ARD values between trials. Statistical significance was set at P ≤ 0.05. The data are presented as means ± SD unless otherwise noted.
RESULTS
Subject Demographics
Eleven obese men and women with physician-diagnosed type 2 diabetes completed this study (M = 3, F = 8). Three women were postmenopausal, and one of these women was taking hormone replacement therapy. While enrolled in the study all subjects maintained their body weight and medication use and dose. The average age was 49 ± 13 yr, height 1.65 ± 0.10 m, weight 101.5 ± 24.1 kg, body mass index 37.0 ± 5.7 kg/m2, body fat 41.5 ± 7.2%, fasting glucose 7.9 ± 2.7 mmol/l, and HbA1c 7.2 ± 0.7%. Medications the subjects were taking included metformin (n = 8), statins (n = 2), lisinopril (n = 2), lipitor (n = 1), glyburide (n = 3), januvia (n = 2), and fenofibrate (n = 1).
Interstitial and Venous Glucose Concentrations
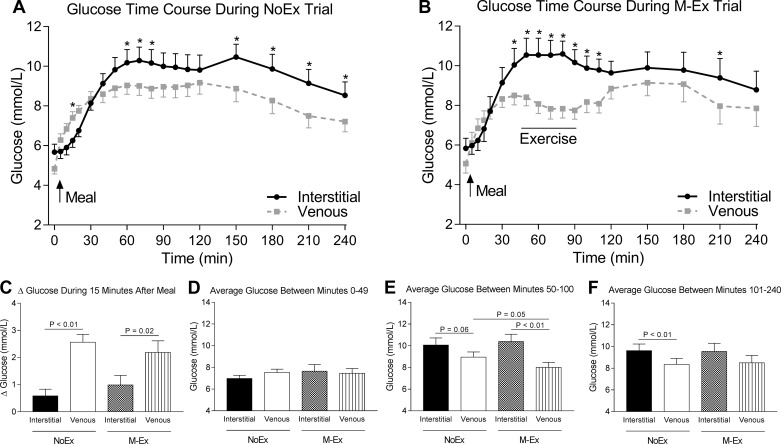
The interstitial and venous glucose time course revealed several differences between compartments (Figs. 1, A and B). During the NoEx trial there was a significant effect of time [F(18,180) = 23.06, P < 0.01], compartment [F(1,10) = 7.72, P = 0.02], and time × compartment interaction [(F(18,180) = 6.01, P < 0.01] with interstitial glucose concentrations being significantly greater compared with venous glucose concentrations at minutes 15, 60, 70, 80, 150, 180, 210, and 240 after the meal (all P < 0.05). During the M-Ex trial there was a significant effect of time [F(18,180) = 10.86, P < 0.01], compartment [F(1,10) = 9.63, P = 0.01], and time × compartment interaction [F(18,180) = 6.20, P < 0.01] with interstitial glucose concentrations being significantly greater compared with venous glucose concentrations at minutes 40, 50, 60, 70, 80, 90, 100, 110, and 210 after the meal (all P < 0.05). Note that during the M-Ex trial, all glucose values during the exercise period (minutes 50–100) were greater in the interstitial compartment compared with the venous compartment, whereas during the NoEx trial, not all interstitial glucose values were greater compared with venous glucose values during this time frame.
Fig. 1.
Glucose time course and average glucose concentrations during exercise. A: glucose time course during the no-exercise (NoEx) trial. B: glucose time course during the postprandial exercise (M-Ex) trial. In A and B, the black line represents interstitial glucose concentrations, whereas the gray line represents venous glucose concentrations. *Interstitial glucose concentrations were significantly (P < 0.05) greater compared with venous glucose concentrations at that specific time point. C: the change in interstitial and venous glucose concentrations during the 15-min period immediately after ingestion of the meal. D–F: average interstitial and venous glucose concentrations between minutes 0–49 (D), 50–100 (E), and 101–240 (F) after the meal was consumed during the NoEx and M-Ex trial. Data in C–F were analyzed using a one-way ANOVA, and the P values shown are from follow-up Holm-Sidak’s post hoc tests that were performed to identify specific differences. A solid line below each P value connects the values that were compared. Values are means ± SE.
To further explore the data set, we next compared the change in glucose concentrations during the initial 15 min after the meal and the average glucose concentrations during the distinct time periods of 0–49 minutes, 50–100 minutes, and 101–240 minutes after the meal. The change in venous glucose within the first 15 min after the meal during both trials was more than twofold greater in venous (NoEx 2.56 ± 0.29 and M-Ex 2.19 ± 0.43 mmol/l) compared with the interstitial compartment (NoEx 0.59 ± 0.25 and M-Ex 0.99 ± 0.35 mmol/l) [F(2.51,25.1) = 8.89, P < 0.01] (Fig. 1C). However, the average interstitial glucose and venous glucose concentration was not different between minutes 0–49 after the meal [F(2.80,22.80) = 0.84, P = 0.46] (Fig. 1D). During exercise (between minutes ~50–100) venous glucose was ~11% (P = 0.05) lower during the M-Ex trial (8.0 ± 0.5 mmol/l) compared with the NoEx trial (9.0 ± 0.5 mmol/l), indicating that venous glucose decreases when exercise is performed [F(2.07,20.7) = 6.90, P < 0.01] (Fig. 1E). When compared with the average interstitial glucose concentration during exercise (10.4 ± 0.7 mmol/l), venous glucose during exercise (8.0 ± 0.5 mmol/l) was 23% lower (P < 0.01). Exercise had no significant effect (P > 0.05) on the average interstitial glucose concentration during exercise (NoEx 10.1 ± 0.7, M-Ex 10.4 ± 0.7 mmol/l). The average venous glucose concentration (8.3 ± 0.6 mmol/l) between minutes 101–240 was 14% lower compared with interstitial glucose (9.6 ± 0.6 mmol/l) during the NoEx trial (P < 0.01). During the M-Ex trial venous glucose (8.5 ± 0.7 mmol/l) tended to be lower (P = 0.09) compared with interstitial glucose (9.5 ± 0.8 mmol/l) [F(1.59,15.89) = 4.42, P = 0.04] (Fig. 1F), indicating that differences between interstitial and venous glucose concentrations during exercise do not persist once exercise stops.
Bland-Altman Plots and Mean and Median Absolute Relative Difference Data
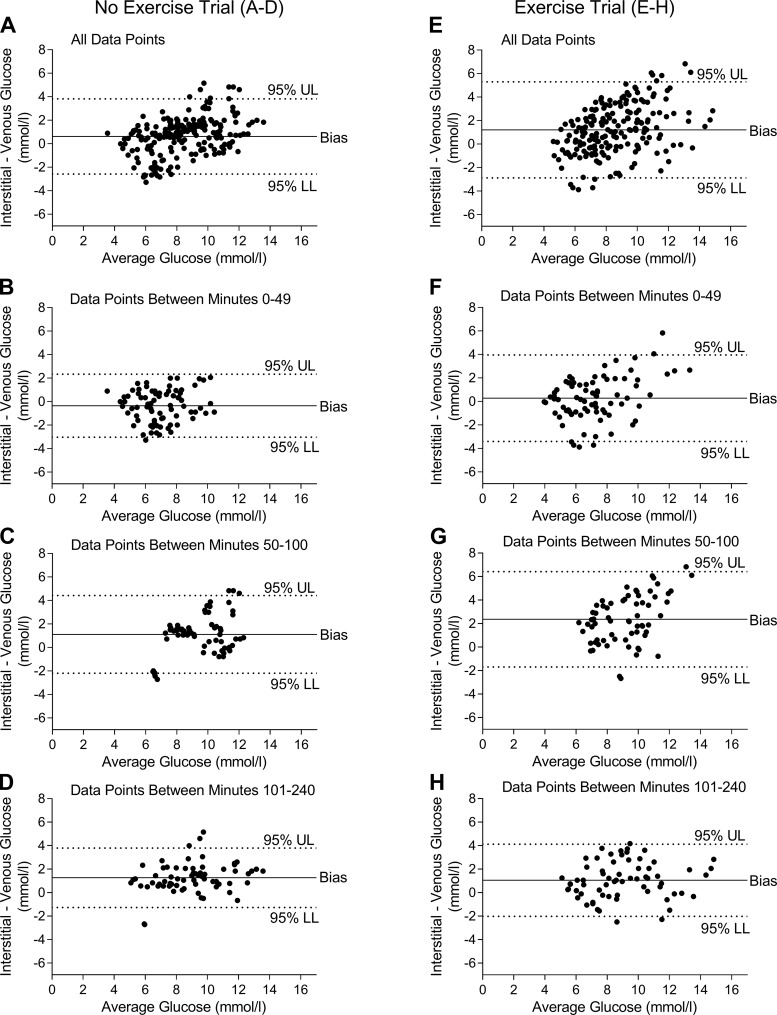
Bland-Altman plots (Fig. 2, A–H) were generated to show all of the glucose values and the average error between interstitial and venous glucose concentrations. This allows a better illustration of the agreement/disagreement between interstitial and venous glucose concentrations, rather than the average glucose values shown in Fig. 1. With all glucose values included in the analysis, the Bland-Altman plots revealed that the difference (bias) between interstitial and venous glucose concentrations was nearly twofold greater during the M-Ex trial (1.20 ± 2.09 mmol/l) compared with the NoEx trial (0.61 ± 1.67 mmol/l) (Table 1). When only the glucose values during exercise (i.e., between minutes 50–100) were used in the analysis, the difference between interstitial and venous glucose concentrations was augmented and more than twofold greater during the M-Ex trial (2.36 ± 2.07 mmol/l) compared with the NoEx trial (1.11 ± 1.69 mmol/l).
Fig. 2.
Bland-Altman plots during each trial. A: Bland-Altman plot including all glucose values during the no exercise (NoEx) trial. B–D: Bland-Altman plot of glucose values between minutes 0–49 (B), 50–100 (C), and 101–240 (D) during the NoEx trial. E: Bland-Altman plot of all glucose values during the postprandial exercise (M-Ex) trial. F–H: Bland-Altman plot of glucose values between minutes 0–49 (F), 50–100 (G), and 101–240 (H) during the Ex trial. In the Bland-Altman plots, concentrations below zero indicate the CGM underestimated glucose concentrations compared with the YSI, whereas concentrations above zero indicate the CGM overestimated glucose concentrations compared with the YSI. UL, upper limit; LL, lower limit. Bias = average error between measurements.
Table 1.
Bland-Altman plot with mean and median absolute relative difference data
| Bland-Altman Plot Data |
|||||
|---|---|---|---|---|---|
| Mean relative difference (bias) | Upper limit | Lower limit | Mean ARD | Median ARD | |
| All glucose values | |||||
| NoEx | 0.61 ± 1.67 | 3.88 | −2.66 | 18.2 ± 2.5 | 16.7 ± 2.7 |
| M-Ex | 1.20 ± 2.09 | 5.29 | −2.88 | 23.6 ± 3.7 | 23.0 ± 4.1 |
| Glucose values, minutes 0–49 | |||||
| NoEx | −0.35 ± 1.37 | 2.33 | −3.04 | 16.3 ± 1.4 | 16.4 ± 2.0 |
| M-Ex | 0.28 ± 1.88 | 3.96 | −3.41 | 20.5 ± 3.4 | 20.5 ± 3.2 |
| Glucose values, minutes 50–100 | |||||
| NoEx | 1.11 ± 1.69 | 4.42 | −2.20 | 19.5 ± 4.1 | 19.5 ± 4.1 |
| M-Ex | 2.36 ± 2.07 | 6.42 | −1.70 | 33.8 ± 6.2* | 34.7 ± 6.3† |
| Glucose values, minutes 101–240 | |||||
| NoEx | 1.27 ± 1.29 | 3.80 | −1.27 | 19.1 ± 4.0 | 18.3 ± 3.8 |
| M-Ex | 1.05 ± 1.56 | 4.12 | −2.01 | 17.1 ± 4.1 | 17.8 ± 4.5 |
Values are means ± SE. NoEx, No-exercise trial, M-Ex, postprandial exercise trial; ARD, absolute relative difference. Note: the mean and median ARD values tended (P = 0.07) to be higher during the M-Ex trial compared with the NoEx trial when all glucose values were included in the analysis.
P = 0.04 compared with mean ARD during NoEx trial within the time frame 50–100 min.
P = 0.04 compared with median ARD during NoEx trial within the time frame 50–100 min.
Mean and median ARD values were calculated to further demonstrate that interstitial and venous glucose concentrations are not impacted equally by exercise. When all glucose values were considered together or the distinct time frame 0–49 minutes or 101–240 minutes after the meal, there was no statistically significant difference (P > 0.05) in mean or median ARD values between NoEx and M-Ex trials (Table 1). On the contrary, when only the glucose values between minutes 50–100 (time frame when exercise was performed) of each trial were used in the analysis, the mean ARD (33.8 ± 6.2) and median ARD (34.7 ± 6.3) values during the M-Ex trial were 73% and 78% greater compared with the mean ARD (19.5 ± 4.1) and median ARD (19.5 ± 4.1) values, respectively, during the NoEx trial. Together, the Bland-Altman plots and ARD data strengthen the observation that interstitial and venous glucose concentrations are not similar during exercise and suggest that exercise has unequal effects on glucose concentrations in different bodily compartments in individuals with type 2 diabetes.
DISCUSSION
Knowledge of how exercise impacts both subcutaneous adipose tissue interstitial glucose concentrations and venous glucose concentrations is beneficial for health care practitioners and scientists. For instance, such information could be used to improve the ability of continuous glucose monitors to more accurately and in a more timely manner detect hypo- and hyperglycemic events during exercise. This, in turn, should enhance the effectiveness of a closed-loop insulin delivery system and provide more precise real-time indication of clinical hyper- or hypoglycemic episodes. From a scientific perspective, this information will enable more precise monitoring of glucose and allow accurate conclusions to be drawn about the impact of exercise on glycemic control. This is the first study, to our knowledge, to characterize how resistance exercise beginning 45 min after a standardized meal simultaneously alters both interstitial and venous glucose concentrations in patients with type 2 diabetes. We show that venous glucose, compared with interstitial glucose, changes more rapidly immediately after a meal, and the addition of exercise reduces venous glucose but not subcutaneous interstitial glucose concentrations in adults with type 2 diabetes. Both eating and exercise have unequal effects on glucose concentrations within different bodily compartments, which is important to consider when designing algorithms for glucose monitors or for the design of clinical trials testing the impact of exercise on glycemic control in adults with type 2 diabetes.
Venous glucose concentrations increased more rapidly within the first 15 min after the meal compared with interstitial glucose concentrations, an observation that is in line with some (1, 5, 16, 17), but not all (6, 30), previously reported data. This finding is consistent with the idea of a biological lag time between the blood and interstitial compartment (1, 5, 7, 16, 17, 25), which is not well understood but likely reflects the diffusion time across the endothelium as well as interstitial microconvection. Similar to some research (10, 11, 15, 22), but contrary to others (32), we show that interstitial glucose concentrations during exercise are greater compared with venous glucose concentrations, although once exercise stopped these differences dissipated. Our findings do not support the idea of a biological lag between interstitial and venous glucose concentrations as a contributing factor to the differences observed during exercise, at least in the abdominal subcutaneous region. Since interstitial glucose concentrations were higher throughout the entire 45-min exercise period, this is an unlikely explanation. There are several other potential explanations. The first explanation is an increase in blood flow to the exercising skeletal muscle at the expense of a reduction in blood flow to the subcutaneous abdominal fat region where the continuous glucose monitor was inserted. Since the subjects were performing several upper body exercises, the decrease in venous glucose concentrations sampled from the forearm vein during exercise may be due to greater glucose uptake from arterial blood into the skeletal muscle of the arm. Another possible explanation for the discrepancy is that exercise reduced glucose uptake in subcutaneous adipose tissue, resulting in no decrease in interstitial glucose during exercise. This idea is supported by data from a study by Simonsen et al. (27) where it was shown that increases in abdominal subcutaneous glycerol and fatty acid output (i.e., increased lipolysis) occur during aerobic cycling exercise in adults with type 2 diabetes. This is significant because increased lipolysis is associated with reduced glucose uptake in adipocytes (12), so this effect may have reduced glucose uptake into the adipocytes that were bathed in the interstitial fluid around the site of measurement. Assuming no rate of change in glucose diffusion into the interstitial compartment, ultimately this effect may have resulted in an accumulation of glucose in the interstitial compartment which would account for the higher measured glucose concentrations during exercise. Alternatively, shifts in interstitial or plasma volume could be the reason that differences exist between interstitial and venous glucose concentrations during exercise. The increased contractile activity and heat production of exercising skeletal muscle increases core body temperature, resulting in a subsequent increase in skin blood flow and fluid loss to transfer heat away from the body (31). This effect could have resulted in unequal shifts in plasma and interstitial volume such that interstitial fluid volume was reduced to a greater extent than plasma, which contributed to the higher interstitial glucose concentrations during exercise as glucose became more concentrated in less volume. These possibilities may be potential explanations, but need further testing.
At the end of exercise, venous glucose concentrations rebounded somewhat to levels that were similar to interstitial glucose. However, during this period average venous glucose concentrations were still lower compared with interstitial glucose concentrations during both trials. Since eating increases insulin concentrations which stimulates glucose uptake, this effect may be due to the fact that the interstitial glucose reflects the arterial blood that has not yet fully traveled through tissues, whereas venous glucose has traveled through insulin sensitive tissues in which glucose has diffused out of the blood and into the interstitial space where it is taken up by cells.
These findings are clinically meaningful for physicians, researchers, and patients with type 2 diabetes. From a technological perspective, these data have important implications in the design of algorithms to better predict hyper- or hypoglycemic events during exercise. It is important to note that we used the Medtronic continuous glucose monitor, and that these findings cannot be extrapolated to continuous glucose monitors from other companies such as Dexcom. Clinically and scientifically, these data have important implications for the accurate assessment of the effects of exercise on glycemic control. Since interstitial glucose concentrations did not change during exercise in the current study, it is possible that the benefits of exercise on glycemia could be underestimated if interstitial glucose is measured under the conditions of this study. Our data suggest that utilizing continuous glucose monitors to assess the effects of exercise on glycemic control should be used with caution, and at least be supplemented with venous glucose measurements. Postprandial hyperglycemia, measured in the venous pool, is a strong predictor of cardiovascular disease (13, 29), whereas no such relationship has been established with subcutaneous interstitial glucose. Thus venous glucose may be the optimal compartment to assess the impact of exercise on glucose concentrations in individuals with type 2 diabetes.
This study has limitations that should be considered when interpreting the results. Our population consisted of obese men and women with type 2 diabetes, and thus extrapolation of our findings to other populations is difficult. In addition, most subjects in this study were taking prescription medications to help manage their diabetes. The most common medication taken was metformin and there is evidence that this drug blunts exercise-induced increases in insulin sensitivity (23, 26), but recent work has shown metformin augments reductions in postprandial glucose with exercise (8, 9). Our subjects also took acetaminophen, which has been shown not to impact postprandial glucose responses (4), but there is evidence that acetaminophen, along with other drugs such as lisinopril, albuterol, atenolol, and red wine, interfere with continuous glucose monitor readings in the fasted state (3). Other studies have shown statins blunt exercise-induced increases in aerobic capacity (24). Whether these drugs impacted our continuous glucose monitor readings in the fed state is not known. To minimize the impact of these exercise and drug interactions on our findings, we instructed all subjects to take their medications at the same dose, time, and frequency, thus limiting the impact of medications on the current findings. We kept the subjects on their medications so that we could gain better insight into the real-world responses to meals and exercise in individuals with type 2 diabetes.
In conclusion, resistance exercise after a meal reduces venous glucose but not subcutaneous interstitial glucose concentrations in patients with type 2 diabetes. These data suggest that resistance exercise has differential effects on postprandial glucose concentrations depending on where glucose is compartmentalized within the body. These findings highlight the importance of identifying the technique used to assess glucose concentrations when comparing the effects of exercise on glucose metabolism across studies. Further research is warranted to elucidate the mechanism by which exercise has differential effects on interstitial and venous glucose concentrations.
GRANTS
Funding for this project was provided by funds from the Department of Nutrition and Exercise Physiology, University of Missouri (J. A. Kanaley). While this project was performed stipend support for T. D. Heden was provided by National Institutes of Health (NIH) Grant AR-048523. During the data analysis, writing, and submission of this manuscript, T. D. Heden was supported by NIH Grants DK-109556 and DK-110338 and J. A. Kanaley was supported by NIH Grant DK-101513.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.D.H. and J.A.K. conceived and designed research; T.D.H., Y.L., and J.A.K. performed experiments; T.D.H. and Y.L. analyzed data; T.D.H. and J.A.K. interpreted results of experiments; T.D.H. prepared figures; T.D.H. drafted manuscript; T.D.H. and J.A.K. edited and revised manuscript; T.D.H. and J.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge N. C. Winn for help with some of the data collection.
This study is registered at https://clinicaltrials.gov as NCT02180620.
REFERENCES
- 1.Aussedat B, Dupire-Angel M, Gifford R, Klein JC, Wilson GS, Reach G. Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab 278: E716–E728, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bantle JP, Thomas W. Glucose measurement in patients with diabetes mellitus with dermal interstitial fluid. J Lab Clin Med 130: 436–441, 1997. doi: 10.1016/S0022-2143(97)90044-5. [DOI] [PubMed] [Google Scholar]
- 3.Basu A, Slama MQ, Nicholson WT, Langman L, Peyser T, Carter R, Basu R. Continuous glucose monitor interference with commonly prescribed medications: a pilot study. J Diabetes Sci Technol 11: 936–941, 2017. doi: 10.1177/1932296817697329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijlani RL, Shukla K, Narain JP, Puri P. Effect of coingestion of paracetamol on glycaemic response. Indian J Physiol Pharmacol 36: 215–218, 1992. [PubMed] [Google Scholar]
- 5.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 52: 2790–2794, 2003. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 6.Caplin NJ, O’Leary P, Bulsara M, Davis EA, Jones TW. Subcutaneous glucose sensor values closely parallel blood glucose during insulin-induced hypoglycaemia. Diabet Med 20: 238–241, 2003. doi: 10.1046/j.1464-5491.2003.00837.x. [DOI] [PubMed] [Google Scholar]
- 7.Cobelli C, Schiavon M, Dalla Man C, Basu A, Basu R. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: insight from an in silico study. Diabetes Technol Ther 18: 505–511, 2016. doi: 10.1089/dia.2016.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson ML, Little JP, Gay JL, McCully KK, Jenkins NT. Effects of postmeal exercise on postprandial glucose excursions in people with type 2 diabetes treated with add-on hypoglycemic agents. Diabetes Res Clin Pract 126: 240–247, 2017. doi: 10.1016/j.diabres.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Erickson ML, Little JP, Gay JL, McCully KK, Jenkins NT. Postmeal exercise blunts postprandial glucose excursions in people on metformin monotherapy. J Appl Physiol (1985) 123: 444–450, 2017. doi: 10.1152/japplphysiol.00213.2017. [DOI] [PubMed] [Google Scholar]
- 10.Fayolle C, Brun JF, Bringer J, Mercier J, Renard E. Accuracy of continuous subcutaneous glucose monitoring with the GlucoDay in type 1 diabetic patients treated by subcutaneous insulin infusion during exercise of low versus high intensity. Diabetes Metab 32: 313–320, 2006. doi: 10.1016/S1262-3636(07)70285-9. [DOI] [PubMed] [Google Scholar]
- 11.Figueira FR, Umpierre D, Ribeiro JP, Tetelbom PS, Henn NT, Esteves JF, Schaan BD. Accuracy of continuous glucose monitoring system during exercise in type 2 diabetes. Diabetes Res Clin Pract 98: e36–e39, 2012. doi: 10.1016/j.diabres.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel A-L, Houssier M, Roussel B, Besse-Patin A, Combes M, Mir L, Monbrun L, Bézaire V, Prunet-Marcassus B, Waget A, Vila I, Caspar-Bauguil S, Louche K, Marques M-A, Mairal A, Renoud M-L, Galitzky J, Holm C, Mouisel E, Thalamas C, Viguerie N, Sulpice T, Burcelin R, Arner P, Langin D. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 11: e1001485, 2013. doi: 10.1371/journal.pbio.1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanefeld M, Koehler C, Henkel E, Fuecker K, Schaper F, Temelkova-Kurktschiev T. Post-challenge hyperglycaemia relates more strongly than fasting hyperglycaemia with carotid intima-media thickness: the RIAD Study. Risk factors in impaired glucose tolerance for atherosclerosis and diabetes. Diabet Med 17: 835–840, 2000. doi: 10.1046/j.1464-5491.2000.00408.x. [DOI] [PubMed] [Google Scholar]
- 14.Heden TD, Winn NC, Mari A, Booth FW, Rector RS, Thyfault JP, Kanaley JA. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J Appl Physiol (1985) 118: 624–634, 2015. doi: 10.1152/japplphysiol.00917.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrington SJ, Gee DL, Dow SD, Monosky KA, Davis E, Pritchett KL. Comparison of glucose monitoring methods during steady-state exercise in women. Nutrients 4: 1282–1292, 2012. doi: 10.3390/nu4091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansson PA, Fowelin J, Smith U, Lönnroth P. Characterization by microdialysis of intracellular glucose level in subcutaneous tissue in humans. Am J Physiol Endocrinol Metab 255: E218–E220, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care 26: 2405–2409, 2003. doi: 10.2337/diacare.26.8.2405. [DOI] [PubMed] [Google Scholar]
- 18.Kumareswaran K, Elleri D, Allen JM, Caldwell K, Nodale M, Wilinska ME, Amiel SA, Hovorka R, Murphy HR. Accuracy of continuous glucose monitoring during exercise in type 1 diabetes pregnancy. Diabetes Technol Ther 15: 223–229, 2013. doi: 10.1089/dia.2012.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen JJ, Dela F, Kjaer M, Galbo H. The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia 40: 447–453, 1997. doi: 10.1007/s001250050699. [DOI] [PubMed] [Google Scholar]
- 20.Larsen JJ, Dela F, Madsbad S, Galbo H. The effect of intense exercise on postprandial glucose homeostasis in type II diabetic patients. Diabetologia 42: 1282–1292, 1999. doi: 10.1007/s001250051440. [DOI] [PubMed] [Google Scholar]
- 21.Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol Endocrinol Metab 253: E228–E231, 1987. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald AL, Philp A, Harrison M, Bone AJ, Watt PW. Monitoring exercise-induced changes in glycemic control in type 2 diabetes. Med Sci Sports Exerc 38: 201–207, 2006. doi: 10.1249/01.mss.0000183852.31164.5a. [DOI] [PubMed] [Google Scholar]
- 23.Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 35: 131–136, 2012. doi: 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol 62: 709–714, 2013. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiavon M, Dalla Man C, Dube S, Slama M, Kudva YC, Peyser T, Basu A, Basu R, Cobelli C. Modeling plasma-to-interstitium glucose kinetics from multitracer plasma and microdialysis data. Diabetes Technol Ther 17: 825–831, 2015. doi: 10.1089/dia.2015.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H, Hirshman MF, Goodyear LJ, Braun B. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab 298: E815–E823, 2010. doi: 10.1152/ajpendo.00517.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonsen L, Henriksen O, Enevoldsen LH, Bülow J. The effect of exercise on regional adipose tissue and splanchnic lipid metabolism in overweight type 2 diabetic subjects. Diabetologia 47: 652–659, 2004. doi: 10.1007/s00125-004-1374-y. [DOI] [PubMed] [Google Scholar]
- 28.Stout PJ, Peled N, Erickson BJ, Hilgers ME, Racchini JR, Hoegh TB. Comparison of glucose levels in dermal interstitial fluid and finger capillary blood. Diabetes Technol Ther 3: 81–90, 2001. doi: 10.1089/152091501750220046. [DOI] [PubMed] [Google Scholar]
- 29.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 23: 1830–1834, 2000. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 30.Thennadil SN, Rennert JL, Wenzel BJ, Hazen KH, Ruchti TL, Block MB. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol Ther 3: 357–365, 2001. doi: 10.1089/15209150152607132. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki F, Sone R. Different vascular responses in glabrous and nonglabrous skin with increasing core temperature during exercise. Eur J Appl Physiol 97: 582–590, 2006. doi: 10.1007/s00421-006-0219-4. [DOI] [PubMed] [Google Scholar]
- 32.Yardley JE, Sigal RJ, Kenny GP, Riddell MC, Lovblom LE, Perkins BA. Point accuracy of interstitial continuous glucose monitoring during exercise in type 1 diabetes. Diabetes Technol Ther 15: 46–49, 2013. doi: 10.1089/dia.2012.0182. [DOI] [PubMed] [Google Scholar]