Abstract
Aging muscle atrophy is in part a neurodegenerative process revealed by denervation/reinnervation events leading to motor unit remodeling (i.e., myofiber type grouping). However, this process and its physiological relevance are poorly understood, as is the wide-ranging heterogeneity among aging humans. Here, we attempted to address 1) the relation between myofiber type grouping and molecular regulators of neuromuscular junction (NMJ) stability; 2) the impact of motor unit remodeling on recruitment during submaximal contractions; 3) the prevalence and impact of motor unit remodeling in Parkinson’s disease (PD), an age-related neurodegenerative disease; and 4) the influence of resistance exercise training (RT) on regulators of motor unit remodeling. We compared type I myofiber grouping, molecular regulators of NMJ stability, and the relative motor unit activation (MUA) requirement during a submaximal sit-to-stand task among untrained but otherwise healthy young (YA; 26 yr, n = 27) and older (OA; 66 yr, n = 91) adults and OA with PD (PD; 67 yr, n = 19). We tested the effects of RT on these outcomes in OA and PD. PD displayed more motor unit remodeling, alterations in NMJ stability regulation, and a higher relative MUA requirement than OA, suggesting PD-specific effects. The molecular and physiological outcomes tracked with the severity of type I myofiber grouping. Together these findings suggest that age-related motor unit remodeling, manifested by type I myofiber grouping, 1) reduces MUA efficiency to meet submaximal contraction demand, 2) is associated with disruptions in NMJ stability, 3) is further impacted by PD, and 4) may be improved by RT in severe cases.
NEW & NOTEWORTHY Because the physiological consequences of varying amounts of myofiber type grouping are unknown, the current study aims to characterize the molecular and physiological correlates of motor unit remodeling. Furthermore, because exercise training has demonstrated neuromuscular benefits in aged humans and improved innervation status and neuromuscular junction integrity in animals, we provide an exploratory analysis of the effects of high-intensity resistance training on markers of neuromuscular degeneration in both Parkinson’s disease (PD) and age-matched older adults.
Keywords: myofiber type grouping, neuromuscular junction
INTRODUCTION
Age-related skeletal muscle atrophy results in reductions in strength, power, and force control. These impairments are preceded and at least partially caused by progressive neuromuscular degeneration. In particular, evidence of age-related motor neuron death (28, 50), neuromuscular junction (NMJ) deterioration (15, 37), and motor unit enlargement (40) points toward motor unit remodeling as a prominent contributor to functional decline. The process of motor unit remodeling involves poorly understood, perhaps continuous denervation and reinnervation that ultimately results in myofiber type grouping within aged skeletal muscle (19, 25). Thus, histopathological detection of myofiber type grouping is a well-established indicator of motor unit remodeling.
Although the overwhelming consensus among skeletal muscle pathology findings is that myofiber type grouping increases with age (16, 24), historically there has been a lack of quantitative data detailing the exact extent or severity of grouping (i.e., relative number of grouped myofibers) between individuals. Recently, we developed methods that allow for both quantitative comparisons and descriptive characteristics of myofiber grouping such as the relative number of grouped myofibers, average myofibers per group, and the relative number of groups present in a sample (21). Using these methods, we showed that type I myofiber grouping prevalence is 2.5 times higher in older adults (66 yr) compared with young adults (26 yr) and that age-related grouping stems from an expansion of myofiber group size (myofibers/group) not number (21). Furthermore, we identified a large range of type I myofiber grouping prevalence among older individuals. Ultimately, these quantitative data are expected to be useful for distinguishing normal aging-related myofiber type grouping from that found in age-related neurodegenerative disease and for characterizing the molecular underpinnings and functional consequences of myofiber type grouping.
Parkinson’s disease (PD) is an example of an age-related neurodegenerative disease in which type I myofiber grouping has been observed (12, 34). In addition, individuals with PD display greater motor unit loss (8, 9) and disrupted motor unit recruitment patterns (14, 20, 43) compared with age-matched non-PD controls. Both motor unit loss and disruption of motor unit recruitment are notable signs of motor unit remodeling that might be indicative of heightened incidence of denervation and myofiber grouping compared with age-matched, non-PD controls.
Denervation in human skeletal muscle is often evaluated using expression levels of developmental proteins/genes such as neural cell adhesion molecule (NCAM) (7, 11, 31, 32), the voltage-gated sodium channel Nav1.5 (44, 45), and the acetylcholine receptor (AChR) γ-subunit (26), which are not expressed in mature, healthy innervated myofibers. Additionally, denervation is known to induce molecular regulators of neuromuscular junction (NMJ) stability such as AChR subunits (α, β, δ, and ε) and signaling components of the agrin-low-density lipoprotein receptor-related protein 4 (Lrp4) muscle-specific kinase (MuSK) signaling pathway (10). Denervation-associated induction of NMJ stability regulators is initiated by myogenin via histone deacetylase 4 (HDAC4) and growth arrest and DNA damage-inducible 45α (Gadd45α) (3, 10). Runt-related transcription factor 1 (RUNX1) has also emerged recently as an indicator of denervation, possibly in a compensatory role to slow denervation-induced atrophy (52). Collectively, these biomarkers provide insight into the extent of denervation and have yet to be comprehensively assessed in human aging, in PD, or across a range of myofiber grouping prevalence.
Although it is well accepted that denervation contributes to motor unit remodeling and, therefore, myofiber type grouping, very little is known about how myofiber grouping progresses. For example, it is unknown whether excessive denervation or more efficient reinnervation is responsible for advanced myofiber type grouping, especially among age-matched older individuals who differ in grouping magnitude. Furthermore, the physiological consequences of varying amounts of myofiber type grouping are not known. Thus, we aimed to characterize the molecular (regulators of NMJ stability/denervation) and physiological (relative motor unit recruitment patterns) correlates of motor unit remodeling within the context of aging, PD, and varied type I myofiber grouping prevalence. Additionally, because exercise training has demonstrated neuromuscular benefits such as motor unit preservation in aged humans (41, 42) and improved innervation status and NMJ integrity in animals (51), we performed an exploratory analysis of the effects of high-intensity resistance training (RT) on markers of neuromuscular degeneration in both PD and age-matched non-PD.
MATERIALS AND METHODS
Human Subjects
Nineteen PD patients (67 ± 6 yr; 3 women, 16 men) were recruited from the University of Alabama at Birmingham (UAB) Department of Neurology Movement Disorders Clinic. We have previously published the effects of high-intensity RT on a host of clinical and physiological variables in a subset of these participants (n = 15). Therefore, detailed recruitment and eligibility information can be found elsewhere (20). Briefly, all PD participants were diagnosed using the UK Brain Bank criteria (18), rated as Hoehn and Yahr stage 2 (n = 12) or 3 (n = 7), and medication stable for ≥4 wk. To provide reference values of type I myofiber grouping data for non-exercise-trained young adults (YA; 26 ± 4 yr; n = 27, 12 women, 15 men) and age-matched non-PD older adults (OA; 66 ± 4 yr; n = 91; 41 women, 50 men), we included previously published data (21). For all other measures, we compared PD to YA and OA performance data and available remaining muscle tissue samples from two previously published RT trials (2, 48).
To minimize the potential impact of differential levels of physical activity, we restricted recruitment to untrained subjects (i.e., no recent resistance training history). As part of the screening process, we also captured physical activity patterns via questionnaires. There were no notable differences in the weekly levels of self-reported physical activity among PD, OA, and YA. All subjects reported levels of activity well below the Department of Health and Human Services physical activity guidelines. All participants gave written, informed consent allowing their samples and data to be used for future research, as approved by the University of Alabama at Birmingham (UAB) Institutional Review Board.
Exercise Training
Participants completed RT interventions ranging from 4 (OA: n = 42) to 16 (YA: n = 27; OA: n = 24; PD: n = 19) wk in duration, which have been published previously (2, 20, 47). Each intervention consisted of progressive high-intensity (8- to 12-repetition maximum each set) RT and incorporated similar movements for activating and mechanically loading the biopsied vastus lateralis muscle (e.g., squat, leg press, knee extension). All participants trained 3 days/wk under the supervision of an experienced trainer in the UAB Center for Exercise Medicine’s Exercise Clinical Trials Facility. Sample availability, particularly for protein and gene analyses, was maximized by combining the 4- and 16-wk RT subjects for OA.
Relative Motor Unit Activation
Surface electromyography (sEMG) was used to determine the magnitude of quadriceps neural activation (relative to maximum) required to perform a sit-to-stand task, which we have published previously (20, 38, 39). sEMG electrodes are placed over the muscle bellies of vastus lateralis (VL), rectus femoris (RF), and vastus medialis (VM), and knee joint angle is monitored by an electrogoniometer (Biometrics, London, UK). Raw sEMG is collected at 1,000 Hz and knee angle at 500 Hz and analyzed via Biometrics DataLink software version 2.00. Maximal voluntary knee extension isometric contraction (MVC) is first assessed on a knee extension dynamometer (at knee angle ≈60° of flexion) via 3 × 5-s contractions separated by 1-min rest. The contraction yielding peak torque is used to quantify maximum voluntary quadriceps motor unit activation (MUA). The sit-to-stand is then performed from a standard bench height (46 cm) with arms crossed for three repetitions at a slow, controlled cadence (2-s ascent, 2-s descent) using an audiovisual metronome.
Analysis of sEMG for both tasks is performed as follows; raw sEMG recordings from each muscle are full-wave rectified and then converted to root mean-squared amplitude (RMS-EMG) using a 100-ms sliding window. For the static MVC, RMS-EMG of each muscle is averaged over a 100-ms window centered on peak torque. For the dynamic sit-to-stand, RMS-EMG of each muscle is averaged over a 100-ms window centered on the MVC knee angle. RMS-EMG of the three muscles is then averaged to represent quadriceps MUA during the given task (MVC or sit-to-stand), and in the case of the sit-to-stand task, quadriceps MUA is then averaged across all three repetitions. Finally, sit-to-stand MUA is divided by MVC MUA to yield relative MUA.
Muscle Histology
All human skeletal muscle biopsy samples were obtained from m. vastus lateralis (VL) in the morning fasted state using our established percutaneous needle biopsy technique via a 5-mm diameter Bergstrom-type needle under suction. Briefly, the trocar enters the VL in the sagittal plane ∼10 cm proximal to the patella, and once in the muscle (through the fascia), the trocar is advanced to a muscle depth of 3–4 cm for tissue collection. Myofiber type distribution (I, IIa, and IIx) was determined via myosin heavy chain (MHC) isoform immunofluorescence on fresh-frozen 6-μm serial cryosections, as established (20–23). Images of each muscle sample were captured (×10) in a grid format and stitched together using Image-Pro Plus (Media Cybernetics) software to render one seamless image of the entire cross-section of the specimen. All cross-sectionally oriented myofibers were typed and counted (myofibers per specimen: PD = 1,448 ± 138 pretraining, 1,149 ± 98 posttraining; OA = 1,643 ± 81; YA = 1,333 ± 86). Using our recently published method (21), each specimen was then assessed for abnormal type I myofiber grouping. All image analyses were performed by a single technician blinded to group (PD, OA, and YA) and time point (pre- or post-RT). Briefly, using the type I myofiber % distribution of each sample, we calculated the expected mean number and standard deviation of like myofibers touching a given myofiber. To qualify as an abnormal type I group, two contiguous type I myofibers (“core myofibers”) must have been bordered by a number of type I myofibers that surpassed the sum of the aforementioned expected mean and standard deviation. Once two core myofibers were identified, all contiguous type I myofibers were tallied as part of the group. This approach yields the percentage of total type I myofibers in abnormal groups, the number of myofibers per group (i.e., group size), and the relative number of type I myofiber groups.
Muscle Protein Isolation and Immunoblotting
Muscle specimens (∼30 mg) were incubated in lysis buffer (6 μl/mg) containing protease and phosphatase inhibitors, homogenized, and centrifuged at (15,000 g for 20 min at 4°C), as previously described (27, 30). The bicinchoninic acid technique with BSA standards was used to assess protein content in the supernatant. All samples were stored at −80°C until future use. As described elsewhere (1, 30), immunoblotting was performed by resolving 35 µg of skeletal muscle mixed protein lysate on 4–12% SDS-PAGE gels (Invitrogen) and transferred to PVDF membranes. Each membrane was stained using Ponceau S solution (Sigma, P7170) to confirm equal protein loading across lanes. The levels of protein biomarkers of myofiber denervation were quantified using primary antibodies against Dok-7 (1:1,000, sc-55169; Santa Cruz Biotechnology), LRP-4 (1:1,000, MAB5948; R & D Systems), nAChRβ (1:250, ab76159; Abcam), p-nAChRβ (1:250, sc-17087-R ;Santa Cruz Biotechnology), MuSK (1:250, ab92950; Abcam), and Rapsyn (1:250, ab156002; Abcam). Following incubation in horseradish peroxidase-conjugated secondary antibody (Pierce, ThermoScientific), membranes were imaged using chemiluminescent detection in a Bio-Rad (Hercules, CA) ChemiDoc imaging system. Finally, band densitometry was performed using Bio-Rad Quantity One (version 4.5.1).
RNA Isolation and Quantitative PCR
Total RNA was isolated from snap-frozen muscle samples (∼30 mg) using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Spectrophotometry (NanoDrop ND-1000; ThermoScientific, Rockford, IL) was used to verify RNA quality and quantity. cDNA was synthesized by reverse transcription using the SuperScript VILO cDNA synthesis kit (Invitrogen, Carlsbad, CA). As we have described in detail previously (30, 49), relative mRNA expression levels were quantified via quantitative (q)RT-PCR using Taqman gene expression assays (Applied Biosystems, Foster City, CA). The following 10 primers for transcripts implicated in myofiber denervation were used: NCAM (NCAM1; Hs00941830_m1), Nav1.5 (SCN5A; Hs00165693_m1), AChRα (ACHRA1; Hs00175578_m1), AChRβ (ACHRB1; Hs00181255_m1), AChRδ (ACHRD; Hs00181284_m1), AChRε (ACHRE; Hs00972485_m1), AChRγ (ACHRG; Hs00183228_m1), myogenin (MYOG; Hs01072232_m1), HDAC4 (Hs01041638_m1), Gadd45α (GADD45A; Hs00169255_m1), and RUNX1 (Hs00231079_m1). Transcript expression of hypoxanthine-guanine phosphoribosyl transferase (HPRT1; Hs01003267_m1) was used as the endogenous control, and the comparative threshold cycle method (StepOne software version 2.3; Applied Biosystems) was used to establish relative target mRNA expression. Results are reported as the relative fold difference among groups.
Clinical Assessments
All participants with PD completed the 39-item Parkinson’s Disease Quality of Life Scale (PDQ-39) and were scored for the Unified Parkinson’s Disease Rating Scale (UPDRS) by the same trained clinician. Only movement-related sections and total scores for the PDQ-39 and UPDRS were considered relevant.
Statistical Analysis
All statistical analyses were performed using Statistica software (StatSoft, Tulsa, OK). Sample sizes varied between experiments due to banked sample availability and common data collection elements across the cohorts (e.g., MUA). Between-group differences at the pre-RT time point were assessed via one-way ANOVA for type I myofiber grouping (YA: n = 27; OA: n = 91; PD: n = 16), MUA (YA: n = 27; OA: n = 59; PD: n = 19), and biomarkers of denervation (YA: n = 16; OA: n = 16; PD: n = 16). The effects of RT on type I myofiber grouping (OA: n = 66; PD: n = 13), MUA (OA: n = 59; PD: n = 17), and biomarkers of denervation (OA: n = 16; PD: n = 12) were evaluated using group × time repeated-measures ANOVA. Significant interactions were followed by Fisher’s least significant difference post hoc tests. Descriptive characteristics are reported as means ± SD; all other data are reported as means ± SE. For all tests, P ≤ 0.05 (2-tailed) was considered statistically significant.
RESULTS
Type I Myofiber Grouping and MUA in PD
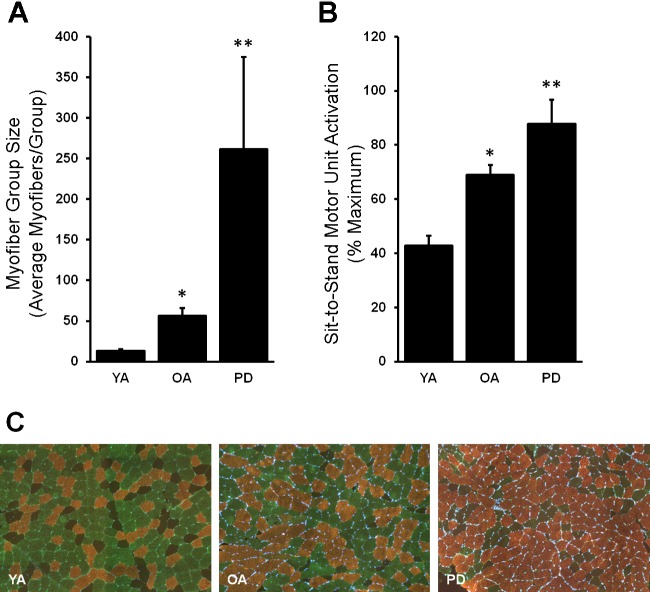
Although PD and OA possess a similar, higher proportion of grouped type I myofibers compared with YA (PD: 57 ± 8%; OA: 52 ± 2 vs. YA: 21 ± 2%; P < 0.05), PD displays a uniquely large type I myofiber group size (P < 0.05; Fig. 1A) and, therefore, lower numbers of type I myofiber groups per 1,000 myofibers (PD vs. OA: 3.3 ± 0.6 vs. 6.5 ± 0.3; YA: 5.7 ± 0.6; P < 0.05). Additionally, the larger type I myofiber group size in PD is accompanied by motor unit overrecruitment during the submaximal sit-to-stand task compared with age-matched OA as well as YA (P < 0.05; Fig. 1B). As we have published previously (20, 38, 39), higher MUA in OA vs. YA (P < 0.05) was expected.
Fig. 1.
Type I myofiber group size and sit-to-stand motor unit activation is abnormally high in Parkinson’s Disease (PD). A and B: both type I myofiber group size (A) and motor unit activation (B) were highest in PD and followed a stepwise pattern: PD > older adults (OA) > young adults (YA). C: representative immunohistological images are shown: type I, copper; type IIa, green; type IIx, black/negative. *Different from YA, P < 0.05; **different from YA and OA, P < 0.05. Values are means + SE.
NMJ Markers of Denervation are Upregulated in OA and PD
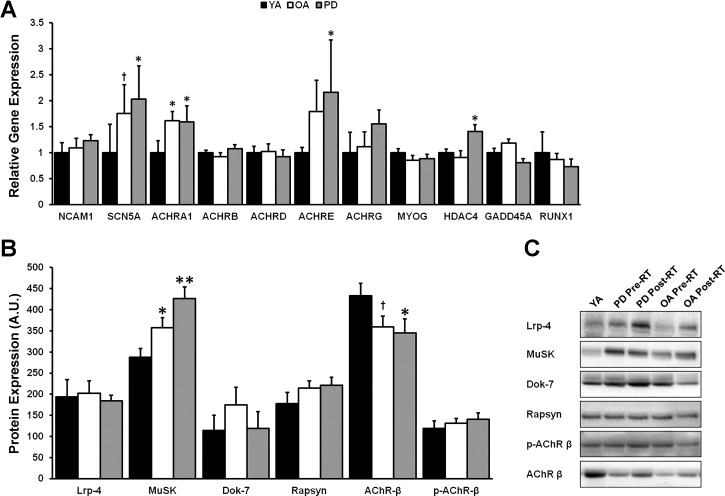
Heightened expression of some denervation-associated mRNA transcripts was detected in OA and PD compared with YA (Fig. 2A). Specifically, OA and PD displayed higher expression of both ACHRA1 (P < 0.05) and SCN5A (PD vs. YA, P < 0.05; OA vs. YA, P = 0.06), whereas PD alone also displayed higher expression of ACHRE (P < 0.05) and HDAC4 (P < 0.05) and a trend for ACHRG (P = 0.12) compared with YA. Furthermore, protein levels of MuSK were elevated in a stepwise fashion with PD greater than OA that was greater than YA (P < 0.05; Fig. 2B). Additionally, total protein levels of AChR-β were suppressed in PD (P < 0.05, PD vs. YA) and tended to also be lower in OA (P = 0.09, OA vs. YA). No other molecular biomarkers differed between groups.
Fig. 2.
Expression levels of skeletal muscle denervation-associated transcripts and proteins are heightened in Parkinson’s disease (PD) and older adults (OA). A: gene expression of acetylcholine receptor subunit-α1 (ACHRA1) and sodium voltage-gated channel α-subunit 5 (SCN5A) was upregulated in both OA and PD compared with young adults (YA), whereas acetylcholine receptor subunit epsilon (ACHRE) and histone deacetylase 4 (HDAC4) were upregulated only in PD compared with YA. B: expression of the postsynaptic-organizing protein muscle-specific kinase (MuSK) was elevated in a stepwise fashion from PD > OA > YA, perhaps in a compensatory manner due to reduced expression of the acetylcholine receptor among PD and OA, as measured by the β-subunit (AChR-β). C: representative immunoblots for proteins of interest in YA, OA, and PD. NCAM1, neural cell adhesion molecule 1; ACHRB, acetylcholine receptor subunit-β; ACHRD, acetylcholine receptor subunit-δ; ACHRG, acetylcholine receptor subunit-γ; MYOG, myogenin; GADD45A, growth arrest and DNA damage inducible-α; RUNX1, runt-related transcription factor 1; Lrp4, low-density lipoprotein receptor-related protein 4; Dok-7, docking protein 7; p-AChR-β, phosphorylated acetylcholine receptor subunit- β. *Different from YA, P < 0.05; †trend toward difference from YA, P < 0.09; **different from YA and OA, P < 0.05. Values are means + SE.
Type I Myofiber Grouping Prevalence is Indicative of Abnormal Group Size and MUA
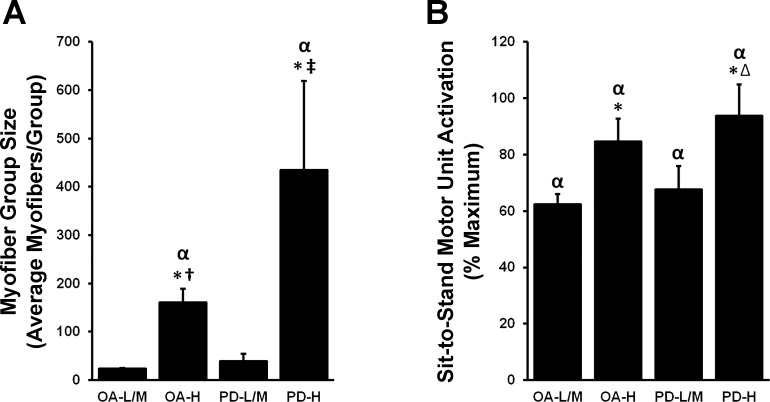
We have previously published a high amount of interindividual variability in type I myofiber grouping prevalence in these OA participants, with K-means cluster analysis yielding three clusters: low (OA-L, n = 31; prevalence: 28 ± 2%), moderate (OA-M, n = 38; prevalence: 54 ± 1%), and high (OA-H, n = 22; prevalence: 81 ± 2%) (21). We applied these clusters here to evaluate potential relationships between type I myofiber grouping prevalence and abnormal MUA or NMJ markers of stability/denervation in both OA and PD. Because of limited muscle sample availability, and no apparent differences in outcomes, we collapsed low and mod clusters in PD to yield PD-L/M (n = 7) and PD-H (n = 9) and in OA to yield OA-L/M (n = 8 for mRNA, n = 8 for protein) and OA-H (n = 10 for mRNA, n = 6 for protein). Differences in type I grouping prevalence were driven primarily by differences in group size, as the average number of type I myofibers per group in both OA-H and PD-H far exceeded YA, OA-L/M, and PD-L/M (P < 0.05; Fig. 3A). Indeed, PD-H clearly had the largest group size, surpassing OA-H as well (P < 0.05). With regard to MUA, OA-H and PD-H also displayed abnormal overrecruitment compared with OA-L/M and PD-L/M (P < 0.05; Fig. 3B); all clusters exceeded YA MUA (P < 0.05).
Fig. 3.
Type I myofiber group size and sit-to-stand motor unit activation (MUA) increase with increasing type I myofiber grouping prevalence. K-means clusters of older adults (OA) and PD individuals displaying low-to-moderate (OA-L/M, PD-L/M) and high (OA-H, PD-H) type I myofiber grouping prevalence were used to assess a relationship between myofiber grouping prevalence and myofiber groups size/MUA. A: those individuals with the most type I myofiber grouping (OA-H, PD-H) displayed the largest myofiber group sizes. B: although OA-L/M and PD-L/M all display heightened MUA compared with YA, OA-H and PD-H display abnormally high MUA. Therefore, overall age and disease effects in OA and PD for group size and MUA appear to have been driven by OA-H and PD-H, respectively. αDifferent from YA, P < 0.05; *different from L/M cluster within groups, P < 0.05; †different from PD-L/M, P < 0.05; ∆different from OA-L/M, P < 0.05; ‡different from OA-L/M/H, P < 0.05. Values are means + SE.
Type I Myofiber Grouping Prevalence is Associated With Molecular Indices of Impaired NMJ Stability/Denervation
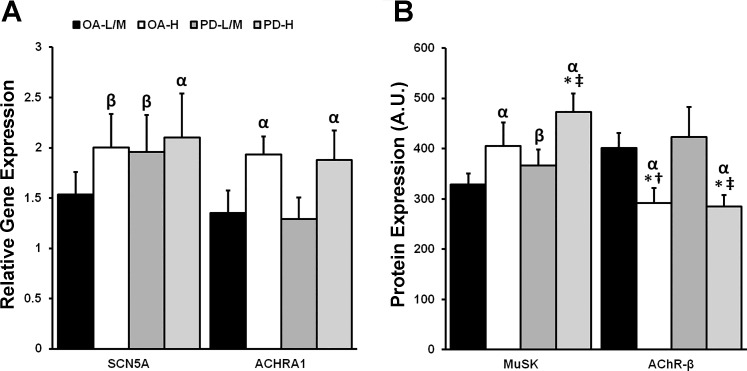
Relative to YA, gene expression of ACHRA1 was upregulated in OA-H and PD-H (P < 0.05; Fig. 4A), whereas SCN5A was upregulated in PD-H (P < 0.05), and tended to be upregulated in PD-L/M (P = 0.07) and OA-H (P = 0.06) as well. Protein expression of MuSK was higher in PD-H than PD-L/M and OA-L/M (P < 0.05; Fig. 4B) but not OA-H, which tended to be higher than OA-L/M (P = 0.11). Both OA-H and PD-H expressed higher levels of MuSK compared with YA (P < 0.05), whereas PD-L/M tended to as well (P = 0.06). Finally, AChR-β protein expression was lower in OA-H and PD-H compared with YA, OA-L/M, and PD-L/M (P < 0.05; Fig. 4B).
Fig. 4.
Expression of markers of neuromuscular junction (NMJ) stability/denervation based on type I myofiber grouping prevalence and PD. As defined by K-means cluster analysis, older adults (OA) and PD individuals with low to moderate (OA-L/M, PD-L/M) and high (OA-H, PD-H) type I myofiber grouping prevalence were evaluated for denervation-associated gene and protein expression. We demonstrate that those denervation-associated NMJ transcripts and proteins that were upregulated due to aging are driven by the OA-H, which displays similarities to PD-H. Specifically, OA-H and PD-H displayed higher gene expression of sodium voltage-gated channel α-subunit 5 (SCN5A) and acetylcholine receptor subunit-α1 (ACHRA1) compared with young adults (YA; YA mean = 1.0) (A) as well as higher protein expression of muscle-specific kinase [MuSK; YA mean = 275 arbitrary units (AU)] and reduced expression of acetylcholine receptor subunit-β (AChR-β;, YA mean = 440 AU) (B). Interestingly, PD-L/M tended to display heightened denervation-associated SCN5A and MuSK expression as well, suggesting a PD-specific effect. There were no other differences in target gene or protein expression among groups. αDifferent from YA, P < 0.05; βtrend toward difference from YA, P < 0.07; *different from L/M cluster within-group, P < 0.05; †different from PD-L/M, P < 0.05; ‡different from OA-L/M, P < 0.05. Values are means + SE.
Type I myofiber grouping prevalence appears related to PD severity
Although limited in statistical power, additional cluster analyses of clinical assessments (Table 1) in PD-L/M and PD-H revealed trends for heightened symptomatic severity among PD-H. For example, PD-H scored worse on the PDQ-39 Mobility subscore (P < 0.05) compared with PD-L/M, and tended to score more poorly on the UPDRS total (P = 0.08) and section III (motor) (P = 0.18) scores.
Table 1.
PD symptom severity pre- and post-RT is associated with myofiber grouping prevalence
| Pre-RT |
Post-RT |
|||
|---|---|---|---|---|
| PD-L/M | PD-H | PD-L/M | PD-H | |
| Hoehn and Yahr | 2.4 ± 0.2 | 2.3 ± 0.2 | — | — |
| UPDRS Section III (motor) | 30.3 ± 3.9 | 36.8 ± 2.7 | 27.9 ± 2.8 | 36.4 ± 3.6 |
| UPDRS total score | 51.0 ± 6.3 | 63.3 ± 3.2 | 48.0 ± 4.1 | 58.1 ± 4.2 |
| PDQ-39 mobility subscore | 10.7 ± 3.1 | 27.5 ± 7.2* | 11.4 ± 3.7 | 15.8 ± 4.5† |
| PDQ-39 index score | 29.3 ± 8.4 | 39.4 ± 7.7 | 27.1 ± 8.0 | 27.0 ± 5.2† |
Values are means ± SE. PD, Parkinson’s disease; RT, resistance training; UPDRS, Unified Parkinson’s Disease Rating Scale; PDQ-39, 39-item Parkinson’s Disease Quality of Life Scale.
Different from PD-L/M, P < 0.05;
different from Pre-RT.
RT-Induced Changes in Type I Myofiber Grouping are Dependent on Grouping Severity
Limited effects of RT when including all OA and PD subjects.
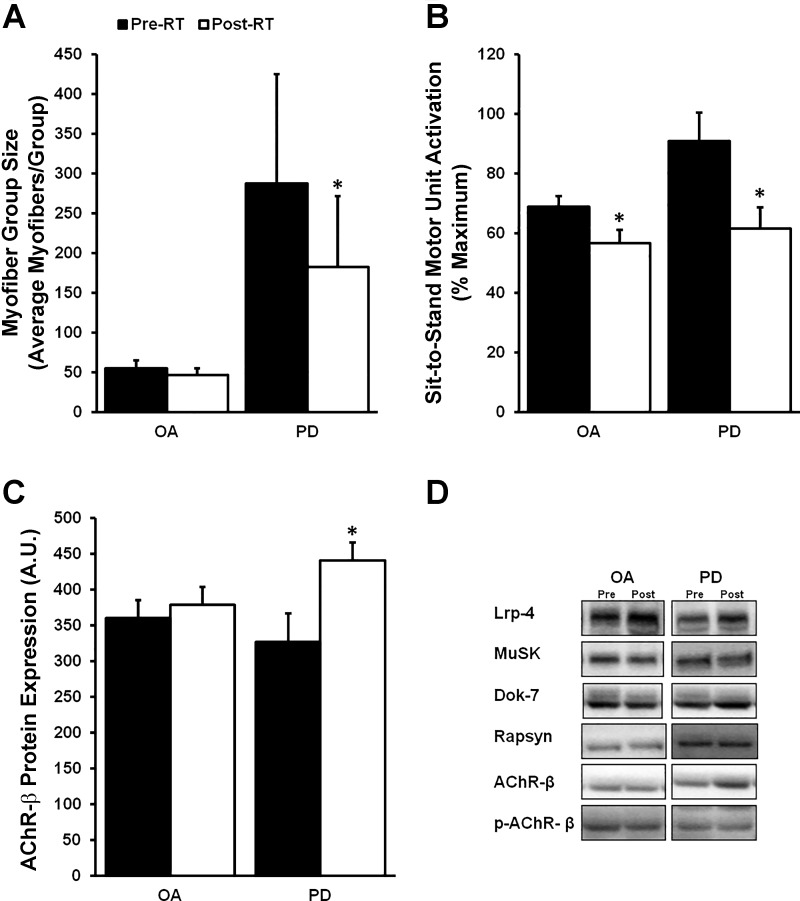
When the entire OA and PD groups were analyzed overall, type I myofiber grouping prevalence (OA pre vs. OA post: 51 ± 3 vs. 50 ± 3%; PD pre vs. PD post: 60 ± 9 vs. 56 ± 7%) and groups per 1,000 myofibers (OA pre vs. OA post: 6.6 ± 0.6 vs. 6.7 ± 0.4; PD pre vs. PD post: 3.4 ± 0.7 vs. 4.5 ± 1.1) did not change for OA or PD after RT. The size of type I myofiber groups also did not change among OA with RT; however, there was a reduction in group size in PD after RT (P < 0.05; Fig. 5A). As expected, RT reduced MUA in both OA and PD (P < 0.05; Fig. 5B). Transcript and protein levels of markers of denervation did not change following RT, with the exception of total AChR-β protein expression, which increased in PD only (P < 0.05; Fig. 5C).
Fig. 5.
The effects of resistance training (RT) on myofiber group size, submaximal motor unit activation (MUA), and acetylcholine receptor (AChR) expression in older adults (OA) and Parkinson’s disease (PD). A: surprisingly, type I myofiber group size decreased in PD after RT. B: expectedly, MUA was reduced post-RT in both groups, likely as a result of neural learning. C: AChR subunit-β (AChR-β) protein expression was upregulated in PD following RT, but no increase was observed in OA. D: representative immunoblots for proteins of interest in OA and PD pre- and post-RT. There were no other differences in target protein expression within groups. *Different from pre-RT, P < 0.05. Values are means + SE.
Prominent effects of RT among subjects with the highest type I grouping.
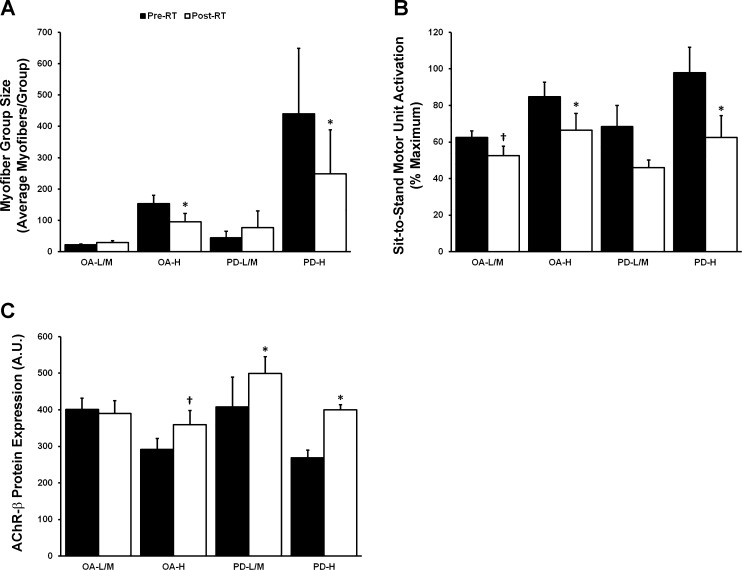
Interestingly, RT-induced adaptations differed considerably based on type I myofiber grouping prevalence. The myofiber group size reduction does not appear to have been a PD-specific phenomenon considering both OA-H and PD-H decreased group size, whereas PD-L/M did not change (P < 0.05; Fig. 6A). It is also important to point out that the reduction in type I group size among OA-H included some subjects from the 4-wk training period and some from the 16-wk training period, suggesting this “reversal” may be quite rapid. Although most clusters tended to decrease MUA, only OA-H and PD-H did so significantly (P < 0.05; Fig. 6B). Increases in AChR-β may occur based on type I myofiber grouping prevalence as well as presence of PD considering that OA-H, PD-L/M, and PD-H all experienced inductions of total AChR-β protein expression (P < 0.05; Fig. 6C). Finally, RT resulted in an improvement on the PDQ-39 mobility subscore (P < 0.05; Table 1) and PDQ-39 index score (P < 0.05), with a trend toward improvement in UPDRS total (P = 0.17) in PD-H only.
Fig. 6.
The effects of resistance training (RT) on myofiber group size, submaximal motor unit activation (MUA), and acetylcholine receptor (AChR) are dependent on type I myofiber grouping prevalence. A and B: only those older adults (OA) with the highest type I myofiber grouping (OA-H, PD-H), as defined by K-means cluster analysis, displayed reductions in type I myofiber group size (A) and MUA post-RT (B). However, both OA-L/M and PD-L/M displayed a trend for MUA reduction post-RT. C: both PD-L/M and PD-H increased AChR-β subunit protein levels post-RT, whereas OA-H displayed a trend toward increase. There were no other RT-induced differences in denervation-associated transcript or protein expression. *Different from pre-RT, P < 0.05; †trend toward difference from pre-RT, P < 0.07. Values are means + SE.
DISCUSSION
In this comparison of type I myofiber grouping in PD compared with age-matched and young non-PD controls, we found that PD has a unique type I myofiber grouping signature that consists of dramatically larger myofiber groups compared with OA and YA. Additionally, abnormally elevated MUA and NMJ markers of denervation followed a stepwise expression pattern with PD > OA > YA. Interestingly, we found that RT decreased type I myofiber group size and increased expression of AChR-β in PD and OA with the most severe type I grouping. Finally, we found that high type I myofiber grouping prevalence in both PD and age-matched OA is associated with both physiological (MUA) and molecular (NMJ markers of denervation) indices of motor unit remodeling as well as symptomatic progression of PD.
Aging is well known to result in motor unit remodeling. Studies using electrophysiological estimates have reported decreases in motor unit number and increases in motor unit size in skeletal muscles from older adults compared with young (40). Because this process involves myofiber denervation/reinnervation events that often lead to myofiber conversion, myofiber type grouping has become a hallmark of age-related motor unit remodeling (16, 21). Thus, it was not surprising that a higher type I myofiber grouping prevalence corresponded with elevated expression of NMJ markers of denervation and MUA. Heightened myofiber denervation would be expected to cause more denervation/reinnervation events and an accumulation of larger motor units (i.e., myofiber grouping prevalence and size), ultimately resulting in overrecruitment of the muscle during submaximal contractions.
The cluster analysis enabled us to further explore PD vs. OA differences and the relationships between type I grouping severity and molecular indices of NMJ denervation. Whereas OA with the highest degree of grouping (OA-H) approached PD on a number of outcomes, PD-H exceeded OA-H type I myofiber group size by more than 2.5-fold and generally displayed a more severe molecular and physiological phenotype than OA-H, indicating PD-specific effects even at more advanced levels of motor unit remodeling among OA. Interestingly, the overall proportion of PD individuals in the high cluster (n = 9/16, 56%) was more than double the proportion of OA in the high cluster (n = 22/91, 24%) using the same type I myofiber grouping prevalence cutoffs. Altogether, these data are suggestive of a PD-specific advancement/acceleration of type I myofiber grouping prevalence as well as heightened neuromuscular degeneration, which exceeds normal age- and myofiber grouping-predicted levels.
Within the PD group, type I myofiber grouping prevalence also seemed to be indicative of symptomatic progression. For example, we found clinically meaningful differences (17, 46) between PD-H and PD-L/M, with higher UPDRS III (motor; +6.5 points) and UPDRS total (+12.3 points) scores in PD-H. Additionally, there were PDQ-39 measures that displayed large differences between PD-H and PD-L/M such as mobility (+16.8 points) and PDQ index (+10.1 points). When combined with the MUA and molecular denervation results, these findings are suggestive of heightened overall neuromuscular degeneration accompanied by compromised mobility status and overall motor function in PD-H compared with PD-L/M despite no difference in Hoehn and Yahr disease staging.
Although the primary pathology of PD, a progressive neurodegenerative disorder, is thought to be initiated by progressive degeneration of dopaminergic neurons within the basal ganglia (in particular the substantia nigra), a number of motor (e.g., tremor, bradykinesia, freezing of gait, stooped posture, etc.) and nonmotor (e.g., impaired olfaction and swallowing, slowed GI motility, sleep disturbances, cognitive decline, mood disorders, hallucinations, vision problems, impulsive behaviors, fatigue, etc.) manifestations indicate changes to the nervous system at multiple levels along with dysfunction of nonneuronal systems (e.g., ubiquitous mitochondrial dysfunction). We do not yet fully understand why PD leads to greater type I myofiber grouping than normal aging, but a few possibilities seem ripe for further study. First, protein aggregation (α-synuclein and/or tau deposition) is a common hallmark of neurodegenerative diseases, including PD, and recent findings of α-synuclein deposition in motor neurons (33, 35) make it attractive to speculate that such deposition may contribute to the progression of type I myofiber grouping and overall neuromuscular degeneration. Second, neuroinflammation appears to be prominent in PD and has become a major research focus area in recent years. It would thus be attractive to determine whether inflammatory upregulation extends to α-motor neurons and impacts NMJ deterioration. Third, the well-established mitochondrial dysfunction in PD, which likely extends to skeletal muscle, as our previous data on subsarcolemmal mitochondria suggest (20), may play an important role in NMJ integrity and should be studied further. Finally, the symptomatic tremors and other motor impairments of PD may themselves influence both NMJ deterioration and the success of renervation. This possibility is of particular interest since the low-force tremors of PD involve type I motor units, which may drive enlargement of such motor units when denervated type II myofibers become available for reinnervation.
Because motor unit remodeling as the result of denervation/reinnervation events precedes functional decline, an intervention aimed at reducing denervation might prove beneficial for altering motor unit remodeling. Exercise training is well-known to improve age-related declines in skeletal muscle function (6). Additionally, exercise studies in animals have shown attenuation and restoration of age-related NMJ degeneration (36, 51). However, very few studies have assessed the impact of exercise training on direct or secondary measures of motor unit remodeling in humans. Current evidence suggests that life-long running preserves motor unit number in a muscle-specific manner (41, 42) and that five months of RT reduces expression of biomarkers of denervation (31). Based on these findings, we pursued an exploratory assessment of the effects of RT on type I myofiber grouping (prevalence, group size, group number), MUA, and markers of NMJ stability/denervation among OA and PD overall as well as the K-means clusters.
We found a surprising RT-induced reduction in type I myofiber group size in PD, which was driven by PD-H, and although there was no reduction in OA, we found that OA-H decreased type I myofiber group size similarly to PD-H. This strongly suggests that in cases of advanced pathological grouping, some of the grouped MHC type I myofibers converted back to MHC type II myofibers. Although conversions between type I and II myofibers are rare and certainly not expected in response to exercise, we believe dual innervation status may offer an explanation. Support for this interpretation comes from animal studies showing that some aged myofibers are dually innervated by two separate motor neurons, and the incidence of dual innervation increases with advancing age (51). It is possible that dually innervated myofibers represent a pool of myofibers captured during the dynamic process of denervation/reinnervation and that MHC expression transition is one of the first events in the conversion process. In fact, we have recently shown that grouped type I myofibers resemble type II myofibers in a number of phenotypic ways and display a disconnect between MHC I expression and the expression of type II-specific sarco(endo)plasmic reticulum calcium ATPase I (SERCA I), implying that MHC I conversion may occur at the earliest stages of reinnervation-induced myofiber conversion (21). Moreover, these findings were most evident in individuals with abnormally high type I myofiber grouping (21). Based on their higher expression levels of NMJ markers of denervation, and likely denervation-reinnervation events, OA-H and PD-H would be expected to possess a higher number of dually innervated myofibers expressing MHC I in this scenario. It is then attractive to hypothesize that high-intensity RT, which activates all motor units in a muscle, may be sufficient to trigger retention of an otherwise deteriorating type II NMJ, thereby restoring the dominant (i.e., original) axonal connection, and thus MHC fiber type (type II), of these transitioning fibers.
The type I myofiber group size results were coupled with RT-induced improvements in MUA in OA-H and PD-H. Although RT-induced MUA adaptations are common (even in young adults) due to neural learnin, and all OA and PD clusters displayed trends of a post-RT MUA reduction, it is interesting that the improvements in OA-H were nearly double those in OA-L/M and that PD-H displayed ∼1.5 times the improvement as PD-L/M. Because we have found abnormally large group size to be indicative of abnormally high MUA (i.e., OA-H and PD-H), this leaves open the possibility that RT-induced reductions in group size accentuate normal RT-induced neural learning. Finally, RT did not appear to alter biomarkers of denervation; however, there was an upregulation of total AChR-β protein levels in PD (both PD-L/M and PD-H) and a trend for upregulation in OA-H after RT. Because transcript levels of AChR-β were not elevated at baseline in any of these groups and did not change in response to RT, this finding could be due to an increase in activity-dependent AChR-β translation. In fact, it has been shown that AChR activation leads to AChR upregulation that is not linked to increased gene expression (29). Notably, the elevated MuSK expression levels before RT in OA (primarily OA-H) and PD (primarily PD-H) did not decrease after RT. This could be due to the time course of RT-induced effects. For example, it is possible that MuSK upregulation is part of a compensatory mechanism to maintain p-AChR-β levels, and thus AChR clustering (4, 5, 13), in the context of denervation, and 4–16 wk might not be a sufficient amount of time to reestablish complete myofiber innervation. However, 4–16 wk of RT may be adequate to upregulate protein expression of AChR-β (e.g., increased protein translation). Therefore, it remains to be determined whether longer RT interventions might result in an eventual normalization of MuSK expression and reductions of other markers of NMJ instability/denervation. We should point out that the histological and molecular changes noted here with RT were in PD and OA subjects in the seventh decade on average, whereas a recent review of the literature suggests that activity- or exercise-mediated influences on motor unit remodeling may lose their efficacy in the eighth decade and beyond (16).
Despite its acceptance as an indicator of denervation/reinnervation events, whether myofiber type grouping is advantageous or deleterious is not clear. High amounts of myofiber type grouping can be interpreted as beneficial for reinnervation-induced myofiber preservation or unfavorable for pathologically heightened levels of denervation, resulting in higher denervation/reinnervation frequency but also higher myofiber loss. The evidence we have shown here suggests that individuals with the highest levels of type I myofiber grouping also display the most dysregulated MUA patterns and heightened disease progression. Further assessments are necessary to more precisely probe the functional consequences associated with varying extents of type I myofiber grouping.
In conclusion, we established the type I myofiber grouping profile of PD, which is characterized by a group size that exceeds that of normal aging. Furthermore, we demonstrate that advanced type I myofiber grouping is associated with disrupted motor unit recruitment and heightened molecular markers of denervation. Finally, our results show that in individuals with the most advanced type I myofiber grouping (i.e., OA-H and PD-H), a portion of grouped type I myofibers may reverse their MHC expression in response to intensive RT. Future research should strive to examine the effects of different modes, intensities, and durations of exercise on myofiber grouping and more thoroughly assess the functional consequences associated with grouping.
GRANTS
This work was supported in part by the UAB Center for Exercise Medicine and National Institutes of Health Grants P2C-HD-086851, R01-AG-017896, R01-AG-046920, R01-HD-084124, and T32-HD-071866 (N. A. Kelly and K. G. Hammond).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.A.K. and M.M.B. conceived and designed research; N.A.K., K.G.H., S.T.W., S.C.T., and M.M.B. performed experiments; N.A.K., K.G.H., and M.M.B. analyzed data; N.A.K., K.G.H., C.S.B., and M.M.B. interpreted results of experiments; N.A.K. and K.G.H. prepared figures; N.A.K. drafted manuscript; N.A.K., K.G.H., C.S.B., S.T.W., S.C.T., and M.M.B. edited and revised manuscript; N.A.K., K.G.H., C.S.B., S.T.W., S.C.T., and M.M.B. approved final version of manuscript.
REFERENCES
- 1.Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol (1985) 97: 1329–1337, 2004. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- 2.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc 43: 1177–1187, 2011. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- 3.Bongers KS, Fox DK, Ebert SM, Kunkel SD, Dyle MC, Bullard SA, Dierdorff JM, Adams CM. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab 305: E907–E915, 2013. doi: 10.1152/ajpendo.00380.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borges LS, Ferns M. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J Cell Biol 153: 1–12, 2001. doi: 10.1083/jcb.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borges LS, Yechikhov S, Lee YI, Rudell JB, Friese MB, Burden SJ, Ferns MJ. Identification of a motif in the acetylcholine receptor beta subunit whose phosphorylation regulates rapsyn association and postsynaptic receptor localization. J Neurosci 28: 11468–11476, 2008. doi: 10.1523/JNEUROSCI.2508-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab 23: 1034–1047, 2016. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashman NR, Covault J, Wollman RL, Sanes JR. Neural cell adhesion molecule in normal, denervated, and myopathic human muscle. Ann Neurol 21: 481–489, 1987. doi: 10.1002/ana.410210512. [DOI] [PubMed] [Google Scholar]
- 8.Caviness JN, Smith BE, Clarke Stevens J, Adler CH, Caselli RJ, Hentz JG, Manfred MS, Muenter D. Motor unit number estimates in idiopathic Parkinson’s disease. Parkinsonism Relat Disord 8: 161–164, 2002. doi: 10.1016/S1353-8020(01)00007-4. [DOI] [PubMed] [Google Scholar]
- 9.Caviness JN, Smith BE, Stevens JC, Adler CH, Caselli RJ, Reiners CA, Hentz JG, Muenter MD. Motor unit changes in sporadic idiopathic Parkinson’s disease. Mov Disord 15: 238–243, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Cohen TJ, Waddell DS, Barrientos T, Lu Z, Feng G, Cox GA, Bodine SC, Yao TP. The histone deacetylase HDAC4 connects neural activity to muscle transcriptional reprogramming. J Biol Chem 282: 33752–33759, 2007. doi: 10.1074/jbc.M706268200. [DOI] [PubMed] [Google Scholar]
- 11.Covault J, Sanes JR. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci USA 82: 4544–4548, 1985. doi: 10.1073/pnas.82.13.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edström L. Selective changes in the sizes of red and white muscle fibres in upper motor lesions and Parkinsonism. J Neurol Sci 11: 537–550, 1970. doi: 10.1016/0022-510X(70)90104-8. [DOI] [PubMed] [Google Scholar]
- 13.Ferns M, Deiner M, Hall Z. Agrin-induced acetylcholine receptor clustering in mammalian muscle requires tyrosine phosphorylation. J Cell Biol 132: 937–944, 1996. doi: 10.1083/jcb.132.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glendinning DS, Enoka RM. Motor unit behavior in Parkinson’s disease. Phys Ther 74: 61–70, 1994. doi: 10.1093/ptj/74.1.61. [DOI] [PubMed] [Google Scholar]
- 15.Gutmann E, Hanzlíková V. Motor unit in old age. Nature 209: 921–922, 1966. doi: 10.1038/209921b0. [DOI] [PubMed] [Google Scholar]
- 16.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horváth K, Aschermann Z, Ács P, Deli G, Janszky J, Komoly S, Balázs É, Takács K, Karádi K, Kovács N. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism Relat Disord 21: 1421–1426, 2015. doi: 10.1016/j.parkreldis.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184, 1992. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol 61: 737–746, 1989. doi: 10.1152/jn.1989.61.4.737. [DOI] [PubMed] [Google Scholar]
- 20.Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol (1985) 116: 582–592, 2014. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 57: E52–E59, 2018. doi: 10.1002/mus.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol (1985) 99: 2149–2158, 2005. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 24.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. doi: 10.1007/BF00293457. [DOI] [PubMed] [Google Scholar]
- 26.Li AM, Ma H, Villarroel A. Acetylcholine receptor gamma-subunits mRNA isoforms expressed in denervated rat muscle. Mol Neurobiol 37: 164–170, 2008. doi: 10.1007/s12035-008-8030-3. [DOI] [PubMed] [Google Scholar]
- 27.Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol 589: 3023–3037, 2011. doi: 10.1113/jphysiol.2010.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 29.Melroy-Greif WE, Stitzel JA, Ehringer MA. Nicotinic acetylcholine receptors: upregulation, age-related effects and associations with drug use. Genes Brain Behav 15: 89–107, 2016. doi: 10.1111/gbb.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messi ML, Li T, Wang ZM, Marsh AP, Nicklas B, Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci 71: 1273–1280, 2016. doi: 10.1093/gerona/glv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Mayr W, Krenn M, Paternostro-Sluga T, Hamar D, Cvecka J, Sedliak M, Tirpakova V, Sarabon N, Musarò A, Sandri M, Protasi F, Nori A, Pond A, Zampieri S. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 73: 284–294, 2014. doi: 10.1097/NEN.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 33.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG; Arizona Parkinson’s Disease Consortium . Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol 72: 119–129, 2013. doi: 10.1097/NEN.0b013e3182801cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG; Arizona Parkinson’s Disease Consortium . Altered pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol 71: 520–530, 2012. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, Adler CH, Shill HA, Caviness JN, Samanta JE, Sue LI, Beach TG; Arizona Parkinson’s Disease Consortium . Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol 72: 614–623, 2013. doi: 10.1097/NEN.0b013e3182965886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 49: 315–324, 2014. doi: 10.1002/mus.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci 66: 327–338, 1984. doi: 10.1016/0022-510X(84)90021-2. [DOI] [PubMed] [Google Scholar]
- 38.Petrella JK, Kim JS, Tuggle SC, Bamman MM. Contributions of force and velocity to improved power with progressive resistance training in young and older adults. Eur J Appl Physiol 99: 343–351, 2007. doi: 10.1007/s00421-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 39.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol (1985) 98: 211–220, 2005. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- 40.Piasecki M, Ireland A, Jones DA, McPhee JS. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 17: 485–496, 2016. doi: 10.1007/s10522-015-9627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Power GA, Dalton BH, Behm DG, Doherty TJ, Vandervoort AA, Rice CL. Motor unit survival in lifelong runners is muscle dependent. Med Sci Sports Exerc 44: 1235–1242, 2012. doi: 10.1249/MSS.0b013e318249953c. [DOI] [PubMed] [Google Scholar]
- 42.Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ, Rice CL. Motor unit number estimates in masters runners: use it or lose it? Med Sci Sports Exerc 42: 1644–1650, 2010. doi: 10.1249/MSS.0b013e3181d6f9e9. [DOI] [PubMed] [Google Scholar]
- 43.Rose MH, Løkkegaard A, Sonne-Holm S, Jensen BR. Effects of training and weight support on muscle activation in Parkinson’s disease. J Electromyogr Kinesiol 23: 1499–1504, 2013. doi: 10.1016/j.jelekin.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One 7: e29082, 2012. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 46.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol 67: 64–70, 2010. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 47.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stec MJ, Thalacker-Mercer A, Mayhew DL, Kelly NA, Tuggle SC, Merritt EK, Brown CJ, Windham ST, Dell’Italia LJ, Bickel CS, Roberts BM, Vaughn KM, Isakova-Donahue I, Many GM, Bamman MM. Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp Gerontol 99: 98–109, 2017. doi: 10.1016/j.exger.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. doi: 10.1152/physiolgenomics.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34: 213–219, 1977. doi: 10.1016/0022-510X(77)90069-7. [DOI] [PubMed] [Google Scholar]
- 51.Valdez G, Tapia JC, Kang H, Clemenson GD Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA 107: 14863–14868, 2010. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, Burden SJ. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev 19: 1715–1722, 2005. doi: 10.1101/gad.1318305. [DOI] [PMC free article] [PubMed] [Google Scholar]