Abstract
We examined the effects of age, sex, and their interaction on mechanical ventilatory constraint and dyspnea during exercise in 22 older (age = 68 ± 1 yr; n = 12 women) and 22 younger (age = 25 ± 1 y, n = 11 women) subjects. During submaximal exercise, older subjects had higher end-inspiratory (EILV) and end-expiratory (EELV) lung volumes than younger subjects (both P < 0.05). During maximal exercise, older subjects had similar EILV (P > 0.05) but higher EELV than younger subjects (P < 0.05). No sex differences in EILV or EELV were observed. We noted that women had a higher work of breathing (Wb) for a given minute ventilation (V̇e) ≥65 l/min than men (P < 0.05) and older subjects had a higher Wb for a given V̇e ≥60 l/min (P < 0.05). No sex or age differences in Wb were present at any submaximal relative V̇e. At absolute exercise intensities, older women experienced expiratory flow limitation (EFL) more frequently than older men (P < 0.05), and older subjects were more likely to experience EFL than younger subjects (P < 0.05). At relative exercise intensities, women and older individuals experienced EFL more frequently than men and younger individuals, respectively (both P < 0.05). There were significant effects of age, sex, and their interaction on dyspnea intensity during exercise at absolute, but not relative, intensities (all P < 0.05). Across subjects, dyspnea at 80 W was significantly correlated with indexes of mechanical ventilatory constraint (all P < 0.05). Collectively, our findings suggest age and sex have significant impacts on Wb, operating lung volumes, EFL, and dyspnea during exercise. Moreover, it appears that mechanical ventilatory constraint may partially explain sex differences in exertional dyspnea in older individuals.
NEW & NOTEWORTHY We found that age and sex have a significant effect on mechanical ventilatory constraint and the perception of dyspnea during exercise. We also observed that the perception of exertional dyspnea is associated with indexes of mechanical ventilatory constraint. Collectively, our results suggest that the combined influences of age and biological sex on mechanical ventilatory constraint during exercise contributes, in part, to the increased perception of dyspnea during exercise in older women.
Keywords: aging, dyspnea, exercise, expiratory flow limitation, operating lung volumes, respiratory mechanics, sex differences, work of breathing
INTRODUCTION
The normative aging of the respiratory system involves significant structural changes to the lungs, airways, chest wall, and respiratory muscles (29), leading to a progressive decline in pulmonary function (36). Consequently, when compared with individuals 20–30 yr of age, those above the age of 60 have a reduced ventilatory capacity, as reflected by the size and shape of their maximum expiratory flow-volume curves (19). It follows that older individuals have a reduced reserve for accommodating increases in ventilatory demand during dynamic exercise (1). Moreover, the ventilatory response to exercise at a given absolute work rate is higher in older individuals relative to their younger counterparts (47). Thus in older individuals it is possible that the ventilatory demand of exercise meets or even exceeds the maximum ventilatory capacity of the respiratory system, resulting in mechanical ventilatory constraint. Several indexes can be used to determine the presence and magnitude of mechanical ventilatory constraint during exercise such as quantifying the work of breathing (Wb), assessing changes in operating lung volumes, and determining the presence of expiratory flow limitation (EFL) (2). Healthy aging of the respiratory system is associated with a progressive increase in mechanical ventilatory constraint to exercise hyperpnea (12), as evidenced by a higher Wb for a given minute ventilation (V̇e), an increase in end-expiratory lung volume (EELV), and a higher propensity toward EFL (30, 31).
Along with age, biological sex is important when considering the mechanical ventilatory response to exercise. When matched for height, women have smaller lungs and lower maximum expiratory flows than men (11). Even when matched for lung size, women have smaller large conducting airways than men, a concept known as dysanapsis (50). Given the aforementioned sex differences in lung size, airway size, and expiratory flow rates, healthy young women appear to be predisposed to greater mechanical ventilatory constraint during exercise compared with men. We recently demonstrated that during exercise young women have a higher Wb for a given V̇e (15, 27) and a higher oxygen cost of breathing for a given V̇e than young men (16). It has also been shown that there are sex differences in the regulation of operating lung volumes, where young women tend to breathe at a higher end-inspiratory lung volume (EILV) for a given submaximal work rate and V̇e (10) and a higher EELV at maximal exercise than young men (15). Furthermore, EFL appears to be more common in young endurance-trained women than in their male counterparts (27).
There is growing evidence suggesting that the magnitude of exertional dyspnea increases during the healthy aging process (28). For example, during cycle exercise, older men and women report a higher intensity of dyspnea for a given absolute work rate than younger men and women (39). Additionally, older women report a higher intensity of dyspnea during exercise at a standardized oxygen uptake (V̇o2) than older men; this is thought to be related to the interaction between the effects of age and sex on ventilatory constraint (46). Indeed, when the magnitude of ventilatory constraint is experimentally increased during exercise, the perception of dyspnea is increased concomitantly (18). Given the effects of age and sex on the mechanical ventilatory response to exercise, it can be surmised that the sex differences in exertional dyspnea noted in older individuals may be explained, at least in part, by mechanical ventilatory constraint.
Several studies have investigated the effects of age (30, 31, 39) and sex (10, 15, 27) on the mechanical ventilatory and perceptual responses to exercise. However, few studies have assessed the combined and potentially interactive effects of age and sex on Wb, operating lung volumes, and EFL during exercise and how they relate to dyspnea. Accordingly, the primary aim of the present study was to assess the effects of biological sex and age as well as their interaction on the mechanical ventilatory and sensory responses to exercise in a group of healthy younger and older, men and women at relative and absolute exercise intensities and ventilations. A secondary aim was to determine if indexes of mechanical ventilatory constraint are related to dyspnea during exercise. Based on the above summary, we hypothesized that biological sex and healthy aging would have a significant interactive effect on indexes of mechanical ventilatory constraint (i.e., Wb, operating lung volumes, and EFL) and dyspnea during exercise. We also hypothesized that, across all subjects, indexes of mechanical ventilatory constraint would be significantly correlated with dyspnea intensity during exercise.
METHODS
Subjects.
After providing written informed consent, 22 older men and women (60–80 yr, n = 12 women) and 22 younger men and women (20–30 yr, n = 11 women) participated in the study. All subjects had normal pulmonary function based on predicted values (5, 7, 11, 44). Additional inclusion criteria were a body mass index of 18–30 kg/m2, peak aerobic power ≥80% predicted, and no evidence of respiratory disease. Subjects were excluded if they were current smokers or had previously smoked more than five pack years; had a history or current symptoms of cardiovascular, metabolic or respiratory disease; were currently taking medication that would interfere with the ventilatory response to exercise; or had any contraindications to exercise testing. Eight of twenty-two older subjects (n = 4 men, n = 4 women) had previously smoked less than five pack years, all of whom had quit smoking >25 yr before participation in the current study. All healthy younger subjects had never smoked. Subjects were divided into four groups based on sex and age: younger women (20–30 yr), younger men (20–30 yr), older women (60–80 yr), and older men (60–80 yr). All study procedures were approved by the University of British Columbia Providence Health Care Research Ethics Board, which adheres to the Declaration of Helsinki.
Experimental overview.
Subjects completed 2 days of testing separated by a minimum of 48 h. On day 1, anthropometric measurements were taken, followed by detailed pulmonary function testing and a symptom-limited incremental cycle exercise test. The incremental exercise test performed on day 1 was intended to familiarize subjects with the exercise protocol. On day 2, subjects were instrumented with a balloon catheter (Guangzhou Yinghui Medical Equipment, Guangzhou, China) that was passed through the naris following the application of a topical anesthetic (Lidocan endotracheal spray; Odan Laboratories, Montreal, QC, Canada) to measure esophageal pressure. Following instrumentation, lung static recoil pressure at 100% of total lung capacity (Pst 100%TLC), lung static recoil pressure at 50% of vital capacity (Pst 50%VC), and static lung compliance were assessed. Subjects then performed a maximal incremental cycle exercise test using the same protocol as on day 1. During the incremental exercise test, EFL was assessed using the negative expiratory pressure (NEP) technique (see Expiratory flow limitation). On day 2, subjects performed a series of forced vital capacity maneuvers at different efforts before and after exercise to construct maximum expiratory flow-volume curves by taking into account exercise-induced bronchodilation and thoracic gas compression (25). All reported resting pulmonary function data, apart from static recoil and lung compliance, were obtained on day 1, whereas all reported exercise data were obtained on day 2.
Pulmonary function testing.
Spirometry, whole body plethysmography, single breath diffusing capacity for carbon monoxide, maximum voluntary ventilation, and maximum inspiratory and expiratory pressures were assessed using a commercially available system (Vmax Encore 229, V62J Autobox; CareFusion, Yorba Linda, CA) according to standard recommendations (21, 38, 42, 53). Pulmonary function measurements were expressed as absolute values and as percentages of predicted (5, 7, 11, 44).
Exercise protocol.
Exercise testing was conducted on an electronically braked cycle ergometer (Ergoselect 200P; Ergoline, Bitz, Germany). Each test began with a 6-min rest period followed by 1 min of unloaded pedaling then 20-W step-wise increases in workload (starting at 20 W) every 2 min until volitional exhaustion. The exercise protocol was selected to allow for comparisons between groups at discrete work rates. Peak work rate was defined as the highest work rate sustained for at least 30 s.
Flow, volume, and pressure.
During the incremental cycle exercise test on day 2, subjects breathed through a low resistance (0.3–0.7 cmH2O·l−1·s−1 at 0.5–8 l/s) circuit with minimal dead space (130 ml). Bidirectional flow was measured using a heated, calibrated pneumotachograph (model 3813; Hans Rudolph, Kansas City, MO). Volume was obtained by numerical integration of the flow signal. Mouth pressure was sampled through a port in the mouthpiece while esophageal pressure was measured using an esophageal balloon catheter. Placement of the catheter was performed as previously described (55), with 0.5 ml of air placed into the esophageal balloon. Validity of the esophageal balloon pressure was verified by performing an occlusion test, as previously described (4). Mouth pressure and esophageal pressure were measured using independent, calibrated differential pressure transducers (DP15-34; Validyne Engineering, Northridge, CA). Flow, volume, and pressures were composite averaged by selecting breaths within a 30-s epoch during rest and at the end of each exercise stage.
Cardiorespiratory responses.
Standard cardiorespiratory measures were recorded on a breath-by-breath basis and averaged over 30-s periods at rest and during exercise. In the younger subjects, heart rate was measured using a heart rate monitor (Polar T34; Polar Electro, Kempele, Finland). In the older subjects, heart rate and electrocardiogram changes were monitored continuously using a 12-lead electrocardiogram (Cardiosoft Diagnostics System v6.71; GE Healthcare). Arterial oxygen saturation was measured in all subjects using a finger-pulse oximeter (Radical-7; Massimo, Irvine, CA). Inspiratory capacity maneuvers were performed at rest and at the end each exercise stage. EILV and EELV were derived from the inspiratory capacity maneuvers (24). Theoretical maximum ventilation (V̇eCAP) was calculated at rest and for each exercise stage based on the maximum expiratory airflow throughout a composite averaged tidal breath at a given lung volume as previously described (33). Fractional utilization of available ventilatory capacity (V̇e/V̇eCAP) was determined as the quotient of V̇e and V̇eCAP.
Work of breathing.
Wb was determined by integrating the area within a composite averaged tidal esophageal pressure-volume loop (17). For each subject, Wb data were plotted as a function of absolute V̇e. To compare the effects of age, sex, and their interaction on Wb for a given absolute V̇e, curves were fit to each individual subject’s data according to the following equation (27):
| (1) |
where, for a given absolute V̇e, aV̇e3 represents the resistive component of Wb and bV̇E2 represents the viscoelastic component of Wb. To determine the total Wb for each subject at discrete levels of absolute V̇e, each subject’s Wb equation (Eq. 1) was solved for successive independent variables in 5 l/min increments up to each subject’s maximal V̇e. The total Wb values for each subject were then normalized to their respective maximal V̇e in 5% increments up to 100%.
Expiratory flow limitation.
At rest and for each stage of exercise on day 2, EFL was determined using the NEP technique (27, 37). Briefly, the NEP technique involves the generation of a negative pressure (between −5 to −10 cmH2O) at the mouth during the expired portion of a breath. Negative pressure was achieved using an electronically controlled Venturi device (207A; Raytech Instruments, Vancouver, BC, Canada) attached to the distal portion of the pneumotachograph. A control flow-volume loop was created by composite averaging the three tidal breaths immediately before each NEP breath to represent spontaneous patterns of flow and volume at a given stage during the exercise test (37). Expiratory flow limitation was considered present when the NEP breath overlapped with the expired portion of the control breath.
Perceptual responses.
At rest and during the last 30 s of each 2-min exercise stage, subjects rated the intensity of “breathing discomfort” (dyspnea) and “leg discomfort” using the modified category-ratio 0–10 Borg scale (6). Dyspnea was defined as “the sensation of labored or difficult breathing” and leg discomfort was defined as the “sensation of leg muscle fatigue.” The end points of the scale were anchored such that 0 represented “no breathing/leg discomfort” and 10 represented “the most severe breathing/leg discomfort ever experienced or imagined.”
Data processing.
All data (see Flow, Pressure, and volume, and Cardiorespiratory responses) were collected using a 16-channel analogue-to-digital data acquisition system (PowerLab/16/35, ADInstruments, Colorado Springs, CO), sampled at 2,000 Hz, and recorded using LabChart 7.3.7 software.
Statistical analysis.
Descriptive characteristics, pulmonary function data, and maximal exercise data were compared using a 2 × 2 ANOVA for age and sex between the four groups. In the case of a significant interaction between age and sex, four pairwise comparisons were performed with Bonferroni corrections where appropriate. To determine the effect of age and sex as well as their interaction on Wb for a given absolute relative V̇e, Wb was compared at discrete levels of absolute V̇e (in 5 l/min increments) or relative V̇e (in 5% increments) using a mixed model analysis of variance. In the case of a significant two-way interaction between V̇e and age, V̇e and sex, or a significant three-way interaction among V̇e, age, and sex, pairwise comparisons were performed with Bonferroni corrections where appropriate. We performed a mixed-model repeated-measures analysis using generalized estimating equations to evaluate the main effects of age and sex as well as their interaction between groups and work rate (absolute and relative) on EFL at rest and during exercise. Cardiorespiratory and perceptual variables were compared at rest and at absolute submaximal work rates up to the highest equivalent work rate achieved by all subjects and at relative work rates in 20% increments from rest to peak exercise using a mixed model analysis of variance. In the case of a significant two-way interaction between work rate and age, work rate and sex, or a significant three-way interaction among work rate, age, and sex, Bonferroni-adjusted post hoc comparisons were conducted where appropriate. Pearson’s product moment correlation analysis was used to determine the relationship between dyspnea and possible physiological contributors. For all analyses, the level of statistical significance was set at P < 0.05. All data are presented as means ± SE.
RESULTS
Subjects.
Subject characteristics and pulmonary function data are shown in Table 1. Resting pulmonary function was within the normal predicted range for all groups. As expected, there were significant age-related differences in maximum expiratory flows, lung volumes (with the exception of total lung capacity, P = 0.97), diffusing capacity, and respiratory muscle strength (all P < 0.05). Furthermore, when expressed in absolute terms, the majority of pulmonary function measures were greater in men than women (all P < 0.05), with the exception of the ratio of forced expired volume in 1 s to forced vital capacity (P = 0.81), forced expired flow between 25 and 75% of forced vital capacity (FEF25–75%) (P = 0.23), and residual volume (P = 0.14). There were no significant interaction effects between age and sex, indicating that the age-related decrement in pulmonary function was similar in both sexes. Regardless of sex, older subjects had lower Pst 100%TLC (P < 0.001) and Pst 50%VC (Table 1; P < 0.001). Moreover, there was a significant correlation between FEF50% and Pst 50%VC (r = 0.54, P < 0.05).
Table 1.
Baseline subject characteristics and pulmonary function data
| Older (n = 22) |
Younger (n = 22) |
||||
|---|---|---|---|---|---|
| Men (n = 10) | Women (n = 12) | Men (n = 11) | Women (n = 11) | P Value | |
| Age, yr | 70 ± 2 | 66 ± 2 | 26 ± 1 | 24 ± 1 | *P < 0.05 |
| Height, cm | 173 ± 2 | 163 ± 2 | 176 ± 2 | 166 ± 2 | †P < 0.05 |
| Body mass, kg | 76 ± 4 | 65 ± 3 | 73 ± 3 | 58 ± 2 | †P < 0.05 |
| BMI, kg/m2 | 25 ± 1 | 25 ± 1 | 23 ± 1 | 21 ± 1 | *P < 0.05 |
| FVC, liter | 4.30 ± 0.12 | 3.46 ± 0.20 | 5.46 ± 0.22 | 4.26 ± 0.18 | *†P < 0.05 |
| FVC, %predicted | 102 ± 2 | 108 ± 3 | 101 ± 3 | 104 ± 3 | |
| FEV1, liter | 3.06 ± 0.13 | 2.49 ± 0.16 | 4.27 ± 0.19 | 3.55 ± 0.13 | *†P < 0.05 |
| FEV1, %predicted | 102 ± 4 | 104 ± 4 | 99 ± 3 | 106 ± 2 | |
| FEV1/FVC | 71 ± 2 | 71 ± 2 | 79 ± 2 | 82 ± 2 | *P < 0.05 |
| FEV1/FVC, %predicted | 99 ± 3 | 96 ± 2 | 94 ± 2 | 95 ± 1 | |
| PEF, l/s | 8.63 ± 0.42 | 6.82 ± 0.38 | 10.79 ± 0.39 | 7.69 ± 0.15 | *†P < 0.05 |
| FEF25–75, l/s | 2.10 ± 0.29 | 1.90 ± 0.12 | 4.18 ± 0.18 | 3.67 ± 0.22 | *P < 0.05 |
| FEF25–75, %predicted | 82 ± 11 | 77 ± 4 | 91 ± 3 | 97 ± 6 | |
| TLC, liter | 6.84 ± 0.31 | 5.57 ± 0.26 | 6.89 ± 0.23 | 5.42 ± 0.20 | †P < 0.05 |
| TLC, %predicted | 102 ± 3 | 108 ± 3 | 102 ± 3 | 103 ± 2 | |
| VC, liter | 4.45 ± 0.14 | 3.57 ± 0.18 | 5.56 ± 0.21 | 4.32 ± 0.17 | *†P < 0.05 |
| VC, %predicted | 101 ± 3 | 109 ± 3 | 103 ± 4 | 102 ± 2 | |
| IC, liter | 3.03 ± 0.26 | 2.39 ± 0.21 | 3.48 ± 0.17 | 2.77 ± 0.15 | *†P < 0.05 |
| IC, %predicted | 100 ± 8 | 100 ± 9 | 99 ± 5 | 115 ± 5 | |
| FRC, liter | 3.90 ± 0.24 | 3.18 ± 0.16 | 3.41 ± 0.17 | 2.64 ± 0.12 | *†P < 0.05 |
| FRC, %predicted | 97 ± 4.25 | 108 ± 4 | 104 ± 6 | 92 ± 4 | |
| RV, liter | 2.39 ± 0.24 | 1.96 ± 0.11 | 1.33 ± 0.12 | 1.07 ± 0.07 | *P < 0.05 |
| RV, % predicted | 95 ± 8 | 100 ± 5 | 90 ± 11 | 81 ± 5 | |
| DLco, ml·min−1·mmHg−1 | 27 ± 2 | 23 ± 1 | 33 ± 2 | 25 ± 1 | *†P < 0.05 |
| DLco, %predicted | 107 ± 6 | 105 ± 4 | 111 ± 4 | 105 ± 4 | |
| MIP, cmH2O | −103 ± 8 | −76 ± 6 | −138 ± 10 | −106 ± 7 | *†P < 0.05 |
| MIP, %predicted | 98 ± 8 | 108 ± 6 | 107 ± 5 | 115 ± 6 | |
| MEP, cmH2O | 151 ± 16 | 109 ± 8 | 178 ± 11 | 142 ± 9 | *†P < 0.05 |
| MEP, % predicted | 78 ± 8 | 81 ± 6 | 74 ± 5 | 90 ± 6 | |
| Cl, l/cmH2O1 | 0.29 ± 0.01 | 0.28 ± 0.03 | 0.31 ± 0.02 | 0.26 ± 0.02 | |
| Pst 100%TLC, cmH2O−1 | 19 ± 2 | 20 ± 2 | 28 ± 1 | 31 ± 1 | *P < 0.05 |
| Pst 50%VC, cmH2O−1 | 4.8 ± 0.4 | 5.5 ± 0.6 | 6.4 ± 0.5 | 6.5 ± 0.2 | *P < 0.05 |
All data are presented as means ± SE. BMI, body mass index; FVC, forced vital capacity; FEV1, forced expired volume in 1 s; PEF, peak expiratory flow; FEF25–75, forced expired flow between 25 and 75% of FVC; TLC, total lung capacity; VC, vital capacity; IC, inspiratory capacity; FRC, functional residual capacity; RV, residual volume; DLco, diffusion capacity of the lung for carbon monoxide; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; Cl, lung compliance; Pst 100%TLC, static recoil pressure of the lungs at 100% of TLC; Pst 50%VC, static recoil pressure of the lungs at 50% of vital capacity.
Older vs. younger subjects.
Men vs. women.
Peak exercise data are shown in Table 2. At peak exercise, there was a significant effect of age and sex on absolute V̇o2, work rate, V̇e, the ventilatory equivalent for carbon dioxide, V̇eCAP, and Wb (all P < 0.05). When V̇o2 at peak exercise was expressed as a percentage of predicted values, there was no significant effect of age or sex, indicating that subjects had statistically similar levels of relative fitness. Independent of sex, there was a significant effect of age on heart rate, breathing frequency, the ventilatory equivalent for oxygen, the ventilatory equivalent for carbon dioxide, and EELV (all P < 0.05). Independent of age, there was a significant effect of sex on tidal volume (P < 0.05). There were no significant interaction effects between age and sex at peak exercise. On average, subjects in each group achieved respiratory exchange ratios >1.10 and near maximum heart rates based on predicted normal values, indicating that maximal effort was exerted across groups. There were no significant differences in the V̇o2-work rate slopes between groups on the basis of age or sex (Table 2; both P > 0.05).
Table 2.
Peak exercise data
| Older (n = 22) |
Younger (n = 22) |
||||
|---|---|---|---|---|---|
| Men (n = 10) | Women (n = 12) | Men (n = 11) | Women (n = 11) | P Value | |
| V̇o2, l/min | 2.63 ± 0.20 | 1.89 ± 0.12 | 3.91 ± 0.29 | 3.01 ± 0.15 | *†P < 0.05 |
| V̇o2, ml·kg−1·min−1 | 34.7 ± 2.0 | 30.6 ± 2.6 | 53.4 ± 3.1 | 51.7 ± 2.8 | *†P < 0.05 |
| V̇o2, %predicted | 119 ± 5 | 122 ± 6 | 123 ± 6 | 140 ± 7 | |
| V̇co2, l/min | 2.92 ± 0.20 | 2.23 ± 0.14 | 4.33 ± 0.27 | 3.34 ± 0.17 | *†P < 0.05 |
| RER | 1.12 ± 0.20 | 1.18 ± 0.02 | 1.12 ± 0.03 | 1.11 ± 0.02 | |
| HR, beats/min | 150 ± 5 | 153 ± 5 | 186 ± 3 | 185 ± 3 | *P < 0.05 |
| HR, %predicted | 100 ± 2.9 | 98 ± 3.2 | 96 ± 1.6 | 95 ± 1.8 | |
| SpO2, % | 98 ± 1 | 98 ± 1 | 97 ± 1 | 98 ± 1 | |
| VT, liters | 2.84 ± 0.18 | 1.95 ± 0.14 | 2.96 ± 0.17 | 2.19 ± 0.08 | †P < 0.05 |
| Fb, breaths/min | 39.5 ± 4.1 | 45.7 ± 3.2 | 51.5 ± 2.5 | 55.1 ± 3.0 | *P < 0.05 |
| V̇e, l/min | 108 ± 8 | 85 ± 4 | 151 ± 10 | 119 ± 5 | *†P < 0.05 |
| V̇e/V̇o2 | 42.4 ± 3.4 | 46.3 ± 2.8 | 39.7 ± 2.2 | 39.8 ± 1.2 | *P < 0.05 |
| V̇e/V̇co2 | 37.6 ± 2.4 | 39.1 ± 2.1 | 35.4 ± 1.7 | 35.9 ± 1.1 | *†P < 0.05 |
| , mmHg | 30.8 ± 0.9 | 32.2 ± 1.4 | 33.3 ± 3.8 | 31.6 ± 1.4 | |
| Work rate, W | 172 ± 10 | 140 ± 11 | 269 ± 21 | 222 ± 12 | *†P < 0.05 |
| V̇o2:work rate slope | 11.7 ± 0.7 | 10.8 ± 0.50 | 11.5 ± 0.3 | 11.2 ± 0.3 | |
| EELV, %TLC | 56 ± 3 | 56 ± 2 | 50 ± 2 | 51 ± 2 | *P < 0.05 |
| EILV, %TLC | 91 ± 1 | 92 ± 1 | 91 ± 2 | 91 ± 1 | |
| Wb, J/min | 257 ± 24 | 236 ± 28 | 335 ± 52 | 307 ± 39 | *†P < 0.05 |
| Resistive Wb, J/min | 76 ± 19 | 101 ± 20 | 61 ± 16 | 140 ± 33 | †P < 0.05 |
| Viscoelastic Wb, J/min | 181 ± 32 | 135 ± 23 | 274 ± 42 | 166 ± 22 | *P < 0.05 |
| V̇eCAP, l/min | 151.1 ± 13.2 | 118.4 ± 8.2 | 220.4 ± 9.1 | 181.1 ± 11.8 | *†P < 0.05 |
| V̇e/V̇eCAP, % | 73.2 ± 4.3 | 75.1 ± 4.9 | 69.6 ± 4.4 | 67.5 ± 3.7 | *P < 0.05 |
| Dyspnea, Borg scale | 4.6 ± 0.7 | 5.3 ± 0.6 | 6.0 ± 0.8 | 6.5 ± 0.9 | *P < 0.05 |
| Leg discomfort, Borg scale | 6.2 ± 0.9 | 6.3 ± 0.8 | 9.2 ± 0.8 | 8.8 ± 0.5 | *P < 0.05 |
All data are presented as means ± SE. V̇o2, oxygen uptake; V̇co2; carbon dioxide output; RER; respiratory exchange ratio; HR, heart rate; SpO2, oxygen saturation by pulse oximetry; VT, tidal volume; Fb, breathing frequency; V̇e, minute ventilation; V̇e/V̇o2, ventilatory equivalent for oxygen; V̇e/V̇co2, ventilatory equivalent for carbon dioxide; , end-tidal carbon dioxide; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; Wb, work of breathing; V̇eCAP, ventilatory capacity.
Older vs. younger subjects.
Men vs. women.
Summary of primary results.
Table 3 summarizes the primary results of the study, which are further described below and illustrated in Figs. 1–8. Variables relating to the ventilatory response to exercise, indexes of mechanical ventilatory constraint, and the perception of dyspnea were compared at absolute submaximal work rates and at relative work rates on the basis of sex, age, and their interaction. The main effects that reached statistical significance and, where appropriate, which interaction effects were statistically significant are highlighted.
Table 3.
Summary of main effects and interaction effects on primary outcome variables during exercise at absolute and relative work rates
| Absolute Work Rates |
Relative Work Rates |
|||||
|---|---|---|---|---|---|---|
| Age (P) | Sex (P) | Interaction (P) | Age (P) | Sex (P) | Interaction (P) | |
| Ventilatory response | ||||||
| V̇e, l/min | P < 0.05 | n.s. | — | n.s. | P < 0.05 | — |
| V̇e/V̇eCAP, % | P < 0.05 | n.s. | — | P < 0.05 | n.s. | — |
| Indexes of mechanical ventilatory constraint | ||||||
| EELV, % TLC | P < 0.05 | n.s. | — | P < 0.05 | n.s. | — |
| EILV, % TLC | P < 0.05 | n.s. | — | P < 0.05 | n.s. | — |
| Wb, J/min | P < 0.05 | P < 0.05 | n.s. | n.s. | n.s. | — |
| EFL | P < 0.05 | — | P < 0.05 | P < 0.05 | P < 0.05 | n.s. |
| Perceptual responses | ||||||
| Dyspnea, Borg scale | P < 0.05 | P < 0.05 | P < 0.05 | n.s. | n.s. | — |
VT, tidal volume; Fb, breathing frequency; V̇E, minute ventilation; V̇E/V̇CO2, ventilatory equivalent for carbon dioxide; V̇ECAP, ventilatory capacity; V̇E/V̇ECAP, fractional utilization of ventilatory capacity; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; Wb, work of breathing; EFL, expiratory flow limitation.
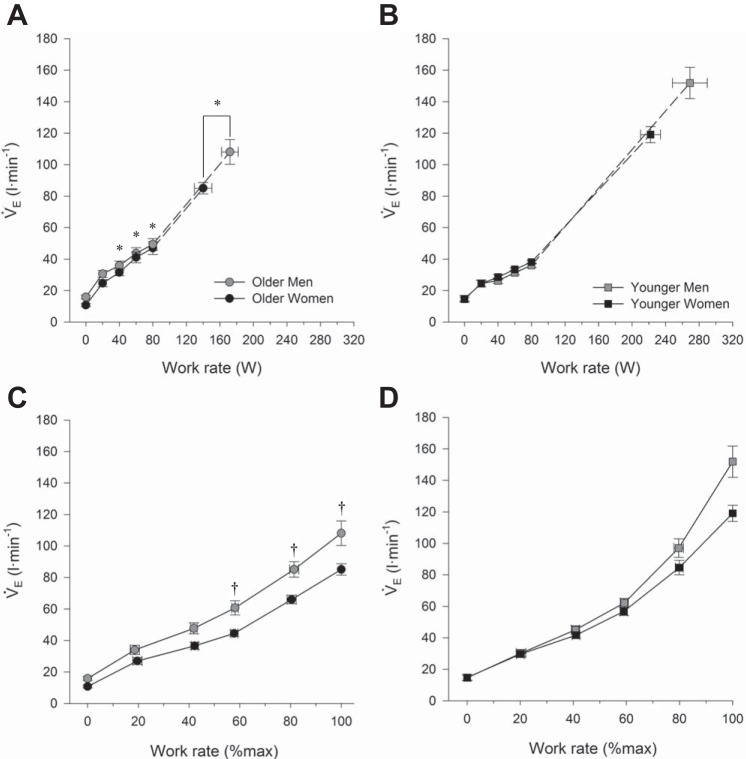
Fig. 1.
Ventilatory response to incremental cycle exercise at absolute and relative work rates in older men and women (A and C) as well as younger men and women (B and D). In C and D, the highest equivalent work rate achieved by all subjects was 80 W. Dashed lines within each group connect the 80 W data point to the peak exercise data point. All data are presented as means ± SE. V̇e, minute ventilation. *P < 0.05, main effect of age, comparisons made between all older and all younger subjects, regardless of sex. †P < 0.05, comparisons made between all men and all women, regardless of age. No significant interaction effect was observed.
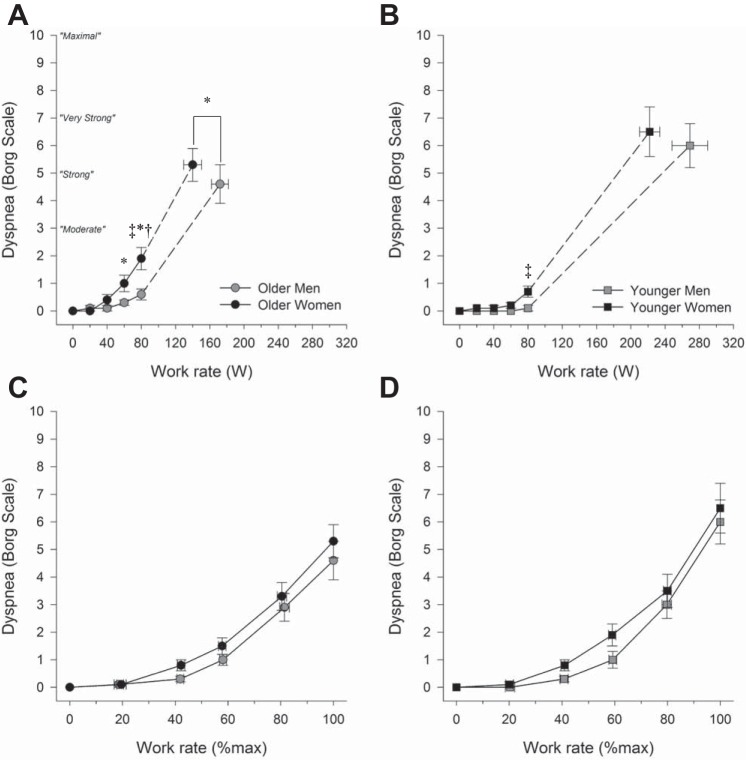
Fig. 8.
Dyspnea intensity responses to incremental cycle exercise at absolute and relative work rates in older men and women (A and C) as well as younger men and women (B and D). In C and D, the highest equivalent work rate achieved by all subjects was 80 W. Dashed lines within each group connect the 80 W data point to the peak exercise data point. All data are presented as means ± SE. *P < 0.05, main effect of age, comparisons made between all older and all younger subjects, regardless of sex. †P < 0.05, comparisons made between all men and all women, regardless of age. ‡P < 0.05, interaction effect between age and sex, men vs. women within each age group.
Ventilatory response to exercise.
Ventilatory responses to exercise are shown in Fig. 1. For a given submaximal absolute work rate ≥40 W, older subjects had a higher absolute V̇e than younger subjects, regardless of sex (all P < 0.05). Men had a higher absolute V̇e than women during exercise at a relative exercise intensity ≥40% of peak work rate, regardless of age (all P < 0.05). There was no significant interaction effect between age and sex on absolute V̇e at rest or during exercise at any absolute or relative work rate (both P > 0.05).
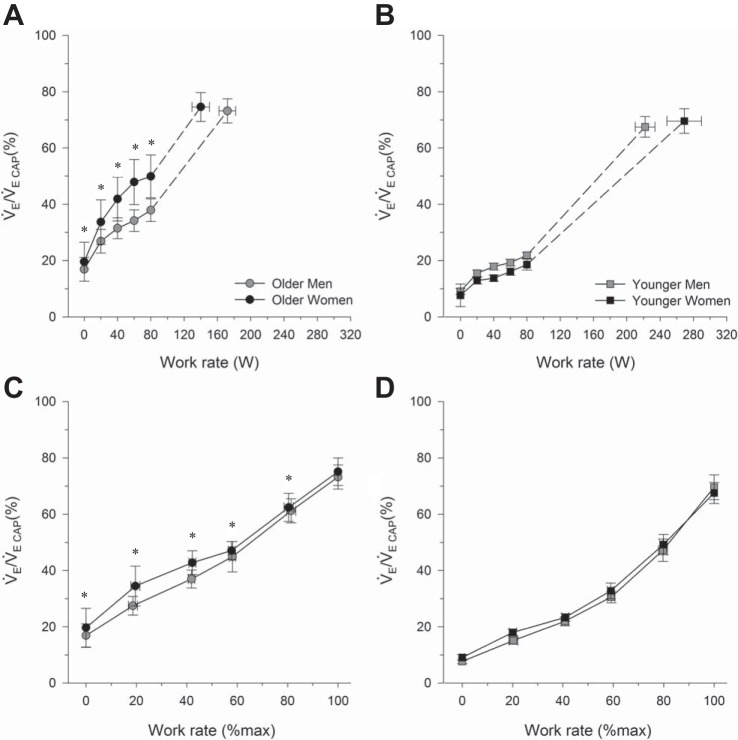
Fractional utilization of V̇ECAP at rest and during exercise are shown in Fig. 2. Older subjects had a significantly higher V̇e/V̇eCAP at rest and throughout submaximal exercise at an absolute work rate (all P < 0.05). The effect of age on V̇e/V̇eCAP was still evident when comparisons were made at relative exercise intensities (all P < 0.05). There was no effect of sex on V̇e/V̇eCAP at rest or during exercise (P > 0.05), and there was no significant interaction effect between age and sex on V̇e/V̇eCAP (P > 0.05).
Fig. 2.
Fractional utilization of ventilatory capacity during incremental cycle exercise at absolute and relative work rates in older men and women (A and C) as well as younger men and women (B and D). In C and D, the highest equivalent work rate achieved by all subjects was 80 W. Dashed lines within each group connect the 80 W data point to the peak exercise data point. All data are presented as means ± SE. V̇e/V̇eCAP, fractional utilization of ventilatory capacity. *P < 0.05, main effect of age, comparisons made between all older and all younger subjects, regardless of sex. No significant interaction effect was observed.
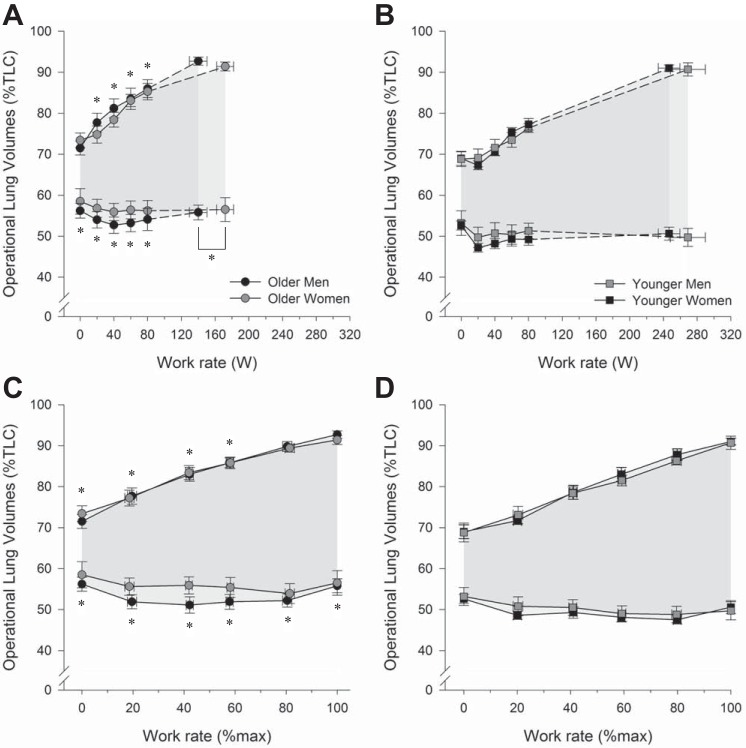
Operating lung volumes at rest and during exercise are shown in Fig. 3. During exercise at a given submaximal absolute work rate, there were no significant differences in EELV or EILV on the basis of sex (both P > 0.05); however, EELV and EILV were both higher in older than in younger subjects (all P < 0.05). The effects of age on relative EELV and EILV were also present at rest and throughout submaximal exercise when comparisons were made at relative exercise intensities (all P < 0.05). Moreover, at peak exercise, older subjects had a higher EELV but a similar EILV than younger subjects (P < 0.05). There was no significant interaction effect between age and sex on EELV or EILV (both P > 0.05).
Fig. 3.
Operating lung volumes during exercise incremental cycle exercise at absolute and relative work rates in older men and women (A and C) as well as younger men and women (B and D). In C and D, the highest equivalent work rate achieved by all subjects was 80 W. Dashed lines within each group connect the 80 W data point to the peak exercise data point. All data are presented as mean ± SE. TLC, total lung capacity. *P < 0.05, main effect of age, comparisons made between all older and all younger subjects, regardless of sex. No significant interaction effect was observed.
Work of breathing.
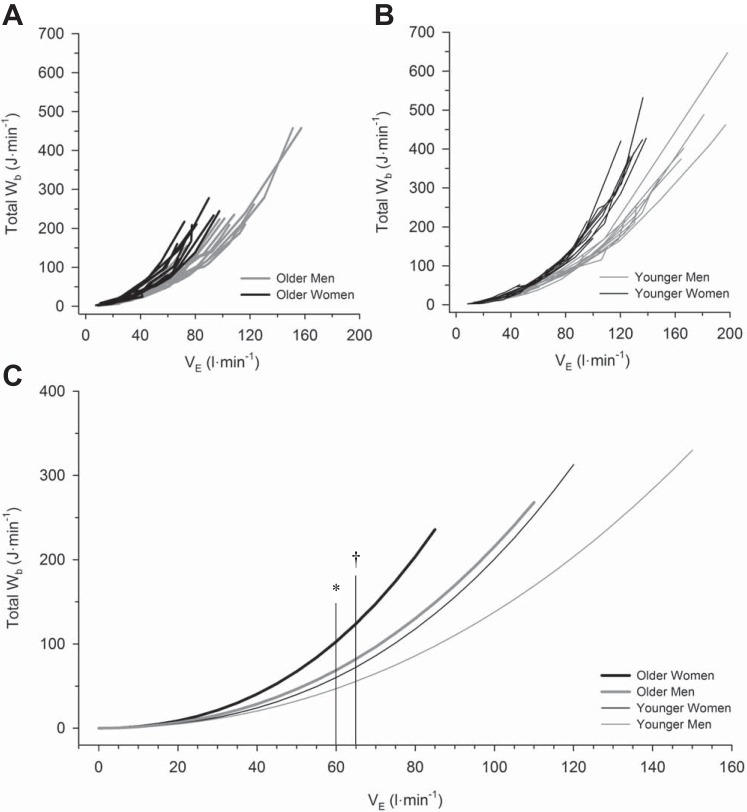
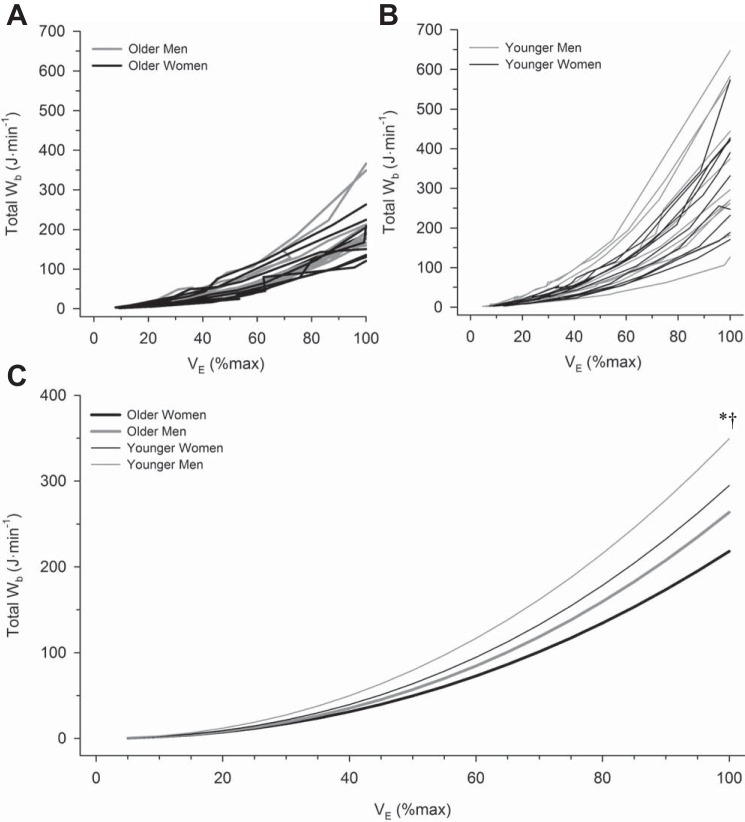
Individual subject values for the Wb are plotted as a function of absolute V̇e in Fig. 4, A and B, and as a function of relative V̇e in Fig. 5, A and B. Each subject’s Wb-V̇e curve was fit to Eq. 1, and without exception there was excellent fit (mean r2: 0.99 ± 0.01). Then, by pooling each individual’s constant a and constant b from Eq. 1, a mean curve was constructed for each group (Figs. 4C and 5C). There were significant main effects of V̇e, sex, and age on Wb (all P < 0.001). There was a significant interaction between V̇e and sex, as well as V̇e and age (both P < 0.001), but no significant interaction effect among V̇e, sex, and age (P > 0.05). Wb was significantly higher in women at and above a V̇e of 65 l/min (P < 0.001) and significantly higher in older subjects at and above a V̇e of 60 l/min (P < 0.001). When Wb was compared at relative fractions of peak exercise V̇e, there was no significant effect of age, sex, or their interaction at any fraction of maximal V̇e below peak exercise (Fig. 5C; both P > 0.05). However, at peak exercise, men had a significantly higher Wb than women, and older subjects had a significantly lower Wb than younger subjects (both P < 0.05, Table 2).
Fig. 4.
The relationship between work of breathing and absolute minute ventilation during incremental cycle exercise. Individual curves of the work of breathing vs. absolute minute ventilation in older men and women (A) and younger men and women (B). Mean curves relating work of breathing to minute ventilation in all groups are shown in C. All mean curves are based on mean values of constants a and b from Eq. 1, and each curve has been extrapolated to the average peak minute ventilation within each group. Data for older men are displayed as thick gray lines, while data for older women are displayed as thick black lines. Data for younger men are displayed as thin gray lines, while data for older women are displayed as thin black lines. Wb, work of breathing; V̇e, minute ventilation. *P < 0.05, main effect of age, comparisons made between all older and all younger subjects, regardless of sex. †P < 0.05, comparisons made between all men and all women, regardless of age. No significant interaction effect was observed.
Fig. 5.
The relationship between work of breathing and relative minute ventilation during incremental cycle exercise. Individual curves of the work of breathing vs. minute ventilation in older men and women (A) and younger men and women (B). Mean curves relating work of breathing to relative minute ventilation in all groups are shown in C. All mean curves are based on mean values of constants a and b from Eq. 1. Data for older men are displayed as thick gray lines, while data for older women are displayed as thick black lines. Data for younger men are displayed as thin gray lines, while data for older women are displayed as thin black lines. Wb, work of breathing; V̇e, minute ventilation. *P < 0.05, main effect of age, comparisons made between all older and all younger subjects, regardless of sex. †P < 0.05, comparisons made between all men and all women, regardless of age. No significant interaction effect was observed.
Expiratory flow limitation.
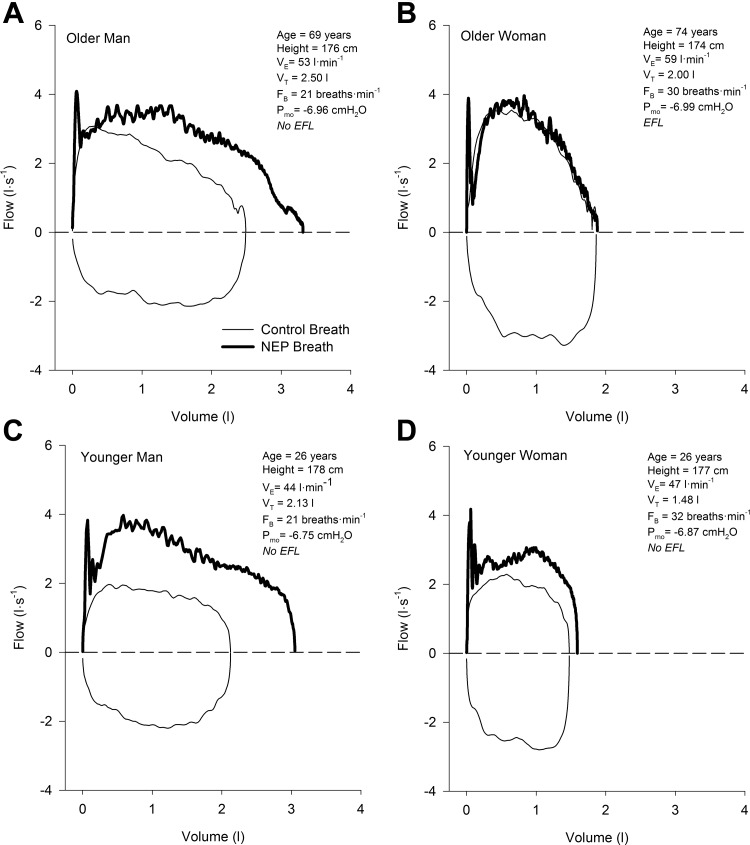
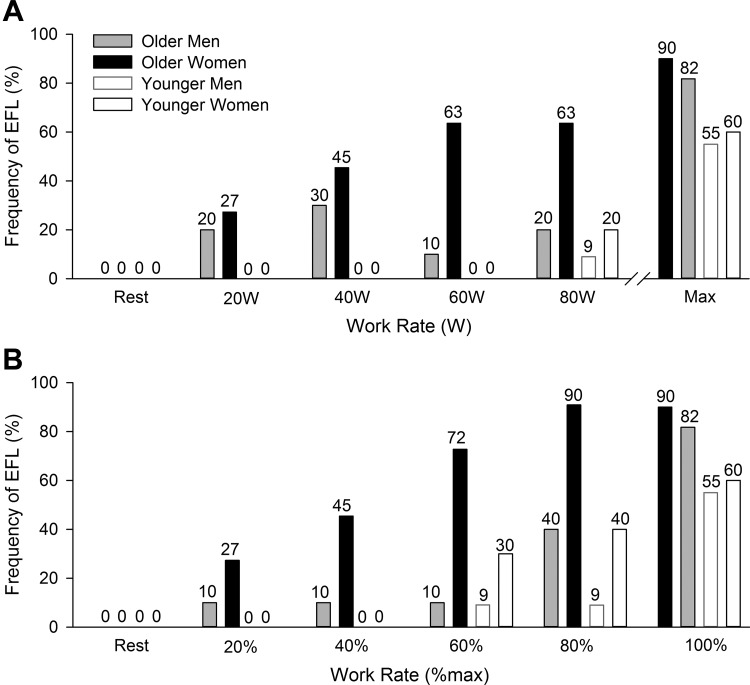
Examples of flow-volume loops in four subjects (1 representative sample from each group) used to determine the presence of EFL using the NEP technique are shown in Fig. 6. Successful NEP maneuvers were obtained at rest and at each exercise stage in all but two subjects (n = 1 older woman, and n = 1 younger woman) whose data were excluded from the analysis since the application of the NEP caused a sustained decrease in expiratory flow relative to the control breath. The frequency of EFL in each group at rest and throughout exercise is shown in Fig. 7. No subjects had EFL at rest, but as exercise intensity increased the fraction of subjects who had EFL increased progressively. Based on our model, there was a significant main effect of age, as well as a significant interaction effect between age and sex on EFL during exercise when comparisons were made at absolute work rates (both P < 0.05). When the analysis was repeated at relative exercise intensities, there were significant main effects of age and sex (both P < 0.05); however, there was no significant interaction effect between age and sex (P = 0.39).
Fig. 6.
Tidal flow-volume loops at a fixed work rate of 100 W in 4 individual subjects closely matched for height: an older man (A), an older woman (B), a younger man (C), and a younger woman (D). Thin black lines represent the control breath and thick black lines represent the negative expiratory pressure breath. All data are raw traces. VT, tidal volume; FB, breathing frequency; Pmo, mouth pressure; NEP, negative expiratory pressure; EFL, expiratory flow limitation.
Fig. 7.
Frequency of EFL at rest and during exercise at absolute (A) and relative (B) work rates. Older men are shown in filled gray bars, older women in filled black bars, younger men open black bars, and younger women open gray bars. In A, the highest equivalent work rate achieved by all subjects was 80 W.
Dyspnea.
Figure 8 shows dyspnea intensity ratings in all groups at rest and during exercise. There were significant effects of age and sex, as well as their interaction on dyspnea during exercise at absolute exercise intensities (all P < 0.05). Specifically, women reported higher levels of dyspnea than men at 80 W regardless of age (P < 0.05), and older subjects reported higher levels of dyspnea at 60 and 80 W, regardless of age (P < 0.05). At an absolute exercise intensity of 80 W, the differences in dyspnea between younger men and younger women were subtle, albeit significant (0.1 ± 0.1 vs. 0.7 ± 0.2, P < 0.001). By contrast, older women reported significantly higher dyspnea at 80 W than older men by 1.3 units on the Borg scale (0.6 ± 0.2 vs. 1.9 ± 0.4, P < 0.001). When dyspnea was compared between groups at relative exercise intensities, there was no significant effect of age, sex, or their interaction (all P > 0.05).
We also found that dyspnea/V̇e slopes showed a significant effect of age (0.093 vs. 0.065 Borg units·l−1·min−1, P < 0.05) and sex (0.092 ± 0.006 vs. 0.064 ± 0.010 Borg units·l−1·min−1, P < 0.05) but not their interaction (P = 0.52). Finally, correlates of dyspnea intensity at a standardized absolute work rate of 80 W are shown in Table 4. The four strongest correlates of dyspnea intensity at 80 W were Wb, V̇e/V̇eCAP, breathing frequency, and V̇e.
Table 4.
Correlates of dyspnea at a standardized absolute work rate during exercise
| Variable | r2 | P Value |
|---|---|---|
| V̇e, l/min | 0.54* | <0.001 |
| V̇e/V̇eCAP, % | 0.56* | <0.001 |
| VT, l | −0.43 | 0.004 |
| VT, %IC | 0.14 | 0.371 |
| VT, %VC | 0.05 | 0.760 |
| FB, breaths/min | 0.69* | <0.001 |
| SpO2, % | −0.34* | 0.024 |
| , mmHg | −0.32* | <0.001 |
| EELV, %TLC | 0.33* | 0.032 |
| IRV, %TLC | −0.23* | 0.014 |
| IC, liter | −0.47* | <0.001 |
| IC, %predicted | −0.01 | 0.792 |
| ΔIC (exercise – rest), liter | −0.03 | 0.315 |
| Wb, J/min | 0.76* | <0.001 |
V̇e, expired minute ventilation; V̇e/V̇eCAP, fractional utilization of ventilatory capacity; VT, tidal volume; IC, inspiratory capacity; VC, vital capacity; Fb, breathing frequency; SpO2, arterial oxygen saturation by pulse oximetry; , end-tidal partial pressure of carbon dioxide; EELV, end-expiratory lung volume; TLC, total lung capacity; IRV, inspiratory reserve volume; Wb, work of breathing.
P < 0.05.
DISCUSSION
Major findings.
We assessed the effects of age and sex on the mechanical ventilatory and perceptual responses to exercise in healthy younger and older, men and women. Our major findings are fivefold. First, women have a higher Wb for a given absolute V̇e ≥65 l/min during exercise compared with men, regardless of age. However, Wb is similar between the sexes for a given relative V̇e during submaximal exercise. Second, older subjects breathe at higher lung volumes during exercise at a given submaximal absolute or relative exercise intensity but do not differ on the basis sex. Third, we observed a significant interaction effect between age and sex on the likelihood of developing EFL during exercise at an absolute work rate. We found that older subjects are more likely to develop EFL than younger subjects and that older women have a higher propensity toward EFL than older men. During exercise at a given relative work rate, older subjects and women are more likely to experience EFL than younger subjects and men, respectively. Fourth, age and sex exert an interactive effect on the perception of dyspnea during exercise at a given absolute work rate ≥80 W. Older women, and to a lesser extent younger women, report higher levels of dyspnea than older men and younger men, respectively. However, the effects of age and sex on the perception of dyspnea are absent at relative exercise intensities. Finally, dyspnea during submaximal exercise is associated with indexes of mechanical ventilatory constraint. Collectively, our findings suggest that age and sex only interactively affect the propensity toward EFL and the perception of dyspnea during exercise when comparisons between groups are made at absolute exercise intensities.
Maximum ventilatory capacity and ventilatory response to exercise.
The primary age-related change to the respiratory system that contributes to decreasing pulmonary function is thought to be the progressive reduction in elastic recoil pressure of the lung (20). As the elastic recoil pressure of the lung decreases, so too does the ability to generate expired flow, thereby reducing maximum ventilatory capacity (54). As expected, older subjects had a significantly lower Pst 100%TLC and Pst 50%VC than younger subjects (Table 1). It follows that despite having similar total lung capacities, older subjects had a reduced capacity to generate expiratory flow, as evidenced by significantly lower mid-expiratory flows than younger subjects (Table 1). Accordingly, we observed a significant linear correlation between Pst 50%TLC and FEF50%. However, it should be noted that we did not detect statistically significant differences in Pst 50%VC, Pst 100%TLC, FEF50%, or FEF25–75% on the basis of sex, indicating that the effect of age on static recoil and expiratory flows was similar between men and women. While the effect of aging on the static recoil pressure of the lung is well characterized (52), evidence of sex differences in static recoil pressure of the lung remains equivocal (9, 20). Overall, the age-related decline in ventilatory capacity observed in our study resulted in a reduction in the available reserve for accommodating increases in ventilatory demand, as evidenced by a significantly lower absolute V̇eCAP at rest and throughout exercise in the older relative to the younger subjects (data not shown). Moreover, due to their relatively smaller lungs, women had a lower absolute V̇eCAP than men at rest and throughout exercise, and this relationship was unaffected by age (data not shown).
During exercise, older subjects had a higher V̇e for a given absolute work rate above 20 W than younger subjects (Fig. 1, C and D), a finding that is in agreement with previous work (47). Given the age-related decline in V̇eCAP, the higher ventilatory response to exercise in older individuals increases the likelihood of reaching the mechanical limits of the respiratory system at a given absolute work rate. Indeed, older subjects utilized a greater fraction of their available ventilatory capacity (V̇e/V̇eCAP) at rest and during submaximal exercise at an absolute work rate than younger subjects (Fig. 2). When comparisons were made at relative exercise intensities, older subjects still had a higher V̇e/V̇eCAP than younger subjects during submaximal exercise (Fig. 2, A and B).
Operating lung volumes.
During incremental exercise, younger subjects reduced EELV below functional residual capacity and increased EILV up to ~90% of total lung capacity (Fig. 4D). In older subjects, the age-related reduction in vital capacity and expiratory flows results in operating lung volumes that are shifted to higher fractions of total lung capacity (46). Compared with younger subjects, we found that older subjects had a higher EELV throughout exercise by 4.9 ± 1.5% of total lung capacity and a higher EILV by 4.7 ± 1.7% of total lung capacity during submaximal exercise (Fig. 3, A and B). These age-related increases in EELV and EIL likely alter the length tension relationship of the respiratory muscles and encroach on inspiratory reserve volume. Furthermore, older subjects decreased EELV during exercise remained below resting EELV. Like their younger counterparts, most older subjects reduced EELV during exercise until they approach EFL, at which point EELV may begin to increase back toward resting EELV to avoid excessive mechanical constraint (30). In some cases, EELV continues to increase to the extent where it exceeds resting EELV, a phenomenon known as dynamic hyperinflation (3). We observed that 7 of 22 (n = 3 men, n = 4 women) older subjects but none of the younger subjects showed evidence of dynamic hyperinflation at maximal exercise, which we defined as an increase in EELV >0.15l above resting EELV. Although EILV was higher in the older subjects than the younger subjects during submaximal exercise, EILV was similar between age groups at maximal exercise. The fact that, regardless of age, the highest EILV reached during exercise was ~90% of total lung capacity is likely due to the sigmoidal shape of the pressure-volume relationship of the respiratory system, whereby any further increase in EILV would substantially increase Wb. Overall, it appears that older individuals regulate their operating lungs volumes during exercise in a similar manner to younger individuals but at a higher fraction of total lung capacity. However, the increase in EILV is constrained due to the age-related reduction in inspiratory reserve volume.
It can be argued that because women have smaller lungs and lower maximum expired flows than men that they have a tendency to breathe at a higher EELV and EILV during exercise. The effect of sex on operating lung volumes has been assessed in several studies, but the results are conflicting (10, 12, 13, 15, 26, 49). In the current study, we did not observe a systematic effect of sex on operating lung volumes when EELV and EILV were expressed as a fraction of total lung capacity. While it is tempting to hypothesize that women are more likely to increase EELV and/or EILV during exercise to avoid EFL, we believe that this is an oversimplification. Although EFL has been shown to increase operating lung volumes under experimental conditions (48), the fact that an individual exhibits EFL does not guarantee that operating lung volumes will increase. For example, it is possible that in the presence of EFL, some individuals preserve relatively low lung volumes to avoid breathing on the flat portion of the pressure-volume relationship of the respiratory system.
Work of breathing.
The mechanical and metabolic cost of maintaining adequate alveolar ventilation during exercise can be substantial and increase exponentially as a function of V̇e (16). Since healthy aging causes a decrease in the compliance of the chest wall (43), and a reduction in airway diameter (45), it would be expected that Wb for a given V̇e would be higher in older relative to younger individuals. We demonstrated that for a given absolute V̇e ≥60 l/min older subjects have a significantly higher Wb than younger subjects (Fig. 4). The relationship between Wb and V̇e during exercise has previously been assessed in highly trained older men (31) and highly trained younger men (32). However, only one study has investigated the effect of age on Wb during exercise within the same study (8). They found that older individuals had a higher Wb than younger individuals during exercise at an absolute V̇o2 of 1.5 l/min as well as at 40 and 60% of cardiac reserve. However, they did not normalize Wb for V̇e, and only included male participants. Thus our study is the first to show that Wb is higher for a given V̇e in older men and women by directly comparing them to younger men and women.
We have previously shown that for a given V̇e above ~55–65 l/min, Wb (15, 27) and respiratory muscle V̇o2 (16) are higher in young women relative to young men. In the present study, we also demonstrate that for a given V̇e ≥65 l/min, women have a significantly higher Wb than men (Fig. 5). Importantly, the effect of sex on Wb appears to be independent of the effect of age. This finding is in keeping with previous work showing that older women have a higher V̇o2 of the respiratory muscles during exercise than older men (51).
When we compared Wb as a function of relative V̇e, there was no significant effect of age, sex, or their interaction on Wb at any submaximal fraction of peak V̇e (Fig. 5). However, it is important to contextualize this relative comparison. If one considers that activities of daily living are performed at similar rates of relative oxygen consumption (i.e., metabolic equivalents) and that age and sex both affect maximal relative V̇o2 (34), it can be surmised that women and older individuals would have to dedicate a higher fraction of whole body V̇o2 to their respiratory muscles to accomplish a given task than men and younger individuals, respectively.
Expiratory flow limitation.
It is well known that older individuals are predisposed to EFL during exercise due to their reduced ventilatory capacity and increased ventilatory response to exercise (12, 29). In the present study, we found that healthy aging had a significant effect on EFL during exercise at absolute work rates (Fig. 7A). We also observed that older women have a higher propensity toward developing EFL than older men but that this apparent sex difference was not present in the younger subjects. We attribute our findings to the interactive influences of healthy aging and biological sex on the structure and function of the respiratory system. When ventilatory demand approaches maximum ventilatory capacity, small sex differences in airway anatomy may play a crucial role in determining the extent of mechanical ventilatory limitation. A corollary to this finding can be drawn from previous work in young endurance-trained athletes, where the maximum capacity of the respiratory system is high but so too is the ventilatory demand associated with the intensity of exercise they are capable of achieving (27). In the context of high ventilatory capacity and high ventilatory demand, the relatively small sex differences in the structure of the respiratory system become important and likely predispose women to EFL. We have shown that young endurance-trained women have a higher propensity toward EFL at maximal exercise than young endurance-trained men (27). While we found a main effect of sex on EFL in the present study, the differences between men and women were only apparent in the older relative to the younger group (Fig. 7), a finding that is in keeping with our previous work in younger recreationally active subjects (15). Based on previous studies (27, 40), it can be argued that young women may be more susceptible to EFL during exercise than young men. However, the factors that determine EFL are complex and multifactorial (14). While differences in lung and airway anatomy play an important contributory role, other factors may supersede these relatively small differences. By contrast, given the reduced ventilatory capacity and increased ventilatory response to exercise, sex differences in respiratory system anatomy are likely an important determinant of EFL in older individuals.
We also observed main effects of age and sex on EFL when comparisons were made at relative exercise intensities (Fig. 7B). However, there was no significant interaction effect between age and sex on EFL at relative exercise intensities. The fact that we observed a greater fraction of older subjects who were flow limited at relative exercise intensities than younger subjects likely reflects the age-associated increase in V̇e/V̇eCAP during exercise (Fig. 2, C and D). By contrast, our observation of a greater fraction of women who experienced EFL at relative exercise intensities than men is perplexing. One would expect that after normalizing for exercise intensity that the effect of sex on EFL would no longer be present. A possible explanation relates to sex differences in airways size and the associated effects on the capacity to generate expired flow (41). However, we did not observe an effect of sex on V̇e/V̇eCAP in women relative to men. Alternatively, since younger women and younger men have a qualitatively similar frequency of EFL during exercise at a given relative intensity, it is possible that the main effect of sex on EFL at relative exercise intensities was driven by the older women. However, given our relatively small sample size, we hesitate to draw definitive conclusions concerning this point. Future studies involving large pools of subjects are required to determine whether women are indeed more likely to experience EFL than men at a given relative exercise intensity.
Dyspnea.
We found that there were significant main effects of age and sex, as well as their interaction on dyspnea at absolute work rates during exercise. Older subjects reported higher dyspnea during submaximal exercise than their younger counterparts (Fig. 8), and women reported significantly higher dyspnea during submaximal exercise than men. The difference in dyspnea between men and women at 80 W was more pronounced in the older subjects. Ofir et al. (46) found that older women reported a higher intensity of dyspnea at a standardized V̇o2 of 20 ml·kg−1·min−1 than older men by ~1 units on the Borg scale. We observed a remarkably similar finding with older women reporting dyspnea ratings that were 1.3 units on the Borg scale higher (1.9 ± 0.4 vs. 0.6 ± 0.2) than older men at 80 W, which corresponded to a relative V̇o2 of 20.5 ± 1.0 and 21.3 ± 0.9 ml·kg−1·min−1 in older men and older women, respectively. Although the perception of dyspnea in older subjects was significantly higher for any given absolute work rate >40 W than in younger subjects, the degree of dyspnea in older subjects was relatively modest throughout exercise and only reached an average of 5.0 ± 0.5 units on the Borg scale at maximal exercise. The relatively low dyspnea ratings observed in the healthy older subjects in our study is in close agreement with previous investigations using incremental cycle exercise (23, 46) and further supports the notion that the respiratory system is unlikely to be the primary locus of exercise limitation in healthy older individuals. In the younger subjects, women also reported a significantly higher intensity of dyspnea than men at 80 W; however, the difference was only to the order of 0.6 units on the Borg scale (0.7 ± 0.2 vs. 0.1 ± 0.1). At maximal exercise, dyspnea ratings were slightly but significantly lower in the older subjects than in the younger subjects, which we attribute to differences in absolute V̇e (Table 2). The effects of age and sex on the perception of dyspnea during exercise were not present when comparisons were made at relative exercise intensities, a finding that is in keeping with previous work (35).
In light of the observed differences in the mechanical ventilatory response to exercise described herein, it stands to reason that respiratory mechanics may explain, at least in part, the age and sex differences in the perception of respiratory sensation observed at absolute exercise intensities. Across all subjects, Wb, breathing frequency, V̇e/V̇eCAP, and V̇e were the strongest correlates of dyspnea at 80 W, each explaining >50% of the variance in dyspnea (Table 4). We speculate that at 80 W those with the highest indexes of mechanical constraint (Wb and V̇e/V̇eCAP) have the highest sensations of dyspnea. In addition, those who had the highest V̇e response and dead-space ventilation (due to the high breathing frequency) also had higher sensations of dyspnea. We emphasize that we are cognizant of the limits of correlative evidence and of the multifactorial causes of dyspnea. In the absence of experimental manipulations, we hesitate to overstate the link between mechanical ventilatory constraint and dyspnea nor argue the primacy of mechanical ventilatory constraint over other factors that cause dyspnea within the context of the present study. Instead, our findings present a hypothesis that awaits experimental testing.
Perspectives on absolute and relative comparisons.
A consistent problem when conducting studies to investigate age or sex differences in the pulmonary physiology of exercise concerns how to most appropriately compare groups. The principal issue revolves around whether to make comparisons in absolute or relative terms. On one hand, making comparisons in absolute terms allows for the assessment of the effects of sex and age but ignores the potential confounding effects of body size and functional capacity. On the other hand, making comparisons in relative terms accounts for differences in body size and functional capacity but potentially obscures important sex differences and overlooks the physical and metabolic requirements of a given task. In the present study, we made comparisons at both absolute and relative work rates or ventilations since both are required to truly determine the influences of age and sex. However, our approach results in a large number of permutations and contributes to interpretive complexities. Thus we offer the following perspectives when interpreting our findings. First, we emphasize that the results of absolute and relative comparisons each have inherent caveats and one should not be favored at the expense the other. Second, in some instances we found some differences between groups when comparisons were made in absolute terms that were absent in relative terms. It follows that the generalizability of our findings will depend on context. For example, if one considers our findings relating to the effects of age and sex on the perception of dyspnea, the confounding influence exercise intensity is of critical importance.
Limitations.
While our study reveals novel findings regarding the mechanical ventilatory and perceptual responses to exercise in older and younger men and women, two important limitations must be considered. First, our measure of Wb (integrated esophageal pressure-volume loops) does not take into account other components of ventilatory work, such as chest-wall distortion and abdominal stabilization (22). Given the age-related changes to the mechanics of the respiratory system, it is possible that age-related differences in chest wall distortion exist and could impact total ventilatory work. However, without measures of respiratory system kinematics or estimates of respiratory muscle V̇o2, this limitation cannot be overcome. Second, EFL can be assessed using a variety of different methods. Second, we chose to use the NEP technique given its numerous advantages (37). However, it should be noted that the NEP technique provides an assessment of EFL at a single point in time rather than a continuous measure or one that is averaged over a longer period of time. It follows that our measures of the frequency of EFL do not represent the entirety of each exercise stage or the dynamic nature of EFL during exercise (3). However, given that the technique was applied consistently within each subject and each group, it is unlikely that this limitation affected the overall results of our study.
Conclusions.
We found that during exercise, age and sex have significant impacts on Wb, operating lung volumes, and EFL. Our results suggest that superimposing the normal age-related changes in respiratory structure and function on innate sex differences in airway anatomy appears to have a significant effect on the mechanical ventilatory responses to exercise in older individuals. Our data also suggest that age and sex affect the perception of dyspnea for a given absolute, but not relative, exercise intensity and that the magnitude of mechanical ventilatory constraint seems to play an important contributory role. However, experimental manipulations of respiratory mechanics are required to directly test this hypothesis. Overall, our study provides new insight into the complexities and interactive effects of biological sex and chronological age on the integrative response to exercise in healthy adults.
GRANTS
This study was supported by the Natural Science and Engineering Research Council of Canada (NSERC) and the British Columbia Lung Association (BCLA). Y. Molgat-Seon, P. B. Dominelli, and A. H. Ramsook were supported by graduate scholarships from NSERC. P. B. Dominelli and M. R. Schaeffer were supported by fellowships from the University of British Columbia and BCLA. J. A. Guenette was supported by a Scholar Award from the Michael Smith Foundation for Health Research, a New Investigator Award from the Providence Health Care Research Institute and St. Paul’s Hospital Foundation, and a Canadian Institutes of Health Research Clinical Rehabilitation New Investigator Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.M.-S., G.E.F., L.M.R., J.D.R., J.A.G., and A.W.S. conceived and designed research; Y.M.-S., P.B.D., A.H.R., M.R.S., and S.M.S. performed experiments; Y.M.-S., P.B.D., G.E.F., and A.W.S. analyzed data; Y.M.-S., P.B.D., A.H.R., M.R.S., S.M.S., G.E.F., L.M.R., J.D.R., J.A.G., and A.W.S. interpreted results of experiments; Y.M.-S., P.B.D., A.H.R., M.R.S., L.M.R., J.D.R., J.A.G., and A.W.S. prepared figures; Y.M.-S., P.B.D., A.H.R., M.R.S., S.M.S., G.E.F., L.M.R., J.D.R., J.A.G., and A.W.S. drafted manuscript; Y.M.-S., P.B.D., A.H.R., M.R.S., S.M.S., G.E.F., L.M.R., J.D.R., J.A.G., and A.W.S. edited and revised manuscript; Y.M.-S., P.B.D., A.H.R., M.R.S., S.M.S., G.E.F., L.M.R., J.D.R., J.A.G., and A.W.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our subjects for enthusiastic participation.
REFERENCES
- 1.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol (1985) 82: 746–754, 1997. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 2.Babb TG. Mechanical ventilatory constraints in aging, lung disease, and obesity: perspectives and brief review. Med Sci Sports Exerc 31, Suppl: S12–S22, 1999. doi: 10.1097/00005768-199901001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Babb TG. Exercise ventilatory limitation: the role of expiratory flow limitation. Exerc Sport Sci Rev 41: 11–18, 2013. doi: 10.1097/JES.0b013e318267c0d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982. [DOI] [PubMed] [Google Scholar]
- 5.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 99: 696–702, 1969. [DOI] [PubMed] [Google Scholar]
- 6.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Burrows B, Kasik JE, Niden AH, Barclay WR. Clinical usefulness of the single-breath pulmonucy diffusing capacity test. Am Rev Respir Dis 84: 789–806, 1961. [DOI] [PubMed] [Google Scholar]
- 8.Chaunchaiyakul R, Groeller H, Clarke JR, Taylor NA. The impact of aging and habitual physical activity on static respiratory work at rest and during exercise. Am J Physiol Lung Cell Mol Physiol 287: L1098–L1106, 2004. doi: 10.1152/ajplung.00399.2003. [DOI] [PubMed] [Google Scholar]
- 9.Colebatch HJ, Greaves IA, Ng CK. Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol Respir Environ Exerc Physiol 47: 683–691, 1979. [DOI] [PubMed] [Google Scholar]
- 10.Cory JM, Schaeffer MR, Wilkie SS, Ramsook AH, Puyat JH, Arbour B, Basran R, Lam M, Les C, MacDonald B, Jensen D, Guenette JA. Sex differences in the intensity and qualitative dimensions of exertional dyspnea in physically active young adults. J Appl Physiol (1985) 119: 998–1006, 2015. doi: 10.1152/japplphysiol.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir 18: 419–425, 1982. [PubMed] [Google Scholar]
- 12.DeLorey DS, Babb TG. Progressive mechanical ventilatory constraints with aging. Am J Respir Crit Care Med 160: 169–177, 1999. doi: 10.1164/ajrccm.160.1.9807045. [DOI] [PubMed] [Google Scholar]
- 13.Deruelle F, Nourry C, Mucci P, Bart F, Grosbois JM, Lensel GH, Fabre C. Difference in breathing strategies during exercise between trained elderly men and women. Scand J Med Sci Sports 18: 213–220, 2008. doi: 10.1111/j.1600-0838.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 14.Dominelli PB, Guenette JA, Wilkie SS, Foster GE, Sheel AW. Determinants of expiratory flow limitation in healthy women during exercise. Med Sci Sports Exerc 43: 1666–1674, 2011. doi: 10.1249/MSS.0b013e318214679d. [DOI] [PubMed] [Google Scholar]
- 15.Dominelli PB, Molgat-Seon Y, Bingham D, Swartz PM, Road JD, Foster GE, Sheel AW. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol (1985) 119: 1105–1113, 2015. doi: 10.1152/japplphysiol.00409.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM, Sheel AW. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593: 1965–1979, 2015. doi: 10.1113/jphysiol.2014.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominelli PB, Sheel AW. Experimental approaches to the study of the mechanics of breathing during exercise. Respir Physiol Neurobiol 180: 147–161, 2012. doi: 10.1016/j.resp.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 18.el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 61: 896–905, 1986. doi: 10.1152/jappl.1986.61.3.896. [DOI] [PubMed] [Google Scholar]
- 19.Fowler RW, Pluck RA, Hetzel MR. Maximal expiratory flow-volume curves in Londoners aged 60 years and over. Thorax 42: 173–182, 1987. doi: 10.1136/thx.42.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson GJ, Pride NB. Lung distensibility. The static pressure-volume curve of the lungs and its use in clinical assessment. Br J Dis Chest 70: 143–184, 1976. doi: 10.1016/0007-0971(76)90027-9. [DOI] [PubMed] [Google Scholar]
- 21.Green M, Road J, Sieck GC, Similowski T. Tests of respiratory muscle strength. Am J Respir Crit Care Med 166: 528–547, 2002. [Google Scholar]
- 22.Grimby G, Bunn J, Mead J. Relative contribution of rib cage and abdomen to ventilation during exercise. J Appl Physiol 24: 159–166, 1968. doi: 10.1152/jappl.1968.24.2.159. [DOI] [PubMed] [Google Scholar]
- 23.Guenette JA, Chin RC, Cheng S, Dominelli PB, Raghavan N, Webb KA, Neder JA, O’Donnell DE. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J 44: 1177–1187, 2014. doi: 10.1183/09031936.00034714. [DOI] [PubMed] [Google Scholar]
- 24.Guenette JA, Chin RC, Cory JM, Webb KA, O’Donnell DE. Inspiratory capacity during exercise: measurement, analysis, and interpretation. Pulm Med 2013: 956081, 2013. doi: 10.1155/2013/956081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guenette JA, Dominelli PB, Reeve SS, Durkin CM, Eves ND, Sheel AW. Effect of thoracic gas compression and bronchodilation on the assessment of expiratory flow limitation during exercise in healthy humans. Respir Physiol Neurobiol 170: 279–286, 2010. doi: 10.1016/j.resp.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Guenette JA, Sheel AW. Physiological consequences of a high work of breathing during heavy exercise in humans. J Sci Med Sport 10: 341–350, 2007. doi: 10.1016/j.jsams.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581: 1309–1322, 2007. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen D, Ofir D, O’Donnell DE. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir Physiol Neurobiol 167: 87–100, 2009. doi: 10.1016/j.resp.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Johnson BD, Dempsey JA. Demand vs. capacity in the aging pulmonary system. Exerc Sport Sci Rev 19: 171–210, 1991. doi: 10.1249/00003677-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Johnson BD, Reddan WG, Pegelow DF, Seow KC, Dempsey JA. Flow limitation and regulation of functional residual capacity during exercise in a physically active aging population. Am Rev Respir Dis 143: 960–967, 1991. doi: 10.1164/ajrccm/143.5_Pt_1.960. [DOI] [PubMed] [Google Scholar]
- 31.Johnson BD, Reddan WG, Seow KC, Dempsey JA. Mechanical constraints on exercise hyperpnea in a fit aging population. Am Rev Respir Dis 143: 968–977, 1991. doi: 10.1164/ajrccm/143.5_Pt_1.968. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol (1985) 73: 874–886, 1992. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 33.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest 116: 488–503, 1999. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 34.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 131: 700–708, 1985. [DOI] [PubMed] [Google Scholar]
- 35.Killian KJ, Summers E, Jones NL, Campbell EJM. Dyspnea and leg effort during incremental cycle ergometry. Am Rev Respir Dis 145: 1339–1345, 1992. doi: 10.1164/ajrccm/145.6.1339. [DOI] [PubMed] [Google Scholar]
- 36.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 127: 725–734, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Koulouris NG, Valta P, Lavoie A, Corbeil C, Chassé M, Braidy J, Milic-Emili J. A simple method to detect expiratory flow limitation during spontaneous breathing. Eur Respir J 8: 306–313, 1995. doi: 10.1183/09031936.95.08020306. [DOI] [PubMed] [Google Scholar]
- 38.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CPM, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735, 2005. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 39.Mahler DA, Fierro-Carrion G, Baird JC. Evaluation of dyspnea in the elderly. Clin Geriatr Med 19: 19–33, 2003. doi: 10.1016/S0749-0690(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 40.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol (1985) 84: 1872–1881, 1998. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- 41.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. [DOI] [PubMed] [Google Scholar]
- 42.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 43.Mittman C, Edelman NH, Norris AH, Shock NW. Relationship between chest wall and pulmonary compliance and age. J Appl Physiol 20: 1211–1216, 1965. doi: 10.1152/jappl.1965.20.6.1211. [DOI] [Google Scholar]
- 44.Morris JF, Koski A, Temple WP, Claremont A, Thomas DR. Fifteen-year interval spirometric evaluation of the Oregon predictive equations. Chest 93: 123–127, 1988. doi: 10.1378/chest.93.1.123. [DOI] [PubMed] [Google Scholar]
- 45.Niewoehner DE, Kleinerman J. Morphologic basis of pulmonary resistance in the human lung and effects of aging. J Appl Physiol 36: 412–418, 1974. doi: 10.1152/jappl.1974.36.4.412. [DOI] [PubMed] [Google Scholar]
- 46.Ofir D, Laveneziana P, Webb KA, Lam YM, O’Donnell DE. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol (1985) 104: 1583–1593, 2008. doi: 10.1152/japplphysiol.00079.2008. [DOI] [PubMed] [Google Scholar]
- 47.Patrick JM, Bassey EJ, Fentem PH. The rising ventilatory cost of bicycle exercise in the seventh decade: a longitudinal study of nine healthy men. Clin Sci (Lond) 65: 521–526, 1983. doi: 10.1042/cs0650521. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrino R, Brusasco V, Rodarte JR, Babb TG. Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J Appl Physiol (1985) 74: 2552–2558, 1993. doi: 10.1152/jappl.1993.74.5.2552. [DOI] [PubMed] [Google Scholar]
- 49.Pellegrino R, Violante B, Nava S, Rampulla C, Brusasco V, Rodarte JR. Expiratory airflow limitation and hyperinflation during methacholine-induced bronchoconstriction. J Appl Physiol (1985) 75: 1720–1727, 1993. doi: 10.1152/jappl.1993.75.4.1720. [DOI] [PubMed] [Google Scholar]
- 50.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol (1985) 107: 1622–1628, 2009. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topin N, Mucci P, Hayot M, Prefaut C, Ramonatxo M. Gender influence on the oxygen consumption of the respiratory muscles in young and older healthy individuals. Int J Sports Med 24: 559–564, 2003. doi: 10.1055/s-2003-43267. [DOI] [PubMed] [Google Scholar]
- 52.Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol 25: 664–671, 1968. doi: 10.1152/jappl.1968.25.6.664. [DOI] [PubMed] [Google Scholar]
- 53.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 54.Yernault JC, De Troyer A, Rodenstein D. Sex and age differences in intrathoracic airways mechanics in normal man. J Appl Physiol Respir Environ Exerc Physiol 46: 556–564, 1979. [DOI] [PubMed] [Google Scholar]
- 55.Zin WA, Milic-Emili J. Esophageal pressure measurement. In: Physiologic Basis of Respiratory Disease, edited by Hamid Q, Shannon J, Martin J. Hamilton, ON, Decker, 2005, p. 639–647. [Google Scholar]