Abstract
Patients with chronic obstructive pulmonary disease (COPD) exhibit an altered skeletal muscle mitochondrial phenotype, which often includes reduced mitochondrial density, altered respiratory function, and elevated oxidative stress. As this phenotype may be explained by the sedentary lifestyle that commonly accompanies this disease, the aim of this study was to determine whether such alterations are still evident when patients with COPD are compared to control subjects matched for objectively measured physical activity (PA; accelerometry). Indexes of mitochondrial density [citrate synthase (CS) activity], respiratory function (respirometry in permeabilized fibers), and muscle oxidative stress [4-hydroxynonenal (4-HNE) content] were assessed in muscle fibers biopsied from the vastus lateralis of nine patients with COPD and nine PA-matched control subjects (CON). Despite performing similar levels of PA (CON: 18 ± 3, COPD: 20 ± 7 daily minutes moderate-to-vigorous PA; CON: 4,596 ± 683, COPD: 4,219 ± 763 steps per day, P > 0.70), patients with COPD still exhibited several alterations in their mitochondrial phenotype, including attenuated skeletal muscle mitochondrial density (CS activity; CON 70.6 ± 3.8, COPD 52.7 ± 6.5 U/mg, P < 0.05), altered mitochondrial respiration [e.g., ratio of complex I-driven state 3 to complex II-driven state 3 (CI/CII); CON: 1.20 ± 0.11, COPD: 0.90 ± 0.05, P < 0.05), and oxidative stress (4-HNE; CON: 1.35 ± 0.19, COPD: 2.26 ± 0.25 relative to β-actin, P < 0.05). Furthermore, CS activity (r = 0.55), CI/CII (r = 0.60), and 4-HNE (r = 0.49) were all correlated with pulmonary function, assessed as forced expiratory volume in 1 s (P < 0.05), but not PA (P > 0.05). In conclusion, the altered mitochondrial phenotype in COPD is present even in the absence of differing levels of PA and appears to be related to the disease itself.
NEW & NOTEWORTHY Chronic obstructive pulmonary disease (COPD) is associated with debilitating alterations in the function of skeletal muscle mitochondria. By comparing the mitochondrial phenotype of patients with COPD to that of healthy control subjects who perform the same amount of physical activity each day, this study provides evidence that many aspects of the dysfunctional mitochondrial phenotype observed in COPD are not merely due to reduced physical activity but are likely related to the disease itself.
Keywords: COPD, mitochondrial dysfunction, muscle dysfunction, physical activity
INTRODUCTION
In addition to pulmonary dysfunction, chronic obstructive pulmonary disease (COPD) is often associated with skeletal muscle dysfunction, typified by reduced skeletal muscle endurance, efficiency, and strength (15, 23, 26, 41). While impaired muscle function may seem trivial in terms of the pulmonary consequences of this disease, reports that skeletal muscle dysfunction serves as an independent predictor of quality of life, health care utilization, and mortality in COPD (11, 26, 43) highlight the importance of this debilitating component of COPD. Although many elements are likely involved in the muscle dysfunction linked to COPD, alterations in the function of skeletal muscle mitochondria are increasingly being recognized as an important contributor (44). Nevertheless, as patients with COPD are often very sedentary (15, 34) and mitochondria are highly responsive to physical activity (PA) (18), it is unclear whether the altered mitochondrial phenotype in COPD is actually related to the disease itself or dependent upon the sedentary lifestyle typically associated with the pulmonary pathology.
One of the most common observations in skeletal muscle of patients with COPD is a reduction in the number of mitochondria per milligram of muscle (i.e., mitochondrial density). Several studies have reported that mitochondrial density, most often assessed by citrate synthase (CS) activity, is reduced by 10–38% in patients with COPD compared with healthy control subjects (1, 32, 37). Importantly, reduced PA, on par with what is typically exhibited by patients with COPD, is also associated with substantial reductions in mitochondrial density (6, 8). However, as PA has not been taken into account in most studies of COPD, it is unclear whether the commonly observed reduction in mitochondrial density is merely an artifact of comparing two groups of participants with very different levels of PA.
Patients with COPD also often exhibit both reduced and altered mitochondrial respiratory function. While much of the attenuated oxidative capacity is likely explained by the reduced density of mitochondria, qualitative alterations in mitochondrial respiratory function have also been reported (15, 29, 40). For example, the mitochondria of patients with COPD exhibit lower complex I (CI)-driven respiration and an increased reliance on the less efficient (i.e., less ATP produced per oxygen consumed) complex II (CII)-driven respiration (15). Importantly, this subtle alteration, which may increase the oxygen cost of exercise, was related to quadriceps exercise intolerance among patients with COPD. Nevertheless, similar alterations have been observed when comparing sedentary to active individuals (10).
The skeletal muscle of patients with COPD also often exhibits elevated levels of oxidative stress [e.g., lipid peroxidation assessed by 4-hydroxynonenal (4-HNE) content] (2), partially derived by increased free radical production from the mitochondria (32, 38), which is thought to contribute to this altered mitochondrial phenotype (7). Importantly, oxidative stress is also influenced by PA (12, 17).
With many of the alterations in skeletal muscle mitochondrial function in COPD potentially being an artifact of comparing two groups of participants with different levels of PA, the aim of this study was to determine whether such alterations are evident when patients with COPD are compared to control subjects matched for objectively measured PA. It was hypothesized that COPD would have a PA-independent effect on mitochondrial phenotype, such that mitochondrial density, respiratory function, and oxidative stress would be altered in patients with COPD, despite exhibiting levels of PA similar to those of control subjects.
METHODS
Subjects.
Patients (1 woman, 8 men) with moderate-to-severe COPD, currently engaged in regular physical activity or pulmonary rehabilitation (e.g., treadmill walking, cycling, etc.), and PA-matched, healthy control subjects (1 woman, 8 men) were recruited for this study based on spirometric evidence of airway obstruction [forced expiratory volume in 1 s (FEV1) < 80% predicted, FEV1/forced vital capacity (FVC) < 0.70] or, for the control subjects, the lack thereof (FEV1 > 80% predicted, FEV1/FVC > 0.70) attained with standard pulmonary function tests (5). As the present study was, in part, a follow-up to a previously published study (15), in addition to several new subjects a number of subjects from the previous study, selected on the basis of PA, were included in this new analysis. All subjects provided written informed consent before participation in this study. The Institutional Review Boards at the University of Utah and the Salt Lake City Department of Veterans Affairs Medical Center approved all protocols employed in this study.
Muscle biopsy.
Subjects reported to the laboratory having refrained from vigorous exercise for 24 h. A muscle sample of the vastus lateralis muscle was obtained by percutaneous needle biopsy ~15 cm proximal to the knee at a depth of ~3.5 cm, under sterile conditions (41). Immediately after the muscle sample (~150 mg) was taken from the leg, part of the sample (~30 mg) was immersed in ice-cold biopsy preservation fluid (BIOPS) for respiratory analyses (31), while the remaining sample was snap frozen and stored at −80°C for biochemical (e.g., CS activity) and protein content (e.g., 4-HNE) analysis.
Citrate synthase activity.
Snap-frozen muscle samples were homogenized with homogenization buffer (in mM: 250 sucrose, 40 KCl, 2 EGTA, and 20 Tris·HCl; Sigma Aldrich, St. Louis, MO). CS activity of the muscle homogenate was then assessed with a spectrophotometer (Synergy 4; Biotek Instruments, Winooski, VT) (32).
Immunoblotting.
The relative abundance of target proteins was determined in samples obtained during skeletal muscle biopsy via Western blot analysis. Briefly, muscle samples were homogenized in lysis buffer, supplemented with a protease/phosphate inhibitor cocktail (10 μM sodium fluoride and 1 mM PMSF; Santa Cruz Biotechnology, Santa Cruz, CA). Protein concentration was determined with the Bradford technique (Sigma). Forty micrograms of homogenate was separated by polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and incubated with primary and secondary antibodies directed against the proteins of interest. Membranes were imaged on a ChemiDoc XRS (Bio-Rad, Hercules, CA) and quantified with Image Laboratory software (Bio-Rad). The expression of complexes I–V of the electron transport chain and OXPHOS, the average expression of the five complexes involved in oxidative phosphorylation, was assessed with the Total OXPHOS Human Western Blot Antibody Cocktail (ab110411; Abcam, Cambridge, MA). 4-HNE (ab46545; Abcam), a marker of lipid peroxidation, and manganese superoxide dismutase (MnSOD) (SC-515068; Santa Cruz Biotechnology), an endogenous mitochondrial antioxidant, were also assessed to gauge the level of intramuscular oxidative stress. The abundance of each protein was normalized to β-actin (ab8227; Abcam), which served as a loading control (21).
Mitochondrial respiration and immunofluorescence.
Muscle samples were prepared and permeabilized for mitochondrial respiration analysis as described by Pesta and Gnaiger (31). Specifically, BIOPS-immersed fibers were carefully separated with fine-tip forceps and subsequently bathed in a BIOPS-based saponin solution (50 µg saponin/ml BIOPS) for 30 min. After saponin treatment, muscle fibers were rinsed twice in ice-cold mitochondrial respiration fluid (MIR05), each rinse for 10 min. After being rinsed for a total of 20 min, fibers were blotted with a paper towel before the weight of each sample (2–4 mg) was measured on a calibrated scale. Muscle fibers were then placed in a temperature-controlled respiration chamber (Oxytherm; Hansatech Instruments. Norfolk, UK) in 2 ml of MIR05 solution and warmed to 37°C. The respiration chamber was calibrated daily, and MIR05 was air saturated with O2 concentrations of ~190 to ~170 µM. After the muscle fibers were allowed 10 min to equilibrate, mitochondrial respiratory function was assessed by using the protocol described in Table 1 to determine peak CI-driven respiration (state 3:CI), peak CII-driven respiration (state 3:CII), and peak CI+CII-driven respiration (state 3:CI+CII). Oxygen consumption not linked to phosphorylation (i.e., state 2) was also assessed. Pilot studies indicated that the concentration of the substrates and inhibitors used were at saturating levels. Only samples that exhibited evidence of mitochondrial membrane integrity (a <10% increase in respiration in response to cytochrome c) were included in this study. Respiration data were acquired as the average respiration for the final minute of steady-state respiration for each step, and samples were run in duplicate and averaged across runs.
Table 1.
Mitochondrial respiration protocol
| Step | Chemical Name (concentration) | Major Site of Action | Respiration State |
|---|---|---|---|
| 1 | Malate (2 mM), glutamate (10 mM) | +Complex I (CI) | State 2 |
| 2 | ADP (5 mM) | +Complex V (CV) | State 3:CI |
| 3 | Succinate (10 mM) | +Complex II (CII) | State 3:CI+CII |
| 4 | Cytochrome c (10 µM) | Test of mitochondrial membrane integrity | |
| 5 | Rotenone (0.5 µM) | −CI | State 3:CII |
Description of the protocol used to assess mitochondrial respiratory function, the site of action of each chemical introduced to the preparation (+, substrate; −, inhibitor), and the respiration state associated with each step. ADP, adenosine diphosphate. Note that steady-state rates were achieved for each step, which took ~3 min, before proceeding to the next step.
Initially, respiration data were examined in terms of O2 flux per milligram of tissue (wet weight) to obtain an indication of mitochondrial capacity per milligram of tissue. Respiration was also normalized relative to CS activity and OXPHOS protein content. To further explore whether the mitochondria of patients with COPD exhibit qualitative changes in mitochondrial respiratory function, respiration was also examined in terms of the ratio of state 3:CI to state 3:CII (i.e., CI/CII). Additionally, the respiratory control ratio (RCR), the ratio between coupled and uncoupled respiration (i.e., the ratio of state 3:CI+CII to state 2) was examined. As described previously (15, 31), these ratios provide insight into the qualitative function of the mitochondria in a density-independent manner, without introducing the additional variance associated with normalizing for mitochondrial density.
Assessment of physical activity and function.
PA was first assessed by self-report and then objectively verified by accelerometry over the course of 7–10 consecutive days (Actigraph GT3X; Actigraph, Pensacola FL), which has been validated for use in COPD patients (39). These accelerometers, which, like pedometers, assess steps per day and additionally assess intensity of PA by summing the number of accelerations (i.e., counts) along three axes, were worn by subjects during all waking hours, except when showering or bathing. Intensity of activity was categorized based upon the Freedson criteria (13) with sedentary, light, and moderate-to-vigorous activity being defined as <99, 100–1,951, and 1,952+ counts/min, respectively. All accelerometer data were screened for non-wear time. Thigh volume and muscle mass were assessed by a series of circumference and skin fold thickness measurements of the upper leg, as previously described (14, 24).
Statistical methods.
Differences in respiration states were identified with two-way repeated-measures ANOVA. Significant main effects or interactions were subsequently analyzed with a Holm-Sidak post hoc test. Independent-sample t-tests were used to assess differences between groups. Correlations were analyzed with Pearson correlations. Data are represented as means ± SE, and α = 0.05 was taken to represent statistical significance.
RESULTS
Subject characteristics.
As documented in Table 2, patients and control subjects were well matched in terms of PA, as evidenced by a similar number of steps and sedentary, light-intensity, and moderate-to-vigorous-intensity PA each day. Patients and control subjects were also not different in terms of age and body mass index (BMI) (P > 0.05). By design, spirometric indexes of lung function were lower in the patients with COPD (P < 0.05). One patient with COPD was a current smoker, and four others identified as former smokers (average elapsed time since quitting: 15 ± 3 yr). While none of the control subjects reported currently smoking, three identified as former smokers and reported an average time elapsed since quitting of 41 ± 8 yr. Medications taken by participants over the previous 6 mo are listed in Table 2.
Table 2.
Subject characteristics
| Control Subjects | COPD | P | |
|---|---|---|---|
| Subjects (women/men) | 9 (1/8) | 9 (1/8) | |
| Age, yr | 70 ± 2 | 62 ± 4 | 0.15 |
| Height, cm | 173 ± 3 | 177 ± 3 | 0.42 |
| Weight, kg | 83 ± 5 | 91 ± 6 | 0.35 |
| BMI, kg/m2 | 28 ± 1 | 29 ± 2 | 0.57 |
| Quadriceps muscle mass, kg | 1.8 ± 0.2 | 1.6 ± 0.1 | 0.25 |
| Lung function | |||
| FVC, liters | 5.1 ± 0.4 | 3.7 ± 0.3* | <0.05 |
| FEV1, liters | 3.6 ± 0.2 | 1.9 ± 0.2* | <0.001 |
| FEV1, % predicted | 124 ± 6 | 54 ± 5* | <0.001 |
| FEV1/FVC, % | 79 ± 5 | 52 ± 4* | <0.001 |
| Physical activity | |||
| Steps per day | 4,596 ± 683 | 4,219 ± 763 | 0.72 |
| Sedentary physical activity, min/day | 1,270 ± 20 | 1,276 ± 83 | 0.24 |
| Light physical activity, min/day | 152 ± 20 | 144 ± 24 | 0.81 |
| Moderate-to-vigorous physical activity, min/day | 18 ± 3 | 20 ± 7 | 0.80 |
| Medication history | |||
| Inhaled β-agonist/cholinergic antagonist, n | 0 | 8* | <0.001 |
| Corticosteroid, n | 0 | 0 | 1.00 |
| Statin-type drugs, n | 2 | 1 | 0.56 |
| Muscle relaxant, n | 0 | 1 | 0.33 |
Values are means ± SE. Medications listed are those reported over the past 6 mo. BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Significantly different from control subjects.
Mitochondrial phenotype.
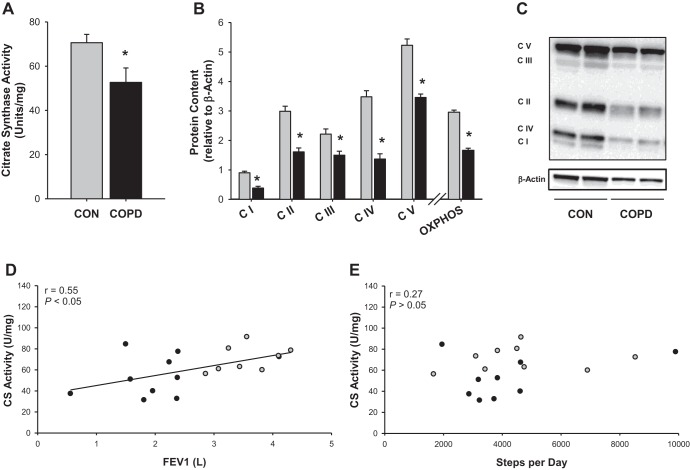
As illustrated in Fig. 1A, mitochondrial density, assessed by CS activity, was significantly lower in the patients with COPD compared with the control subjects (CON 70.6 ± 3.8, COPD 52.7 ± 6.5 U/mg, P < 0.05). Additionally, the relative content of each of the five mitochondrial electron transport chain complexes was reduced in patients with COPD (P < 0.05; Fig. 1, B and C). Ultimately, when the expression of all five complexes was averaged to determine OXPHOS proteins, the patients exhibited a 43% reduction in OXPHOS protein expression (CON 2.96 ± 0.07, COPD 1.67 ± 0.07 U, P < 0.05). Notably, CS activity (r = −0.55, P < 0.05; Fig. 1D) and OXPHOS protein expression (r = 0.79, P < 0.05) were both related to FEV1, an index of lung function, but not PA in terms of steps per day (Fig. 1E) or daily moderate-to-vigorous activity.
Fig. 1.
Indexes of mitochondrial density in patients with chronic obstructive pulmonary disease (COPD) and physical activity-matched control subjects (CON). A: citrate synthase (CS) activity. B: protein content of the electron transport chain complexes and average protein for all electron transport chain complexes (OXPHOS). C: representative gel illustrating electron transport chain complexes relative to β-actin. D: relationship between lung function, assessed by forced expiratory volume in 1 s (FEV1), and CS activity. E: relationship between physical activity, assessed by steps per day, and CS activity. Gray bars or circles represent control subjects, while black bars or circles represent patients with COPD. Data are presented as means ± SE. *Significantly different from CON.
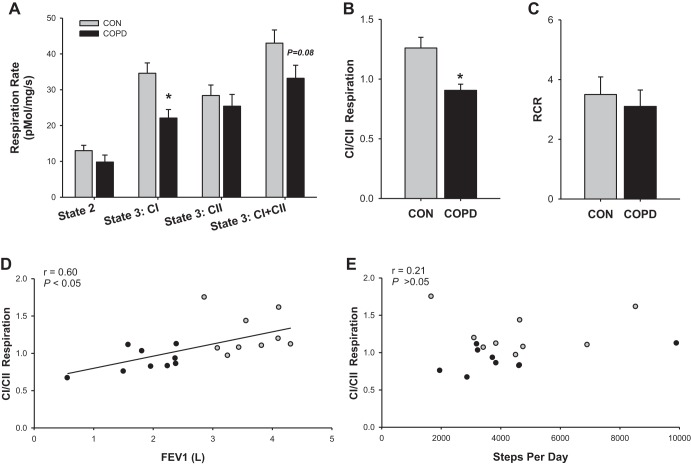
In terms of mitochondrial respiration per milligram of muscle, a significant interaction existed between the subject groups and respiration states (P < 0.05; Fig. 2A), such that the impact of COPD on respiration depended upon which respiration state was being examined. Notably, compared with control subjects, state 3:CI was significantly lower in patients with COPD (CON: 34.7 ± 2.9 pmol·mg−1·s−1, COPD: 22.1 ± 2.4 pmol·mg−1·s−1, P < 0.05). Although not reaching the threshold for statistical significance, state 3:CI+CII also tended to be lower in patients with COPD than in control subjects (CON: 42.7 ± 3.7 pmol·mg−1·s−1, COPD: 33.2 ± 3.6 pmol·mg−1·s−1, P = 0.08). Meanwhile, state 3:CII (CON: 28.4 ± 2.9 pmol·mg−1·s−1, COPD: 25.4 ± 3.3 pmol·mg−1·s−1, P > 0.05) and state 2 (CON: 13.0 ± 1.5 pmol·mg−1·s−1, COPD: 9.8 ± 2.0 pmol·mg−1·s−1, P > 0.05) were not different between groups. As wet weight muscle respiration indicated altered utilization of the CI and CII pathways in COPD, the ratio of state 3:CI to state 3:CII (i.e., CI/CII) was examined and a significant reduction was observed in patients compared with control subjects (CON: 1.27 ± 0.09, COPD: 0.90 ± 0.05, P < 0.05; Fig. 2B). As illustrated in Fig. 2, D and E, CI/CII respiration was significantly related to FEV1 but not PA assessed by steps per day. Daily time spent in moderate-to-vigorous activity was also unrelated to CI/CII (r = 0.01, P > 0.05). Notably, the ratio of coupled respiration (state 3:CI+CII) to uncoupled respiration (state 2), the RCR, was not different between groups (CON: 3.5 ± 0.6, COPD: 3.1 ± 0.6, P > 0.05; Fig. 2C).
Fig. 2.
Mitochondrial respiratory function of vastus lateralis muscle from patients with chronic obstructive pulmonary disease (COPD) and physical activity-matched healthy control subjects (CON). A: mitochondrial O2 flux per milligram of muscle. B: complex I (CI)-driven state 3 respiration relative to complex II (CII)-driven state 3 respiration (CI/CII respiration). C: state 3:CI+CII relative to state 2 [i.e., respiratory control ratio (RCR)]. D: relationship between lung function, assessed by forced expiratory volume in 1 s (FEV1), and CI/CII respiration. E: relationship between physical activity, assessed by steps per day, and CI/CII respiration. Gray bars or circles represent control subjects, while black bars or circles represent patients with COPD. Data are presented as means ± SE.*Significantly different from healthy control.
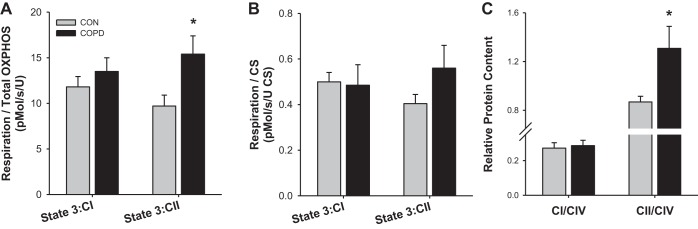
In an attempt to further explore the qualitative function of the mitochondria, state 3:CI and state 3:CII respiration were normalized for mitochondrial density with both CS activity or OXPHOS protein content. As illustrated in Fig. 3, A and B, while normalizing respiration by OXPHOS protein content revealed a significant increase in state 3:CII in patients with COPD (CON: 9.7 ± 1.2, COPD: 15.4 ± 2.0 pmol·s−1·U−1; P < 0.05), respiration normalized by CS activity did not reveal any significant differences, with state 3:CII only weakly tending to be greater in COPD (CON: 0.40 ± 0.04, COPD: 0.56 ± 0.13 pmol·s−1·U−1 CS activity, P = 0.19). To explore the possibility that differences in the relative expression of CI and CII within the mitochondria may contribute to this observed alterations in CI/CII respiratory function, the expression of CI and CII relative to CIV was examined. As illustrated in Fig. 3C, while the ratio of CI to CIV was not different between groups (CON: 0.27 ± 0.03, COPD: 0.29 ± 0.03, P > 0.05), the ratio of CII to CIV was significantly greater in the patients with COPD (CON: 0.87 ± 0.05, COPD: 1.31 ± 0.18, P < 0.05).
Fig. 3.
Comparison of complex I (CI)- and complex II (CII)-related factors in patients with chronic obstructive pulmonary disease (COPD) and physical activity-matched control subjects (CON). A: CI-driven respiration (i.e., state 3:CI) and CII-driven respiration (i.e., state 3:CII) respiration normalized for average protein content of all 5 electron transport chain complexes (i.e., OXPHOS). B: state 3:CI and state 3:CII respiration normalized by citrate synthase (CS) activity. C: complex I (CI) and complex II (CII) protein content relative to complex IV (CIV) protein content. Data are presented as means ± SE. *Significantly different from CON.
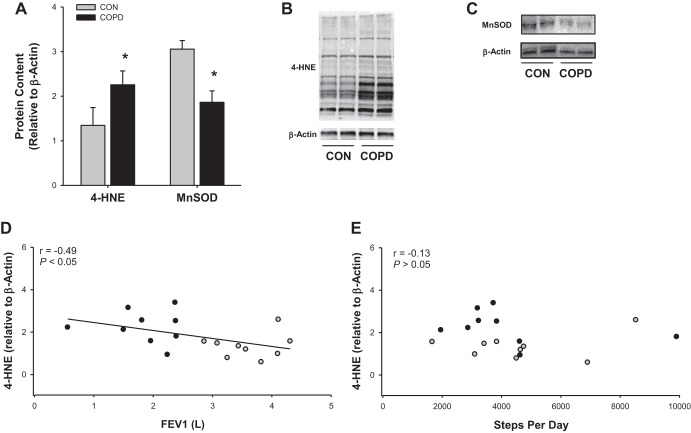
In terms of indexes of muscle oxidative stress, patients with COPD exhibited greater levels of the lipid peroxidation marker 4-HNE (CON: 1.35 ± 0.19, COPD: 2.26 ± 0.25 relative to β-actin, P < 0.05), which was accompanied by a significant reduction in the mitochondria-specific antioxidant MnSOD (CON: 3.05 ± 0.40, COPD: 1.86 ± 0.25 relative to β-actin, P < 0.05). Moreover, 4-HNE protein content was related to FEV1 (r = −0.49, P < 0.05; Fig. 4D) but not any index of PA (r = 0.13–0.25, P > 0.05; Fig. 4E). MnSOD exhibited a nonsignificant relationship with FEV1 (r = 0.41, P = 0.09).
Fig. 4.
Markers of antioxidant capacity and oxidative stress in skeletal muscle of patients with chronic obstructive pulmonary disease (COPD) and physical activity-matched control subjects (CON). A: protein content of the mitochondria-specific antioxidant manganese superoxide dismutase (MnSOD) and the lipid peroxidation and oxidative stress marker 4-hydroxynonenal protein content (4-HNE). B: representative gel of 4-HNE and β-actin. C: representative gel of MnSOD and β-actin. D: relationship between lung function, assessed by forced expiratory volume in 1 s (FEV1), and 4-HNE protein content. E: relationship between physical activity, assessed by steps per day, and 4-HNE protein content. Gray circles represent control subjects, while black circles represent patients with COPD. Data are presented as means ± SE. *Significantly different from CON.
DISCUSSION
This study aimed to determine whether commonly observed alterations in the skeletal muscle mitochondrial phenotype of patients with COPD are evident when patients with COPD are compared to control subjects matched for objectively measured PA. This approach revealed that even when patients with COPD and control subjects were matched for PA, reduced mitochondrial density, altered respiratory function, and elevated oxidative stress were still evident in patients with COPD. As addressed in detail in the following paragraphs, these findings provide useful insight into the etiology and therefore the potential treatment of skeletal muscle mitochondrial dysfunction in COPD.
COPD, physical activity, and mitochondrial density.
One of the most commonly reported alterations in mitochondrial phenotype of patients with COPD is a decrease in mitochondrial density (1, 32, 37). For example, utilizing techniques similar to those employed in the present study, Picard et al. (32), Allaire et al. (1), and Puente-Maestu et al. (37) all reported a reduction in CS activity of 10–40%, a marker of mitochondrial density (22), in patients with COPD. Similarly, the present data revealed a ~20% reduction in CS activity (Fig. 1A), which was substantiated by a reduction in the expression of each electron transport chain complex (I–V) and, therefore, OXPHOS (Fig. 1, B and C). This is in agreement with data from Puente-Maestu et al. (37), who reported that patients with COPD exhibited approximately an 11% reduction in CS activity compared with control subjects matched for PA, based upon a PA questionnaire. Moreover, Mattson et al. (27) reported similar findings with an animal model of COPD, in which PA was well matched.
As illustrated in Fig. 1, D and E, in these two groups CS activity was significantly related to FEV1, an index of lung function, while it was entirely unrelated to PA, further supporting a PA-independent link between COPD and reduced mitochondrial density. Similarly, in an examination of 29 patients with COPD, van den Borst et al. (3) found no relationship between objectively measured PA and CS activity or any marker of mitochondrial function. Although the mechanism responsible for this blunted mitochondrial density in patients with COPD, in the face of a level of PA similar to that of control subjects, is unknown, it is possible that oxidative stress may play a role. Crane et al. (9) reported that mice genetically altered to express less MnSOD exhibited greater oxidative stress and a blunted exercise-induced increase in CS activity. Moreover, Puente-Maestu et al. (36) reported that exercise-induced changes in mitochondrial DNA content, a marker of mitochondrial density, were related to the magnitude of oxidative stress present in the muscle of patients with COPD. Similarly, the present data indicate an inverse relationship between 4-HNE, a marker of lipid peroxidation/oxidative stress, and CS activity (r = −0.53, P < 0.05). Thus, it is conceivable that, even in the absence of differences in PA, oxidative stress may contribute to the decreased mitochondrial density observed in COPD.
COPD, physical activity, and mitochondrial respiratory function.
Skeletal muscle from patients with COPD exhibited significant alterations in mitochondrial respiratory function, including a specific decrease in state 3:CI that contributed to a reduction in peak respiration (i.e., state 3:CI+CII) (15). In the present study (Fig. 2A), a similar decrease in state 3:CI (per mg tissue) was determined to persist among patients with COPD compared with PA-matched control subjects. A decrease in mitochondrial density may contribute to the attenuated state 3:CI; however, if lower mitochondrial density were the sole culprit, state 3:CI and state 3:CII, theoretically, should be equally affected. As illustrated in Fig. 2A, and consistent with previous findings (4, 15), CI- and CII-driven respiration are not equally affected in COPD. This uneven impact of COPD on CI- and CII-driven respiration is further highlighted by the lower CI-to-CII respiration ratio, which gauges the relative capacity of state 3:CI to state 3:CII in patients with COPD compared with control subjects, independent of mitochondrial density (Fig. 2B) (15, 31). As discussed previously (15), since CII-driven respiration yields less ATP per oxygen consumed, this reduction in CI/CII respiration may contribute to the increased oxygen cost of exercise reported in patients with COPD (28, 41).
That this type of mitochondrial inefficiency is present in patients with COPD, even when matched for PA, is consistent with the findings of Richardson et al. (41), who reported that, despite being matched for PA, patients with COPD required more oxygen to perform a given amount of exercise than their healthy counterparts. Importantly, as illustrated in Fig. 2, D and E, while CI/CII respiration was significantly related to FEV1, it was completely unrelated to PA. Thus, although similar patterns of mitochondrial respiration have been observed in healthy sedentary individuals and attributed to inactivity (10, 17), the attenuated CI/CII of patients with COPD does not appear to simply be a product of PA.
While it is not possible to conclude from the CI-to-CII respiration ratio whether a reduction in CI-driven respiration or an increase in CII-driven respiration drives this phenomenon, evidence from other data may provide clues in this regard. As illustrated in Fig. 3A, after normalization of each respiration rate for OXPHOS protein content (i.e., average expression of all 5 complexes), state 3:CI was not different between groups while state 3:CII was elevated in COPD. Potentially due to variability in the measurement of CS activity, this trend did not reach significance when respiration was normalized for CS activity (Fig. 3B). Nevertheless, as illustrated in Fig. 3C, when considering the protein expression of CI relative to CIV or CII relative to CIV, to gain an idea of the relative expression of each complex within the mitochondria, it again appears that an increase in the relative expression of CII rather than a decrease of CI may be responsible for the altered CI-to-CII respiration ratio.
In agreement with an altered ratio of CII to CIV in patients with COPD, Konokhova et al. (19) reported an increase in ratio of CII to CIV activity in the mitochondria of these patients. Moreover, this increase in CII/CIV activity was accompanied by an increase in the level of oxidative DNA damage and severely impaired mitochondrial biogenesis leading to decreased mitochondrial density. Since CII is the only complex of the electron transport chain entirely encoded by nuclear DNA, the reduced CIV-to-CII ratio was interpreted as evidence of mitochondrial DNA damage, potentially due to oxidative stress. Indeed, with COPD patients experiencing a decrease in mitochondrial density (Fig. 1), in which CII is less affected than CI (Fig. 2A, Fig. 3C), the present data support the idea put forward by Konokhova et al. (19), that, regardless of PA, oxidative damage to mitochondrial DNA may contribute to the altered mitochondrial phenotype in COPD.
COPD, physical activity, and markers of oxidative stress.
COPD is associated with high levels of oxidative stress and is thought to play a substantial role in the development of muscle dysfunction in COPD (32, 35, 44). Nevertheless, with physical inactivity also being associated with oxidative stress, it has been unclear whether the COPD, or the relative inactivity associated with COPD, actually drives the elevated oxidative stress. As illustrated in Fig. 4A, muscle from patients with COPD exhibited a significant reduction in the abundance of the mitochondria-specific antioxidant MnSOD. As the mitochondria of patients with COPD reportedly produce more free radicals than controls (32, 36, 38), it is not surprising that the decrease in MnSOD was accompanied by an increase in muscle oxidative stress/lipid peroxidation, assessed by 4-HNE (Fig. 4B). Moreover, as illustrated in Fig. 4, D and E, 4-HNE content was related to lung function but not PA. While the mitochondria are a potential source of oxidative stress, other factors (e.g., NADPH oxidase) may also be involved (20). Thus, with patients being matched to control subjects for PA, the present data support the concept that the oxidative stress observed in COPD is not exclusively an artifact of the relative inactivity of patients with COPD but may be related to the disease itself.
Treating mitochondrial dysfunction in COPD with increased PA.
Together, the findings of this study indicate that some of the hallmarks of the COPD mitochondrial phenotype can occur independently from differences in PA. Nevertheless, it should not be concluded that exercise interventions would be of no use in COPD, since most patients with COPD tend to be very inactive (34) and inactivity may elicit additional impairments in mitochondrial function (6, 8, 12), which may further exacerbate the overall dysfunction of the patient. Thus, while the present data indicate that one may not expect a complete amelioration of the mitochondrial phenotype with exercise training, an exercise intervention may prove useful in minimizing any potential contribution of physical inactivity to the overall disability in this population. For example, Maltais et al. (25) and Brønstad et al. (4) have reported that endurance exercise training results in a significant increase in CS activity in patients with COPD. Furthermore, Brønstad et al. (4), who also observed a specific reduction in state 3:CI in COPD, reported that 6 wk of high-intensity, single-leg knee extension training, which is rarely limited by the capacity of the heart and lungs, restored state 3:CI to normal levels. While the present data suggest that decreased PA may not be the cause of the attenuated CI/CII, the data of Brønstad et al. (4) suggest that high-intensity training free of central limitations, which is more intense than traditional exercise training performed by patients with COPD, may provide an approach to mitigate the impairment. It may be that central limitations during more traditional exercise prevent the muscle from reaching an intensity sufficient to stimulate the necessary improvement in mitochondrial function.
Experimental considerations.
Building upon previous studies documenting alterations in the mitochondrial phenotype of patients with COPD compared with more physically active control subjects (15, 29), the present study provides evidence that several alterations in the mitochondrial phenotype in COPD persist even when physical activity is matched between groups. Based upon criteria described by Tudor-Locke (45), with daily step counts < 5,000 steps/day, despite being relatively active for patients with COPD, on average the patients and control subjects in the present study exhibited sedentary-to-low activity levels. Nevertheless, as a more physically active control group was not included in the present study, it would not be appropriate to make definitive conclusions about how reductions in PA impacted the mitochondrial phenotype in this study. It should also be noted that while subjects were matched for current habitual PA, lifelong history of PA was not considered. Future investigations may investigate the effect of lifelong PA (e.g., 10 yr of disuse vs. 2 yr) on the mitochondrial phenotype in COPD. It is also important to note that body composition (e.g., body fat percentage) was not directly assessed in this study. Nevertheless, participants did not differ in terms of height, weight, BMI, or quadriceps muscle mass, suggesting that body composition was likely similar between groups.
The correlations presented in Figs. 1, 2, and 4 indicate a relationship between lung function (FEV1) and many facets of mitochondrial phenotype. Nevertheless, it is important to note that the relationships presented are correlations with both patients and control subjects included. While correlations only among the patients with COPD would have allowed for more precise examination of the relationship between disease severity and the mitochondrial phenotype, the limited sample size hindered such an analysis.
Conclusions.
Patients with COPD, who are typically relatively inactive, often exhibit an altered mitochondrial phenotype with lower mitochondrial density, decreased respiratory function, and elevated oxidative stress. As the patients with COPD in the present study exhibited all of these factors, despite being compared to PA-matched control subjects, it appears that these commonly reported alterations in mitochondrial function in patients with COPD are not entirely dependent on the relative inactivity that typically accompanies this pathology but are likely related to the progression of the disease itself.
GRANTS
This study was funded by Flight Attendant Medical Research Institute (FAMRI), NIH National Heart, Lung, and Blood Institute Grant PO1 HL-09830 and K99HL125756, Department of Veterans Affairs (VA) Merit Awards E6910-R and E1697-R, VA SPiRe Award E1433-P, and VA Senior Career Scientist Award E9275-L.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.G., J.D.T., O.S.K., G.L., R.S.G., S.Y.P., A.D.N., and R.S.R. conceived and designed research; J.R.G., J.D.T., O.S.K., G.L., R.S.G., S.Y.P., A.D.N., and R.S.R. performed experiments; J.R.G., O.S.K., G.L., R.S.G., S.Y.P., and R.S.R. analyzed data; J.R.G., J.D.T., O.S.K., G.L., R.S.G., S.Y.P., A.D.N., and R.S.R. interpreted results of experiments; J.R.G., O.S.K., and R.S.R. prepared figures; J.R.G., O.S.K., and R.S.R. drafted manuscript; J.R.G., J.D.T., O.S.K., G.L., R.S.G., S.Y.P., A.D.N., and R.S.R. edited and revised manuscript; J.R.G., J.D.T., O.S.K., G.L., R.S.G., S.Y.P., A.D.N., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the subjects for their gracious participation.
REFERENCES
- 1.Allaire J, Maltais F, Doyon J-F, Noël M, LeBlanc P, Carrier G, Simard C, Jobin J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax 59: 673–678, 2004. doi: 10.1136/thx.2003.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreiro E, Gea J, Corominas JM, Hussain SNA. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 29: 771–778, 2003. doi: 10.1165/rcmb.2003-0138OC. [DOI] [PubMed] [Google Scholar]
- 3.van den Borst B, Slot IG, Hellwig VA, Vosse BA, Kelders MC, Barreiro E, Schols AM, Gosker HR. Loss of quadriceps muscle oxidative phenotype and decreased endurance in patients with mild-to-moderate COPD. J Appl Physiol (1985) 114: 1319–1328, 2013. doi: 10.1152/japplphysiol.00508.2012. [DOI] [PubMed] [Google Scholar]
- 4.Brønstad E, Rognmo Ø, Tjonna AE, Dedichen HH, Kirkeby-Garstad I, Håberg AK, Ingul CB, Wisløff U, Steinshamn S. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J 40: 1130–1136, 2012. doi: 10.1183/09031936.00193411. [DOI] [PubMed] [Google Scholar]
- 5.Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PM, Chavannes N, Dillard T, Fahy B, Fein A, Heffner J, Lareau S, Meek P, Martinez F, McNicholas W, Muris J, Austegard E, Pauwels R, Rennard S, Rossi A, Siafakas N, Tiep B, Vestbo J, Wouters E, ZuWallack R. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23: 932–946, 2004. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 6.Chi MM, Hintz CS, Coyle EF, Martin WH 3rd, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol 244: C276–C287, 1983. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 7.Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J 26: 703–719, 2005. doi: 10.1183/09031936.05.00139904. [DOI] [PubMed] [Google Scholar]
- 8.Coyle EF, Martin WH 3rd, Sinacore DR, Joyner MJ, Hagberg JM, Holloszy JO. Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol Respir Environ Exerc Physiol 57: 1857–1864, 1984. doi: 10.1152/jappl.1984.57.6.1857. [DOI] [PubMed] [Google Scholar]
- 9.Crane JD, Abadi A, Hettinga BP, Ogborn DI, MacNeil LG, Steinberg GR, Tarnopolsky MA. Elevated mitochondrial oxidative stress impairs metabolic adaptations to exercise in skeletal muscle. PLoS One 8: e81879, 2013. doi: 10.1371/journal.pone.0081879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daussin FN, Zoll J, Ponsot E, Dufour SP, Doutreleau S, Lonsdorfer E, Ventura-Clapier R, Mettauer B, Piquard F, Geny B, Richard R. Training at high exercise intensity promotes qualitative adaptations of mitochondrial function in human skeletal muscle. J Appl Physiol (1985) 104: 1436–1441, 2008. doi: 10.1152/japplphysiol.01135.2007. [DOI] [PubMed] [Google Scholar]
- 11.Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J 10: 417–423, 1997. doi: 10.1183/09031936.97.10020417. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo PA, Powers SK, Ferreira RM, Amado F, Appell HJ, Duarte JA. Impact of lifelong sedentary behavior on mitochondrial function of mice skeletal muscle. J Gerontol A Biol Sci Med Sci 64: 927–939, 2009. doi: 10.1093/gerona/glp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30: 777–781, 1998. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Gifford JR, Garten RS, Nelson AD, Trinity JD, Layec G, Witman MA, Weavil JC, Mangum T, Hart C, Etheredge C, Jessop J, Bledsoe A, Morgan DE, Wray DW, Rossman MJ, Richardson RS. Symmorphosis and skeletal muscle V̇o2max: in vivo and in vitro measures reveal differing constraints in the exercise-trained and untrained human. J Physiol 594: 1741–1751, 2016. doi: 10.1113/JP271229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gifford JR, Trinity JD, Layec G, Garten RS, Park SY, Rossman MJ, Larsen S, Dela F, Richardson RS. Quadriceps exercise intolerance in patients with chronic obstructive pulmonary disease: the potential role of altered skeletal muscle mitochondrial respiration. J Appl Physiol (1985) 119: 882–888, 2015. doi: 10.1152/japplphysiol.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gram M, Vigelsø A, Yokota T, Helge JW, Dela F, Hey-Mogensen M. Skeletal muscle mitochondrial H2O2 emission increases with immobilization and decreases after aerobic training in young and older men. J Physiol 593: 4011–4027, 2015. doi: 10.1113/JP270211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56: 831–838, 1984. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 19.Konokhova Y, Spendiff S, Jagoe RT, Aare S, Kapchinsky S, MacMillan NJ, Rozakis P, Picard M, Aubertin-Leheudre M, Pion CH, Bourbeau J, Hepple RT, Taivassalo T. Failed upregulation of TFAM protein and mitochondrial DNA in oxidatively deficient fibers of chronic obstructive pulmonary disease locomotor muscle. Skelet Muscle 6: 10, 2016. doi: 10.1186/s13395-016-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 47: 333–343, 2009. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Kwon OS, Tanner RE, Barrows KM, Runtsch M, Symons JD, Jalili T, Bikman BT, McClain DA, O’Connell RM, Drummond MJ. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. Am J Physiol Endocrinol Metab 309: E11–E21, 2015. doi: 10.1152/ajpendo.00124.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layec G, Haseler LJ, Hoff J, Richardson RS. Evidence that a higher ATP cost of muscular contraction contributes to the lower mechanical efficiency associated with COPD: preliminary findings. Am J Physiol Regul Integr Comp Physiol 300: R1142–R1147, 2011. doi: 10.1152/ajpregu.00835.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layec G, Venturelli M, Jeong EK, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum: from able-bodied adults to individuals with a spinal cord injury. J Appl Physiol (1985) 116: 1142–1147, 2014. doi: 10.1152/japplphysiol.01120.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maltais F, LeBlanc P, Simard C, Jobin J, Bérubé C, Bruneau J, Carrier L, Belleau R. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 154: 442–447, 1996. doi: 10.1164/ajrccm.154.2.8756820. [DOI] [PubMed] [Google Scholar]
- 26.Man WD, Kemp P, Moxham J, Polkey MI. Skeletal muscle dysfunction in COPD: clinical and laboratory observations. Clin Sci (Lond) 117: 251–264, 2009. doi: 10.1042/CS20080659. [DOI] [PubMed] [Google Scholar]
- 27.Mattson JP, Poole DC. Pulmonary emphysema decreases hamster skeletal muscle oxidative enzyme capacity. J Appl Physiol (1985) 85: 210–214, 1998. doi: 10.1152/jappl.1998.85.1.210. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros WM, Fernandes MC, Azevedo DP, de Freitas FF, Amorim BC, Chiavegato LD, Hirai DM, O’Donnell DE, Neder JA. Oxygen delivery-utilization mismatch in contracting locomotor muscle in COPD: peripheral factors. Am J Physiol Regul Integr Comp Physiol 308: R105–R111, 2015. doi: 10.1152/ajpregu.00404.2014. [DOI] [PubMed] [Google Scholar]
- 29.Naimi AI, Bourbeau J, Perrault H, Baril J, Wright-Paradis C, Rossi A, Taivassalo T, Sheel AW, Rabøl R, Dela F, Boushel R. Altered mitochondrial regulation in quadriceps muscles of patients with COPD. Clin Physiol Funct Imaging 31: 124–131, 2011. doi: 10.1111/j.1475-097X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- 31.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25–58, 2012. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 32.Picard M, Godin R, Sinnreich M, Baril J, Bourbeau J, Perrault H, Taivassalo T, Burelle Y. The mitochondrial phenotype of peripheral muscle in chronic obstructive pulmonary disease: disuse or dysfunction? Am J Respir Crit Care Med 178: 1040–1047, 2008. doi: 10.1164/rccm.200807-1005OC. [DOI] [PubMed] [Google Scholar]
- 34.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 972–977, 2005. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 35.Puente-Maestu L, Lázaro A, Humanes B. Metabolic derangements in COPD muscle dysfunction. J Appl Physiol (1985) 114: 1282–1290, 2013. doi: 10.1152/japplphysiol.00815.2012. [DOI] [PubMed] [Google Scholar]
- 36.Puente-Maestu L, Lázaro A, Tejedor A, Camaño S, Fuentes M, Cuervo M, Navarro BO, Agustí A. Effects of exercise on mitochondrial DNA content in skeletal muscle of patients with COPD. Thorax 66: 121–127, 2011. doi: 10.1136/thx.2010.153031. [DOI] [PubMed] [Google Scholar]
- 37.Puente-Maestu L, Pérez-Parra J, Godoy R, Moreno N, Tejedor A, González-Aragoneses F, Bravo JL, Villar Álvarez F, Camaño S, Agustí A. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J 33: 1045–1052, 2009. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]
- 38.Puente-Maestu L, Tejedor A, Lázaro A, de Miguel J, Alvarez-Sala L, González-Aragoneses F, Simón C, Agustí A. Site of mitochondrial reactive oxygen species production in skeletal muscle of chronic obstructive pulmonary disease and its relationship with exercise oxidative stress. Am J Respir Cell Mol Biol 47: 358–362, 2012. doi: 10.1165/rcmb.2011-0382OC. [DOI] [PubMed] [Google Scholar]
- 39.Rabinovich RA, Louvaris Z, Raste Y, Langer D, Van Remoortel H, Giavedoni S, Burtin C, Regueiro EM, Vogiatzis I, Hopkinson NS, Polkey MI, Wilson FJ, Macnee W, Westerterp KR, Troosters T. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J 42: 1205–1215, 2013. doi: 10.1183/09031936.00134312. [DOI] [PubMed] [Google Scholar]
- 40.Rabinovich RA, Bastos R, Ardite E, Llinàs L, Orozco-Levi M, Gea J, Vilaró J, Barberà JA, Rodríguez-Roisin R, Fernández-Checa JC, Roca J. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J 29: 643–650, 2007. doi: 10.1183/09031936.00086306. [DOI] [PubMed] [Google Scholar]
- 41.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med 169: 89–96, 2004. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 43.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, Moore AJ, Moxham J, Polkey MI. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 62: 115–120, 2007. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taivassalo T, Hussain SN. Contribution of the mitochondria to locomotor muscle dysfunction in patients with COPD. Chest 149: 1302–1312, 2016. doi: 10.1016/j.chest.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Tudor-Locke C, Hatano Y, Pangrazi RP, Kang M. Revisiting “how many steps are enough?” Med Sci Sports Exerc 40, Suppl: S537–S543, 2008. doi: 10.1249/MSS.0b013e31817c7133. [DOI] [PubMed] [Google Scholar]