Abstract
Blood pressure (BP) reactivity is predictive of the development of cardiovascular disease. We hypothesized that the BP response at the onset of isometric handgrip exercise would occur earlier and to a lesser degree in individuals who underwent bariatric surgery compared with obese adults and that the reliance on total peripheral resistance (TPR) would be attenuated. Twenty-six individuals (7 nonobese, 11 obese, 8 postbariatric surgery) completed isometric handgrip exercise (40% maximum voluntary contraction) to exhaustion. Heart rate (HR, ECG) and arterial BP (brachial catheter) were measured continuously. Stroke volume was estimated from the pressure waveform, and cardiac output (CO) and TPR were calculated. Peak change, time to peak, and rate of rise in BP were assessed during the first 30 s of exercise. Obese adults exhibited a slower rise in BP and higher peak BP at exercise onset compared with nonobese controls (P < 0.05). Peak BP and the rate of rise were not different between individuals who underwent bariatric surgery and nonobese controls (P > 0.05). Nonobese controls exhibited an exercise-mediated increase in CO, whereas obese adults increased TPR (P < 0.05). The increases in CO and TPR were less apparent in individuals who underwent bariatric surgery (P > 0.05). In contrast to obese adults, individuals who underwent bariatric surgery exhibit a rapid rise in BP at exercise onset. This rapid increase in BP is associated with a fall in TPR and results in lower peak BP at the onset of isometric exercise. These data suggest that bariatric surgery improves BP reactivity via changes in the time course of hemodynamic responses.
NEW & NOTEWORTHY Bariatric surgery has been shown to reduce the blood pressure (BP) response to isometric handgrip exercise. By examining the time course of the BP response to exercise, we found, in contrast to obese adults, individuals who underwent bariatric surgery exhibit a rapid rise in BP at exercise onset, which is associated with a fall in total peripheral resistance and results in lower peak BP at the onset of isometric exercise. These data suggest that bariatric surgery improves BP reactivity via reflex autonomic adjustments.
Keywords: autonomic, baroreflex sensitivity, gastric bypass, heart rate variability
INTRODUCTION
The prevalence of extreme obesity is ~5% in the United States and is associated with significant cardiovascular morbidity, including hypertension, cardiac hypertrophy, and heart failure (4, 21, 34). Bariatric surgery results in dramatic and sustained weight loss and resolution or improvement in many of these associated cardiovascular complications (1, 4, 19, 21, 34). Given the known effectiveness of bariatric surgery on weight loss and the increasingly popular nature of bariatric procedures (5), it is important to understand mechanisms contributing to the cardiovascular effects of bariatric surgery (33, 38).
Exaggerated blood pressure reactivity to stress is predictive of the development of cardiovascular disease (5a, 11, 16, 22, 23, 32), particularly hypertension. Data from Dipla and colleagues (10) suggest the mechanisms contributing to blood pressure reactivity (e.g., stroke volume vs. total peripheral resistance) differ between lean and obese individuals. Bariatric surgery has been shown to reduce the blood pressure response to isometric handgrip exercise and postexercise ischemia (9); however, the physiological mechanisms contributing to this attenuated response were not directly examined. Recently, El Sayed and colleagues (12) showed that the early blood pressure rise strongly influences the neurovascular response to stress. Central command is activated at exercise onset, and, via vagal withdraw, heart rate is increased. The rapid increase in heart rate at exercise onset “resets” the baroreflex to higher blood pressures, thereby allowing sympathetic activity and total peripheral resistance to remain low. However, if a rapid increase in heart rate does not occur, an increase in sympathetic activity and total peripheral resistance (which is characteristically slower) may be needed to raise blood pressure to its new operating point (17, 28, 29) These distinctive differences in vascular control have important implications for overall blood pressure regulation and cardiovascular risk because the ability of the baroreflex to “buffer” large swings in pressure may be decreased in conditions where blood pressure fluctuations are slower. Thus, by examining the time course of the hemodynamic components of the blood pressure response to an acute stressor, we can gain insight into the mechanisms important in the cardiovascular response to stress and potential areas where adaptation may occur.
With this information in mind, we sought to examine mechanisms for increasing blood pressure in response to stress in individuals who underwent bariatric surgery. We used isometric handgrip exercise as a stressor that consistently causes an acute pressor and sympathoexcitatory response (30, 31). We hypothesized that the blood pressure response at the onset of isometric handgrip exercise would be smaller and occur more quickly in individuals who had undergone bariatric surgery and that the reliance on total peripheral resistance would be attenuated compared with obese adults.
MATERIALS AND METHODS
We completed a cross-sectional study in three groups of individuals (nonobese, n = 7; obese, n = 11; postbariatric surgery, n = 8). The data presented are a subset of a larger study, and results from the larger group were published previously (8, 26, 27). All subjects were between 18 and 45 yr of age, nonpregnant, nonsmokers, and not taking any medications known to affect metabolic, neurological, or cardiovascular function. Individuals were excluded if they had known renal insufficiency, diabetes, or a history of serious psychiatric, pulmonary, or cardiovascular disease (e.g., heart failure, previous myocardial infarction, angina, significant cardiac arrhythmias). Female subjects had a confirmed negative pregnancy test within 48 h of any experimental procedure and were studied during the early follicular phase of the menstrual cycle or days 3–7 of the placebo phase of oral contraceptive therapy (oral contraceptive use, n = 6; lean n = 2, bariatric n = 4).
Nonobese adults had a body mass index (BMI) less than 25 kg/m2, and obese adults had a BMI >35 kg/m2. Individuals in the bariatric surgery group were recruited from a database of patients who had open or laparoscopic proximal Roux-en-Y (limb length ~150 cm) gastric bypass operations at Mayo Clinic for medically complicated obesity refractory to behavioral modification. Individuals were >12 mo postbariatric surgery with a presurgery BMI between 35 and 45 kg/m2 followed by a 25- to 75-kg weight loss or BMI <30 kg/m2. Exclusion criteria included active participation in an endurance-type training program (aerobic exercise >60 min/day and >5 days/week for >1 mo). All experiments were performed in the Clinical Research Unit (CRU) at Mayo Clinic and were approved by the Institutional Review Board at the Mayo Clinic. On a screening visit, each subject gave written informed consent, followed by a review of medical history and a physical exam performed by a laboratory physician, blood sampling (for fasting measures of glucose, insulin, and lipid panel), and measurement of body composition (duel-energy X-ray absorptiometry, DPX-IQ; GE Medical Systems Lunar, Madison, WI).
For 3 days preceding the study visit, subjects received weight-maintenance meals provided by the CRU metabolic kitchen with a macronutrient distribution of 50% carbohydrate, 20% protein, and 30% fat. On the study day, subjects arrived to the laboratory at 0700 h after an overnight fast and after abstaining from exercise, caffeine, and alcohol for at least 24 h. Subjects were semirecumbent and were instrumented with a three-lead electrocardiogram to measure heart rate. A 20-gauge, 5-cm catheter was placed in the brachial artery under aseptic conditions after local anesthesia (2% lidocaine) to measure arterial blood pressure and for blood sampling (epinephrine, norepinephrine). Standard blood assays were performed by the Immunochemistry Core Laboratory of the CRU of the Mayo Clinic CTSA.
Resting muscle sympathetic nerve activity (MSNA) was recorded as previously described using the technique of microneurography (8). Multiunit MSNA was recorded with a tungsten microelectrode in the peroneal nerve, posterior to the fibular head. The recorded signal was amplified 80,000-fold, band pass filtered (700–2,000 Hz), rectified, and integrated (resistance-capacitance integrator circuit, time constant 0.1 s) by a nerve-traffic analyzer. Following instrumentation, baroreflex sensitivity was evaluated using the modified Oxford technique as previously described (2). Briefly, heart rate, MSNA, and arterial blood pressure were measured continuously during a 5-min quiet resting period. An intravenous bolus of sodium nitroprusside (100 µg) was then administered, followed by an intravenous bolus of phenylephrine (150 µg) 1 min later. Following a standard 20-min wash-out period, the modified Oxford technique was repeated. Resting hemodynamics (Table 1) are reported as an average of data collected during the two 5-min quiet resting periods. The relationships between MSNA and diastolic blood pressure and between R-R interval (RRI) and systolic blood pressure during the boluses of nitroprusside and phenylephrine were used as indices of baroreflex control of sympathetic activity and of the heart, respectively. Results from the two modified Oxford tests were averaged.
Table 1.
Subject demographics
| Nonobese (n = 7) | Obese (n = 11) | Bariatric (n = 8) | P Value | |
|---|---|---|---|---|
| Subject demographics | ||||
| Sex, M/F | 5/2 | 5/6 | 0/8 | |
| Age, yr | 28 ± 3 | 33 ± 2 | 35 ± 2 | 0.17 |
| Height, cm | 174 ± 3 | 174 ± 2 | 166 ± 2 | 0.04 |
| Weight, kg | 71 ± 3 | 118 ± 3*† | 79 ± 3 | <0.01 |
| BMI, kg/m2 | 23 ± 1 | 39 ± 1*† | 29 ± 1 | <0.01 |
| Total body fat, % | 26 ± 3 | 48 ± 3* | 40 ± 2* | <0.01 |
| Baseline metabolic health | ||||
| Glucose, mg/dl | 78 ± 5 | 94 ± 3* | 86 ± 5 | 0.045 |
| Insulin, mIU/l | 7.9 ± 1.5 | 15.3 ± 2.8† | 3.9 ± 0.8 | <0.01 |
| Cholesterol, mg/dl | 170 ± 16 | 189 ± 7 | 173 ± 9 | 0.39 |
| Triglycerides, mg/dl | 116 ± 14 | 127 ± 18 | 90 ± 13 | 0.21 |
| HDL, mg/dl | 53 ± 3 | 42 ± 4† | 69 ± 5 | <0.01 |
| LDL, mg/dl | 94 ± 14 | 122 ± 3† | 86 ± 10 | 0.03 |
| Baseline cardiovascular health | ||||
| Heart rate, beats/min | 58 ± 3 | 71 ± 3* | 63 ± 1 | 0.02 |
| Systolic blood pressure, mmHg | 129 ± 4 | 137 ± 4 | 133 ± 7 | 0.41 |
| Diastolic blood pressure, mmHg | 71 ± 2 | 79 ± 2 | 72 ± 4 | 0.08 |
| Mean blood pressure, mmHg | 91 ± 2 | 100 ± 4 | 96 ± 5 | 0.41 |
| Baseline neurovascular control | ||||
| Epinephrine, pg/ml | 46 ± 7 | 35 ± 8 | 24 ± 5 | 0.15 |
| Norepinephrine, pg/ml | 102 ± 29 | 193 ± 24*† | 82 ± 15 | <0.01 |
| Burst frequency, bursts/min | 15 ± 3 | 21 ± 4 | 11 ± 2 | 0.054 |
| Bursts incidence, bursts/100 heart beats | 27 ± 5 | 31 ± 5 | 17 ± 3 | 0.10 |
| Sympathetic baroreflex sensitivity, % bursts/mmHg | −5.5 ± 0.7 | −6.2 ± 0.7 | −5.3 ± 0.5 | 0.63 |
| Mean NN, ms | 1041 ± 29 | 904 ± 20* | 953 ± 18* | <0.01 |
| SDNN, ms | 65 ± 3 | 46 ± 7 | 55 ± 5 | 0.13 |
| RMSSD, ms | 65 ± 4 | 44 ± 8* | 47 ± 7 | 0.03 |
| Cardiac baroreflex sensitivity, ms/mmHg | 26.0 ± 3.5 | 10.9 ± 1.4* | 15.7 ± 3.4 | 0.01 |
Data are reported as means ± SE from nonobese (n = 7), obese (n = 11), and bariatric (n = 8) groups, unless otherwise noted (insulin, epinephrine, norepinephrine: nonobese, n = 6; cardiac output: nonobese, n = 6, obese, n = 10; cardiac baroreflex sensitivity: obese, n = 10). One-way ANOVA was performed to determine the main effect of group (nonobese, obese, bariatric). BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NN, mean time between normal cardiac cycles; SDNN, standard deviation of normal cardiac cycles; RMSSD, root mean square of successive heart beat internal differences.
P < 0.05 vs. nonobese.
P < 0.05 vs. bariatric.
After a minimum of 10 min of quiet rest, when hemodynamic variables returned to baseline, subjects completed isometric handgrip exercise at 40% of maximal voluntary contraction to exhaustion to assess cardiovascular reactivity to stress. Maximal voluntary contraction was calculated as an average of three attempts using a handgrip dynamometer, and subjects were verbally encouraged to keep the generated force at the calculated value (40% of average calculated maximum). Failure was defined as an inability of the subject to maintain grip force at 40% of maximum despite verbal encouragement. Forearm blood flow in the contralateral forearm was measured with venous occlusion plethysmography in a subset of subjects (nonobese n = 5, obese n = 10, bariatric n = 8). Heart rate, arterial blood pressure, and forearm blood flow were measured continuously throughout baseline and the contraction period. MSNA was not measured during isometric handgrip exercise because of methodological challenges and the secondary nature of the research questions (8, 26, 27). Stroke volume was estimated from the arterial pressure waveform (LabChart; ADInstruments, Sydney, Australia), and cardiac output and total peripheral resistance were calculated.
Data were recorded at 250 Hz using a computer data acquisition system (WinDaq; DATAQ Instruments, Akron, OH) and stored for offline analysis. Heart rate variability (time domain) was assessed (LabChart, ADInstruments) during the last 5 min of quiet rest before the modified Oxford test, and data reported included mean time between normal cardiac cycles (NN interval, reported in ms), standard deviation of normal cardiac cycles (SDNN), and root mean square of successive heart beat internal differences (RMSSD). MSNA data were analyzed during rest only, and sympathetic bursts in the integrated neurogram were identified using a custom-manufactured automated analysis program as published previously (8). Resting MSNA is reported as bursts per minute (burst frequency) and bursts per 100 heartbeats (burst incidence) (8).
Hemodynamic data were analyzed beat by beat during exercise, and changes in systolic blood pressure, diastolic blood pressure, mean arterial blood pressure, cardiac output, stroke volume, and total peripheral resistance responses from resting baseline were determined across 15-s intervals throughout the task (12). Data are reported as a percentage of time to fatigue. Area under the curve (AUC) was calculated for changes in each hemodynamic variable from exercise to exhaustion. Peak systolic, diastolic, and mean blood pressure were determined during the first 30 s of the task based on previous work (12). The peak change was defined as the highest blood pressure value during the first 30 s of the task minus the blood pressure of the first cardiac cycle of the task. The time of the peak in blood pressure during the first 30 s of the task was defined as the number of seconds to reach the peak blood pressure from the start of the task. The rate of rise was defined as peak change divided by time to peak (12).
Statistical analyses were conducted using SigmaPlot Version 14.0 (Systat Software, San Jose, CA). Repeated-measures ANOVA was performed to determine the main effect of group (nonobese, obese, bariatric) and/or time for each variable. Post hoc multiple comparisons were made to determine which time points were significantly different between groups. The relationships between main outcome variables were also assessed using Pearson product moment correlation with data from all groups pooled. All data are presented as means ± SE. P values ≤0.05 were considered statistically significant.
RESULTS
Subject demographics.
Individuals who underwent bariatric surgery were studied 47 ± 8 mo from surgery and had a presurgery weight of 126 ± 6 kg and presurgery BMI of 46 ± 1 kg/m2 (Table 1). At the time of study, obese adults had greater weight and BMI compared with nonobese controls and individuals who underwent bariatric surgery (P < 0.01). However, there was no difference in percentage of body fat between obese adults and individuals who underwent bariatric surgery, and both groups had higher body fat than nonobese controls (P < 0.01). Obese individuals also exhibited higher fasting glucose levels and higher fasting insulin compared with nonobese individuals and individuals who underwent bariatric surgery, respectively (P < 0.05).
Resting neurovascular control.
Resting heart rate was higher in obese adults compared with nonobese controls and/or individuals who underwent bariatric surgery (P = 0.02) (Table 1). RMSSD was lower in obese adults compared with nonobese controls (P = 0.03). No group differences in resting blood pressure were observed; however, there was a trend for higher diastolic blood pressure in obese adults (~7 mmHg) compared with nonobese controls and those who underwent bariatric surgery (P = 0.08). As shown previously in this cohort (8), obese adults exhibited higher plasma norepinephrine (P < 0.01), and MSNA tended to be higher (P = 0.054) than nonobese controls and/or individuals who underwent bariatric surgery. No group differences in sympathetic baroreflex sensitivity were detected (P = 0.63); however, cardiac baroreflex sensitivity was lower in obese adults compared with nonobese controls (P = 0.01).
Isometric handgrip exercise to exhaustion.
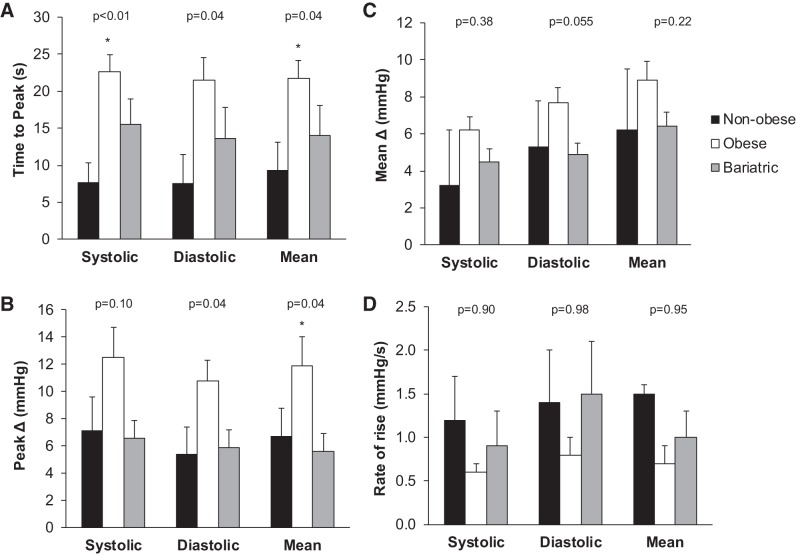
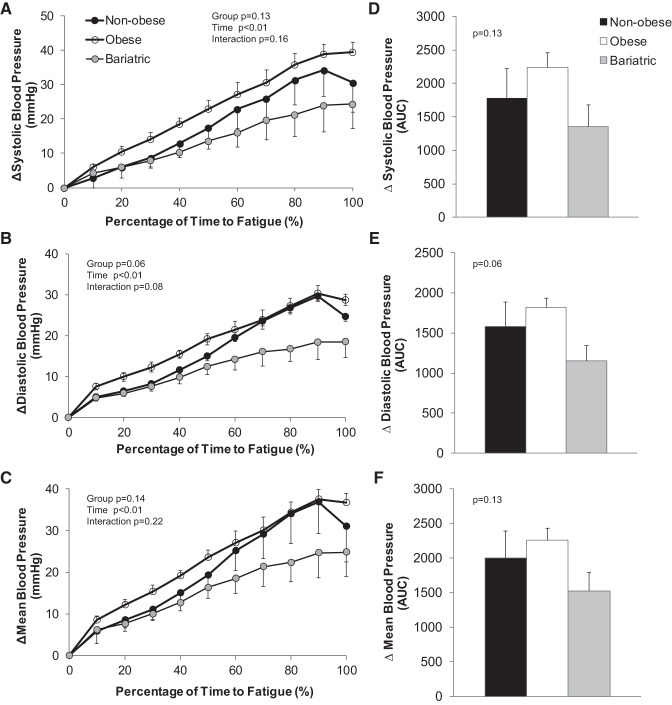
The time to exhaustion did not differ between groups (nonobese 216 ± 40 s, obese 155 ± 13 s, bariatric 169 ± 25 s; P > 0.05). Obese adults exhibited a slower initial rise in blood pressure and higher early peak blood pressure compared with nonobese controls (P < 0.05, Fig. 1); no differences were observed between nonobese controls and individuals who underwent bariatric surgery. Isometric handgrip exercise to exhaustion elicited large (~20 mmHg) increases in blood pressure across groups (P < 0.01; Fig. 2), and the increase in blood pressure tended to be lower in individuals who underwent bariatric surgery compared with nonobese controls and obese adults (P value range 0.06–0.14; Fig. 2).
Fig. 1.
Peak blood pressure at the onset of isometric handgrip exercise. Data are reported as means ± SE. One-way ANOVA was performed to determine the main effect of group (nonobese, obese, bariatric). Obese adults exhibited a slower initial rise in blood pressure (A) and higher early peak blood pressure (B) compared with nonobese controls; no differences were observed between nonobese controls and individuals who underwent bariatric surgery. No group differences in the mean change (C) or rate of rise (D) in blood pressure were observed. *P < 0.05 vs. nonobese.
Fig. 2.
Change in blood pressure during isometric handgrip exercise to fatigue. Data are reported as a change from baseline relative to the total time to fatigue (A–C: absolute change 15-s averages; D–F: area under the curve, AUC). Repeated-measures ANOVA was performed to determine the main effect of group (nonobese, obese, bariatric), time, and the interaction of group and time. Isometric handgrip exercise elicited large increases in blood pressure across groups (P < 0.01). The increase in blood pressure tended to be lower in individuals who underwent bariatric surgery compared with nonobese controls and obese adults (P value range 0.06–0.14).
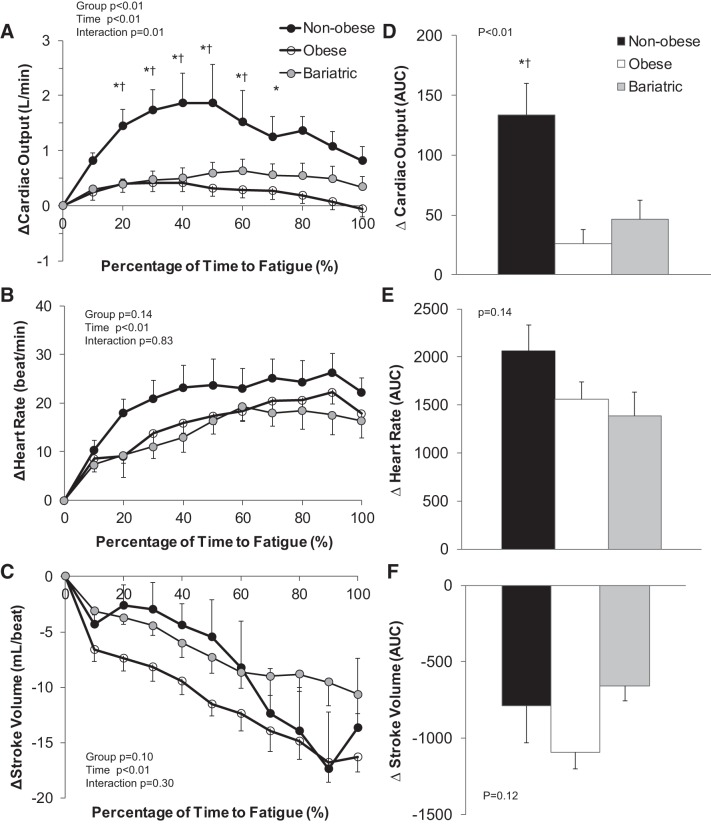
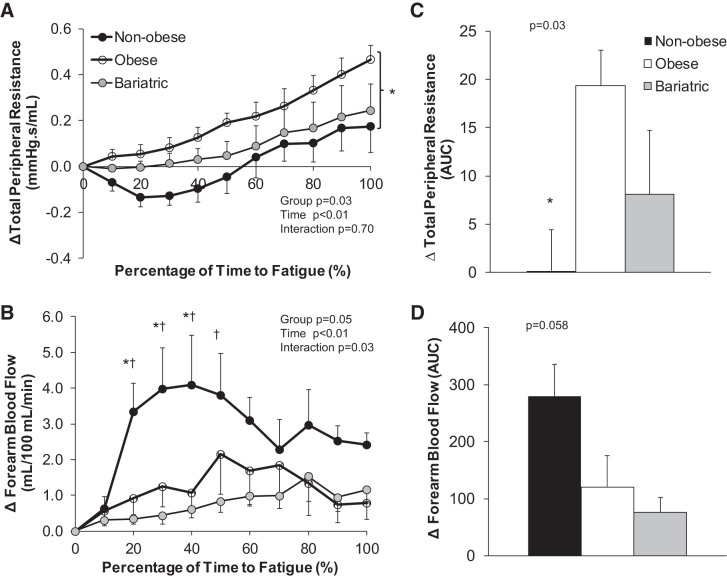
Nonobese controls exhibited a greater exercise-mediated increase in cardiac output compared with obese adults and individuals who underwent bariatric surgery (P < 0.01, Fig. 3). The increase in cardiac output was achieved primarily by increasing heart rate, given that stroke volume was reduced in all groups over time (P < 0.01, Fig. 3). Nonobese controls also exhibited a substantially larger increase in forearm blood flow in the nonexercising arm compared with obese adults and individuals who underwent bariatric surgery (P = 0.03). Obese adults exhibited an increase in total peripheral resistance with isometric handgrip exercise that was significantly greater compared with nonobese controls (P = 0.03, Fig. 4); the increase in peripheral resistance was less apparent in individuals who underwent bariatric surgery (P = 0.88 vs. nonobese, Fig. 4).
Fig. 3.
Change in central hemodynamic variables during isometric handgrip exercise to fatigue. Data are reported as a change from baseline relative to the total time to fatigue (A–C: absolute change 15-s averages; D–F: area under the curve, AUC). Repeated-measures ANOVA was performed to determine the main effect of group (nonobese, obese, bariatric), time, and the interaction of group and time. *P < 0.05 nonobese vs. obese, †P < 0.05 nonobese vs. bariatric. Nonobese controls exhibited a greater exercise-mediated increase in cardiac output compared with obese adults and individuals who underwent bariatric surgery (P < 0.01). The increase in cardiac output was achieved primarily by increasing heart rate, given that stroke volume was reduced in all groups over time (P < 0.01).
Fig. 4.
Change in peripheral hemodynamic variables during isometric handgrip exercise to fatigue. Data are reported as a change from baseline relative to the total time to fatigue (A and B: absolute change 15-s averages; C and D: area under the curve, AUC). Repeated-measures ANOVA was performed to determine the main effect of group (nonobese, obese, bariatric), time, and the interaction of group and time. *P < 0.05 nonobese vs. obese, †P < 0.05 nonobese vs. bariatric. Nonobese controls exhibited an increase in forearm blood flow in the nonexercising arm and a fall in total peripheral resistance during exercise. In contrast, obese adults exhibited an increase in total peripheral resistance with isometric handgrip exercise that was significantly greater compared with nonobese controls; the increase in peripheral resistance was less apparent in individuals who underwent bariatric surgery.
Relationships between main outcome variables.
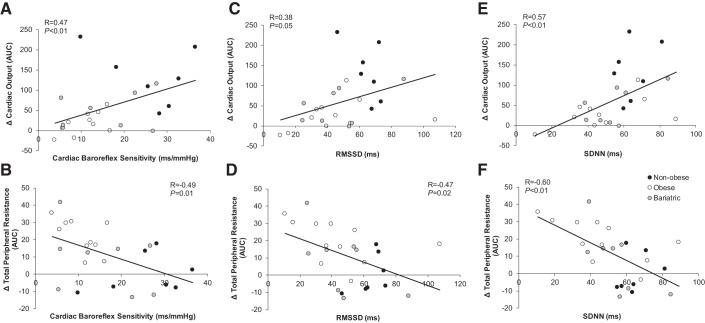
A greater increase in total peripheral resistance was related to a greater pressure response to isometric handgrip exercise (greater blood pressure reactivity) (systolic AUC R = 0.69, P < 0.01; diastolic AUC R = 0.63, P < 0.01; mean AUC R = 0.64, P < 0.01). A significant relationship was observed between the time to reach peak blood pressure at exercise onset and the increase in total peripheral resistance (systolic R = 0.58, P < 0.01; diastolic R = 0.54, P < 0.01; mean R = 0.59, P < 0.01), such that a longer time to reach peak blood pressure (slower response) was related to a greater increase in total peripheral resistance. The time to achieve peak blood pressure was also related to measures of resting heart rate variability (diastolic and SDNN R = −0.44, P = 0.03; mean and SDNN R = −0.36, P = 0.07), showing that individuals with high resting heart rate variability achieved peak blood pressure faster than individuals with low heart rate variability. Low cardiac baroreflex sensitivity and heart rate variability at rest were also related to a smaller increase in cardiac output and greater increase in total peripheral resistance during exercise (Fig. 5); these relationships were not seen with sympathetic baroreflex sensitivity (cardiac output: R = −0.01, P = 0.98; total peripheral resistance: R = −0.16, P = 0.42).
Fig. 5.
Relationships between main outcome variables. The relationships between main outcome variables were assessed using Pearson product moment correlation with data from all groups pooled. Low cardiac baroreflex sensitivity (A and B) and heart rate variability (C–F) at rest were related to a smaller increase in cardiac output and greater increase in total peripheral resistance during exercise. AUC, area under the curve; RMSSD, root mean square of successive heart beat internal differences; SDNN, standard deviation of normal cardiac cycles.
DISCUSSION
The present study is the first to investigate the time course of the systemic hemodynamic responses to physical stress in individuals who had previously undergone bariatric surgery compared with groups of healthy nonobese and obese subjects. Our major findings provide novel insight into three main areas of cardiovascular control mechanisms important in the response to stress in these populations. First, we show that, compared with nonobese controls, obese adults increased blood pressure more slowly at the onset of isometric handgrip exercise and reached a higher peak blood pressure via an increase in total peripheral resistance. Obese individuals also exhibited lower baroreflex sensitivity and heart rate variability compared with nonobese controls. In individuals who underwent bariatric surgery, impairments in baroreflex sensitivity and heart rate variability were not observed, and the blood pressure responses to exercise (blood pressure reactivity) were more similar to that of nonobese controls. These distinctive differences in hemodynamic responsiveness to an acute physical stress, and resultant autonomic reflex responses, have important implications for the day-to-day regulation of arterial blood pressure in obese individuals and in those who have undergone bariatric surgery. Thus, by examining hemodynamic responses at exercise onset, we are able to uncover impairments and/or compensations in key cardiovascular regulatory variables before the development of overt disease and identify how they may be reversed/modified with bariatric surgery.
Blood pressure increases rapidly in response to stress. Exaggerated blood pressure reactivity to stress is associated with an increased risk of cardiovascular disease (23) and is attenuated following bariatric surgery (9). We speculate that the rapid rise in blood pressure at the onset of stress, which was observed in both nonobese adults and individuals who underwent bariatric surgery, resulted in a reflex decrease in MSNA via the baroreflex. Although MSNA was not directly measured during exercise, suppression of MSNA was mirrored in an attenuated increase in total peripheral resistance in nonobese individuals at exercise onset. On the other hand, a slow rise in blood pressure at exercise onset, like that seen in obese individuals, likely contributed to the higher peripheral resistance observed. When the baroreceptors reset to higher blood pressures, in the absence of an immediate increase in heart rate, MSNA and total peripheral resistance will increase to reinforce these higher blood pressures (12, 17, 28). Thus what sets the groups apart in the present investigation is the rate at which the early blood pressure is achieved.
Although we did not observe group differences in the time to achieve peak heart rate (data not shown), we found that individuals with higher resting heart rate variability were able to elicit more rapid increases in blood pressure via an immediate increase in heart rate (i.e., greater vagal tone at baseline and hence greater vagal withdraw). This rapid rise in heart rate allowed these individuals to rely primarily on cardiac output rather than total peripheral resistance to increase blood pressure during isometric handgrip exercise, ultimately resulting in lower peak pressures (lower blood pressure reactivity). The observed increase in total peripheral resistance and higher peak pressures in response to stress in obese adults are likely the result of changes in autonomic control, such that reflex sympathoinhibition was minimized in this group. Consistent with this, our group and others have observed lower cardiac baroreflex sensitivity and heart rate variability in obese individuals (2, 13–15), which in the present study was related to individual increases in total peripheral resistance and higher peak pressures with exercise. On the other hand, both baroreflex sensitivity and heart rate variability may be improved following bariatric surgery (3, 7, 18, 24, 25), and our data suggest that such adaptations likely contributed to an attenuated blood pressure response to stress in this group. Specifically, we observed lower heart rate variability and cardiac baroreflex in obese individuals compared with nonobese controls, and this impairment was not observed in individuals who had undergone bariatric surgery. However, given the small number of individuals per group (n = 7–11), correlational analyses should be interpreted with caution.
Although improvements in baroreflex sensitivity following laparoscopic adjustable gastric banding have been attributed to a reduction in waist circumference (18), other reports have shown that individuals who have undergone bariatric surgery have decreased cardiovascular disease risk despite remaining obese (4, 21, 34). Our present data extend these previous findings and provide novel evidence that individuals who had undergone bariatric surgery improve in a number of key areas in cardiovascular control despite exhibiting significantly higher body fat than nonobese controls.
Interestingly, the large increases in cardiac output and forearm blood flow during isometric exercise seen in nonobese controls in the present study were not observed in individuals who had undergone bariatric surgery. Trombetta and colleagues (37) showed that weight loss alone (achieved by changes in diet) was not sufficient to improve the forearm blood flow response to stress; rather, only conditions in which diet was combined with an exercise intervention did improvements in peripheral vasodilation occur. Consistent with this idea, we have shown previously that physical activity levels did not differ between the obese adults and individuals who had undergone bariatric surgery (27). Thus it is possible, or even likely, that bariatric surgery alone is unable to completely reverse the deleterious effects of obesity on cardiovascular health. It is also important to note that Trombetta and colleagues (37) found that the tachycardic response to exercise was attenuated after weight loss. This phenomenon may have also contributed to the smaller increase in cardiac output in the present cohort of individuals who had undergone bariatric surgery. Taken together, our data and previous works suggest that, although bariatric surgery attenuates the increase in total peripheral resistance at the onset of isometric handgrip exercise, parasympathetic withdrawal (i.e., leading to increases in cardiac output) and/or endothelial function (i.e., leading to increases in forearm blood flow) are not completely restored.
Experimental considerations.
Strengths of the present investigation include studying steady-state conditions after bariatric surgery, extensive cardiovascular phenotyping, and thorough analysis of cardiovascular function during physical stress; however, there are some important limitations to consider. First, this is a cross-sectional study. Although a longitudinal study design may be superior, the control groups chosen allowed us to determine the physiological effects of bariatric surgery compared with obese individuals and individuals who maintain a healthy weight. Second, ~80% of individuals who undergo bariatric surgery are women (1, 19), and this is mirrored in our study population. Thus additional studies are necessary to determine whether similar observations can be made in men. This is especially important given that neurovascular transduction may differ by sex (6) and previous data suggesting that improvements in heart rate variability following bariatric surgery may be more evident in men (20). Furthermore, the effects of age were not considered in the present investigation, and the effects of advancing age on autonomic and hemodynamic responses to exercise differ between aging men and women (36); thus this is an important area for future investigation. Third, direct measures of MSNA were not available in the individuals studied. The inclusion of MSNA would have provided valuable information regarding acute autonomic adjustments and is thus an important limitation. Last, we examined cardiovascular function during physical stress, and thus our findings cannot be directly applied to other forms of stress (e.g., psychological stressors).
Conclusions.
Individuals who undergo bariatric surgery exhibit a rapid increase in blood pressure and lower peak change in blood pressure at the onset of isometric handgrip exercise. This attenuation in blood pressure reactivity is indicative of a reduction in cardiovascular disease risk and is associated with improvements in cardiac baroreflex sensitivity and heart rate variability. Despite these improvements, our data suggest that mechanisms important in heart rate and peripheral blood flow control during isometric exercise are not completely restored by bariatric surgery alone. There is a strong body of literature that suggests that improvements in these areas require concomitant exercise interventions for complete reversal of the obesity phenotype. Importantly, such improvements are likely to occur despite individuals remaining overweight. Nonetheless, our and others’ data show promising cardiovascular results for individuals who undergo bariatric surgery to treat obesity.
GRANTS
This study was funded by the National Institutes of Health (NIH) HL-130339 (J. Limberg), NIH HL-083947 (M. Joyner), NIH DK-82424 (T. Curry), NIH UL1 RR-024150 (Mayo), Mayo Clinic Department of Anesthesiology, and Mayo Foundation for Medical Education and Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L., M.J.J., N.C., and T.B.C. conceived and designed research; J.K.L., W.G., and T.B.C. analyzed data; J.K.L., W.G., M.J.J., N.C., and T.B.C. interpreted results of experiments; J.K.L. and W.G. prepared figures; J.K.L. and W.G. drafted manuscript; J.K.L., W.G., M.J.J., N.C., and T.B.C. edited and revised manuscript; J.K.L., W.G., M.J.J., N.C., and T.B.C. approved final version of manuscript; M.J.J., N.C., and T.B.C. performed experiments.
ACKNOWLEDGMENTS
We thank all our research volunteers, in addition to Madhuri Somaraju, Casey Hines, and Farah Ramirez-Marrero for technical and intellectual contributions.
REFERENCES
- 1.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, Strong MB, Vinik R, Wanner NA, Hopkins PN, Gress RE, Walker JM, Cloward TV, Nuttall RT, Hammoud A, Greenwood JL, Crosby RD, McKinlay R, Simper SC, Smith SC, Hunt SC. Health benefits of gastric bypass surgery after 6 years. JAMA 308: 1122–1131, 2012. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Khateeb AA, Limberg JK, Barnes JN, Joyner MJ, Charkoudian N, Curry TB. Acute cyclooxygenase inhibition and baroreflex sensitivity in lean and obese adults. Clin Auton Res 27: 17–23, 2017. doi: 10.1007/s10286-016-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam I, Lewis MJ, Lewis KE, Stephens JW, Baxter JN. Influence of bariatric surgery on indices of cardiac autonomic control. Auton Neurosci 151: 168–173, 2009. doi: 10.1016/j.autneu.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 321: 225–236, 2001. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 5.American Society for Metabolic and Bariatric Surgery Estimate of Bariatric Surgery Numbers, 2011–2015. July, 2016 https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
- 5a.Berger A, Grossman E, Katz M, Kivity S, Klempfner R, Segev S, Goldenberg I, Sidi Y, Maor E. Exercise blood pressure and the risk for future hypertension among normotensive middle-aged adults. J Am Heart Assoc 4: 4, 2015. doi: 10.1161/JAHA.114.001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briant LJ, Burchell AE, Ratcliffe LE, Charkoudian N, Nightingale AK, Paton JF, Joyner MJ, Hart EC. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol 594: 4753–4768, 2016. doi: 10.1113/JP272167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casellini CM, Parson HK, Hodges K, Edwards JF, Lieb DC, Wohlgemuth SD, Vinik AI. Bariatric surgery restores cardiac and sudomotor autonomic C-fiber dysfunction towards normal in obese subjects with type 2 diabetes. PLoS One 11: e0154211, 2016. doi: 10.1371/journal.pone.0154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curry TB, Somaraju M, Hines CN, Groenewald CB, Miles JM, Joyner MJ, Charkoudian N. Sympathetic support of energy expenditure and sympathetic nervous system activity after gastric bypass surgery. Obesity (Silver Spring) 21: 480–485, 2013. doi: 10.1002/oby.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva RP, Martinez D, Faria CC, de Carli LA, de Souza WI, Meinhardt NG, Souto KE, Trindade MR, Ribeiro JP. Improvement of exercise capacity and peripheral metaboreflex after bariatric surgery. Obes Surg 23: 1835–1841, 2013. doi: 10.1007/s11695-013-0988-x. [DOI] [PubMed] [Google Scholar]
- 10.Dipla K, Zafeiridis A, Koidou I, Geladas N, Vrabas IS. Altered hemodynamic regulation and reflex control during exercise and recovery in obese boys. Am J Physiol Heart Circ Physiol 299: H2090–H2096, 2010. doi: 10.1152/ajpheart.00087.2010. [DOI] [PubMed] [Google Scholar]
- 11.Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 106: 316–320, 1983. doi: 10.1016/0002-8703(83)90198-9. [DOI] [PubMed] [Google Scholar]
- 12.El Sayed K, Macefield VG, Hissen SL, Joyner MJ, Taylor CE. Rate of rise in diastolic blood pressure influences vascular sympathetic response to mental stress. J Physiol 594: 7465–7482, 2016. doi: 10.1113/JP272963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens 22: 2363–2369, 2004. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995. doi: 10.1161/01.HYP.25.4.560. [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998. doi: 10.1161/01.CIR.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 16.Jae SY, Franklin BA, Choo J, Choi YH, Fernhall B. Exaggerated exercise blood pressure response during treadmill testing as a predictor of future hypertension in men: a longitudinal study. Am J Hypertens 28: 1362–1367, 2015. doi: 10.1093/ajh/hpv036. [DOI] [PubMed] [Google Scholar]
- 17.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- 18.Lambert EA, Rice T, Eikelis N, Straznicky NE, Lambert GW, Head GA, Hensman C, Schlaich MP, Dixon JB. Sympathetic activity and markers of cardiovascular risk in nondiabetic severely obese patients: the effect of the initial 10% weight loss. Am J Hypertens 27: 1308–1315, 2014. doi: 10.1093/ajh/hpu050. [DOI] [PubMed] [Google Scholar]
- 19.Lent MR, Benotti PN, Mirshahi T, Gerhard GS, Strodel WE, Petrick AT, Gabrielsen JD, Rolston DD, Still CD, Hirsch AG, Zubair F, Cook A, Carey DJ, Wood GC. All-cause and specific-cause mortality risk after Roux-en-Y gastric bypass in patients with and without diabetes. Diabetes Care 40: 1379–1385, 2017. doi: 10.2337/dc17-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado MB, Velasco IT, Scalabrini-Neto A. Gastric bypass and cardiac autonomic activity: influence of gender and age. Obes Surg 19: 332–338, 2009. doi: 10.1007/s11695-008-9665-x. [DOI] [PubMed] [Google Scholar]
- 21.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L, Rhodes S, Morton SC, Shekelle PG. Meta-analysis: surgical treatment of obesity. Ann Intern Med 142: 547–559, 2005. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 22.Manolio TA, Burke GL, Savage PJ, Sidney S, Gardin JM, Oberman A. Exercise blood pressure response and 5-year risk of elevated blood pressure in a cohort of young adults: the CARDIA study. Am J Hypertens 7: 234–241, 1994. doi: 10.1093/ajh/7.3.234. [DOI] [PubMed] [Google Scholar]
- 23.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 110: 74–78, 2004. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 24.Nault I, Nadreau E, Paquet C, Brassard P, Marceau P, Marceau S, Biron S, Hould F, Lebel S, Richard D, Poirier P. Impact of bariatric surgery–induced weight loss on heart rate variability. Metabolism 56: 1425–1430, 2007. doi: 10.1016/j.metabol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Pontiroli AE, Merlotti C, Veronelli A, Lombardi F. Effect of weight loss on sympatho-vagal balance in subjects with grade-3 obesity: restrictive surgery versus hypocaloric diet. Acta Diabetol 50: 843–850, 2013. doi: 10.1007/s00592-013-0454-1. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez-Marrero FA, Edens KL, Joyner MJ, Curry TB. Predicted vs. actual resting energy expenditure and activity coefficients: post-gastric bypass, lean and obese women. Obes Control Ther 1: 1–7, 2014. [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Marrero FA, Miles J, Joyner MJ, Curry TB. Self-reported and objective physical activity in postgastric bypass surgery, obese and lean adults: association with body composition and cardiorespiratory fitness. J Phys Act Health 11: 145–151, 2014. doi: 10.1123/jpah.2012-0048. [DOI] [PubMed] [Google Scholar]
- 28.Raven PB. Recent advances in baroreflex control of blood pressure during exercise in humans: an overview. Med Sci Sports Exerc 40: 2033–2036, 2008. doi: 10.1249/MSS.0b013e318180bc41. [DOI] [PubMed] [Google Scholar]
- 29.Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol (1985) 69: 407–418, 1990. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 30.Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J Appl Physiol (1985) 64: 2197–2203, 1988. doi: 10.1152/jappl.1988.64.5.2197. [DOI] [PubMed] [Google Scholar]
- 31.Seals DR, Enoka RM. Sympathetic activation is associated with increases in EMG during fatiguing exercise. J Appl Physiol (1985) 66: 88–95, 1989. doi: 10.1152/jappl.1989.66.1.88. [DOI] [PubMed] [Google Scholar]
- 32.Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation 99: 1831–1836, 1999. doi: 10.1161/01.CIR.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 33.Sjöström CD, Lystig T, Lindroos AK. Impact of weight change, secular trends and ageing on cardiovascular risk factors: 10-year experiences from the SOS study. Int J Obes 35: 1413–1420, 2011. doi: 10.1038/ijo.2010.282. [DOI] [PubMed] [Google Scholar]
- 34.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 36.Trinity JD, Layec G, Hart CR, Richardson RS. The sex-specific impact of aging on the blood pressure response to exercise. Am J Physiol Heart Circ Physiol 314: H95–H104, 2017. doi: 10.1152/ajpheart.00505.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrão CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol 285: H974–H982, 2003. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- 38.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 98: 1763–1777, 2012. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]