Abstract
There is evidence suggesting diaphragmatic fatigue (DF) occurs relatively early during high-intensity exercise; however, studies investigating the temporal characteristics of exercise-induced DF are limited by incongruent methodology. Eight healthy adult males (25 ± 5 yr) performed a maximal incremental exercise test on a cycle ergometer on day 1. A constant-load time-to-exhaustion (TTE) exercise test was conducted on day 2 at 60% delta between the calculated gas exchange threshold and peak work rate. Two additional constant-load exercise tests were performed at the same intensity on days 3 and 4 in a random order to either 50 or 75% TTE. DF was assessed on days 2, 3, and 4 by measuring transdiaphragmatic twitch pressure (Pdi,tw) in response to cervical magnetic stimulation. DF was present after 75 and 100% TTE (≥20% decrease in Pdi,tw). The magnitude of fatigue was 15.5 ± 5.7%, 23.6 ± 6.4%, and 35.0 ± 12.1% at 50, 75, and 100% TTE, respectively. Significant differences were found between 100 to 75 and 50% TTE (both P < 0.01), and 75 to 50% TTE (P < 0.01). There was a significant relationship between the magnitude of fatigue and cumulative diaphragm force output (r = 0.785; P < 0.001). Ventilation, the mechanical work of breathing (WOB), and pressure-time products were not different between trials (P > 0.05). Our data indicate that exercise-induced DF presents a relatively late onset and is proportional to the cumulative WOB; thus the ability of the diaphragm to generate pressure progressively declines throughout exercise.
NEW & NOTEWORTHY The notion that diaphragmatic fatigue (DF) occurs relatively early during exercise is equivocal. Our results indicate that DF occurs during high-intensity endurance exercise in healthy men and its magnitude is strongly related to the amount of pressure and work generated by respiratory muscles. Thus we conclude that the work of breathing is the major determinant of exercise-induced DF.
Keywords: diaphragm fatigue, dyspnea, metaboreflex, work of breathing
INTRODUCTION
Muscular fatigue is defined as a temporary condition in which there is a decrease in the capacity of muscle force generation that is reversible by rest (49). It has been purported that diaphragmatic fatigue (DF) may occur in healthy adults during whole body endurance exercise at intensities above 80–85% of maximal oxygen uptake (V̇o2) (27, 41) due to the combination of high respiratory muscle work (27), elevated levels of circulating metabolites (acid-base unbalance) (19), and reduced blood flow to the diaphragm (such as during whole body exercise) (4). These factors are prerequisite for DF to occur, the consequences of which may limit exercise tolerance and performance, particularly in highly trained individuals. Increased sensory perceptions of breathlessness and the hastening of peripheral locomotor muscle fatigue through activation of the respiratory muscle metaboreflex are examples of mechanisms believed to contribute to exercise limitation (22, 39). The respiratory muscle metaboreflex is triggered by the stimulation of group III/IV phrenic afferents, which reflexively increases sympathetic efferent discharge, resulting in active limb muscle vasoconstriction and a preferential redistribution of blood flow (10, 11). The hyperpnea of exercise is accountable for 10–15% of total V̇o2 (1, 16), which is met by a 14–16% reduction in limb blood flow (assumed to be redirected toward the fatiguing respiratory muscles) (14, 22, 23). As a result, oxygen delivery to limb muscles is compromised and peripheral locomotor muscle fatigue accelerated, ultimately impairing exercise tolerance (24, 39).
Few studies have examined the time course of exercise-induced DF, and some contradictory outcomes have been postulated. Johnson and coauthors (27) suggest that DF occurrence and its magnitude are intrinsically related to exercise intensity, whereas others suggest that diaphragmatic strength increases throughout exercise, and fatigue only takes place after exercise cessation (28). Finally, there is evidence suggesting that DF occurs relatively early during high-intensity exercise with no further decrease in contractile force as exercise continues toward exhaustion (46), a notion that has been replicated by the same group during voluntary isocapnic hyperpnea (31, 38). Importantly, the aforementioned studies present methodological considerations and limitations that must be acknowledged. In one study, exercise was not maintained until exhaustion, and the workload was not constant (28), making it difficult to ascertain the mechanisms contributing to DF. Both studies (28, 46) performed measurements of transdiaphragmatic twitch pressure (Pdi,tw) during exercise, frequently pausing the test to deliver a single unpotentiated stimulus; as a result, exercise economy was compromised and neuromuscular function poorly examined. Nevertheless, previous work from our laboratory has reported a reduction in diaphragm contribution to total inspiratory muscle force production that begins within the first third of exhaustive exercise (21). It is posited that extradiaphragmatic muscles are recruited to relieve diaphragmatic work, potentially due to the onset of DF.
Therefore, we sought to investigate the temporal characteristics of exercised-induced DF. We hypothesized that during constant-load submaximal exercise, diaphragm force output will decrease progressively until a relatively early threshold (approximately two-thirds time to exhaustion), at which point the magnitude of DF will plateau (nonsignificant change of Pdi,tw) and no further reductions in diaphragm force production will occur.
METHODS
Participants.
Eight healthy, recreationally active adult males were recruited and provided written informed consent to participate in the study. Exclusion criteria consisted of the following: history of asthma, smoking or cardiopulmonary disease, metallic implants, ulcer or esophageal tumor, recent nasopharyngeal surgery, and allergies to latex or lidocaine. All procedures conformed to the Declaration of Helsinki and were approved by the Clinical Research Ethics Board at the University of British Columbia (approval no.: H16–01178).
Study design.
Participants completed four testing days separated by a minimum of 48 h and scheduled at the same time of day to control for possible circadian influences (42). All subjects abstained from exercise for a minimum of 24 h, and from stimulant or caffeinated beverages for 4 h before testing. A light meal was recommended 1.5–2 h before testing commenced. On day 1, a standardized incremental workload cycle test was performed to assess aerobic fitness. On day 2, a time-to-exhaustion (TTE) constant-load exercise test was completed using the 60% delta method (details below). On days 3 and 4, the participants performed the same constant-load exercise test, but exercise was terminated at 50 or 75% TTE in a randomized and equally assigned order. DF was assessed via cervical magnetic stimulation before and after exercise on days 2, 3, and 4.
Resting pulmonary function.
Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), the ratio of FEV1 to FVC, forced expiratory flow at 25–75% of FVC, and peak expiratory flow were measured using a digital portable spirometer (Spirolab II, Medical International Research, Vancouver, BC, Canada). All measures were performed according to ATS guidelines and compared with predicted reference values (18).
Incremental exercise test (day 1).
After familiarization with the testing procedures and a 5-min resting period, participants performed a 5-min warmup at a fixed workload on an electronically braked cycle ergometer (Velotron, Racermate, Seattle, WA). The test started at 60 W and the intensity increased in a ramp fashion by 30 W/min. Participants cycled at their preferred cadence, but were encouraged to maintain a cycle cadence above 80 rpm at all times. The test was terminated and peak workload registered when cadence dropped below 60 rpm despite verbal encouragement. V̇o2 was measured by having participants breathe into a mouthpiece connected to a nonrebreathing valve (2700B, Hans-Rudolph, Kansan City, MO). Mixed expired gases were measured at a port located within a mixing chamber and sampled through calibrated O2 and CO2 gas analyzers (S-3-A/I and CD-3A, respectively, Applied Electrochemistry, Pittsburgh, PA). End-tidal partial pressure of CO2 () was measured via a mouthpiece port connected to a CO2 analyzer (CD-3A, Applied Electrochemistry, Pittsburgh, PA). Inspiratory and expiratory flows were measured by having participants breathe through a mouthpiece attached to two pneumotachographs (no. 3813, Hans Rudolph) located on inspiratory and expiratory sides of the breathing circuit. All metabolic data during exercise testing were recorded continuously at 200 Hz (PowerLab/16SP ML795, AD Instruments, Colorado Springs, CO) and stored on a computer for subsequent analysis (LabChart v8, AD Instruments). Metabolic parameters were averaged every 30 s throughout exercise. Maximal values were obtained using the final 30-s average of exercise data.
Constant-load exercise tests (days 2–4).
A 5-min warm-up at a fixed workload was completed before the constant-load TTE test completed on day 2. Exercise intensity was prescribed at 60% of the delta between the calculated gas exchange threshold and peak workload (9). In brief, the gas exchange threshold was identified from the incremental exercise test completed on day 1 by dual inspection of v-slope and ventilatory equivalents for O2 and CO2. The 60% delta method has been shown to reduce TTE variability between subjects by taking into consideration interindividual differences in O2 uptake kinetics (33). Exercise was maintained until cycling cadence dropped below 60 rpm despite verbal encouragement. Procedures were repeated on days 3 and 4; however, exercise ceased at either 50 or 75% TTE. Days 3 and 4 were randomized and groups equally assigned. Metabolic variables were measured constantly during exercise as described previously. In addition, perceptual responses to exercise (breathing and leg discomfort) were registered every 2 min using the modified Borg scale (CR-10) (8).
Cervical magnetic stimulation.
Magnetic stimulation of the phrenic nerve roots was used to nonvolitionally assess DF (35). Participants were seated with the neck flexed. A circular 90-mm coil attached to a magnetic stimulator (MagStim 200 Mono Pulse, MagStim, Whitland, Wales, UK) was placed on the spinous process between the fifth and seventh cervical vertebrae to optimize stimulation of the phrenic nerves. Phrenic nerve activation was assessed using a ramp protocol, which consisted of multiple stimulations delivered at ever-increasing intensities (ranging from 60 to 100% of the maximal power output). Three stimuli were delivered at each intensity stimulus, separated by 30 s each. The phrenic nerves were deemed maximally activated if a plateau in Pdi,tw was observed. The confounding effect of lung volume was suppressed by performing all stimulations at functional residual capacity by monitoring esophageal pressure. The same coil position was used during all stimulations delivered within and between days.
Pdi was estimated by calculating the difference between gastric (Pga) and esophageal (Pes) pressure with the use of balloon-tipped catheters (47–9005, Ackrad Laboratory, Cranford, NJ) carefully placed via nasopharyngeal introduction in the stomach and lower third of esophagus, respectively (36). Topical anesthetic (viscous lidocaine) was applied to the nasal and pharyngeal passages to minimize discomfort during catheter placement. Each catheter was connected to a piezoelectric pressure transducer (201A, Raytech Instruments, Vancouver, BC, Canada) that was calibrated using a digital manometer (2021P, Digitron, Torquay, UK). After both balloons were inserted, air was completely evacuated by having the participant perform a Valsalva maneuver, after which esophageal and gastric balloons were filled with 1 and 2 ml of air, respectively, using a glass syringe. Correct placement was confirmed using the occlusion technique (7). Mouth pressure (Pm) was assessed through a port in the mouthpiece and connected to the same pressure transducer. DF was assessed using a protocol previously described elsewhere (21). A block of 6–8 potentiated twitches was performed before and 5, 15, and 30 min after each trial of constant-load exercise. Each twitch was preceded by maximal inspiratory maneuver lasting ~5 s.
Electromyography.
Surface EMG was recorded using electrodes (H59P, Kendall–LTP, Chicopee, MA) placed between the sixth and eighth intercostal spaces along the anterior-axillary line for the left and right costal diaphragm as previously demonstrated (48). Electrodes were placed after skin preparation (Nuprep, Weaver, Aurora, CO) to minimize electrical impedance. EMG signals were amplified (×200), band-pass filtered (0.1 Hz to 3 kHz) and sampled at a rate of 10 kHz. Analog signals were converted to digital (Powerlab/16SP ML795, AD Instruments) and recorded simultaneously on a computer (Labchart v8, AD Instruments). M-wave onset latency, duration, peak-to-peak amplitude, and total rectified area were subsequently analyzed for each participant using a custom algorithm (MATLAB R2015a, MathWorks, Natick, MA) (48).
Data analysis.
The mechanical work of breathing (WOB) was determined using esophageal pressure-volume loop integration (17). Pressure-time products were calculated during periods of inspiratory flow for esophageal (PTPes), gastric (PTPga), and transdiaphragmatic (PTPdi) pressure at distinct time points during exercise (10% increments). Nonrepresentative breaths (e.g., coughs, swallows, sighs, etc.) were not included. Previous studies from our group (21, 44) and others (4, 32) have considered fatigue of the diaphragm if a ≥15% reduction in Pdi,tw relative to the preexercise baseline values at any time point after exercise is present. In the current study, the criterion used for DF was a ≥20% reduction in Pdi,tw based upon our within-day coefficient of variation for twitch pressures of 9.4%. To be confident of a significant reduction in diaphragmatic force output, a value twofold greater than the coefficient of variation was chosen as a conservative threshold for detection of fatigue. The first two twitches were excluded from analysis to ensure adequate potentiation, and subsequent twitches were excluded for any of the following reasons: 1) the subject was not resting at functional residual capacity immediately before stimulation; 2) if esophageal peristalsis was evident at the time of the twitch; 3) if a cardiac artifact was superimposed upon a stimulus; 4) if there was lack of diaphragmatic relaxation evidenced by noticeable diaphragm EMG; and 5) if the preceding maximal inspiratory maneuver was <70% of the maximal value obtained at any time point.
Statistical analysis.
Normality of the data was determined by visual inspection and Shapiro-Wilk tests. A one-way repeated-measures ANOVA with a Tukey post hoc test was performed to verify differences between exercise-related variables on days 2, 3, and 4. A one-way ANOVA with a Dunnett post hoc test was performed to identify differences in Pdi,tw between submaximal and maximal (100%) stimulation intensities during the supramaximal diaphragmatic stimulation protocol. A two-way repeated-measures ANOVA was used to determine if differences existed in DF between different exercise trials (50, 75, and 100% TTE) and time points (5, 15, and 30 min after exercise). The Holm-Sidak post hoc test was used in the event of main effects. Pearson product-moment correlations were performed between the magnitude of fatigue and cumulative diaphragmatic force output; correlations were interpreted as moderate (r = 0.40–0.59), strong (r = 0.60–0.79), and very strong (r = 0.80–0.99). A significant difference was considered when P < 0.05. Values are presented as means ± SD unless otherwise stated.
RESULTS
Sample characteristics, pulmonary function, and maximal incremental exercise data.
Table 1 summarizes participant demographics, pulmonary function, and aerobic fitness. Subjects had normal spirometric values. Aerobic fitness ranged from 44.9 to 75.6 ml·kg−1·min−1 (118.9 ± 16.2% predicted) (37).
Table 1.
Participant characterization, pulmonary function, and maximal incremental exercise data
| Value | |
|---|---|
| Age, yr | 25 ± 5 |
| Anthropometrics | |
| Height, cm | 182 ± 8 |
| Body mass, kg | 74.5 ± 5.8 |
| BMI, kg/m2 | 22.4 ± 0.8 |
| Pulmonary function | |
| FVC, liters | 5.8 ± 0.5 |
| FVC, % predicted | 105.9 ± 10.9 |
| FEV1, liters | 4.8 ± 0.4 |
| FEV1, % predicted | 104.6 ± 5.8 |
| FEV1/FVC, % | 83.3 ± 5.4 |
| FEV1/FVC, % predicted | 100.1 ± 6.9 |
| FEF25–75, l/s | 4.7 ± 0.8 |
| FEF25–75, % predicted | 91.9 ± 15.6 |
| PEF, l/s | 11.6 ± 0.8 |
| PEF, % predicted | 111.6 ± 12.3 |
| Maximal incremental exercise | |
| HR, beats/min | 188 ± 11 |
| V̇o2, ml·min–1·kg–1 | 58.8 ± 9.5 |
| V̇o2, l/min | 4.4 ± 0.5 |
| V̇co2, l/min | 5.2 ± 0.6 |
| RER | 1.20 ± 0.04 |
| V̇e, l/min | 178.6 ± 25.4 |
| fb, breaths/min | 57 ± 11 |
| Workload, W | 373.9 ± 45.1 |
| V̇o2/workload, ml·min–1·W—1 | 10.5 ± 0.9 |
Values are means ± SD; n = 8. BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEF25–75, forced expiratory flow in 25–75% of pulmonary volume; PEF, peak expiratory flow; HR, heart rate; V̇o2, oxygen consumption; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; V̇e, minute ventilation; fb, breathing frequency.
Supramaximal stimulation.
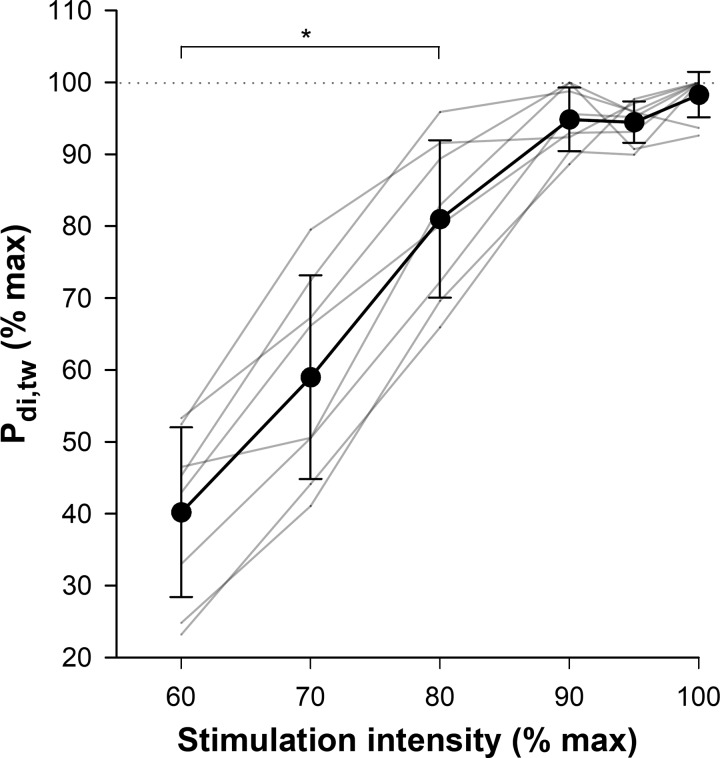
A plateau in Pdi,tw was observed at 90 and 95% of the peak stimulator intensity (P > 0.05). Upon inspection of individual data, clear evidence of a plateau occurred in all participants (Fig. 1). The between-day coefficient of variation for twitch pressures was 11.9%.
Fig. 1.
Relative transdiaphragmatic twitch pressure (Pdi,tw) responses to increasing cervical magnetic stimulation intensity. Gray lines represent individual values and the thick black line represents group mean ± SD *P < 0.01 vs. 100% stimulation intensity.
Time-to-exhaustion trials.
Results of the TTE test (day 2) and subsequent two constant-load exercise tests (days 3 and 4) are presented in Table 2. The workload was 299.7 ± 39.5 W, corresponding to 86 ± 1% of maximal V̇o2. TTE was 08:18 ± 01:11 min (range = 06:15–09:47 min). There was no significant difference between trials in relative values of V̇o2, absolute values of carbon dioxide production, or cycling cadence (P > 0.05). Minute ventilation (V̇e) progressively increased with exercise duration, concomitant with a reduction in the respiratory exchange ratio and (P < 0.05). Perceived leg and breathing discomfort was significantly greater at 100% TTE compared with 75 (P = 0.001 and 0.027, respectively) and 50% TTE (P = 0.001 and 0.006, respectively).
Table 2.
Exercise data from 50, 75, and 100% time-to-exhaustion (TTE) trials
| 50% TTE | 75% TTE | 100% TTE | |
|---|---|---|---|
| Time, s | 249.0 ± 35.8 | 373.5 ± 53.7 | 498.1 ± 71.5* |
| HR, beats/min | 178 ± 8 | 179 ± 14 | 182 ± 9* |
| V̇o2, ml·min–1·kg–1 | 55.0 ± 11.3 | 57.8 ± 9.9 | 57.5 ± 9.6 |
| V̇o2, l/min | 4.1 ± 0.7 | 4.3 ± 0.6 | 4.3 ± 0.6 |
| V̇co2, l/min | 4.6 ± 0.7 | 4.7 ± 0.5 | 4.5 ± 0.6 |
| RER | 1.14 ± 0.04 | 1.10 ± 0.07* | 1.05 ± 0.04*† |
| V̇e, l/min | 137.7 ± 32.2 | 157.3 ± 26.0* | 168.7 ± 36.5* |
| , mmHg | 38.7 ± 6.5 | 32.8 ± 4.7* | 28.7 ± 4.4*† |
| fb, breaths/min | 40 ± 9 | 47 ± 6* | 56 ± 10*† |
| VT, liters | 3.2 ± 0.4 | 3.1 ± 0.4 | 2.8 ± 0.4*† |
| Cadence, rpm | 102 ± 13 | 99 ± 11 | 97 ± 12 |
| Breathing discomfort, points | 4.3 ± 2.3 | 5.1 ± 2.4 | 7.9 ± 2.4*† |
| Leg discomfort, points | 5.4 ± 1.9 | 7.3 ± 1.4* | 9.9 ± 0.4*† |
| PTPes, cmH2O/s | 2,232.2 ± 569.1 | 3,925.9 ± 1,073.6* | 5,931.8 ± 1388.6*† |
| PTPdi, cmH2O/s | 1,704.2 ± 405.3 | 2,974.1 ± 658.2* | 4,266.7 ± 1,246.0*† |
| PTPga, cmH2O/s | 503.1 ± 187.3 | 813.3 ± 377.1* | 1,135.4 ± 426.7*† |
Values are means ± SD. HR, heart rate; V̇o2, oxygen consumption; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; V̇e, minute ventilation; , end-tidal pressure of carbon dioxide; fb, breathing frequency; VT, tidal volume; PTPes, cumulative esophageal pressure-time product; PTPdi, cumulative diaphragmatic pressure-time product; PTPga, cumulative gastric pressure-time product.
P < 0.05 from 50% TTE trial;
P < 0.05 from 75% TTE trial.
Diaphragmatic fatigue.
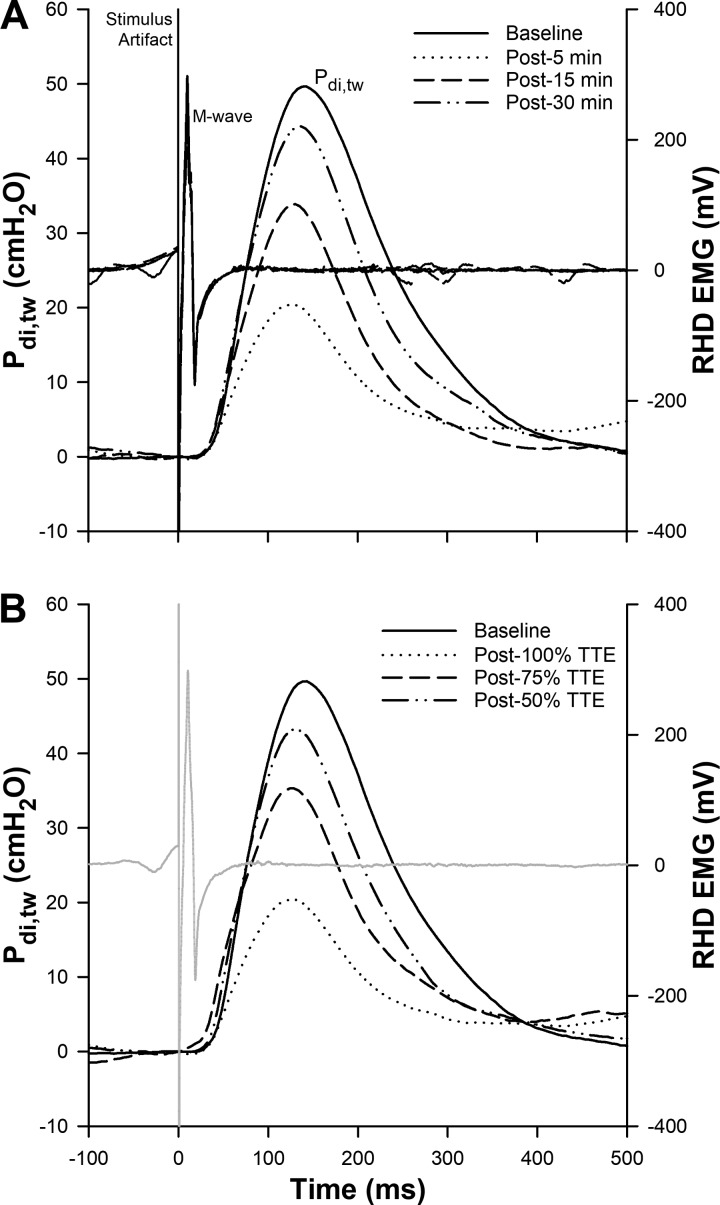
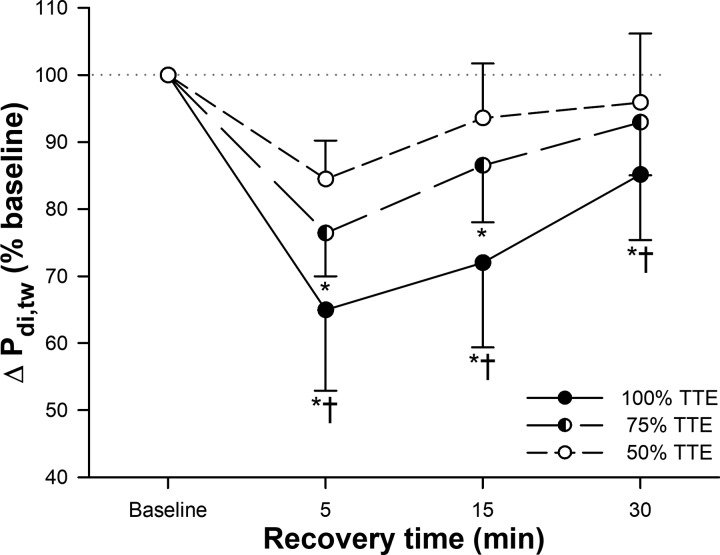
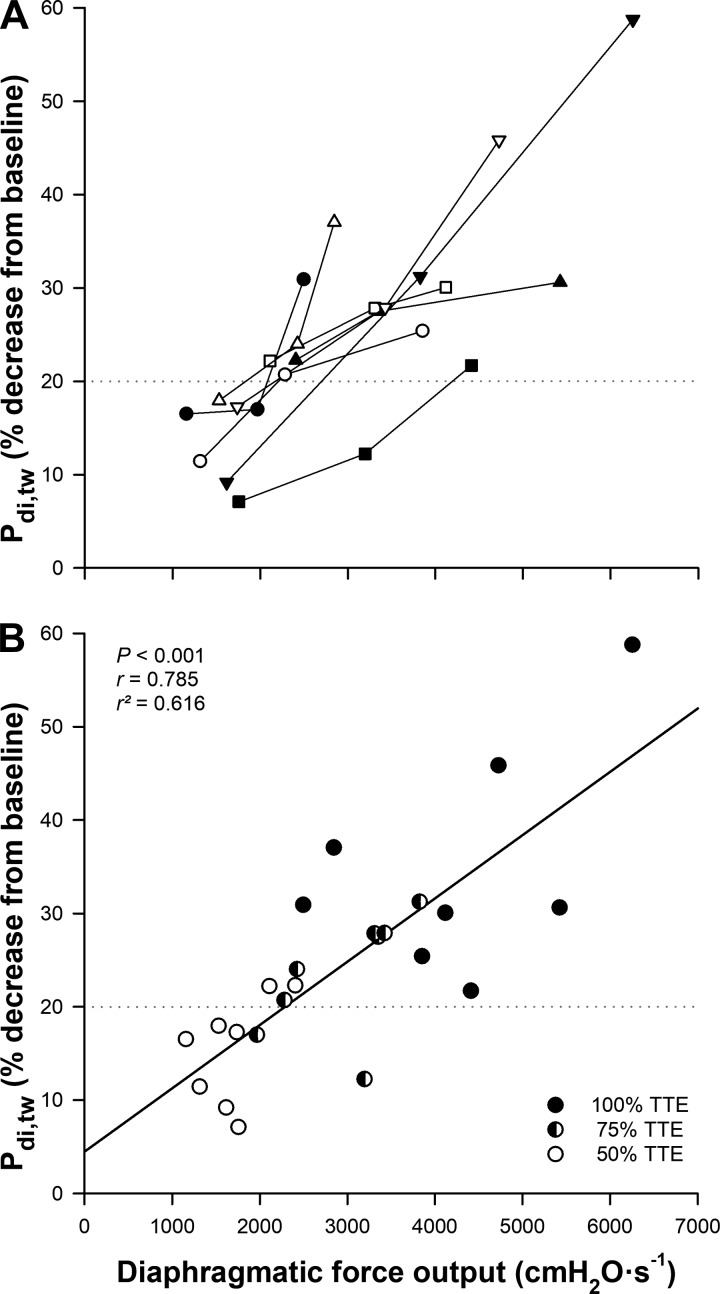
An illustration of the mechanical (Pdi,tw) and electrical (M-wave) responses of the diaphragm to phrenic nerve stimulation is presented in Fig. 2, A and B (one representative subject). DF was present after 75% (in 6 of 8 participants) and 100% (in all participants) TTE trials; however, the magnitude of reduction in Pdi,tw was progressively larger as exercise continued. The percent decrease in Pdi,tw was 15.5 ± 5.7%, 23.6 ± 6.4%, and 35.0 ± 12.1% at 50, 75, and 100% TTE, respectively (Fig. 3). DF was significantly greater following the 100% TTE than both 50 and 75% (both P < 0.001) TTE trials, and 75% TTE than 50% (P = 0.009) TTE. After 15 min of recovery, DF was still significantly greater in 100% TTE compared with 50 and 75% (both P < 0.001) TTE trials, and 75 compared with 50% (P = 0.020) TTE. Moreover, after 30 min recovery, DF presented higher only in 100% TTE compared with 50 (P = 0.002) and 75% (P = 0.022) TTE trials. The magnitude of fatigue was significantly correlated with the cumulative diaphragmatic force output (P < 0.001, r = 0.785 and r2 = 0.616) [Fig. 4A (individual values) and Fig. 4B]. End-expiratory Pes was unchanged at the time of stimulation, and M-wave characteristics were not different relative to baseline measures (P > 0.05) (Table 3).
Fig. 2.
One subject representative raw data traces from within-day [100% time-to-exhaustion (TTE) trial] (A), and between-days (50, 75, and 100% TTE trials) (B) of transdiaphragmatic twitch pressures (Pdi,tw) and right hemidiaphragm (RHD) electromyography (EMG). Note that on A, there are 4 EMG traces superimposed for each time point; however, on B, there is only one representative EMG trace in light gray from baseline.
Fig. 3.
Transdiaphragmatic twitch pressure (Pdi,tw) responses during recovery after 50, 75, and 100% time-to-exhaustion (TTE) trials of exercise. Values are means ± SD. *P < 0.01 vs. 50% TTE trial; †P < 0.01 vs. 75% TTE trial.
Fig. 4.
A: individual relationship between total diaphragmatic force output and the decrease of transdiaphragmatic twitch pressure (Pdi,tw). Each participant represents one symbol linked three times, which corresponds to 50, 75, and 100% of time-to-exhaustion (TTE). B: correlation of this relationship from 50 (open circles), 75 (half-open circles), and 100% (closed circles) TTE trials. The dashed horizontal lines in both panels represents the criterion used to consider diaphragmatic fatigue present (≥20% reduction in Pdi,tw).
Table 3.
End-expiratory esophageal pressure (Pes) before stimuli, and M-wave data
| End-Expiratory Pes | 50% TTE | 75% TTE | 100% TTE |
|---|---|---|---|
| Baseline, cmH2O | −1.9 ± 0.7 | −2.3 ± 1.1 | −2.5 ± 0.6 |
| Post 5 min, cmH2O | −3.1 ± 0.6 | −4.0 ± 0.9 | −3.7 ± 0.7 |
| Post 15 min, cmH2O | −2.9 ± 0.2 | −2.9 ± 1.0 | −3.5 ± 0.6 |
| Post 30 min, cmH2O | −2.1 ± 0.3 | −2.5 ± 0.3 | −2.8 ± 0.7 |
| Left Hemidiaphragm |
Right Hemidiaphragm |
|||
|---|---|---|---|---|
| M-Wave Characteristic | Baseline | Postexercise | Baseline | Postexercise |
| Latency, ms | 5.1 ± 0.5 | 4.9 ± 0.9 | 4.4 ± 0.2 | 4.3 ± 0.2 |
| Duration, ms | 40.6 ± 8.3 | 40.8 ± 4.1 | 40.6 ± 8.4 | 44.4 ± 2.2 |
| Amplitude, mV | 4.5 ± 1.1 | 4.6 ± 0.9 | 4.4 ± 1.4 | 4.7 ± 1.4 |
| Area, mV/ms | 38.4 ± 7.6 | 41.1 ± 8.2 | 37.5 ± 9.3 | 41.8 ± 10.8 |
Values are means ± SD.
Work of breathing.
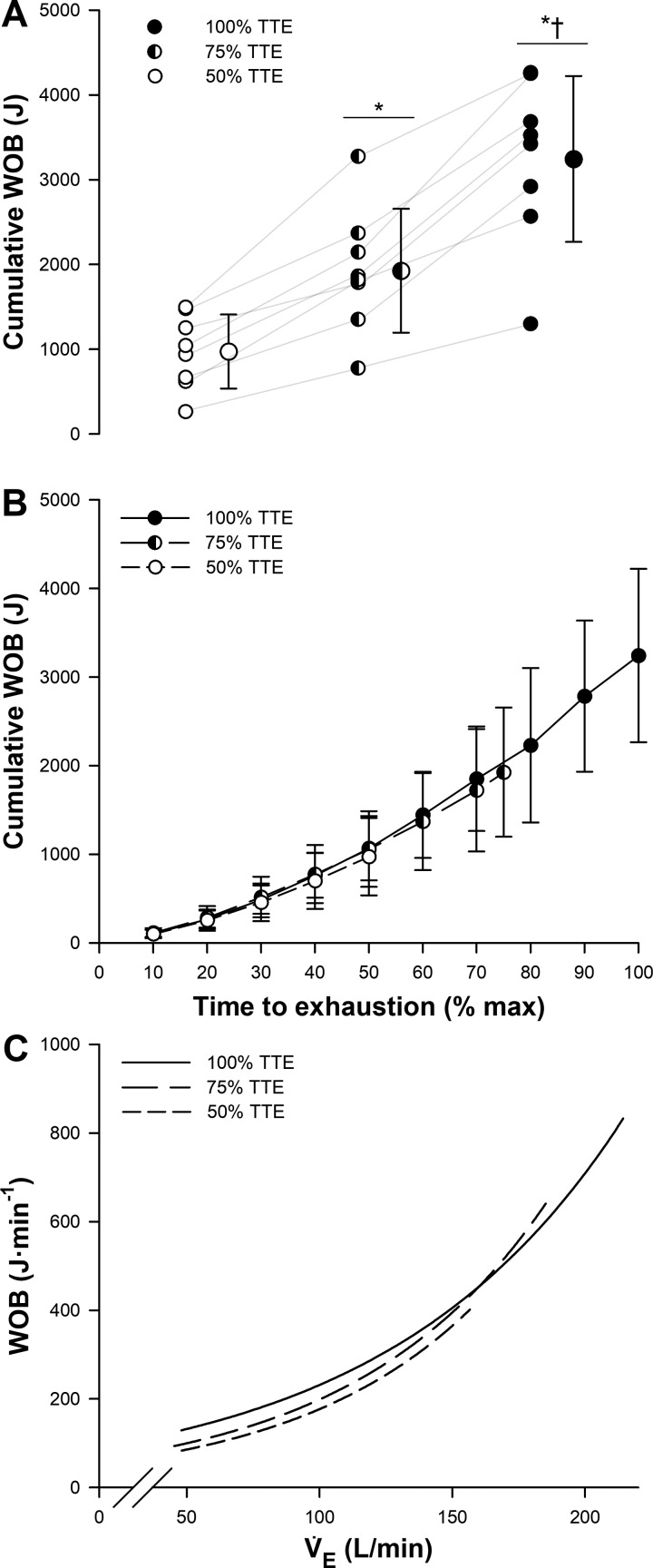
The cumulative WOB (Fig. 5A) was significantly greater after 100% TTE compared with both 50 and 75% (both P < 0.001) TTE trials, and after 75% TTE compared with 50% (P < 0.001) TTE. Importantly, the cumulative WOB presented a similar increasing pattern throughout all trials (P > 0.05) (Fig. 5B), and the relationship between WOB and V̇e was also similar between trials (Fig. 5C); thus the progressive decrease in Pdi,tw was not caused by disparities in the WOB.
Fig. 5.
A: accumulated mechanical work of breathing (WOB) after 50, 75, and 100% time-to-exhaustion (TTE) trials. Individual values are linked through gray lines along trials. Group means ± SD are also presented for each trial. B: cumulative WOB throughout exercise trials. Values are means ± SD. C: relationship between WOB and minute ventilation (V̇e) during 50, 75, and 100% TTE trials (mean values of WOB and V̇e were calculated in 10% increments throughout exercise). *P < 0.05 vs. 50% TTE trial; †P < 0.05 vs. 75% TTE trial.
Pressure-time products.
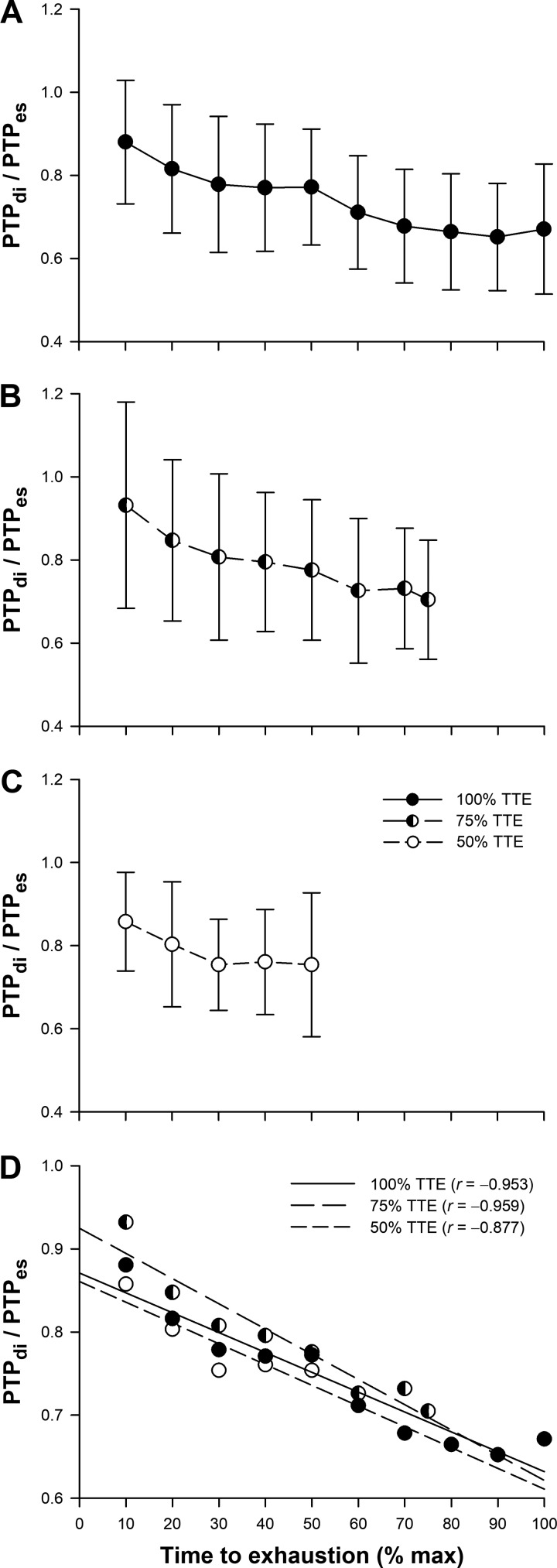
All trials demonstrated a similar time course of respiratory muscle pressure generation. A progressive increase in PTPes, PTPdi, and PTPga (Table 2) was observed throughout exercise. No significant differences were found for any variable between trials (P > 0.05). Additionally, Fig. 6, A–D, shows the diaphragmatic contribution to total inspiratory muscle force output in all trials. A linear fall in PTPdi/PTPes was observed as exercise continued (Fig. 6D); no significant differences were found between trials at any time point during exercise (P > 0.05).
Fig. 6.
Ratio between diaphragmatic (PTPdi) and esophageal (PTPes) pressure-time products during 100% time-to-exhaustion (TTE) (A), 75% TTE (B), and 50% TTE exercise trials (C). Values are means ± SD. D: all-trials correlations between PTPdi/PTPes and TTE.
DISCUSSION
Main findings.
We investigated the temporal onset of exercise-induced DF in healthy men. We found that DF is present after as little as 6 min high-intensity endurance exercise and progressively worsens as exercise continues toward exhaustion. Moreover, we found a strong relationship between diaphragm force output and the magnitude of DF. This suggests that DF is intrinsically linked to the amount of generated pressure and work performed by the respiratory muscles. To our knowledge, this is the first study to show that diaphragm contractile strength (i.e., Pdi,tw) progressively decreases during high-intensity endurance exercise in healthy men.
Exercise-induced diaphragmatic fatigue.
It is well documented that DF occurs under certain conditions of exercise; in particular, DF is dependent upon the intensity and duration of exercise. In addition, diaphragm function is unimpaired unless a limitation is placed upon respiratory muscle perfusion which creates metabolic milieu (41). Several studies have demonstrated the presence of DF after submaximal endurance exercise above 80–85% of maximal V̇o2 (2–4, 6, 27); conversely, others found that DF does not occur after incremental exercise (34, 40, 45). Thus the intensity (constant vs. incremental) and duration of whole body exercise appears to play an important role in the etiology of DF. Our findings are in agreement that constant high-intensity and duration of exercise are important determinants of exercise-induced DF. Significant increases in diaphragm lactate concentration have been observed during high-intensity exercise in rodents (19). Although glycogen content was unchanged, the accumulation of metabolites within the muscle likely impaired crossbridge formation. However, diaphragm contractile strength declined progressively; therefore, exercise intensity cannot fully explain our observations due to workload being unchanged throughout. Thus exercise duration and concurrent changes in the cumulative WOB contribute to the progressive decline in diaphragm contractility.
Another key variable in the development of exercise-induced DF relates to energy supply and demand. Here, the adequacy of respiratory muscle perfusion and oxygenation are paramount. Babcock and colleagues (4) demonstrated that mimicking exercise-related diaphragmatic work at rest does not result in DF. Therefore, factors not associated with exercise duration or intensity must be accountable. Mimicking exercise hyperpnea at rest fails to replicate the competition for finite cardiac output that takes place between the respiratory and locomotor muscles. In other words, the work done by the respiratory muscles during exercise is insufficient alone to cause DF unless a limitation is placed upon its blood supply. What remains equivocal, however, is at what time point during exercise does diaphragm fatigue occur.
Temporal characteristics.
The time course of DF onset has only been examined by a select few studies. Our study measured DF on three separate occasions where exercise intensity and, most importantly, the WOB were not different between trials at a given time point. Furthermore, phrenic nerve activation was maximal in all subjects, and potentiated twitches were delivered at functional residual capacity. Thus factors known to affect diaphragm contractility such as lung volumes (length-tension relationship) and degree of potentiation were monitored and did not differ between trials. We reason that the magnitude of DF is time dependent of the increasing WOB in conditions of high-intensity endurance exercise. This theory is further supported by analyzing the ratio between PTPdi and PTPes where a linear fall represents an increase in the activation of accessory inspiratory muscles, implying that the diaphragm becomes fatigued over time.
On the other hand, Kabitz and colleagues (28) concluded that diaphragmatic muscle contractility/strength increases progressively with exercise workload, and DF only occurs after exercise rather than during. Some specific methodological limitations should be pointed out: first, the degree of phrenic nerve activation was not assessed, and thus it is not known if the phrenic nerves were maximally stimulated; second, exercise was not performed to exhaustion and workload was not constant, which infers that the participants did not perform the same relative amount of exercise; third, phrenic nerve stimulation was delivered during exercise (subjects were instructed to stop cycling for 1 s and regain momentum after stimulation delivery) without any indication of consistent or effective potentiation. The first conclusion made by Kabitz and coworkers can be explained by poorly controlled nonpotentiated twitches that were delivered throughout exercise. As exercise workload increases, the diaphragm becomes progressively potentiated as the pressure generated by the muscle increases over time to achieve the required alveolar ventilation; consequently, Pdi,tw will be larger than at rest. When nonpotentiated twitches are assessed again after exercise, Pdi,tw is reduced as calcium ions are pumped back to the sarcoplasmic reticulum, thereby decreasing the ability of the muscle to generate force. Other studies performed by the same group presented similar limitations (29, 30, 46).
Relationship between the work of breathing and diaphragmatic fatigue.
Our findings suggest that exercise-induced DF is determined primarily by the WOB. To our knowledge, the only study to make this inference comes from the work of Babcock et al. (2). By reducing the WOB during high-intensity exercise via proportional assist ventilation, the relationship between DF and the WOB was revealed. Under control conditions (no assist), a 20% decrease in Pdi,tw was observed. Conversely, mechanically unloading the respiratory muscles prevented DF. The authors attributed their findings to a lower breathing frequency, PTPdi, and PTPdi/PTPes during ventilatory-assisted exercise; they concluded that the required diaphragmatic force output during high-intensity endurance exercise plays a major role in exercise-induced DF. In agreement with this postulate, we observed a strong relationship between the cumulative WOB and magnitude of DF. Increased respiratory rate elevates the mechanical WOB by virtue of the force-velocity relationship. The WOB is believed to have a profound effect on blood flow distribution during exercise, which may also affect DF development.
Harms and colleagues (22, 23) demonstrated that the amount of work performed by the respiratory muscles is inversely related to the magnitude of limb blood flow, representing a 14–16% redirection of total cardiac output from locomotor to respiratory muscles. The authors found that alleviating the WOB by ~50% during high-intensity exercise equals ~2 l/min of cardiac output diverted from the legs; accordingly, leg vascular resistance and V̇o2 were also impaired by the augmented WOB. The reduction in limb blood flow, modulated by the WOB, coincides with the progressive fall in diaphragm force output observed in the present study. In a similar experiment using near-infrared spectroscopy and indocyanine green dye, our group recently demonstrated how respiratory and locomotor blood flow is moderated by the WOB (14). Clearly, when the WOB is decreased, less blood flow is required to the respiratory muscles; conversely, increasing the WOB leads to an increase in respiratory muscle perfusion at the expense of active locomotor muscles. Therefore, the magnitude of work done by the respiratory muscles and thus the occurrence of DF have major implications concerning hemodynamics, ultimately affecting the ventilatory response and tolerance to high-intensity exercise. However, the increase in WOB and V̇e over time make it difficult to differentiate between the cumulative or instantaneous level of WOB.
Perspectives: consequences of exercise-induced diaphragmatic fatigue.
DF is associated with many functional consequences and adaptations that may affect exercise performance. For example, as exercise continues toward exhaustion, the diaphragm is assisted by extradiaphragmatic muscles (such as the sternocleidomastoid, external intercostal muscles, and parasternal muscles) to accommodate further increases in ventilation, while expiratory muscles are recruited to lower end-expiratory lung volume. Doing so alters chest wall mechanics leading to an increased oxygen cost of breathing and heightened sensory perception of breathing discomfort (i.e., dyspnea) (13, 20, 47). In our study, dyspnea was significantly greater at exhaustion (i.e., 100% TTE) relative to 50 and 75% TTE exercise trials. On the other hand, 50 and 75% TTE exercise trials were not different from each other, suggesting that high levels of dyspnea are reached near exhaustion and are “linked” to the presence of DF. The recruitment of extradiaphragmatic muscles may have contributed to the disassociation between sensory input and mechanical output.
Another consequence of exercise-induced DF is the respiratory muscle metaboreflex. Activation of mechanically and metabolically sensitive thin fiber phrenic nerve afferents reflexly increases sympathetic outflow resulting in vasoconstriction and decreased vascular conductance in the active limb (12, 25, 26, 43). There is evidence that blood flow is redistributed, at least in part, to the fatigued respiratory muscles (14, 22, 23), although it is still unclear if there is also vasoconstriction in the respiratory musculature in response to metaboreflex sympathetic outflow. As a result of the reduction in active limb blood flow, quadriceps muscle fatigue is hastened which, in turn, induces central fatigue and the termination of exercise (15, 39). In our study, subjective assessment of leg fatigue (i.e., leg discomfort) was significantly higher after 100% TTE compared with 50 and 75% TTE exercise trials, and also greater after 75% TTE compared with 50% TTE exercise trials. The respiratory muscle metaboreflex is time-dependent; our results conform to this mandate and suggest that a respiratory metaboreflex may have augmented limb fatigue. Therefore, the consequences associated with DF may influence exercise tolerance through dyspnea or metaboreflex-induced alterations in limb flow and fatigue.
Limitations.
Some limitations in our study must be acknowledged. We did not measure blood acidity and/or metabolite concentration during trials. It is highly likely that as exercise continued toward exhaustion, changes in blood pH, arterial Po2 and Pco2, , and K+ (i.e., strong-ion difference) created an acidic environment that catalyzed the development of DF. Methodologically, magnetic stimulation of the phrenic nerve roots is unavoidably hindered by the activation of nondiaphragmatic muscles, which may contribute to contamination of Pdi,tw and EMG. Last, operating lung volumes were not measured during exercise; therefore, the presence of expiratory flow limitation and dynamic hyperinflation may have influenced findings. However, any shift in operational lung volumes would also affect lung and chest wall compliance that would be reflected in pressure-volume relationships and WOB. There was no change in WOB at any given time point during exercise; as a result, we believe that lung volumes were similar between trials.
Conclusions.
Our results indicate that the development and severity of exercise-induced DF are directly related to the cumulative WOB. Building upon previous observations, our findings further support the notion that high-intensity submaximal exercise is sufficient to induce DF in healthy men. We provide evidence that diaphragm contractility progressively declines throughout high-intensity whole body exercise. Recruitment of extradiaphragmatic muscles aids the diaphragm in meeting ventilatory demand without compromising respiratory muscle function.
GRANTS
This study was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada. B. Archiza was supported by the São Paulo Research Foundation (FAPESP) Postgraduate Scholarship (Grant 2016/08999–5). J. F. Welch was supported by a University of British Columbia fellowship. C. M. Geary and G. P. Allen were supported by NSERC Student Research Awards.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F.W., G.P.A., and A.W.S. conceived and designed research; B.A., J.F.W., C.M.G., G.P.A., and A.W.S. performed experiments; B.A., J.F.W., C.M.G., and A.W.S. analyzed data; B.A., J.F.W., C.M.G., G.P.A., A.B.-S., and A.W.S. interpreted results of experiments; B.A. and J.F.W. prepared figures; B.A., J.F.W., C.M.G., G.P.A., A.B.-S., and A.W.S. drafted manuscript; B.A., J.F.W., A.B.-S., and A.W.S. edited and revised manuscript; B.A., J.F.W., C.M.G., G.P.A., A.B.-S., and A.W.S. approved final version of manuscript.
REFERENCES
- 1.Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol (1985) 72: 1818–1825, 1992. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- 2.Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol (1985) 93: 201–206, 2002. doi: 10.1152/japplphysiol.00612.2001. [DOI] [PubMed] [Google Scholar]
- 3.Babcock MA, Pegelow DF, Johnson BD, Dempsey JA. Aerobic fitness effects on exercise-induced low-frequency diaphragm fatigue. J Appl Physiol (1985) 81: 2156–2164, 1996. doi: 10.1152/jappl.1996.81.5.2156. [DOI] [PubMed] [Google Scholar]
- 4.Babcock MA, Pegelow DF, McClaran SR, Suman OE, Dempsey JA. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol (1985) 78: 1710–1719, 1995. doi: 10.1152/jappl.1995.78.5.1710. [DOI] [PubMed] [Google Scholar]
- 6.Babcock MA, Pegelow DF, Taha BH, Dempsey JA. High frequency diaphragmatic fatigue detected with paired stimuli in humans. Med Sci Sports Exerc 30: 506–511, 1998. doi: 10.1097/00005768-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 8.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Casaburi R, Storer TW, Ben-Dov I, Wasserman K. Effect of endurance training on possible determinants of V̇o2 during heavy exercise. J Appl Physiol (1985) 62: 199–207, 1987. doi: 10.1152/jappl.1987.62.1.199. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol 151: 242–250, 2006. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol 130: 3–20, 2002. doi: 10.1016/S0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 12.Derchak PA, Sheel AW, Morgan BJ, Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol (1985) 92: 1539–1552, 2002. doi: 10.1152/japplphysiol.00790.2001. [DOI] [PubMed] [Google Scholar]
- 13.Dodd DS, Yarom J, Loring SH, Engel LA. O2 cost of inspiratory and expiratory resistive breathing in humans. J Appl Physiol (1985) 65: 2518–2523, 1988. doi: 10.1152/jappl.1988.65.6.2518. [DOI] [PubMed] [Google Scholar]
- 14.Dominelli PB, Archiza B, Ramsook AH, Mitchell RA, Peters CM, Molgat-Seon Y, Henderson WR, Koehle MS, Boushel R, Sheel AW. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102: 1535–1547, 2017. doi: 10.1113/EP086566. [DOI] [PubMed] [Google Scholar]
- 15.Dominelli PB, Molgat-Seon Y, Griesdale DEG, Peters CM, Blouin J-S, Sekhon M, Dominelli GS, Henderson WR, Foster GE, Romer LM, Koehle MS, Sheel AW. Exercise-induced quadriceps muscle fatigue in men and women: effects of arterial oxygen content and respiratory muscle work. J Physiol 595: 5227–5244, 2017. doi: 10.1113/JP274068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM, Sheel AW. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593: 1965–1979, 2015. doi: 10.1113/jphysiol.2014.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominelli PB, Sheel AW. Experimental approaches to the study of the mechanics of breathing during exercise. Respir Physiol Neurobiol 180: 147–161, 2012. doi: 10.1016/j.resp.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J; ERS Global Lung Function Initiative . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 40: 1324–1343, 2012. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fregosi RF, Dempsey JA. Effects of exercise in normoxia and acute hypoxia on respiratory muscle metabolites. J Appl Physiol (1985) 60: 1274–1283, 1986. doi: 10.1152/jappl.1986.60.4.1274. [DOI] [PubMed] [Google Scholar]
- 20.Goldman MD, Grimby G, Mead J. Mechanical work of breathing derived from rib cage and abdominal V-P partitioning. J Appl Physiol 41: 752–763, 1976. doi: 10.1152/jappl.1976.41.5.752. [DOI] [PubMed] [Google Scholar]
- 21.Guenette JA, Romer LM, Querido JS, Chua R, Eves ND, Road JD, McKenzie DC, Sheel AW. Sex differences in exercise-induced diaphragmatic fatigue in endurance-trained athletes. J Appl Physiol (1985) 109: 35–46, 2010. doi: 10.1152/japplphysiol.01341.2009. [DOI] [PubMed] [Google Scholar]
- 22.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 82: 1573–1583, 1997. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 23.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol (1985) 85: 609–618, 1998. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 24.Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol (1985) 89: 131–138, 2000. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SN, Chatillon A, Comtois A, Roussos C, Magder S. Chemical activation of thin-fiber phrenic afferents. 2. Cardiovascular responses. J Appl Physiol (1985) 70: 77–86, 1991. doi: 10.1152/jappl.1991.70.1.77. [DOI] [PubMed] [Google Scholar]
- 26.Jammes Y, Balzamo E. Changes in afferent and efferent phrenic activities with electrically induced diaphragmatic fatigue. J Appl Physiol (1985) 73: 894–902, 1992. doi: 10.1152/jappl.1992.73.3.894. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol 460: 385–405, 1993. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabitz HJ, Walker D, Schwoerer A, Sonntag F, Walterspacher S, Roecker K, Windisch W. New physiological insights into exercise-induced diaphragmatic fatigue. Respir Physiol Neurobiol 158: 88–96, 2007. doi: 10.1016/j.resp.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Kabitz HJ, Walker D, Sonntag F, Walterspacher S, Kirchberger A, Burgardt V, Roecker K, Windisch W. Post-exercise diaphragm shielding: a novel approach to exercise-induced diaphragmatic fatigue. Respir Physiol Neurobiol 162: 230–237, 2008. doi: 10.1016/j.resp.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Kabitz HJ, Walker D, Walterspacher S, Sonntag F, Schwoerer A, Roecker K, Windisch W. Independence of exercise-induced diaphragmatic fatigue from ventilatory demands. Respir Physiol Neurobiol 161: 101–107, 2008. doi: 10.1016/j.resp.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Kabitz HJ, Walker DJ, Schwoerer A, Schlager D, Walterspacher S, Storre JH, Roecker K, Windisch W, Vergès S, Spengler CM. Biometric approximation of diaphragmatic contractility during sustained hyperpnea. Respir Physiol Neurobiol 176: 90–97, 2011. doi: 10.1016/j.resp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Kufel TJ, Pineda LA, Junega RG, Hathwar R, Mador MJ. Diaphragmatic function after intense exercise in congestive heart failure patients. Eur Respir J 20: 1399–1405, 2002. doi: 10.1183/09031936.02.00016702. [DOI] [PubMed] [Google Scholar]
- 33.Lansley KE, Dimenna FJ, Bailey SJ, Jones AM. A “new” method to normalise exercise intensity. Int J Sports Med 32: 535–541, 2011. doi: 10.1055/s-0031-1273754. [DOI] [PubMed] [Google Scholar]
- 34.Levine S, Henson D. Low-frequency diaphragmatic fatigue in spontaneously breathing humans. J Appl Physiol (1985) 64: 672–680, 1988. doi: 10.1152/jappl.1988.64.2.672. [DOI] [PubMed] [Google Scholar]
- 35.Man WD-C, Moxham J, Polkey MI. Magnetic stimulation for the measurement of respiratory and skeletal muscle function. Eur Respir J 24: 846–860, 2004. doi: 10.1183/09031936.04.00029004. [DOI] [PubMed] [Google Scholar]
- 36.Milic-Emili J, Orzalesi MM, Cook CD, Turner JM. Respiratory thoraco-abdominal mechanics in man. J Appl Physiol 19: 217–223, 1964. doi: 10.1152/jappl.1964.19.2.217. [DOI] [PubMed] [Google Scholar]
- 37.Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E, Arena R. A reference equation for normal standards for V̇o2max: analysis from the fitness registry and the importance of exercise national database (FRIEND registry). Prog Cardiovasc Dis 60: 21–29, 2017. doi: 10.1016/j.pcad.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Renggli AS, Verges S, Notter DA, Spengler CM. Development of respiratory muscle contractile fatigue in the course of hyperpnoea. Respir Physiol Neurobiol 164: 366–372, 2008. doi: 10.1016/j.resp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol 571: 425–439, 2006. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romer LM, Miller JD, Haverkamp HC, Pegelow DF, Dempsey JA. Inspiratory muscles do not limit maximal incremental exercise performance in healthy subjects. Respir Physiol Neurobiol 156: 353–361, 2007. doi: 10.1016/j.resp.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol (1985) 104: 879–888, 2008. doi: 10.1152/japplphysiol.01157.2007. [DOI] [PubMed] [Google Scholar]
- 42.Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA 107: 20541–20546, 2010. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529: 493–504, 2000. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomczak SE, Guenette JA, Reid WD, McKenzie DC, Sheel AW. Diaphragm fatigue after submaximal exercise with chest wall restriction. Med Sci Sports Exerc 43: 416–424, 2011. doi: 10.1249/MSS.0b013e3181ef5e67. [DOI] [PubMed] [Google Scholar]
- 45.Verin E, Ross E, Demoule A, Hopkinson N, Nickol A, Fauroux B, Moxham J, Similowski T, Polkey MI. Effects of exhaustive incremental treadmill exercise on diaphragm and quadriceps motor potentials evoked by transcranial magnetic stimulation. J Appl Physiol (1985) 96: 253–259, 2004. doi: 10.1152/japplphysiol.00325.2003. [DOI] [PubMed] [Google Scholar]
- 46.Walker DJ, Walterspacher S, Schlager D, Ertl T, Roecker K, Windisch W, Kabitz HJ. Characteristics of diaphragmatic fatigue during exhaustive exercise until task failure. Respir Physiol Neurobiol 176: 14–20, 2011. doi: 10.1016/j.resp.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Ward ME, Eidelman D, Stubbing DG, Bellemare F, Macklem PT. Respiratory sensation and pattern of respiratory muscle activation during diaphragm fatigue. J Appl Physiol (1985) 65: 2181–2189, 1988. doi: 10.1152/jappl.1988.65.5.2181. [DOI] [PubMed] [Google Scholar]
- 48.Welch JF, Mildren RL, Zaback M, Archiza B, Allen GP, Sheel AW. Reliability of the diaphragmatic compound muscle action potential evoked by cervical magnetic stimulation and recorded via chest wall surface EMG. Respir Physiol Neurobiol 243: 101–106, 2017. doi: 10.1016/j.resp.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 49.NHLBI Respiratory Muscle Fatigue Workshop Group NHLBI Workshop summary. Respiratory muscle fatigue. Report of the Respiratory Muscle Fatigue Workshop Group. Am Rev Respir Dis 142: 474–480, 1990. doi: 10.1164/ajrccm/142.2.474. [DOI] [PubMed] [Google Scholar]