Abstract
Respiratory muscles such as the diaphragm are active across a range of behaviors including ventilation and higher-force behaviors necessary for maintenance of airway patency, and minimal information is available regarding anesthetic effects on the capacity of respiratory muscles to generate higher forces. The purpose of the present study was to determine whether diaphragm EMG activity during lower-force behaviors, such as eupnea and hypoxia-hypercapnia, is differentially affected compared with higher-force behaviors, such as a sigh, in lightly anesthetized animals. In adult male rats, chronically implanted diaphragm EMG electrodes were used to measure the effects of low-dose ketamine (30 mg/kg) and xylazine (3 mg/kg) on root mean square (RMS) EMG amplitude across a range of motor behaviors. A mixed linear model was used to evaluate the effects of ketamine-xylazine anesthesia on peak RMS EMG and ventilatory parameters, with condition (awake vs. anesthetized), behavior (eupnea, hypoxia-hypercapnia, sigh), side (left or right hemidiaphragm), and their interactions as fixed effects and animal as a random effect. Compared with the awake recordings, there was an overall reduction of peak diaphragm RMS EMG across behaviors during anesthesia, but this reduction was more pronounced during spontaneous sighs (which require ~60% of maximal diaphragm force). Respiratory rates and duty cycle during eupnea and hypoxia-hypercapnia were higher in awake compared with anesthetized conditions. These results highlight the importance of identifying anesthetic effects on a range of respiratory motor behaviors, including sighs necessary for maintaining airway patency.
NEW & NOTEWORTHY Respiratory muscles accomplish a range of motor behaviors, with forces generated for ventilatory behaviors comprising only a small fraction of their maximal force generating capacity. Induction of anesthesia exerts more robust effects on the higher-force diaphragm motor behaviors such as sighs compared with eupnea. This novel information on effects of low, sedative doses of a commonly used anesthetic combination (ketamine-xylazine) highlights the importance of identifying anesthetic effects on a range of respiratory motor behaviors.
Keywords: anesthesia, diaphragm muscle, electromyography, force, ketamine
INTRODUCTION
Respiratory muscles such as the diaphragm are important in both sustaining ventilation and performing higher-force nonventilatory behaviors necessary for maintaining airway patency (39, 58). In mammals, including humans, ventilatory behaviors (i.e., eupnea and response to hypoxia-hypercapnia) can be achieved with a fraction of maximal transdiaphragmatic pressure (Pdi), usually measured during bilateral phrenic nerve stimulation (5, 40, 57–59, 61). Indeed, in rats ventilatory behaviors require only 30% of maximal Pdi, whereas sighs (i.e., spontaneous deep breaths) require forces ~60% of maximal Pdi (16, 32, 40). Near-maximal forces are only generated by the diaphragm muscle during expulsive behaviors such as sneezing or coughing. Recent studies suggest that these higher-force behaviors are accomplished via activation of distinct neural pathways, e.g., by converting normal breaths into higher-force efforts (34). Anesthetic effects on complex behaviors such as sighs and other higher-force motor behaviors that require coactivation of various respiratory muscles are poorly understood but presumably will be more pronounced compared with less complex, lower-force motor behaviors. Accordingly, sighs would be preferentially affected by anesthetics compared with room air breathing.
The effects of anesthesia on ventilation are well known (8, 67). Ketamine is a widely used dissociative anesthetic agent with effects on ventilation when used both singly (30, 65) and in combination with xylazine (54). In a variety of mammalian species, ketamine and xylazine exhibit variable effects on minute ventilation but generally decrease respiratory rate, duty cycle, and blood oxygen levels (7, 12, 28, 30, 63). It is important to recognize that considerations of anesthetic effects on ventilation and neural control of breathing do not usually include higher-force behaviors that are necessary for airway clearance (3, 43, 66). Previous studies on the respiratory effects of anesthetics have focused almost exclusively on ventilatory parameters (e.g., tidal volume, respiratory rate, minute ventilation) while ignoring measures of respiratory muscle activity, and currently minimal information is available regarding anesthetic effects on the capacity of respiratory muscles to generate higher-force behaviors.
Neuromotor control of respiratory muscles is reflected in muscle electrical activity, which is usually recorded with electromyography (EMG) (40, 55, 68). Previous studies in anesthetized rats have shown a robust correlation between Pdi and peak diaphragm muscle root mean square (RMS) EMG, suggesting that diaphragm EMG measurements may be used as a surrogate of muscle force generation, permitting assessment of respiratory muscle activity across a range of motor behaviors in both awake and anesthetized conditions in rats (16, 40). The purpose of the present study was to determine whether a commonly used anesthetic regimen in animal surgery (ketamine-xylazine) affects diaphragm EMG activity differentially during lower-force behaviors such as eupnea and hypoxia-hypercapnia compared with a higher-force behavior such as a sigh. We hypothesized that after induction of low-dose ketamine-xylazine anesthesia diaphragm EMG activity is depressed to a greater extent during sighs than during ventilatory behaviors.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (n = 17) with a body weight of 301 ± 6 g were used in the present study. All procedures were approved by the Institutional Animal Care and Use Committee and were in accordance with American Physiological Society animal care guidelines.
Diaphragm muscle electrode placement.
As previously reported, diaphragm EMG activity was recorded after implantation of bilateral diaphragm muscle electrodes (38, 40, 68). Briefly, animals were anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg) via intramuscular injection. A pair of fine-wire (AS631; Cooner Wire, Chatsworth, CA) electrodes were bilaterally implanted and fixed into the midcostal diaphragm muscle in a manner such that an uninsulated 3-mm segment was embedded within muscle fibers of the diaphragm with an interelectrode distance of ~3 mm. The loose ends were tunneled subcutaneously to the dorsum of the rat, externalized, and fixed for use during EMG recording sessions. Animals were provided with carprofen (50 mg/ml) in their drinking water ~48 h before and until ~72 h after surgery. The muscle and skin layers were sutured with 3-0 absorbable VICRYL (polyglactin 910). Animals were allowed to recover for 3 days to avoid possible laparotomy-induced inhibition of neural activity of the diaphragm, which peaks ∼6–8 h after laparotomy and returns to baseline by ~48 h (11, 14, 50). The skin incision remained sutured, and the animals moved around without impairment and performed normal grooming behaviors by this time.
Diaphragm muscle activity during respiratory motor behaviors.
Animals were placed in a prone position inside a custom-built Plexiglas restraint cylinder for several days to facilitate animal tolerance of awake diaphragm EMG recording sessions. Animals were maintained prone after induction of anesthesia with intramuscular administration of low doses of ketamine (30 mg/kg) and xylazine (3 mg/kg) for anesthetized EMG recordings. Rats remained calm and sedate but displayed an intact corneal reflex and responded to toe pinch, consistent with expected effects at the selected low dose of this anesthetic combination. For the awake and anesthetized EMG recordings, externalized diaphragm electrodes were connected with gold pin connectors to differential amplifiers (model EMG100C; Biopac Systems, Goleta, CA), and EMG signals were amplified (2,000×), band-pass filtered (100–5,000 Hz), and digitally sampled at 2 kHz with a Powerlab 4/35 (ADInstruments, Colorado Springs, CO), as in previous studies (2, 9, 10, 19–21, 27, 36–38, 42, 48, 60, 68). This is consistent with previous studies in humans showing a centroid frequency of ~100 Hz with surface electrodes placed on the chest (53, 62). The centroid frequency is higher with intramuscular EMG electrodes. Filtering at 100 Hz substantially reduces ECG contamination, movement-related EMG artifacts, and 60-Hz electrical noise without introducing additional filters (53).
Recordings were obtained during ventilatory behaviors under awake and anesthetized conditions in the following order: 1) awake eupnea ~2 min, 2) awake hypoxia-hypercapnia (10% O2 and 5% CO2) ~5 min, 3) anesthetized eupnea ~2 min, 4) anesthetized hypoxia-hypercapnia ~5 min. For hypoxia-hypercapnia measurements, animals were placed in a large-volume container with a high-flow fresh gas rate of the appropriate gas mixture and recordings were conducted after an appropriate washin period. We previously used capnometry to indicate reliable, stable measures of 5% CO2 in the container, which confirm the intended composition of the gas (2). In all cases, ~5 min was allowed between behaviors, during which a return to baseline respiratory rate was verified. Sporadic deep breaths (“sighs”) were evident during spontaneous ventilatory behaviors in awake and anesthetized conditions. Sighs were defined as individual breaths with peak RMS EMG at least twofold greater than the average eupneic breaths (2, 27, 38, 42, 48, 55).
Diaphragm EMG analysis.
To assess diaphragm EMG activity, RMS EMG signals (computed with a 50-ms window) were analyzed with custom-made software in MATLAB (MathWorks, Natick, MA) (38, 40, 56, 60). The peak RMS EMG signal was measured during awake and anesthetized conditions for all behaviors (eupnea, hypoxia-hypercapnia, sigh). Although sighs were present during eupnea and hypoxia-hypercapnia, peak RMS EMG was measured only during hypoxia-hypercapnia-induced sighs. As in previous studies, neural drive was estimated by the RMS EMG value at 75 ms after the onset of activity (RMS75) (16, 48, 55). In several previous studies from our laboratory we analyzed diaphragm EMG during sighs under both eupneic and hypoxic-hypercapnic conditions (32, 48, 55). The peak RMS EMG and RMS75 of sighs during both eupnea and hypoxia-hypercapnia are similar.
Respiratory parameters.
Respiratory rate and burst duration were obtained from the RMS EMG signal (2, 19–21, 27, 36, 42, 48). Respiratory rate in awake animals appeared variable, consistent with sniffing and other exploratory behaviors in gently restrained rats. Analysis of the distribution of the instantaneous respiratory rates allowed for evaluation across a range of motor behaviors and evidence of respiratory activity (~85 breaths/min) compared with nonrespiratory activity such as sniffing (>200 breaths/min), which is expected to be particularly evident in awake animals (6). Respiratory rate during eupnea and hypoxia-hypercapnia for each animal was determined as the mode of the respiratory frequency distribution (19–21, 27, 36, 38, 42). Duty cycle (ratio of active to inactive time of the diaphragm muscle) was calculated as the ratio of burst duration to respiratory period and expressed as a percentage (38).
Transdiaphragmatic pressure.
In a separate set of animals, simultaneous Pdi and EMG measurements were performed under deeply anesthetized conditions (100 mg/kg ketamine and 10 mg/kg xylazine), to be consistent with previous studies reporting Pdi and EMG activity across a range of behaviors including maximal Pdi obtained during bilateral phrenic nerve stimulation (16, 24, 25, 32). The surgical conditions necessary for bilateral phrenic nerve stimulation require higher doses of anesthetics, but the relationship between Pdi and EMG is expected to be unaffected by anesthetic depth since muscle force and EMG activity are directly related during isometric conditions (35). As described previously, Pdi was measured as the difference in the pressures recorded from intrathoracic and abdominal compartments. Two 3.5-Fr Millar solid-state pressure catheters (SPR-524; Millar Instruments, Houston, TX) were inserted through the mouth into the esophagus and stomach, and correct catheter position was determined on the basis of the direction of signal deflection during real-time measurements. Intrathoracic and abdominal pressures were recorded and digitized (400 Hz) with PowerLab 4/35 and visualized in real time with LabChart 8. The Pdi signal was band-pass filtered between 0.3 and 30 Hz with a digital filter to remove the DC offset and high-frequency noise. Data were exported for post hoc analysis with a custom-designed semiautomated script in MATLAB. The selection of RMS EMG as a quantitative measure of the extent of respiratory motor activity was examined by comparing Pdi and RMS EMG amplitudes in anesthetized conditions.
Statistical analyses.
Peak diaphragm RMS EMG and RMS75 values were normalized to the peak RMS EMG value during awake sigh within the same animal. Differences in peak RMS EMG and RMS75 across motor behaviors as well as respiratory rate, burst duration, and duty cycle were evaluated with a mixed linear model with condition (awake vs. anesthetized), behavior (eupnea, hypoxia-hypercapnia, sigh), side (left or right hemidiaphragm), and their interactions as fixed effects and animal as a random effect. The absolute difference in normalized peak RMS EMG between awake and anesthetized conditions was evaluated for each behavior by one-way ANOVA. Frequency of sighs during awake and anesthetized conditions was evaluated with a matched-pairs t-test. When appropriate, post hoc analyses were conducted with the Tukey-Kramer honestly significant difference (HSD) test. All statistical evaluations were performed with JMP statistical software (JMP Pro 10.0; SAS Institute, Cary, NC). Experimental data are presented as means ± SE unless otherwise specified. Statistical significance was established at the P < 0.05 level.
RESULTS
A total of 17 rats were implanted with EMG electrodes bilaterally in the midcostal diaphragm muscle for EMG recordings. All rats tolerated implantation of electrodes without complication. All EMG measurements were performed at least 3 days after electrode implantation. In eight rats, simultaneous Pdi and EMG measurements were performed to confirm linearity between these two measures. In a separate cohort of nine rats, EMG activity was recorded during awake and anesthetized conditions across different motor behaviors. Electrode dislodgement in one hemidiaphragm (left in both cases) occurred in two rats, and thus recordings on this side were not available for analyses. Representative raw diaphragm EMG recordings and RMS EMG tracings from a single rat obtained across behaviors during awake and anesthetized conditions are shown in Fig. 1. Note increased EMG amplitude during the awake condition compared with anesthetized condition.
Fig. 1.
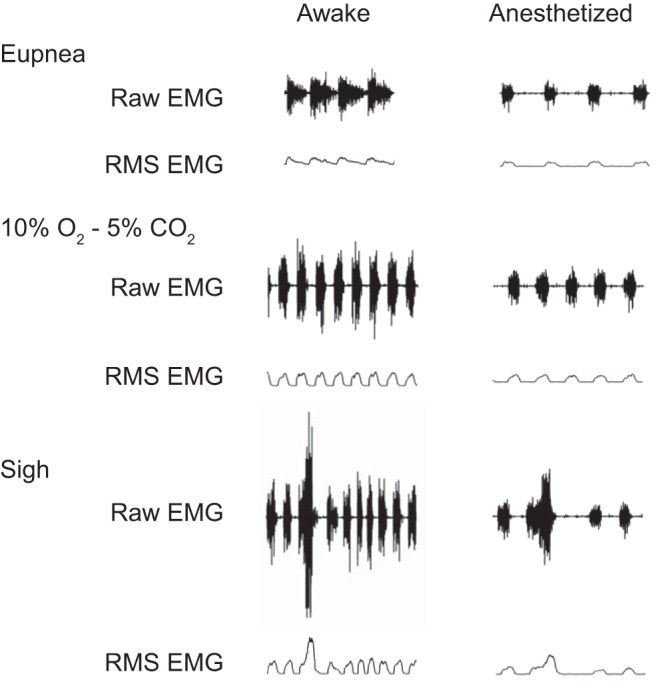
Representative raw and root mean square (RMS) diaphragm EMG recordings in a single rat obtained during awake and anesthetized (ketamine-xylazine) conditions. All tracings are at same scale.
Linearity of Pdi amplitude and peak diaphragm RMS EMG.
Pdi and EMG were recorded simultaneously to determine the correlation between these two measures of respiratory activity across motor behaviors. As shown in previous studies, sigh amplitude represents 63% of the maximum amplitude obtained by bilateral phrenic nerve stimulation (16, 32, 40). As is evident from Fig. 2, there is a strong, linear correlation between Pdi and peak RMS EMG (R2 > 0.99). Accordingly, peak RMS EMG was used as the measure of respiratory activity in awake animals.
Fig. 2.
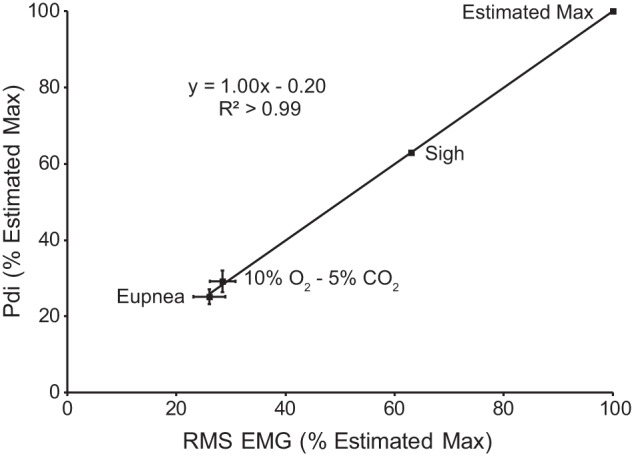
Relationship between diaphragm peak RMS EMG and Pdi (n = 8). Both measures were normalized to the estimated maximum based on previous work and the assumption that Pdi during sighs was ~63% of the maximum Pdi obtained by bilateral phrenic nerve stimulation (40). The relationship between peak RMS EMG and Pdi shows a strong linear correlation, suggesting that peak RMS EMG is a reasonable surrogate for Pdi across diaphragm motor behaviors that require varying levels of force.
Effect of anesthesia on peak diaphragm RMS EMG.
Average peak RMS EMG values across motor behaviors during awake and anesthetized conditions are shown in Fig. 3. The mixed linear model revealed no significant effect of the side of the diaphragm from which EMG recordings were obtained (F1,68 = 1; P = 0.902); thus activities of the two hemidiaphragms were averaged for each animal in subsequent analyses. Significant effects on normalized peak RMS EMG of both behavior (F2,37 = 157; P < 0.001) and condition (F1,37 = 51; P < 0.001) as well as the behavior × condition interaction (F2,37 = 20; P < 0.001) were evident. Overall, peak RMS EMG was higher in awake compared with anesthetized recordings.
Fig. 3.
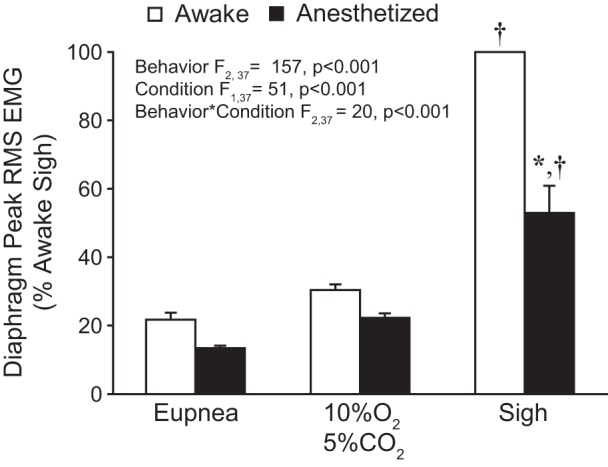
Bilateral peak RMS EMG across behaviors during awake and anesthetized conditions. Peak RMS EMG was normalized to the awake sigh amplitude for each hemidiaphragm within each animal (n = 9). A main effect of behavior, condition, and their interaction was observed. Post hoc comparisons: *P < 0.05 compared with awake normalized peak RMS EMG; †P < 0.05 compared with other behaviors/conditions.
In the awake condition, peak RMS EMG during eupnea and hypoxia-hypercapnia was 22% (95% CI [17, 27]) and 30% (95% CI [27, 34]) of the awake sigh peak RMS EMG, respectively. In anesthetized animals, the peak RMS EMG during eupnea, hypoxia-hypercapnia, and sigh was 13% (95% CI [8, 11]), 22% (95% CI [12, 15]), and 53% (95% CI [10, 14]) of the awake sigh peak RMS EMG, respectively. In post hoc comparisons (Tukey-Kramer HSD; P < 0.05), peak RMS EMG during sighs was significantly higher than during hypoxia-hypercapnia, which was significantly higher than during eupnea, regardless of anesthetized or awake condition. In post hoc comparisons peak RMS EMG was significantly lower during sighs in anesthetized vs. awake condition but was not significantly different during eupnea or hypoxia-hypercapnia. A significant difference in the relative change in RMS EMG between awake and anesthetized conditions was evident (F2,26 = 32; P < 0.001) and was greater during sighs (53.1% ± 6.6) than during eupnea (7.8% ± 3.6) or hypoxia-hypercapnia (8.4% ± 2.6). Taken together, these data indicate that compared with the awake condition low-dose ketamine-xylazine anesthesia results in a greater reduction in respiratory muscle activity during sighs than during lower-force behaviors such as eupnea and response to hypoxia-hypercapnia.
Effect of anesthesia on descending premotor drive (RMS75) to respiratory muscles.
As in previous studies (16, 48, 55), the RMS75 EMG value, normalized to peak RMS EMG obtained during sighs in the awake condition, was used as an estimate of neural drive. Figure 4 shows RMS75 values during awake and anesthetized conditions across behaviors. The mixed linear model revealed no significant effect of the side of the diaphragm from which EMG recordings were obtained (F1,71 = 1; P = 0.281), and values for the two hemidiaphragms were averaged for each animal in subsequent analyses. The model further revealed significant effects on normalized RMS75 of both behavior (F2,39 = 9; P < 0.001) and condition (F1,39 = 164; P < 0.001) but not their interaction (F2,39 = 2; P = 0.196). Overall, RMS75 values were higher during awake compared with anesthetized conditions.
Fig. 4.
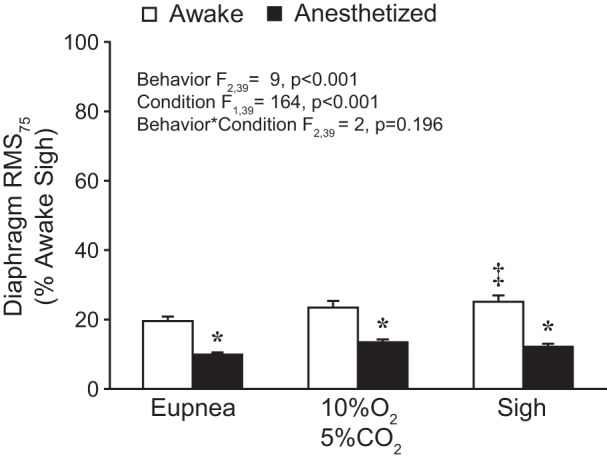
Bilateral average amplitude of the RMS EMG signal at 75 ms (RMS75) across behaviors during awake and anesthetized conditions. RMS75 values were normalized to the awake sigh peak RMS EMG for each hemidiaphragm within each animal (n = 9). A main effect of behavior and condition was observed. Post hoc comparisons: *P < 0.05 compared with awake RMS75 for the same behavior; ‡P < 0.05 compared with awake eupnea.
In awake recordings, RMS75 values during eupnea, hypoxia-hypercapnia, and sigh were 20% (95% CI [17, 23]), 23% (95% CI [19, 28]), and 25% (95% CI [21, 29] of the awake sigh peak RMS EMG, respectively. In anesthetized animals, RMS75 values during eupnea, hypoxia-hypercapnia, and sigh were 10% (95% CI [8, 11]), 13% (95% CI [12, 15]), and 12% (95% CI [10, 14]) of the awake sigh peak RMS EMG, respectively. In post hoc comparisons (Tukey-Kramer HSD; P < 0.05), RMS75 values during all behaviors were greater in awake compared with anesthetized conditions and greater during sigh or hypoxia-hypercapnia than during eupnea. Taken together, these results indicate that the differences in diaphragm RMS75 values between awake and anesthetized conditions reflect reduced respiratory drive across motor behaviors during low-dose anesthesia.
Effect of anesthesia on respiratory parameters in spontaneously breathing rats.
Respiratory rate, burst duration, and duty cycle were measured from diaphragm EMG recordings during eupnea and hypoxia-hypercapnia in awake and anesthetized conditions (Table 1). The mixed linear model revealed significant effects on respiratory rate of behavior (F1,23 = 37; P < 0.001) and condition (F1,23 = 78; P < 0.001) but not their interaction (F1,23 = 4; P = 0.052). Overall, respiratory rates were higher during awake compared with anesthetized conditions. Additionally, the distributions of instantaneous respiratory rates across awake and anesthetized conditions during eupnea and hypoxia-hypercapnia are shown in Fig. 5. The histograms reflect the full range of respiratory rates across motor behaviors including resting breathing (~100 breaths/min) as well as sniffing (~400 breaths/min). During anesthesia there was a loss of high-frequency sniffing evidenced by the bimodal distribution present during eupnea in the awake condition (Fig. 5A) and a leftward shift of respiratory rates compared with the awake condition (Fig. 5B), particularly in response to hypoxia-hypercapnia.
Table 1.
Respiratory parameters across ventilatory behaviors during awake and anesthetized conditions
| Condition |
|||
|---|---|---|---|
| Behavior | Respiratory Parameters | Awake | Anesthetized |
| Eupnea | Respiratory rate, min−1 | 111 ± 13 | 71 ± 6* |
| Burst duration, ms | 404 ± 43 | 362 ± 34 | |
| Duty cycle, % | 74 ± 9 | 43 ± 5* | |
| Hypoxia-hypercapnia | Respiratory rate, min−1 | 158 ± 8† | 94 ± 6*† |
| Burst duration, ms | 271 ± 27† | 346 ± 24 | |
| Duty cycle, % | 69 ± 4 | 54 ± 5 | |
| Frequency of sighs, min−1 | 0.9 ± 0.1 | 0.6 ± 0.1* | |
Data are presented as means ± SE. Duty cycle was calculated as % of total time corresponding to inspiration. Main effects of condition were present for respiratory rate and duty cycle (P ≤ 0.001). Main effects of behavior were present for respiratory rate and burst duration (P ≤ 0.006). See text for details. Post hoc comparisons are shown:
different from awake during same behavior (P < 0.05);
different from eupnea during same condition (P < 0.05).
Fig. 5.

Histograms of instantaneous respiratory rate for all animals during eupnea (A; n = 9) and hypoxia-hypercapnia (B; n = 9). Histograms reflect the full range of respiratory rates across various motor behaviors including resting breathing (usually ~100 breaths/min) as well as sniffing (usually ~400 breaths/min). Sniffing behavior was apparent during eupnea in all awake animals and ceased after induction of anesthesia. Note the blunting of the response to hypoxia-hypercapnia during ketamine-xylazine anesthesia.
For burst duration, the mixed linear model revealed a significant effect of behavior (F1,23 = 9; P = 0.006) and behavior × condition interaction (F1,23 = 6; P = 0.025) but not condition itself (F1,23 = 1; P = 0.562). Overall, burst duration was longer during eupnea than during hypoxia-hypercapnia, and anesthesia had no effect on burst duration. For duty cycle, the mixed linear model revealed a significant effect of condition (F1,23 = 18; P < 0.001) but not behavior (F1,23 = 1; P = 0.600) or the behavior × condition interaction (F1,23 = 2; P = 0.150). Overall, duty cycle activity was higher during awake compared with anesthetized conditions. Finally, the frequency of sighs was measured from diaphragm EMG recordings during hypoxia-hypercapnia in awake and anesthetized conditions (Table 1), and a matched-pairs t-test showed that anesthesia significantly reduced the frequency of sighs [t(8) = −3; P = 0.038].
DISCUSSION
The present study demonstrates that the motor drive of respiratory muscles such as the diaphragm is significantly reduced during low-dose ketamine-xylazine anesthesia in rats, particularly during higher-force behaviors. Specifically, there was an overall reduction in peak diaphragm RMS EMG across various motor behaviors during anesthesia, and this reduction was more pronounced during spontaneous deep breaths, i.e., sighs (which require ~60% of maximal diaphragm force compared with only ~30% for eupnea) (16, 32, 40). Central respiratory drive (assessed by RMS75) was reduced to a similar extent across all behaviors. Respiratory rates and duty cycle during eupnea and hypoxia-hypercapnia were significantly reduced with anesthesia, as well as the frequency of sighs. The present study provides novel, proof-of-principle information regarding the effects of low, sedative doses of ketamine-xylazine and highlights the importance of identifying anesthetic effects on a range of motor behaviors.
Selection of anesthetic agents.
In the present study, ketamine and xylazine were selected because of their wide use in preclinical research. Ketamine is an anesthetic agent acting mainly as an NMDA receptor antagonist (67), with broad use given a favorable safety profile (45, 47, 65, 70). Xylazine is an α2-adrenergic agonist that provides pain relief and muscle relaxation (64). Since ketamine may increase skeletal muscle tone and elicit muscle spasms, the combination with xylazine facilitates adequate anesthetic conditions including muscle relaxation (22). These agents are commonly used in combination in animal studies and have known effects on respiratory parameters and arterial blood gas levels (7, 12, 28, 30, 63, 74).
The purpose of the present study was to determine whether low doses of ketamine (30 mg/kg) and xylazine (3 mg/kg) would reduce respiratory muscle activity preferentially during higher-force behaviors, such as a sigh. Previous studies assessed the effects of anesthetics on the respiratory system, focusing primarily on respiratory parameters such as , , and and pH or ventilation as determined by plethysmography (i.e., tidal volume, respiratory rate, and inspiratory time) (1, 28, 30, 43, 44, 71). In rats, ketamine (40 mg/kg) and xylazine (5 mg/kg) anesthesia caused a 23% and 32% decline in and mean arterial blood pressure compared with preanesthetic values, respectively (71). High doses of ketamine (125 mg/kg) and xylazine (10 mg/kg) caused a significant, albeit small, reduction in respiratory rate in rats (17). Similar effects of ketamine-xylazine on respiratory rate and blood oxygen levels are found in mice, rabbits, and guinea pigs (13, 52, 54, 72). It is important to note that the effects of ketamine are less prominent compared with other anesthetic agents such as volatile anesthetics, barbiturates, and propofol (26, 67, 73) and available evidence does not provide information regarding the ability to generate higher forces.
Peak RMS EMG and diaphragm muscle force across motor behaviors.
Respiratory muscles such as the diaphragm are highly active in order to sustain ventilation throughout life. The diaphragm muscle comprises different muscle fiber types that vary in their contractile and fatigue properties (4, 15, 57). Previous studies across multiple species show that ventilatory behaviors (i.e., eupnea and the response to hypoxia-hypercapnia) can be accomplished by recruitment of the fatigue-resistant motor units (15, 16, 40, 58, 59, 61). Nonventilatory behaviors like swallowing, vocalization, and expulsive behaviors require the recruitment of fatigable motor units capable of generating higher forces. In rats, ventilatory behaviors in anesthetized rats require ~30% of the maximal Pdi generating capacity and, in contrast, sighs and response to airway occlusion require ~60% of the maximal Pdi generating capacity (16, 40, 41, 58, 61).
Previous studies assessed diaphragm muscle force by measuring Pdi (16, 23, 25, 29, 32, 40, 61, 69). Measurement of Pdi involves insertion of catheters in the esophagus and stomach as well as the implementation of a restriction method to prevent outward movements of the abdomen during inspiration and can be readily applied in humans. However, the invasiveness of the catheters limits the ability to obtain Pdi measurements in awake animals. Importantly, diaphragm EMG measurements may be used as a surrogate of muscle force generation, since there is a strong, positive correlation between Pdi and diaphragm peak RMS EMG across motor behaviors in rat as shown previously (40) and recapitulated in the present investigation (Fig. 2). Of note, the number of diaphragm motor behaviors over which we could assess this relationship is relatively small. It is possible that the linearity of this relationship may not hold with certain higher-force behaviors, particularly those involving more intercostal activity as well as those involving ballistic, highly nonisometric contractions. Nevertheless, previous studies including Pdi during behaviors of interest in the present study (eupnea, hypoxia-hypercapnia, and sigh) as well as during airway occlusion and maximal Pdi elicited by bilateral phrenic nerve stimulation document a highly linear relationship (R2 0.78–0.99) (16, 40). Indeed, EMG measurements have been used successfully in a number of studies using chronically implanted electrodes in the diaphragm muscle to measure changes in muscle activity over time (9, 38, 40, 46, 60, 68). In the present study, EMG measurements were used for assessment of diaphragm muscle forces generated in awake and anesthetized animals. We observed a significant reduction in peak RMS EMG across behaviors, with post hoc analysis showing that peak RMS EMG during sighs was preferentially reduced after ketamine-xylazine anesthesia (Fig. 3). We cannot exclude the possibility that the effect of ketamine-xylazine on peak RMS EMG is more pronounced as the amplitude of the EMG signal increases (e.g., during sighs). We do not expect maximum force generation by the diaphragm muscle to be significantly affected given the short time frame of anesthetic exposure (minutes). Although there is evidence for minimal effects of volatile anesthetics on muscle force and neuromuscular transmission (18, 31, 33), no such effects appear to be present after ketamine or xylazine (51).
RMS75 and central drive.
The magnitude of descending respiratory central drive to phrenic motoneurons was estimated by the RMS EMG value at 75 ms after the onset of EMG activity (RMS75) (16, 32, 48, 55). Motor unit recruitment across behaviors is thus reflected in both the peak RMS EMG and RMS75. Higher levels of RMS75 are evident during ballistic behaviors such as the response to airway occlusion (16, 48, 55), which results in similar forces generated by the diaphragm muscle compared with sigh. Consistent with previous studies in rats, there were no differences in central drive across these motor behaviors in anesthetized conditions (16, 32, 39, 55, 56). Recent evidence suggests that sighing may be accomplished by the augmentation of a normal breath via a peptidergic control circuit (34), and, in general agreement, RMS75 values were higher during sighs compared with eupnea. Overall, central respiratory drive was only slightly lower during anesthetized compared with awake conditions across all motor behaviors (Fig. 4). These findings are consistent with the minor effects of low-dose ketamine and xylazine anesthesia on ventilation across various species, including rats (13, 17, 52, 54, 72).
Ventilatory parameters.
Previous reports document primarily a blunting of the ventilatory response to hypoxia-hypercapnia following ketamine and xylazine (28, 44) and are in general agreement with the results of the present study on respiratory parameters such as respiratory rate, burst duration, and duty cycle during eupnea or exposure to hypoxia-hypercapnia. Respiratory rates were reduced during both eupnea and hypoxia-hypercapnia after anesthesia, compared with the awake values, and this reduction was similar to that reported in a previous study in Sprague-Dawley rats with higher doses of ketamine (80 mg/kg) and xylazine (10 mg/kg) (3). Of note, a lower dose of ketamine and xylazine (35 mg/kg and 5 mg/kg, respectively) also decreased respiratory rate during eupnea in rabbits (52). As expected, higher respiratory rates during hypoxia-hypercapnia compared with during eupnea were observed in both awake and anesthetized conditions (Fig. 5), with the distribution of respiratory rates revealing a blunted response to hypoxia-hypercapnia after anesthesia. A previous study using ketamine (100 mg/kg) and xylazine (10 mg/kg) in rats showed generally similar effects (44). Overall, these results support minor effects on ventilation (1, 28, 30, 43, 44, 71), Finally, it is worth noting that ketamine-xylazine also decreased the frequency of sighs by ~30% (Table 1). A previous study reported a reduction of sighs during exposure to hypoxia-hypercapnia with administration of ketamine (80 mg/kg) and xylazine (10 mg/kg) in rats (3). Whereas previous studies had documented effects on the frequency of sighs, the present report substantiates preferential effects of low, sedative doses of a ketamine-xylazine combination on the higher forces generated by respiratory muscles.
Summary.
The present study highlights the importance of evaluating respiratory muscle activity across a range of motor behaviors and identifying anesthetic effects on this activity. Even low doses of ketamine and xylazine, insufficient to provide surgical conditions, exerted a predominant depressant effect on the amplitude and frequency of spontaneous deep breaths (i.e., sighs), an important observation considering that sighs are essential protective behaviors (49) and represent higher levels of force generation by the diaphragm muscle (32, 40).
GRANTS
This work was supported by National Institutes of Health Grants R01 AG-044615, R01 AG-057052, R01 HL-096750, and T32 HL-105355 and the Mayo Clinic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.J.-R., O.U.K., H.M.G., G.C.S., and C.B.M. conceived and designed research; F.J.-R. and W.-Z.Z. performed experiments; F.J.-R., O.U.K., H.M.G., and C.B.M. analyzed data; F.J.-R., O.U.K., H.M.G., G.C.S., and C.B.M. interpreted results of experiments; F.J.-R., O.U.K., H.M.G., G.C.S., and C.B.M. drafted manuscript; F.J.-R., O.U.K., H.M.G., G.C.S., and C.B.M. edited and revised manuscript; F.J.-R., O.U.K., W.-Z.Z., H.M.G., G.C.S., and C.B.M. approved final version of manuscript; O.U.K., H.M.G., and C.B.M. prepared figures.
REFERENCES
- 1.Allen DG, Dyson DH, Pascoe PJ, O’Grady MR. Evaluation of a xylazine-ketamine hydrochloride combination in the cat. Can J Vet Res 50: 23–26, 1986. [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Argote S, Gransee HM, Mora JC, Stowe JM, Jorgenson AJ, Sieck GC, Mantilla CB. The impact of midcervical contusion injury on diaphragm muscle function. J Neurotrauma 33: 500–509, 2016. doi: 10.1089/neu.2015.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell HJ, Azubike E, Haouzi P. The “other” respiratory effect of opioids: suppression of spontaneous augmented (“sigh”) breaths. J Appl Physiol (1985) 111: 1296–1303, 2011. doi: 10.1152/japplphysiol.00335.2011. [DOI] [PubMed] [Google Scholar]
- 4.Burke RE, Levine DN, Tsairis P, Zajac FE 3rd. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234: 723–748, 1973. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J Physiol 518: 907–920, 1999. doi: 10.1111/j.1469-7793.1999.0907p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnevali L, Sgoifo A, Trombini M, Landgraf R, Neumann ID, Nalivaiko E. Different patterns of respiration in rat lines selectively bred for high or low anxiety. PLoS One 8: e64519, 2013. doi: 10.1371/journal.pone.0064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung A, Fishman M, Dasenbrook EC, Loparo KA, Dick TE, Jacono FJ. Isoflurane and ketamine anesthesia have different effects on ventilatory pattern variability in rats. Respir Physiol Neurobiol 185: 659–664, 2013. doi: 10.1016/j.resp.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahan A, Teppema LJ. Influence of anaesthesia and analgesia on the control of breathing. Br J Anaesth 91: 40–49, 2003. doi: 10.1093/bja/aeg150. [DOI] [PubMed] [Google Scholar]
- 9.Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc 1: 1204–1207, 2006. doi: 10.1109/IEMBS.2006.260688. [DOI] [PubMed] [Google Scholar]
- 10.Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf Proc IEEE Eng Med Biol Soc 2009: 404–407, 2009. doi: 10.1109/IEMBS.2009.5334892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dureuil B, Viirès N, Cantineau JP, Aubier M, Desmonts JM. Diaphragmatic contractility after upper abdominal surgery. J Appl Physiol (1985) 61: 1775–1780, 1986. doi: 10.1152/jappl.1986.61.5.1775. [DOI] [PubMed] [Google Scholar]
- 12.Eikermann M, Grosse-Sundrup M, Zaremba S, Henry ME, Bittner EA, Hoffmann U, Chamberlin NL. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology 116: 35–46, 2012. doi: 10.1097/ALN.0b013e31823d010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blümel G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res Exp Med (Berl) 184: 159–169, 1984. doi: 10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- 14.Ford GT, Whitelaw WA, Rosenal TW, Cruse PJ, Guenter CA. Diaphragm function after upper abdominal surgery in humans. Am Rev Respir Dis 127: 431–436, 1983. doi: 10.1164/arrd.1983.127.4.431. [DOI] [PubMed] [Google Scholar]
- 15.Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- 16.Gill LC, Mantilla CB, Sieck GC. Impact of unilateral denervation on transdiaphragmatic pressure. Respir Physiol Neurobiol 210: 14–21, 2015. doi: 10.1016/j.resp.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giroux MC, Hélie P, Burns P, Vachon P. Anesthetic and pathological changes following high doses of ketamine and xylazine in Sprague Dawley rats. Exp Anim 64: 253–260, 2015. doi: 10.1538/expanim.14-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gissen AJ, Karis JH, Nastuk WL. Effect of halothane on neuromuscular transmission. JAMA 197: 770–774, 1966. doi: 10.1001/jama.1966.03110100078019. [DOI] [PubMed] [Google Scholar]
- 19.Gransee HM, Gonzalez Porras MA, Zhan WZ, Sieck GC, Mantilla CB. Motoneuron glutamatergic receptor expression following recovery from cervical spinal hemisection. J Comp Neurol 525: 1192–1205, 2017. doi: 10.1002/cne.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma 32: 185–193, 2015. doi: 10.1089/neu.2014.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8: e64755, 2013. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green CJ, Knight J, Precious S, Simpkin S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10 year experience. Lab Anim 15: 163–170, 1981. doi: 10.1258/002367781780959107. [DOI] [PubMed] [Google Scholar]
- 23.Greising SM, Mantilla CB, Medina-Martínez JS, Stowe JM, Sieck GC. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol 309: L46–L52, 2015. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greising SM, Mantilla CB, Sieck GC. Functional measurement of respiratory muscle motor behaviors using transdiaphragmatic pressure. Methods Mol Biol 1460: 309–319, 2016. doi: 10.1007/978-1-4939-3810-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol 188: 56–59, 2013. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedenstierna G, Edmark L. Effects of anesthesia on the respiratory system. Best Pract Res Clin Anaesthesiol 29: 273–284, 2015. doi: 10.1016/j.bpa.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Torres V, Gransee HM, Mantilla CB, Wang Y, Zhan WZ, Sieck GC. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury. J Neurophysiol 117: 537–544, 2017. doi: 10.1152/jn.00654.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirshman CA, McCullough RE, Cohen PJ, Weil JV. Hypoxic ventilatory drive in dogs during thiopental, ketamine, or pentobarbital anesthesia. Anesthesiology 43: 628–633, 1975. doi: 10.1097/00000542-197512000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Hubmayr RD, Sprung J, Nelson S. Determinants of transdiaphragmatic pressure in dogs. J Appl Physiol (1985) 69: 2050–2056, 1990. doi: 10.1152/jappl.1990.69.6.2050. [DOI] [PubMed] [Google Scholar]
- 30.Jaspar N, Mazzarelli M, Tessier C, Milic-Emili J. Effect of ketamine on control of breathing in cats. J Appl Physiol Respir Environ Exerc Physiol 55: 851–859, 1983. doi: 10.1152/jappl.1983.55.3.851. [DOI] [PubMed] [Google Scholar]
- 31.Karis JH, Gissen AJ, Nastuk WL. The effect of volatile anesthetic agents on neuromuscular transmission. Anesthesiology 28: 128–134, 1967. doi: 10.1097/00000542-196701000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Khurram OU, Sieck GC, Mantilla CB. Compensatory effects following unilateral diaphragm paralysis. Respir Physiol Neurobiol 246: 39–46, 2017. doi: 10.1016/j.resp.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kochi T, Ide T, Isono S, Mizuguchi T, Nishino T. Different effects of halothane and enflurane on diaphragmatic contractility in vivo. Anesth Analg 70: 362–368, 1990. doi: 10.1213/00000539-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL. The peptidergic control circuit for sighing. Nature 530: 293–297, 2016. doi: 10.1038/nature16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippold OC. The relation between integrated action potentials in a human muscle and its isometric tension. J Physiol 117: 492–499, 1952. doi: 10.1113/jphysiol.1952.sp004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol 247: 101–109, 2013. doi: 10.1016/j.expneurol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol (1985) 114: 380–386, 2013. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol 177: 176–182, 2011. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantilla CB, Seven YB, Sieck GC. Convergence of pattern generator outputs on a common mechanism of diaphragm motor unit recruitment. Prog Brain Res 209: 309–329, 2014. doi: 10.1016/B978-0-444-63274-6.00016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martínez-Gálvez G, Zambrano JM, Diaz Soto JC, Zhan WZ, Gransee HM, Sieck GC, Mantilla CB. TrkB gene therapy by adeno-associated virus enhances recovery after cervical spinal cord injury. Exp Neurol 276: 31–40, 2016. doi: 10.1016/j.expneurol.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massey CA, Richerson GB. Isoflurane, ketamine-xylazine, and urethane markedly alter breathing even at subtherapeutic doses. J Neurophysiol 118: 2389–2401, 2017. doi: 10.1152/jn.00350.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore J, Haouzi P, Van de Louw A, Bell HJ. Hypocapnia-dependent facilitation of augmented breaths: observations in awake vs. anesthetized rats. Respir Physiol Neurobiol 180: 105–111, 2012. doi: 10.1016/j.resp.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Morgan CJ, Curran HV; Independent Scientific Committee on Drugs . Ketamine use: a review. Addiction 107: 27–38, 2012. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 46.Navarrete-Opazo A, Mitchell GS. Recruitment and plasticity in diaphragm, intercostal, and abdominal muscles in unanesthetized rats. J Appl Physiol (1985) 117: 180–188, 2014. doi: 10.1152/japplphysiol.00130.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radvansky BM, Puri S, Sifonios AN, Eloy JD, Le V. Ketamine—a narrative review of its uses in medicine. Am J Ther 23: e1414–e1426, 2016. doi: 10.1097/MJT.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 48.Rana S, Sieck GC, Mantilla CB. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J Neurophysiol 117: 545–555, 2017. doi: 10.1152/jn.00727.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds LB., Jr Characteristics of an inspiration-augmenting reflex in anesthetized cats. J Appl Physiol 17: 683–688, 1962. doi: 10.1152/jappl.1962.17.4.683. [DOI] [PubMed] [Google Scholar]
- 50.Road JD, Burgess KR, Whitelaw WA, Ford GT. Diaphragm function and respiratory response after upper abdominal surgery in dogs. J Appl Physiol Respir Environ Exerc Physiol 57: 576–582, 1984. doi: 10.1152/jappl.1984.57.2.576. [DOI] [PubMed] [Google Scholar]
- 51.Rydqvist B, Faijerson B. Ketamine: effects on the mechanical properties of the frog sartorius muscle. Acta Anaesthesiol Scand 27: 68–71, 1983. doi: 10.1111/j.1399-6576.1983.tb01907.x. [DOI] [PubMed] [Google Scholar]
- 52.Sanford TD, Colby ED. Effect of xylazine and ketamine on blood pressure, heart rate and respiratory rate in rabbits. Lab Anim Sci 30: 519–523, 1980. [PubMed] [Google Scholar]
- 53.Schweitzer TW, Fitzgerald JW, Bowden JA, Lynne-Davies P. Spectral analysis of human inspiratory diaphragmatic electromyograms. J Appl Physiol Respir Environ Exerc Physiol 46: 152–165, 1979. doi: 10.1152/jappl.1979.46.1.152. [DOI] [PubMed] [Google Scholar]
- 54.Schwenke DO, Cragg PA. Comparison of the depressive effects of four anesthetic regimens on ventilatory and cardiovascular variables in the guinea pig. Comp Med 54: 77–85, 2004. [PubMed] [Google Scholar]
- 55.Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol (1985) 117: 1308–1316, 2014. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med 9: 195–210, 1988. [PubMed] [Google Scholar]
- 58.Sieck GC. Neural control of the inspiratory pump. News Physiol Sci 6: 260–264, 1991. doi: 10.1152/physiologyonline.1991.6.6.260. [DOI] [Google Scholar]
- 59.Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med 15: 641–659, 1994. [PubMed] [Google Scholar]
- 60.Sieck GC, Fournier M. Changes in diaphragm motor unit EMG during fatigue. J Appl Physiol (1985) 68: 1917–1926, 1990. doi: 10.1152/jappl.1990.68.5.1917. [DOI] [PubMed] [Google Scholar]
- 61.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol (1985) 66: 2539–2545, 1989. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 62.Sieck GC, Mazar A, Belman MJ. Changes in diaphragmatic EMG spectra during hyperpneic loads. Respir Physiol 61: 137–152, 1985. doi: 10.1016/0034-5687(85)90121-5. [DOI] [PubMed] [Google Scholar]
- 63.Soliman MG, Brindle GF, Kuster G. Response to hypercapnia under ketamine anaesthesia. Can Anaesth Soc J 22: 486–494, 1975. doi: 10.1007/BF03004864. [DOI] [PubMed] [Google Scholar]
- 64.Stephenson JC, Blevins DI, Christie GJ. Safety of Rompun/Ketaset combination in dogs: a two-year study. Vet Med Small Anim Clin 73: 303–306, 1978. [PubMed] [Google Scholar]
- 65.Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med 26: 985–1028, 2008. doi: 10.1016/j.ajem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Sumitra M, Manikandan P, Rao KV, Nayeem M, Manohar BM, Puvanakrishnan R. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar rats—a comparative analysis. Life Sci 75: 1887–1896, 2004. doi: 10.1016/j.lfs.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Teppema LJ, Baby S. Anesthetics and control of breathing. Respir Physiol Neurobiol 177: 80–92, 2011. doi: 10.1016/j.resp.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Trelease RB, Sieck GC, Harper RM. A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr Clin Neurophysiol 53: 459–462, 1982. doi: 10.1016/0013-4694(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 69.Watchko JF, Mayock DE, Standaert TA, Woodrum DE. Postnatal changes in transdiaphragmatic pressure in piglets. Pediatr Res 20: 658–661, 1986. doi: 10.1203/00006450-198607000-00016. [DOI] [PubMed] [Google Scholar]
- 70.White PF, Way WL, Trevor AJ. Ketamine—its pharmacology and therapeutic uses. Anesthesiology 56: 119–136, 1982. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Wixson SK, White WJ, Hughes HC Jr, Lang CM, Marshall WK. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab Anim Sci 37: 736–742, 1987. [PubMed] [Google Scholar]
- 72.Wyatt JD, Scott RA, Richardson ME. The effects of prolonged ketamine-xylazine intravenous infusion on arterial blood pH, blood gases, mean arterial blood pressure, heart and respiratory rates, rectal temperature and reflexes in the rabbit. Lab Anim Sci 39: 411–416, 1989. [PubMed] [Google Scholar]
- 73.Younes M, Youssef M. Effect of five human anesthetics on respiratory control in cats. J Appl Physiol Respir Environ Exerc Physiol 44: 596–606, 1978. doi: 10.1152/jappl.1978.44.4.596. [DOI] [PubMed] [Google Scholar]
- 74.Zsigmond EK, Matsuki A, Kothary SP, Jallad M. Arterial hypoxemia caused by intravenous ketamine. Anesth Analg 55: 311–314, 1976. doi: 10.1213/00000539-197605000-00005. [DOI] [PubMed] [Google Scholar]


