Abstract
Background:
Androgenetic alopecia (AGA) is the most common cause of hair loss. Although it is a medically benign condition, it can have a significant psychosocial impact on patients. “Metabolic syndrome” (MetS) is a collection of clinical signs that focus on cardiovascular and diabetes-related parameters. Despite the high burden of AGA and MetS in India, specific data on the participants are relatively sparse.
Aim of the Study:
The aim of is to study the association of AGA with MetS and its parameters.
Materials and Methods:
A case–control study was undertaken in a tertiary care hospital from December 2015 to November 2016 with 100 cases and controls in the age group of 20–50 years. Diagnosis of MetS was based on the National Cholesterol Education Program Adult Treatment Panel III. Independent t-test was used as a test of significance. Categorical data were assessed using Chi-square test of significance. P <0.05 was considered to be significant.
Results:
MetS was seen in 53% of cases and 17% of controls (P = 0.001). The mean serum triglyceride level (P = 0.015, P < 0.05), mean systolic blood pressure (P = 0.003, P < 0.05), high-density lipoprotein levels in males (P < 0.001), and waist circumference in males (P = 0.022, P < 0.05) were statistically significant in patients with androgenetic alopecia when compared to healthy controls.
Conclusion:
A higher prevalence of MetS was noted in androgenic alopecia. Early screening for MetS is beneficial in patients with androgenic alopecia.
Keywords: Metabolic syndrome, prevention, screening
INTRODUCTION
Androgenetic alopecia (AGA) is the most common cause of hair loss.[1,2] Although it is a medically benign condition, it can have a significant psychosocial impact on patients. It is a very common condition encountered in almost all dermatology outpatient departments across the world.
A population-based study in India of 1005 participants showed 58% AGA in males aged 30–50 years.[1]
Male pattern baldness is found to be associated with coronary artery disease[3] hypertension,[4] insulin resistance,[5] abnormal serum lipid profile,[6] obesity,[7] prostate cancer,[8] benign prostatic hyperplasia,[9] scalp pain, and smoking; high androgen levels can contribute to the development of atherosclerosis and thrombosis leading to hypercholesterolemia and hypertension. Metabolic syndrome (MetS) is a cluster of interrelated risk factors that increase the risk for atherosclerotic cardiovascular disease.[10] The risk increases with the number of components of MetS.[11]
”Metabolic syndrome” (MetS) is a collection of clinical signs that focus on cardiovascular and diabetes-related parameters including increased waist circumference, elevated triglycerides (TGs), low high-density lipoprotein-cholesterol (HDL-C), elevated fasting blood glucose, and blood pressure.
Despite the increasing burden of AGA[12] and MetS[13] in India, specific data on the participants are relatively sparse. Very few studies are available in medical literature, and there are no exact data available regarding this association in Indian population.
This study was intended to evaluate the association of AGA with MetS and to compare with healthy control group.
MATERIALS AND METHODS
One-year long case–control study was conducted on patients attending as outpatients and inpatients to the Department of Dermatology of Rajarajeswari Medical College, Mysore road, Bengaluru. Ethical clearance for the study was taken from Institutional Ethics Committee. A total of 100 patients with AGA between 20 and 50 years’ age group and both sexes were included in the study. The control group included 100 age- and sex-matched individuals without any history of AGA. A written informed consent was obtained from all the study patients.
A detailed history was taken, and a thorough systemic and cutaneous examination was done. Diagnosis of AGA was made through clinical findings.
Clinical assessment of AGA, the Hamilton-Norwood classification,[14] a standard classification scheme with good test–retest reliability, was used in male patients, and for women, the Ludwig classification (Grade I–III) was used.[15]
The cases of AGA were further divided into two categories, normal-to-mild AGA which was considered as severity 1 Hamilton-Norwood Types I–III and Ludwig classification Type I and moderate or severe AGA which was considered as severity 2 Hamilton Norwood Types IV–VII and Ludwig classification Grade II-III, respectively, to assess its association with other potential risk factors.
Diagnosis of MetS was based on the National Cholesterol Education Program Adult Treatment Panel III[16] by the presence of 3 or more of the following criteria.
Relevant investigations were sent, and reports were recorded. TG value ≥150 mg/dl and HDL ≤40 mg/dl in males and HDL ≤50 mg/dl in females were the cutoff points of dyslipidemia.
Fasting blood sugar (FBS) ≥110 mg/dl was the cutoff point of impaired fasting glycemia.
Data were entered into Excel and analyzed using the Statistical Package for the Social Sciences Statistical Package for the Social Sciences (SPSS) version 17.00 (SPSS Inc. Chicago, IL). Quantitative data were presented as mean ± standard deviation, median, and range. Qualitative data were expressed in terms of frequency and percentage. Independent t-test was used as a test of significance. Categorical data were assessed using Chi-square test of significance. P < 0.05 was considered to be significant.
RESULTS
In our study, males outnumbered the females. About 64% of the cases and the controls studied were males and 36% were females. The male:female ratio in cases and controls was 1.78:1. A maximum number of cases and controls belonged to the age group of 20–29 years (43%). The mean value of the age of the patients studied was 33.560 ± 8.533, and the mean value of the age of onset was 29.750 ± 7.736. Nearly 77% of the cases studied belonged to severity 1 (normal-to-mild AGA) and 23% of the cases studied belonged to severity 2 (moderate or severe AGA).
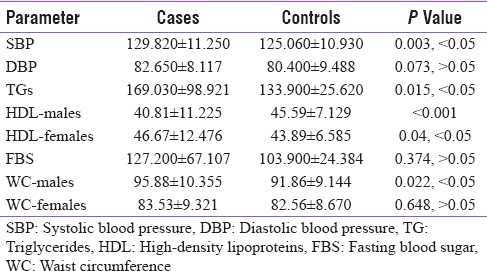
The value of systolic blood pressure, TG, HDL levels in males and waist circumference in males was statistically significant between cases and controls as shown in Table 1. The prevalence of MetS in cases (53%) was statistically very highly significant when compared to the controls (17%) (P < 0.001).
Table 1.
Mean±standard deviation and P value of metabolic syndrome parameters in cases and controls
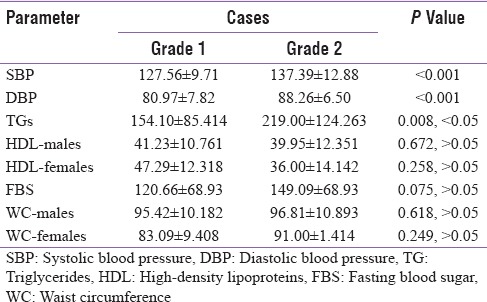
The value of systolic blood pressure, diastolic blood pressure, and TG levels in severity 2 cases was statistically significant when compared to severity 1 cases as shown in Table 2. The prevalence of MetS in severity 2 cases (73.9%) was statistically significant when compared to severity 1 cases (46.8%) (P = 0.022, P < 0.05).
Table 2.
Mean±standard deviation and P value of metabolic syndrome parameters in severity 1 and severity 2 cases
DISCUSSION
In our study, 100 cases diagnosed with AGA and 100 controls without AGA were investigated for other conditions such as hypertension, hyperlipidemia, fasting blood glucose levels, and the various grades of AGA according to the Hamilton-Norwood classification in males and according to Ludwig classification in females.
They were subjected to blood investigations for measuring HDL levels, TG levels, and FBS levels, and anthropometric measurements were taken from both the cases and controls. In our study, the age of onset was around 29.750 ± 7.736 and the age group involved was 33.560 ± 8.533 which was similar to the study conducted by Acibucu et al.,[17] where the age group was 36.280 ± 7.740.
In our study, the value of systolic blood pressure was statistically significant between cases and controls. There is presence of androgen-mediated receptors in the arterial wall endothelium. Increase in serum androgen levels in AGA cases[18] leads to the proliferation of smooth muscle cells in vessels[19] and thereby increasing the tendency to hypertension.[20] Androgens decrease HDL-C levels which were seen in few experimental studies.[21] Low values of HDL-C and high values of TGs were associated with the conversion of atheroma to atherothrombosis. Hence, investigation of lipid profiles in patients with AGA is important to reduce this risk.[22]
In our study, the value of TG in cases was statistically significant (55%) when compared to the controls. Similar results were seen in studies conducted by Acibucu et al.,[17] Chakrabarty et al.,[23] and Ola Ahmed Bakry et al.[24] In males, the value of high-density lipoprotein in cases (48.43%) was lower and statistically very highly significant when compared to the controls.
In our study, in males, the value of waist circumference in cases (45.31%) was statistically significantly higher when compared to the controls (12.5%) P = 0.022, P < 0.05. Similar results were reported by Arias-Santiago et al.,[25] Acibucu et al.,[17] and Ola Ahmed Bakry et al.,[24] regarding waist circumference parameter. Other parameters of MetS values of diastolic blood pressure, FBS levels, and HDL in females and waist circumference in females were not statistically significant.
In our study, MS was significantly associated with AGA cases. The prevalence of MetS in cases (53%) was statistically very highly significant when compared to the control (17%) (P < 0.001). Similar results were seen in studies conducted by Acibucu et al.,[17] Chakrabarty et al.,[23] Arias-Santiago et al.,[25] and Ola Ahmed Bakry et al.[24] In contrast to our study results reported by Mumcuoglu et al.,[26] there was no statistically significant difference between the cases and controls.
To our knowledge, this is the first comparative study among Indian population to evaluate the association of MetS and its parameters with respect to severity of AGA.
The value of systolic blood pressure in severity 2 cases (82.60%) was statistically very highly significant when compared to severity 1 cases (53.24%), P < 0.001, and the value of diastolic blood pressure in severity 2 cases (69.56%) was statistically very highly significant when compared to severity 1 cases (27.27%), P < 0.001. The value of TG levels in severity 2 cases (65.21%) was statistically highly significant when compared to the severity 1 cases (51.19%), P = 0.008, P < 0.05. Other parameters of MetS values of FBS levels, HDL levels in both males and females, and waist circumference in both males and females were not statistically significant. The prevalence of MetS in severity 2 cases (73.9%) was statistically significant when compared to severity 1 cases (46.8%), P = 0.022, P < 0.05.
In a similar study conducted by Yi et al.,[27] among men, the difference between the two groups was not statistically significant, but the number of cases was more in severity 2 when compared with severity 1 cases. In women, the difference was statistically significant when severity 2 cases were compared with that of severity 1.
CONCLUSION
A higher prevalence of MetS is seen in androgenic alopecia cases when compared with that of controls. A significant association was seen between the severity of AGA and MetS. This may suggest an association of AGA with MetS, and early screening for MetS is beneficial in patients with androgenic alopecia to prevent future unforeseen complications by early lifestyle modifications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Banger HS, Malhotra SK, Singh S, Mahajan M. Is early onset androgenic alopecia a marker of metabolic syndrome and carotid artery atherosclerosis in young Indian male patients? Int J Trichology. 2015;7:141–7. doi: 10.4103/0974-7753.171566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupa Shankar D, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichology. 2009;1:131–3. doi: 10.4103/0974-7753.58556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su LH, Chen LS, Lin SC, Chen HH. Association of androgenetic alopecia with mortality from diabetes mellitus and heart disease. JAMA Dermatol. 2013;149:601–6. doi: 10.1001/jamadermatol.2013.130. [DOI] [PubMed] [Google Scholar]
- 4.Ahouansou S, Le Toumelin P, Crickx B, Descamps V. Association of androgenetic alopecia and hypertension. Eur J Dermatol. 2007;17:220–2. doi: 10.1684/ejd.2007.0152. [DOI] [PubMed] [Google Scholar]
- 5.Matilainen V, Koskela P, Keinänen-Kiukaanniemi S. Early androgenetic alopecia as a marker of insulin resistance. Lancet. 2000;356:1165–6. doi: 10.1016/S0140-6736(00)02763-X. [DOI] [PubMed] [Google Scholar]
- 6.Sadighha A, Zahed GM. Evaluation of lipid levels in androgenetic alopecia in comparison with control group. J Eur Acad Dermatol Venereol. 2009;23:80–1. doi: 10.1111/j.1468-3083.2008.02704.x. [DOI] [PubMed] [Google Scholar]
- 7.Mosley JG, Gibbs AC. Premature grey hair and hair loss among smokers: A new opportunity for health education? BMJ. 1996;313:1616. doi: 10.1136/bmj.313.7072.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawk E, Breslow RA, Graubard BI. Male pattern baldness and clinical prostate cancer in the epidemiologic follow-up of the first national health and nutrition examination survey. Cancer Epidemiol Biomarkers Prev. 2000;9:523–7. [PubMed] [Google Scholar]
- 9.Oh BR, Kim SJ, Moon JD, Kim HN, Kwon DD, Won YH, et al. Association of benign prostatic hyperplasia with male pattern baldness. Urology. 1998;51:744–8. doi: 10.1016/s0090-4295(98)00108-3. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, et al. American Heart Association. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Eberly LE, Prineas R, Cohen JD, Vazquez G, Zhi X, Neaton JD, et al. Metabolic syndrome: Risk factor distribution and 18-year mortality in the multiple risk factor intervention trial. Diabetes Care. 2006;29:123–30. doi: 10.2337/diacare.29.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian Indians: A community study from urban Eastern India. J Cardiovasc Dis Res. 2012;3:204–11. doi: 10.4103/0975-3583.98895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra A, Khurana L. The metabolic syndrome in south Asians: Epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 14.Messenger AG, De Berker DA, Sinclair RD. Disorders of hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. West Sussex: Wiley-Blackwell Publication; 2010. pp. 16–31. 66. [Google Scholar]
- 15.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247–54. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 16.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). II-Rationale for intervention. Table II.6–1. Clinical Identification of theMetabolic Syndrome. II-27 [Google Scholar]
- 17.Acibucu F, Kayatas M, Candan F. The association of insulin resistance and metabolic syndrome in early androgenetic alopecia. Singapore Med J. 2010;51:931–6. [PubMed] [Google Scholar]
- 18.Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S, et al. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169–74. doi: 10.1016/0960-0760(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan PJ, McGill HC, Jr, Aufdemorte TB, Triplett RG, Holt RG. Heart contains receptors for dihydrotestosterone but not testosterone: Possible role in the sex differential in coronary heart disease. Anat Rec. 1989;223:414–9. doi: 10.1002/ar.1092230410. [DOI] [PubMed] [Google Scholar]
- 21.Greger NG, Insull W, Jr, Probstfield JL, Keenan BS. High-density lipoprotein response to 5-alpha-dihydrotestosterone and testosterone in Macaca fascicularis: A hormone-responsive primate model for the study of atherosclerosis. Metabolism. 1990;39:919–24. doi: 10.1016/0026-0495(90)90301-r. [DOI] [PubMed] [Google Scholar]
- 22.Sharrett AR, Sorlie PD, Chambless LE, Folsom AR, Hutchinson RG, Heiss G, et al. Relative importance of various risk factors for asymptomatic carotid atherosclerosis versus coronary heart disease incidence: The atherosclerosis risk in communities study. Am J Epidemiol. 1999;149:843–52. doi: 10.1093/oxfordjournals.aje.a009900. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarty S, Hariharan R, Gowda D, Suresh H. Association of premature androgenetic alopecia and metabolic syndrome in a young Indian population. Int J Trichology. 2014;6:50–3. doi: 10.4103/0974-7753.138586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakry OA, Shoeib MA, El Shafiee MK, Hassan A. Androgenetic alopecia, metabolic syndrome, and insulin resistance: Is there any association. A case-control study? Indian Dermatol Online J. 2014;5:276–81. doi: 10.4103/2229-5178.137776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol. 2010;63:420–9. doi: 10.1016/j.jaad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Mumcuoglu C, Ekmekci TR, Ucak S. The investigation of insulin resistance and metabolic syndrome in male patients with early-onset androgenetic alopecia. Eur J Dermatol. 2011;21:79–82. doi: 10.1684/ejd.2010.1193. [DOI] [PubMed] [Google Scholar]
- 27.Yi SM, Son SW, Lee KG, Kim SH, Lee SK, Cho ER, et al. Gender-specific association of androgenetic alopecia with metabolic syndrome in a middle-aged korean population. Br J Dermatol. 2012;167:306–13. doi: 10.1111/j.1365-2133.2012.10978.x. [DOI] [PubMed] [Google Scholar]


