Abstract
Objectives:
Glycemic excursions are commonly seen in patients admitted to the Intensive Care Unit (ICU) and are related to adverse outcomes. Glycemic gap is a marker of this excursion in patients with diabetes. It can be used to predict adverse outcomes in patients with diabetes admitted to the ICU. It is calculated by subtracting A1C-derived average glucose (ADAG) = ([28.7 × HbA1c]-46.7) from plasma glucose at admission. Objective of this study was to correlate glycemic gap and adverse outcomes in patients with type 2 diabetes mellitus (DM) admitted to the ICU.
Materials and Methods:
We conducted an ambispective study to include patients with type 2 DM admitted to the ICUs from July 2015 to June 2016. The following adverse outcomes were recorded: Multiorgan dysfunction syndrome (MODS), acute respiratory distress syndrome (ARDS), shock, upper gastrointestinal (UGI) bleed, acute kidney injury (AKI), and acute respiratory failure (ARF).
Results:
A total of 200 patients were enrolled, with a mean age ± standard deviation of 62 ± 11.24 years, and 64.5% were males. The median (interquartile range) duration of hospital stay and ICU stay were 8 (6–12) days and 4 (3–7) days, respectively. The most common primary diagnosis was cardiovascular (39.5%) followed by neurological (16.5%), infection at diagnosis (16.5%), respiratory (14%), gastrointestinal (7.5%), and others (6%). A higher glycemic gap was associated with occurrence of MODS (P < 0.01), ARDS (P = 0.026), shock (P = 0.043), UGI bleed (P = 0.013), AKI (P = 0.01), and ARF (P < 0.01). Glycemic gap cutoffs of 43.31, 45.26, and 39.12 were found to be discriminatory for predicting ICU mortality (area under the receiver operating characteristic [AUROC]=0.631, P = 0.05), MODS (AUROC = 0.725, P < 0.001), and ARF (AUROC = 0.714, P < 0.001).
Conclusion:
This study showed that higher glycemic gap levels were associated with an increased risk of MODS, ARDS, shock, UGI bleed, AKI, and ARF. Glycemic gap is a tool that can be used to determine prognosis in patients with diabetes admitted to the ICU.
Keywords: A1C-derived average glucose, glycemic gap, Intensive care unit mortality, outcomes
INTRODUCTION
Blood glucose levels at the time of admission to hospital are associated with adverse outcomes.[1,2] Studies have shown that the spikes in blood glucose over and above the previous existing values have more bearing on the outcomes.[3] This is due to increased levels of counterregulatory hormones and cytokines[4] through their effect on the glucose metabolism.
There are numerous markers and scores for prognosticating patients with acute illness. Not many of these take into account plasma glucose levels or glycemic variability. It is hypothesized that measuring the rise in glucose levels above the existing average (HbA1c) would help in assessing the stress levels in acute illness.
To derive average ambient glucose levels, the formula from A1C-derived average glucose study (ADAG)[5] may be used. The glycemic gap is calculated as a difference between the ADAG and the admission glucose and may be a better reflector of outcomes.
In the current study, we studied if higher levels of glycemic gap can be used as a tool to predict adverse outcomes in patients with diabetes mellitus (DM) admitted with critical illness.
MATERIALS AND METHODS
It is an ambispective study; patients with type 2 DM admitted to the Intensive Care Units (ICUs) were included in this study. The Institutional Ethical Review Board clearance was taken.
The following data were collected: age, sex, underlying comorbidities, previous medications for diabetes, plasma glucose level at the time of admission, HbA1c levels at the time of admission, level of mentation, respiratory rate, blood pressure at admission, outcomes, length of mechanical ventilation, and length of ICU stay. The following adverse outcomes were recorded: Multiorgan dysfunction syndrome (MODS– dysfunction of more than one organ), acute respiratory distress syndrome (ARDS– PaO2 (mmHg)/FiO2 <200 mmHg), shock (defined as persistent hypotension despite adequate fluid resuscitation), upper gastrointestinal (UGI) bleed, acute kidney injury (AKI-defined as serum creatinine elevated >0.3 mg/dL or 50% from baseline), acute respiratory failure (ARF-defined as the need for ventilatory support).
Admission blood glucose was the finger stick glucose value at the point of care testing in the accident and emergency department. The average glucose for the past 8–12 weeks was derived from the formula for ADAG, calculated as such: ADAG = ([28.7 × HbA1c]-46.7).[5] The glycemic gap was calculated as the difference between admission blood glucose and the ADAG. HbA1c was measured using HPLC method (BIORAD).
The following were excluded age <18 years, hypoglycemia at admission, admission diagnosis of diabetic ketoacidosis/hyperosmolar hyperglycemic syndrome, treatment with corticosteroids, death within 24 h of admission, renal failure, acute and chronic blood loss, hemolytic anemia, known hemoglobin variants, pregnancy, patients with incomplete data, and hospital stay >180 days.
Statistical methods
All the quantitative variables such as point-of-care blood glucose (GRBS), HbA1c were summarized in terms of descriptive statistics. Chi-square test was employed to test for differences in the proportion while Students -t test will be employed from testing differences in mean value. Area under curve-ROC was calculated to determine the relation between the glycemic gap and the oucomes. Youden's index[6] was used to calculate the best possible value of glycemic gap which can predict adverse outcomes. The data were calculated using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
A total of 200 patients were enrolled, with a mean age ± standard deviation of 62 ± 11.24 years and 64.5% (129) were males. Majority of the patients (62%) belonged to the age group of 51-70 years. Comparatively lesser number of patients (38.5%) were on insulin. The median (interquartile range) duration of hospital stay and ICU stay were 8 (6–12) days and 4 (3–7) days, respectively. A total of 45 (22.5%) patients required mechanical ventilation [Table 1, 2].
Table 1.
Baseline characteristics-I
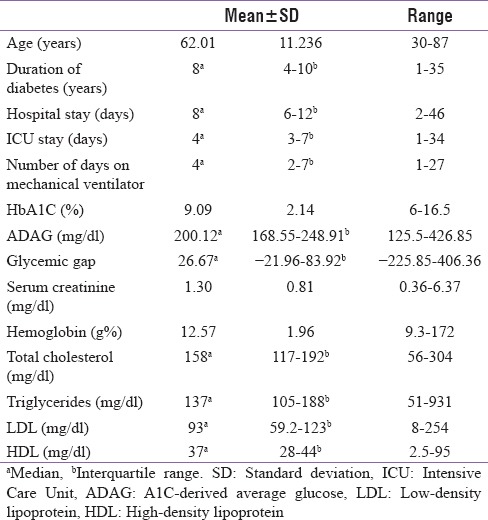
Table 2.
Baseline characteristics-II

The most common primary diagnosis was cardiovascular (39.5%) followed by neurological (16.5%), infection at diagnosis (16.5%), respiratory (14%), gastrointestinal (7.5%), and others (6%) [Table 3].
Table 3.
Distribution of patients by primary diagnosis

Glycemic gap distribution was not significantly different across age (P = 0.418), gender (P = 0.165), lifestyle (P = 0.465), or primary diagnosis (P = 0.733).
A higher glycemic gap was associated with occurrence of MODS (P < 0.01), ARDS (P = 0.026), shock (P = 0.043), UGI bleed (P = 0.013), AKI (P = 0.01), and ARF (P < 0.01).
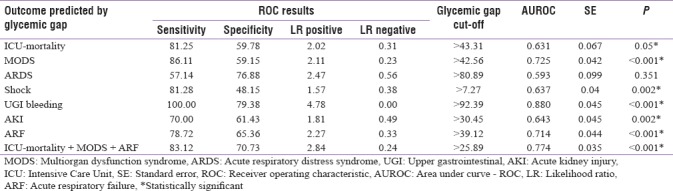
Glycemic gap cutoffs of 43.31mg/dL, 45.26 mg/dL, and 39.12 mg/dL were found to be discriminatory for predicting ICU mortality (area under the receiver operating characteristic [AUROC]=0.631, P = 0.05), MODS (AUROC=0.725, P < 0.001) and ARF (AUROC = 0.714, P < 0.001), respectively, A glycemic gap cutoff of 25.89 mg/dL was predictive for combined occurrence of mortality, MODS, and ARF [Table 4].
Table 4.
Receiver operating characteristic analysis for glycemic gap cut-offs
DISCUSSION
The findings of our study showed that glycemic gap was a good predictor of increased adverse outcomes (MODS, ARDS, shock, UGI bleed, AKI, and ARF) and mortality in patients with diabetes admitted to the ICU. Glycemic gap cutoffs of 43.31 mg/dL, 45.26 mg/dL and 39.12 mg/dL were associated with increased likelihood of ICU mortality (AUROC = 0.631, P = 0.05), MODS (AUROC = 0.725, P < 0.001) and ARF (AUROC = 0.714, P < 0.001). Further, a cutoff as low as 25.89 mg/dL was associated with combined occurrence of mortality, MODS, and ARF.
Stress-induced hyperglycemia has been linked to increased mortality in hospitalized patients.[1] It occurs due to the release of counter-regulatory hormones and cytokines[4] leading to increased gluconeogenesis and insulin resistance. Admission glucose levels are associated with higher mortality in patients coming to the emergency department.[7,8] In some studies, this link was found to be weaker in patients with diabetes.[9] Measurement of admission glucose in patients with diabetes may not correlate with stress levels as many have previously high-glucose levels in the blood. To counter this fallacy, glycemic gap takes into account the HbA1c. HbA1c is not affected by stress or infection but can be affected by anemia and hemoglobinopathies.[10]
Previously, studies have showed that glycemic gap is associated with adverse outcomes in patients having diabetes admitted with liver abscess;[11] community-acquired pneumonia (CAP),[12] and acute myocardial infarction.[13] In a study by Liao et al.,[14] patients with a glycemic gap of ≥80 mg/dL had higher ICU mortality, whereas in our study, it was 43.31 mg/dL. In another study, a glycemic gap of ≥72 was found to significantly correlate with adverse outcomes in diabetic patients with pyogenic liver abscess.[11] In a study by Chen et al.,[12] a glycemic gap of ≥40 mg/dL was found to be discriminatory for adverse outcomes in patients with CAP. Moreover, it was found to be comparable to clinical scoring systems in CAP.
CONCLUSION
Our study showed that higher glycemic gap levels were significantly associated with an increased risk of MODS, ARDS, shock, UGI bleed, AKI, ARF, and ICU mortality. Glycemic gap is a tool that can be used to determine prognosis in patients with type 2 DM admitted with critical illness.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zelihic E, Poneleit B, Siegmund T, Haller B, Sayk F, Dodt C, et al. Hyperglycemia in emergency patients – Prevalence and consequences: Results of the GLUCEMERGE analysis. Eur J Emerg Med. 2015;22:181–7. doi: 10.1097/MEJ.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 2.Martin WG, Galligan J, Simpson S, Jr, Greenaway T, Burgess J. Admission blood glucose predicts mortality and length of stay in patients admitted through the emergency department. Intern Med J. 2015;45:916–24. doi: 10.1111/imj.12841. [DOI] [PubMed] [Google Scholar]
- 3.Sharma J, Chittawar S, Maniram RS, Dubey TN, Singh A. Clinical and epidemiological study of stress hyperglycemia among medical Intensive Care Unit patients in central India. Indian J Endocrinol Metab. 2017;21:137–41. doi: 10.4103/2230-8210.196011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonidou L, Mouzaki A, Michalaki M, DeLastic AL, Kyriazopoulou V, Bassaris HP, et al. Cytokine production and hospital mortality in patients with sepsis-induced stress hyperglycemia. J Infect. 2007;55:340–6. doi: 10.1016/j.jinf.2007.05.177. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–8. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Sung J, Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM, et al. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;59:80–3. doi: 10.1097/01.ta.0000171452.96585.84. [DOI] [PubMed] [Google Scholar]
- 8.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39:105–11. doi: 10.1097/CCM.0b013e3181feb5ea. [DOI] [PubMed] [Google Scholar]
- 9.Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001–9. doi: 10.1097/CCM.0b013e3181b083f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG, et al. The effect of anaemia and abnormalities of erythrocyte indices on hbA1c analysis: A systematic review. Diabetologia. 2015;58:1409–21. doi: 10.1007/s00125-015-3599-3. [DOI] [PubMed] [Google Scholar]
- 11.Liao WI, Sheu WH, Chang WC, Hsu CW, Chen YL, Tsai SH, et al. An elevated gap between admission and A1C-derived average glucose levels is associated with adverse outcomes in diabetic patients with pyogenic liver abscess. PLoS One. 2013;8:e64476. doi: 10.1371/journal.pone.0064476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen PC, Liao WI, Wang YC, Chang WC, Hsu CW, Chen YH, et al. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with community-acquired pneumonia. Medicine (Baltimore) 2015;94:e1456. doi: 10.1097/MD.0000000000001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao WI, Lin CS, Lee CH, Wu YC, Chang WC, Hsu CW, et al. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with acute myocardial infarction. Sci Rep. 2016;6:27770. doi: 10.1038/srep27770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao WI, Wang JC, Chang WC, Hsu CW, Chu CM, Tsai SH, et al. Usefulness of glycemic gap to predict ICU mortality in critically ill patients with diabetes. Medicine (Baltimore) 2015;94:e1525. doi: 10.1097/MD.0000000000001525. [DOI] [PMC free article] [PubMed] [Google Scholar]


