Abstract
Introduction:
Due to the high prevalence of Vitamin D deficiency in spite of abundant sunshine and scarcity of studies investigating Vitamin D status in Indian children from rural and semirural areas, the objectives of this cross-sectional study were to: (1) assess the Vitamin D status of school-children in a semi-rural setting and (2) identify the determinants of Vitamin D status in these children.
Materials and Methods:
Data collected included anthropometric measurements (height and weight), body composition, three-one-day dietary recall method, demographic data, and sunlight exposure. Serum 25-hydroxyVitamin D (25(OH)D) was estimated by enzyme-linked immunosorbent assay (ELISA) technique. SPSS software was used for statistical analysis.
Results:
Anthropometric characteristics of the children were similar and mean serum 25(OH)D concentration was 58.5 ± 10.3 nmol/L with no significant differences between genders. Around 80% children reported sunlight exposure of 2 h or more. A majority (71%) of children were Vitamin D insufficient with serum 25(OH)D concentrations between 50 and 74.9 nmol/L. Determinants of Vitamin D identified were duration of sunlight exposure and body fat percent. Significant (P < 0.05) positive association of duration of sunlight exposure was observed with serum 25(OH)D concentrations, while BF% showed a negative association with serum 25(OH)D (β = –0.307; standard error = 0.1388; P < 0.05).
Discussion:
We have reported a high prevalence of Vitamin D insufficiency in school-children aged 6–12 years, from a semirural setting, in spite of a majority (80%) reporting >2 h of sunlight exposure. We have also demonstrated that duration of sunlight exposure and body fat percentage are the two important determinants of serum 25(OH)D concentrations in these children.
Keywords: Body fat percent, semirural, sunlight exposure, Vitamin D status
INTRODUCTION
Vitamin D is a steroid hormone that plays a major role in maintaining skeletal health. A number of factors have been identified as determinants of Vitamin D with adequate exposure to sunlight occupying the prime position. The minimum daily sunlight exposure recommended for Indians who have the Fitzpatrick skin type V is >45 min.[1] However, there have been reports of a high prevalence of Vitamin D deficiency even among Indians who enjoy adequate exposure to sunlight. While most of these studies focus on adults, very few studies have looked into the Vitamin D status of young schoolchildren (6–12 years), especially in rural and semirural areas.[2] This necessitates investigations into Vitamin D status of school-children in rural and semirural areas.
Other important factors identified as determinants of Vitamin D status are dietary intake of Vitamin D and use of Vitamin D supplements, dietary calcium intake, adiposity (as Vitamin D gets sequestrated into the adipose tissue) and skin pigmentation, use of sunscreens, and increasing age.[1,3,4,5]
The reasons being put forth for the widespread prevalence of Vitamin D deficiency among Indians are negligible dietary vitamin D intake because of primarily vegetarian diets and lack of guidelines and programs for the fortification of food with Vitamin D. Urbanization leading to increasing pollution as well as increased time being spent indoors have also been implicated.[6] However, there are very few studies investigating the determinants of Vitamin D status in Indian school-children.
Thus, the objectives of our study were to: (1) assess the Vitamin D status of school-children in a semirural setting in Western India (18°N) and (2) identify the determinants of Vitamin D status in these children.
MATERIALS AND METHODS
This cross-sectional study was carried out in school-children aged 6–12 years from a Government-run primary school (Elementary School Grade 1–4) in a semirural district located about 65 km from Pune at 18°N. Rural and semirural areas around Pune city were surveyed before this particular site was chosen. The local governing body was approached, and a list of schools in the chosen area was obtained. Ten schools with children in the required age group were shortlisted. Of the eight schools which consented to allow the study to be carried out on their premises, one school was randomly selected. The inclusion criteria were children aged 6.0–12.0 years, children who were not consuming Vitamin D or any mineral supplements, and children with no chronic systemic illnesses or congenital abnormalities. Parents of all the children (544) who met the age criteria in the selected school were approached for consent, and 474 parents consented to allow their children to participate in the study. Written informed consents were obtained from the parents, while the children gave a written informed assent. Pediatricians carried out a general medical examination for all the children to rule out chronic disease conditions and congenital abnormalities. After screening and clinical examination of 474 children, 30 were excluded due to medical conditions and 9 did not come for blood collection. Thus, 435 children were enrolled and final results have been presented on 359 children (192 boys) for whom complete data were available. The Institutional Ethics Committee gave ethics approval for the study.
Demographic data, details of parents’ education, and sunlight exposure of the children were recorded by administering a detailed, validated questionnaire[7] to the primary caregiver and the participant.
Anthropometric measurements collected included height and weight. Standing height was measured using a portable stadiometer (Seca 213 Portable Stadiometer, Germany). Body weight and body composition were measured using the Tanita Body Composition Analyzer (Model BC-420MA) after the children were asked to empty their pockets and stand barefoot on the scale. Body mass index (BMI) was calculated by dividing the weight in kilograms by height in meters squared. The Z-scores for height (HAZ), weight (WAZ), and BMI (BAZ) were computed using Indian growth references.[8]
Venous blood sample was collected in fasting condition for estimation of serum 25-hydroxy Vitamin D (25(OH)D) by ELISA technique using standard kits (DLD diagnostics GMBH, intra-assay coefficient of variation [CV] 5%; interassay CV 7.8%). Vitamin D status was then classified according to the Endocrine Society Practice Guidelines: vitamin D deficiency as serum 25(OH) D below 50 nmol/L; insufficiency as 25(OH) D of 50.0–74.9 nmol/L; and sufficiency as concentrations ≥75 nmol/L.[9]
Dietary data were recorded using the three-one-day dietary recall method, over three non-consecutive days including one holiday or a Sunday. Trained nutritionists interviewed the children along with their primary caregivers to get an accurate estimate of the foods consumed. Nutrient intakes were then computed using the cooked food database software, C-Diet.[10] Adequacies of nutrient intakes were estimated by computing the following measures: (1) percentage of the recommended dietary allowance (RDA) for Indian children for each nutrient consumed and (2) nutrient density as the amount of nutrient consumed for every 1000 kcals consumed by the participant.
Statistical analysis was carried out using the software IBM SPSS Statistics for Windows (version 21.0.2012, IBM Corp, Armonk, NY, USA). All results have been expressed as mean ± standard deviation. Differences between genders were tested using the Student's t-test. Univariate analysis was used to test associations of various factors independently with serum 25(OH)D. The following factors were identified and entered into the generalized linear model to identify the determinants of Vitamin D concentrations: gender, body fat percent (BF%), duration of sunlight exposure, surrogates of socioeconomic status (i.e., type of house the participant lived in, father's and mother's education), and dietary calcium density.
RESULTS
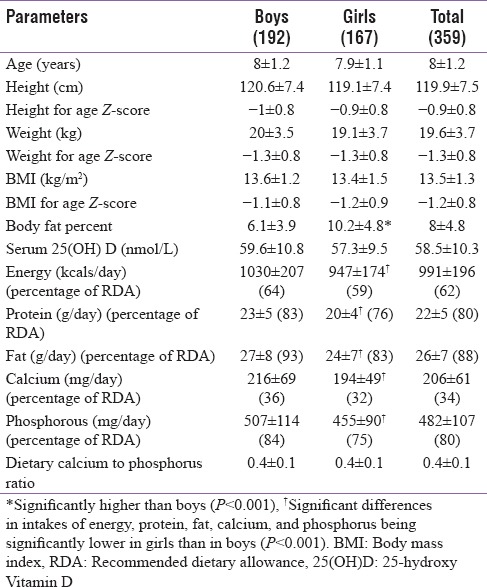
The general characteristics of the children were similar between boys and girls with no significant differences found in age, height, weight, BMI, and anthropometric Z-scores [Table 1]. The exception was body fat percentage which was significantly higher in girls than in boys (P < 0.001). The mean serum 25(OH) D concentration for the children was 58.5 ± 10.3 nmol/L and these concentrations were similar between the genders. Nineteen percent of children reported sunlight exposure of <2 h, 51% between 2 and 2.5 h, and 30% of more than 2.5 h. The diets of the children were deficient in calcium and had low calcium to phosphorus ratio of 0.4:1. The children did not consume any dietary source of Vitamin D.
Table 1.
General characteristics of the children
Classification of Vitamin D status according to the endocrine society guidelines revealed that a majority (71%) of the children were Vitamin D insufficient with serum 25(OH)D concentrations between 50 and 74.9 nmol/L. Twenty-four percent of children were deficient (serum concentrations <50 nmol/L) and only 5% were Vitamin D sufficient (serum concentrations ≥75 nmol/L).
Figure 1 is an illustration of Vitamin D status according to the sunlight exposure of the children. No participant with <2 h of sunlight exposure was found to be Vitamin D sufficient compared to 11% children who reported more than 2.5 h of sunlight exposure (P < 0.01). Among children reporting <2 h of sunlight exposure, 35% were found to be deficient as compared to 21% children reporting more than 2.5 h of sunlight exposure (P < 0.01).
Figure 1.
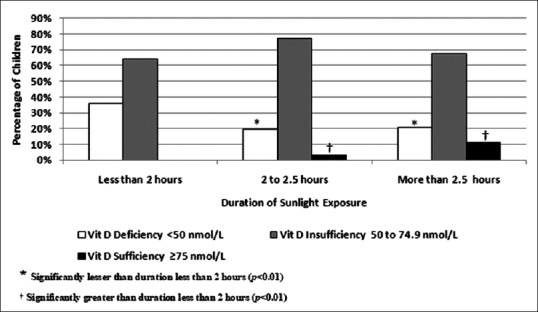
Vitamin D status according to sunlight exposure of the children
Determinants of Vitamin D in the children were identified by fitting a generalized linear model [Table 2]. A significant (P < 0.05) positive association of duration of sunlight exposure was observed with serum 25(OH)D concentrations, while BF% showed a negative association with serum 25(OH)D (β = −0.307; standard error = 0.1388; P < 0.05). All the other factors that were entered into the model, that is, gender, type of house, father's and mother's education, and dietary calcium density, did not show any significant influence on serum 25(OH)D concentrations.
Table 2.
Determinants of serum Vitamin D concentrations

DISCUSSION
Our study in school-children aged 6–12 years from a semirural setting suggests that duration of sunlight exposure and body fat percentage were the two important determinants of serum 25(OH) D concentrations. Duration of sunlight exposure positively influenced Vitamin D status, while BF% negatively influenced 25(OH)D concentrations in these children. However, in spite of an average of 2 h of sunlight exposure, a majority of the children had insufficient 25(OH)D concentrations, according to the classification by the Endocrine Society Clinical Practice Guidelines.
Abu Shady et al. have suggested that low sun exposure, obesity, and low level of maternal education were significant determinants of Vitamin D insufficiency in Egyptian school-children aged 9–11 years.[11] Wakayo et al. have reported that duration of sunlight exposure, body fatness, maternal education, and socioeconomic status were among some of the significant determinants of Vitamin D deficiency in Ethiopian children aged 11–18 years.[12] Similar to the reports above, we found that duration of exposure to sunlight and body fat percentage were important determinants of Vitamin D status in our children. However, we did not find any significant influence of gender, socioeconomic status surrogates (type of house, father's and mother's education), and dietary calcium density on 25(OH)D concentrations. Similar findings have been reported in children aged 0–15 years from Qatar, where gender and parent's education were not found to influence Vitamin D status in the children.[13]
Increased duration of sunlight exposure was found to have a positive influence on the serum 25(OH) D concentrations in this group. However, a majority of the children (71%) continued to remain insufficient (serum 25(OH) D between 50 and 75 nmol/L) in spite of approximately 2 h or more of daily sunlight exposure. In accordance with our results, Harinarayan et al., too reported that rural children had higher serum 25(OH)D concentrations as compared to their urban counterparts, and this was attributed to the longer duration of sunlight exposure in them. However, despite the extended duration of exposure to sunlight, most of their rural study children (76% males and 72% females) were found to be deficient.[2]
Body fat percentage was found to negatively affect serum 25(OH)D concentrations in these children, with higher body fat percent being associated with lower serum 25(OH)D concentrations. This has been ascribed to the sequestration of Vitamin D (both synthesized as well as ingested) into adipocytes before it is transported to the liver for conversion into 25(OH)D. Similar negative association of adiposity measures (BMI and body fat percent) with serum 25(OH)D has been reported in a cohort of Cypriot adolescents.[14]
Poor dietary calcium intake was proposed as one of the reasons for low 25(OH)D concentrations in populations with adequate exposure to sunlight.[15] Clements et al. demonstrated through rat models the mechanism of how calcium intake could influence 25(OH)D concentrations. They suggested that a diet low in calcium induces mild hyperparathyroidism which elevates 1,25-dihydroxy Vitamin D; this reduces the half-life of 25(OH)D and thus, indirectly reduces 25(OH)D concentrations.[15,16] Nutritional intakes of calcium in the current study group were very poor with diets contributing a mean of 34% (205 ± 61 mg/day) of the RDA for calcium. The RDA of calcium for children aged 6–12 years ranges from 600 to 800 mg/day.[17] Nevertheless, we did not find calcium intake to be a determinant of 25(OH)D concentrations in the present study. We speculate that this may be due to the lack of variation in calcium intake with all children consuming calcium-deficient diets.
Another important reason may be pollution due to the proximity of the site to an industrial zone. While we have neither assessed pollution concentrations nor haze scores, which are considered as a surrogate for ultraviolet B reaching the ground, at this point, we can only speculate that air pollution due to factory fumes may have contributed to some extent to the Vitamin D insufficiency observed in this group. There have been reports of significantly lower concentrations of 25(OH)D in infants living in a part of New Delhi with considerably higher pollution (Mori Gate) as compared to infants from Gurgaon, a lesser polluted part of Delhi city.[18]
Apart from this study being a cross-sectional study where we were able to study associations but not causations, we also acknowledge the limitation that we assessed sunlight exposure by questionnaire, and objective measures such as polysulfone badges or measuring haze scores were not used. However, the questionnaire used has been validated in Indian school-children using PSU badges and these results have been reported.[7]
CONCLUSION
The present study demonstrates a high prevalence of Vitamin D insufficiency among school-children in a semirural setting in India. Duration of sunlight exposure and body fat percentage are important determinants of serum 25(OH)D concentrations in these children. Further studies are required to investigate the determinants of Vitamin D status in children from rural areas to aid policy-making to tackle the problem of inadequate 25(OH)D concentrations among children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Rubina Mandlik was funded by a Fellowship Grant from the University Grants Commission, Government of India. We wish to express our sincere thanks to all the children and parents who participated in this study. We also wish to thank the school principals, teachers, and school staff.
REFERENCES
- 1.Ritu G, Gupta A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients. 2014;6:729–75. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D. Vitamin D status in Andhra Pradesh: A population based study. Indian J Med Res. 2008;127:211–8. [PubMed] [Google Scholar]
- 3.Webb AR. Who, what, where and when-influences on cutaneous Vitamin D synthesis. Prog Biophys Mol Biol. 2006;92:17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Lips P, van Schoor NM, de Jongh RT. Diet, sun, and lifestyle as determinants of Vitamin D status. Ann N Y Acad Sci. 2014;1317:92–8. doi: 10.1111/nyas.12443. [DOI] [PubMed] [Google Scholar]
- 5.Abboud M, Rybchyn MS, Rizk R, Fraser DR, Mason RS. Sunlight exposure is just one of the factors which influence Vitamin D status. Photochem Photobiol Sci. 2017;16:302–13. doi: 10.1039/c6pp00329j. [DOI] [PubMed] [Google Scholar]
- 6.Babu US, Calvo MS. Modern India and the Vitamin D dilemma: Evidence for the need of a national food fortification program. Mol Nutr Food Res. 2010;54:1134–47. doi: 10.1002/mnfr.200900480. [DOI] [PubMed] [Google Scholar]
- 7.Patwardhan V, Khadilkar A, Chiplonkar S, Mughal Z, Khadilkar V. Varying relationship between 25-hydroxyvitamin D, high density lipoprotein cholesterol, and serum 7-dehydrocholesterol reductase with sunlight exposure. Journal of Clinical Lipidology. 2015;9:652–7. doi: 10.1016/j.jacl.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Indian Academy of Pediatrics Growth Charts Committee. Khadilkar V, Yadav S, Agrawal KK, Tamboli S, Banerjee M, et al. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr. 2015;52:47–55. doi: 10.1007/s13312-015-0566-5. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 10.Kajale NA, Khadilkar VV, Mughal Z, Chiplonkar SA, Khadilkar AV. Changes in body composition of Indian lactating women: A longitudinal study. Asia Pac J Clin Nutr. 2016;25:556–62. doi: 10.6133/apjcn.092015.16. [DOI] [PubMed] [Google Scholar]
- 11.Abu Shady MM, Youssef MM, Salah El-Din EM, Abdel Samie OM, Megahed HS, Salem SM, et al. Predictors of serum 25-Hydroxyvitamin D concentrations among a sample of Egyptian schoolchildren. Scientific World Journal. 2016;2016:8175768. doi: 10.1155/2016/8175768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakayo T, Belachew T, Vatanparast H, Whiting SJ. Vitamin D deficiency and its predictors in a country with thirteen months of sunshine: The case of school children in central Ethiopia. PLoS One. 2015;10:e0120963. doi: 10.1371/journal.pone.0120963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bener A, Al-Ali M, Hoffmann GF. Vitamin D deficiency in healthy children in a sunny country: Associated factors. Int J Food Sci Nutr. 2009;60(Suppl 5):60–70. doi: 10.1080/09637480802400487. [DOI] [PubMed] [Google Scholar]
- 14.Kolokotroni O, Papadopoulou A, Yiallouros PK, Raftopoulos V, Kouta C, Lamnisos D, et al. Association of Vitamin D with adiposity measures and other determinants in a cross-sectional study of Cypriot adolescents. Public Health Nutr. 2015;18:112–21. doi: 10.1017/S1368980013003480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettifor JM. Nutritional rickets: Deficiency of Vitamin D, calcium, or both? Am J Clin Nutr. 2004;80:1725S–9S. doi: 10.1093/ajcn/80.6.1725S. [DOI] [PubMed] [Google Scholar]
- 16.Clements MR, Johnson L, Fraser DR. A new mechanism for induced Vitamin D deficiency in calcium deprivation. Nature. 1987;325:62–5. doi: 10.1038/325062a0. [DOI] [PubMed] [Google Scholar]
- 17.Indian Council of Medical Research. Nutrient Requirements and Recommended Dietary Allowances for Indians – A Report of the Expert Group of the Indian Council of Medical Research. Expert Group of the Indian Council of Medical Research. 2009:1–334. [Google Scholar]
- 18.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM, et al. The impact of atmospheric pollution on Vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002;87:111–3. doi: 10.1136/adc.87.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]


