Abstract
Background:
Homocysteine (HCY) interferes with collagen cross-linking in bones and stimulates osteoclast activity. The activated osteoclasts secrete cathepsin K (CathK), a cysteine protease, in eminent quantity during bone resorption. Hyperhomocysteinemia may effect bone mineral density (BMD) through CathK. We, therefore, examined the relation between HCY and BMD along with CathK, 25-hydroxyvit-D (25[OH]D), intact parathyroid hormone (iPTH), and Vitamin B12.
Materials and Methods:
We recruited a total of 93 postmenopausal women between the age group of 45–60 years, attending the Endocrinology outpatient department at King George's Medical University, Lucknow. BMD was done by DXA scan using Hologic QDR1000 system. Based on the WHO criteria, patients were segregated into three groups as follows; normal bone mass, osteopenia, and osteoporosis. All women underwent routine biochemical laboratory parameters, HCY, Vitamin B12, and CathK levels.
Results:
Among 93 postmenopausal women, 56% (52) had osteoporosis. Nineteen percent (18) had normal BMD (mean age, 53.22 ± 8.5 years) and 23 (25%) had osteopenia (mean age 52.86 ± 6.67 years). The mean age in the osteoporetic group was 56.2 ± 6.9 years. The median (interquartile range) levels of HCY in the three groups were 14.5 μmol/L (12.2–24.7), 15.05 μmol/L (12.1–19.9) and 13.2 μmol/L (10.3–17.0), respectively. CathK levels were similar in three groups 7.6 ng/ml (7.0–80.5), 8.3 ng/ml (7.3–8.5), and 8.6 ng/ml (7.2–8.9). Both HCY and CathK were found positively associated with serum phosphorus (r = 0.584, P < 2.01 and r = 0.249, P < 0.05, respectively). Levels of HCY positively correlate with PTH (r = 0.303, P < 0.01) and inversely with Vitamin B12 (r = −0.248, P < 0.05). No significant association was seen between CathK level and 25(OH) D, iPTH, serum calcium.
Conclusion:
Low bone mass by DXA is a significant problem in postmenopausal females. HCY and CathK do not reliably correlate with bone loss in postmenopausal women although phosphorus metabolism may play a role.
Keywords: Bone mineral density, cathepsin K, homocysteine, osteoporosis, postmenopausal
INTRODUCTION
Osteoporosis is common in women after menopause with increased bone fragility.[1,2] Besides the known risk factors, other potential risk factors have been identified.
Association of hyperhomocysteinemia with lower bone mineral density (BMD)[3,4] and risk of osteoporotic fractures[5,6] has been shown with contrasting results.[7,8] Homocysteine (HCY) decreases bone blood flow, increases matrix metalloproteinases,[9] and interferes with collagen cross-linking.[10,11] Collagen cross-links provide strength and stability to collagen network in bone matrix. Cathepsin-K (CathK) is mainly expressed in osteoclasts and is essential for bone resorption.[12] High levels of CathK are seen in osteoporotic women.[13,14] We studied the correlation between BMD, HCY, and CathK in postmenopausal women.
MATERIALS AND METHODS
Ethics statement
Ethical approval for the study was obtained from the Institutional Ethics Committee of King George's Medical University, Lucknow (Uttar Pradesh), India (No. 9329/ethics/R. Cell-16). Before entering the study, all the patients were given a verbal presentation of information on the study, and a written consent was obtained from all the subjects enrolled in the study.
Study participants
In this cross-sectional study, postmenopausal women aged ≥45 years whose menses had stopped for more than 1 year were recruited from the Endocrinology outpatient department, King George's Medical University, Lucknow. For study, we took the definition of menopause as cessation of menses for more than 1 year.[15] Osteoporosis and osteopenia were classified according to the World Health Organization classification system.[16] Premature menopause, patients with secondary osteoporosis and women who were suffering from any other medical complication such as hyperparathyroidism or rheumatoid arthritis which might affect bone metabolism was excluded from the study. Patients receiving Vitamin B12 or folic acid supplements were also excluded from the study.
Lifestyle factors and anthropometric measurement
All subjects included in the study underwent detailed history concerning their lifestyle and general health information such as duration of menopause, history of fracture, family history and other details regarding the intake of any other drug in the past, mainly focusing on antitubercular treatment, antiretroviral therapy, antiepileptics, food fortification with Vitamin D, calcium supplementation, and steroids.
Anthropometric measures, such as height, weight, and body mass index (BMI) was measured. Height was measured using a standard stadiometer, and weight was measured using an electronic scale.
Bone mineral density
BMD was measured at hip, lumbar spine, and forearm in all subjects by dual-energy X-ray absorptiometry (DEXA) using the Hologic QDR1000 machine and was expressed in absolute values as grams of mineral content per square centimeters of the bone area (g/cm2).
Bone markers assessment
Blood samples were collected from all the participants and serum was immediately separated and stored in-20°C until the level of specific serum bone-related markers were measured. Serum HCY, 25-hydroxyvit-D (25[OH] D), Calcium, phosphorus, intact parathyroid hormone, SGOT/SGPT, alkaline phosphatase (ALP), protein/albumin, creatinine, Vitamin B12, folic acid, and CathK were measured using standard analytical techniques.
Statistical analysis
Descriptive statistics were examined for all study variables. Data analysis was using statistical software package SPSS 20.0 (SPSS, Chicago, IL). In subjects demographic and biochemical parameters, differences in baseline between the three groups were analyzed using Kruskal–Wallis test. Baseline characteristics of each study group were examined using Chi-square test for categorical variables. Data are presented as the mean ± standard deviation. Nonparametric tests were applied because some variables did not present a normal distribution (Gaussian); due to data dispersion, the null hypothesis was rejected according to the Shapiro–Wilk test. The Pearson correlation coefficient was calculated as a measure of association between BMD and study variables. Multiple linear regression analysis was performed to determine the predictors of BMD at different regions (hip, spine, and forearm). BMD was entered as the dependent variable with serum ALP, calcium, phosphorus, 25(OH) D, parathyroid hormone (PTH), HCY, Vitamin B12, folic acid, and CathK simultaneously as predictor variables. A value of P < 0.05 was considered statistically significant for all analysis.
RESULTS
On the basis of BMD, the enrolled cases were put into three groups as follows: Postmenopausal women with osteoporosis (t-score <−2.5), postmenopausal women with osteopenia (t score between − 2.5 and − 1), and postmenopausal women with normal BMD values (t score >−1). Table 1 illustrates that mean values of age, height, weight, and BMI were comparable between the three groups. The mean value of the duration of menopause was significantly higher (P < 0.05) in women with osteoporosis. No significant differences were observed between mean values of hemoglobin, SGOT, SGPT, SALP, creatinine, and protein. The Vitamin D and PTH levels were found similar between the osteoporotic, osteopenic, and normal group while significant difference was detected between mean values of phosphorus among the groups. Conversely, the calcium level in osteoporotic and osteopenic group was significantly higher (P = 0.029) than the normal group.
Table 1.
Physical and biochemical characteristics of subjects
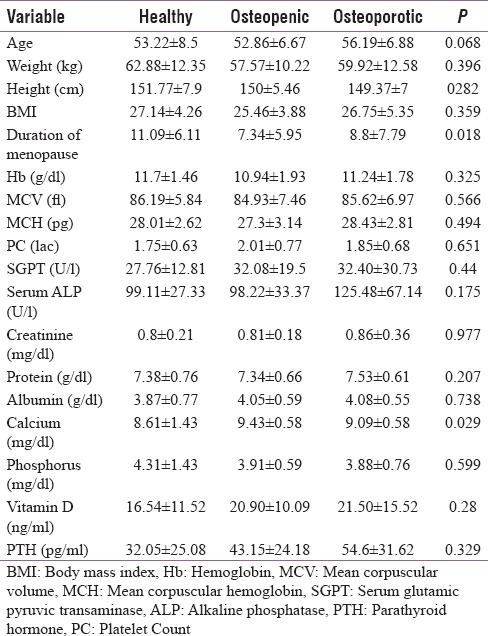
Table 2 provides the detailed information about the distribution of cath K, folic acid, Vitamin B12 and HCY in three groups. An enlarged level of HCY was observed in the osteopenic group while lowest in osteoporosis and no clinical reasons were found for such variation among the two groups. There was no significant difference in Cath K level between the groups (P = 0.421). Pooling all healthy women and comparing them with the osteoporotic also did not show significant differences (P = 0.181). Similarly, no significant differences were observed in HCY, Vitamin B12 and folic acid between these three groups.
Table 2.
Distribution of homocysteine, Vitamin B12, cathepsin K postmenopausal healthy, postmenopausal osteopenic and postmenopausal osteoporotic, respectively, given as median and interquartile range
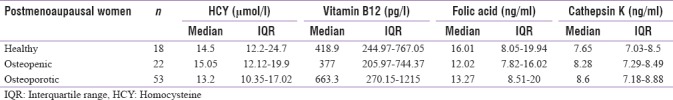
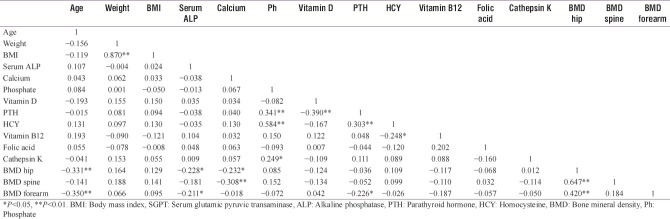
Table 3 illustrates the Pearson correlation coefficients of the parameters measured in all subjects. Age was negatively correlated with BMD (hip and forearm). No statistically significant correlations were detected between Vitamin D and BMD at the three sites as well as with age, weight, and BMI. PTH was found to be negatively correlated with Vitamin D (r = −0.39, P < 0.01) while positively correlated with phosphate level (r = 0.341, P < 0.01) in all the subjects.
Table 3.
Pearson correlation between different bone-remodelling parameters related to osteoporosis
BMD at hip was negatively correlated with calcium (r = −0.232, P < 0.05) and ALP (r = −0.228, P < 0.05). BMD at spine was also negatively correlated with calcium level (r = −0.018, P < 0.01). No significant correlations were seen between calcium and Vitamin D, calcium and PTH, BMI and Vitamin D, BMI and PTH, BMI and age, BMI and BMD at the three sites. No significant correlation was found between BMD values and HCY, Vitamin B12, folic acid, and cathepsin.
Levels of HCY were related positively to serum phosphate (r = 0.584, P < 0.01) and PTH (r = 0.303, P < 0.01) and inversely with Vitamin B12 (r = −0.248, P < 0.05). No significant relation was observed between levels of Vitamin B12 and folic acid. Similarly, except serum phosphate level, there are no correlations between CathK levels and other biomarkers such as Vitamin D, PTH calcium as well as BMD and age, which is shown in Table 2.
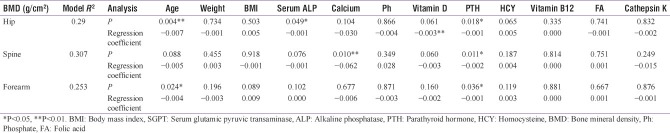
Multiple regressions were also performed on all variables to determine which variables could predict the BMD level when controlling for the effect of others at each of the tested locations. Results in Table 4 shows that age of the participants was a significant determinant of BMD at hip and forearm, whereas it lost its correlation strength with BMD at spine. All other variables did not show any association with BMD at any tested location, except for calcium which appeared to have an effect on the lumbar spine and PTH which appeared to have an effect on BMD at all the three sites.
Table 4.
Association of bone marker variables with bone mineral density by location using multiple regression analysis
DISCUSSION
In this study, no significant differences of HCY as well as CathK levels were found between the three groups based on WHO criteria for the diagnosis of osteoporosis. Both these parameters had poor indicative power to distinguish between osteoporotic and nonosteoporotic subjects. In addition, both these markers did not correlate with the established marker of bone resorption such as ALP and Vitamin D.
There have been a few studies on Hcy and osteoporosis. Some have shown a correlation of increased HCY levels with lower BMD,[3,4] while others have failed to find any such correlation.[7,8] Taken into consideration the controversial findings of other researchers, this study would support the reporting of no association of Hcy and CathK with osteoporosis. It is also not clear whether higher HCY levels may be due to a state of increased bone resorption rather than having a causative role in osteoporosis. Although it generally seems that high Hcy level contributes to osteoporosis development, while few studies reveal its direct effect on bone. HCY seems to stimulate osteoclast formation and activity leading to an inhibitory effect of HCY on bone formation.[17] Several studies reported that the increased HCY level was a risk factor for osteoporotic fractures.[18] van Meurs et al.[5] did not find any association between HCY and BMD at spine but found a strong relationship of HCY and fracture incidence. They speculated that the mechanism underlying the association between the HCY level and the risk of fracture may involve interference of HCY in collagen cross-linking independent of the amount of mineral in the bone which would account for the lack of association between HCY and BMD. Similarly, in present study HCY levels were similar in all three groups without any significant correlation between HCY and BMD. Thus, we suggest that HCY may not play a significant role in the osteoporosis.
Considering the relationship between Vitamin B12 and BMD, studies have shown Vitamin B12 as an independent predictor of BMD at total hip. According to previous researches, it was found that osteoporotic postmenopausal women had lower plasma Vitamin B12 than those with no osteoporosis. A recent study suggested that osteoporotic postmenopausal women had a higher plasma Vitamin B12 than those with no osteoporosis,[19,20] which is further supported by the present study. Tucker et al., found that both men and women with Vitamin B12 concentrations below 148 pmol/l had lower average BMD than those with higher concentrations, but with significance varying with the site of BMD measurement (men at the hip, women at the spine).[21] The difference in findings of the association of serum Vitamin B12 with BMD may, therefore, be explained by population demographic differences, and more particularly, the site of assessment of BMD. Since the majority of Indian population follows vegetarianism that predisposes them to Vitamin B12 deficiency. Studies had clearly shown that vegetarians in India have low Vitamin B12 levels. Since Vitamin B12 deficiency is related to high levels of HCY. Therefore, the prevalence of hyperhomocysteinemia in Indian population is high.[22]
There are controversial results regarding the CathK level as a potential biomarker for the prediction of osteoporosis. Various groups found significant differences of CathK levels between osteoporotic and nonosteoporotic subjects.[14,23] In contrast, Adolf et al. identified no significant differences between the postmenopausal osteoporotic and healthy women by testing the CathK levels.[24] The present study is in line with the study conducted by Adolf et al., where the data showed no difference in CathK level among the osteoporotic and healthy postmenopausal women. Indeed, because the circulating concentration of CathK is very low and currently available assays lack sensitivity, accurate determination of the serum concentration of CathK remains challenging.[25] Moreover thus, could not be used as a diagnostic marker for osteoporosis.
Strengths of this study include the use of same DEXA machine (Hologic) QDR1000 system and single operator in the investigation of BMD, few dropout rate (consistent with our group) and the application of our findings to Indian postmenopausal women. Its weakness includes our possibly limited ability to enrol more healthy control postmenopausal women.
The present study showed no direct correlation between BMD and HCY, CathK, folic acid, and Vitamin B12. There were also no differences in mean values of HCY and CathK between control and osteoporosis postmenopausal women. The apparent detrimental effect of age, duration of menopause and PTH on osteoporosis was observed on postmenopausal women.
CONCLUSION
More than half of the postmenopausal females had osteoporosis. Less than one-fourth of the postmenopausal females had normal, BMD. There was no significant difference found in HCY and CathK levels between the three groups. BMD neither correlated with HCY nor with CathK. Hence, our data suggest that HCY and CathK do not reliably correlate with bone loss in postmenopausal women. Phosphorus levels correlating with HCY and CathK could be an area of further research in bone loss pathophysiology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tuck SP, Datta HK. Osteoporosis in the aging male: Treatment options. Clin Interv Aging. 2007;2:521–36. doi: 10.2147/cia.s820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral efficacy with risedronate therapy (VERT) study group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 3.Bahtiri E, Islami H, Rexhepi S, Qorraj-Bytyqi H, Thaçi K, Thaçi S, et al. Relationship of homocysteine levels with lumbar spine and femur neck BMD in postmenopausal women. Acta Reumatol Port. 2015;40:355–62. [PubMed] [Google Scholar]
- 4.Gerdhem P, Ivaska KK, Isaksson A, Pettersson K, Väänänen HK, Obrant KJ, et al. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. J Bone Miner Res. 2007;22:127–34. doi: 10.1359/jbmr.061003. [DOI] [PubMed] [Google Scholar]
- 5.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–41. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 6.Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Meyer HE, Tell GS, et al. Plasma homocysteine, folate, and Vitamin B 12 and the risk of hip fracture: The hordaland homocysteine study. J Bone Miner Res. 2007;22:747–56. doi: 10.1359/jbmr.070210. [DOI] [PubMed] [Google Scholar]
- 7.Kim JI, Moon JH, Chung HW, Kong MH, Kim HJ. Association between homocysteine and bone mineral density according to age and sex in healthy adults. J Bone Metab. 2016;23:129–34. doi: 10.11005/jbm.2016.23.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green TJ, McMahon JA, Skeaff CM, Williams SM, Whiting SJ. Lowering homocysteine with B Vitamins has no effect on biomarkers of bone turnover in older persons: A 2-y randomized controlled trial. Am J Clin Nutr. 2007;85:460–4. doi: 10.1093/ajcn/85.2.460. [DOI] [PubMed] [Google Scholar]
- 9.Vacek TP, Kalani A, Voor MJ, Tyagi SC, Tyagi N. The role of homocysteine in bone remodeling. Clin Chem Lab Med. 2013;51:579–90. doi: 10.1515/cclm-2012-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fratoni V, Brandi ML. B Vitamins, homocysteine and bone health. Nutrients. 2015;7:2176–92. doi: 10.3390/nu7042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubec B, Fang-Kircher S, Lubec T, Blom HJ, Boers GH. Evidence for mcKusick's hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta. 1996;1315:159–62. doi: 10.1016/0925-4439(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 12.Jahn O, Wex T, Klose S, Kropf S, Adolf D, Piatek S, et al. Cathepsin K in treatment monitoring following intravenous zoledronic acid. Biomed Rep. 2014;2:915–7. doi: 10.3892/br.2014.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holzer G, Noske H, Lang T, Holzer L, Willinger U. Soluble cathepsin K: A novel marker for the prediction of nontraumatic fractures? J Lab Clin Med. 2005;146:13–7. doi: 10.1016/j.lab.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz-Torres M, Reyes-García R, Mezquita-Raya P, Fernández-García D, Alonso G, Luna Jde D, et al. Serum cathepsin K as a marker of bone metabolism in postmenopausal women treated with alendronate. Maturitas. 2009;64:188–92. doi: 10.1016/j.maturitas.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Borker SA, Venugopalan PP, Bhat SN. Study of menopausal symptoms, and perceptions about menopause among women at a rural community in Kerala. J Midlife Health. 2013;4:182–7. doi: 10.4103/0976-7800.118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Genant HK, Shepherd J, Zhao S, Mathur A, Fuerst TP, et al. Classification of osteoporosis based on bone mineral densities. J Bone Miner Res. 2001;16:901–10. doi: 10.1359/jbmr.2001.16.5.901. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann M, Widmann T, Colaianni G, Colucci S, Zallone A, Herrmann W, et al. Increased osteoclast activity in the presence of increased homocysteine concentrations. Clin Chem. 2005;51:2348–53. doi: 10.1373/clinchem.2005.053363. [DOI] [PubMed] [Google Scholar]
- 18.Cagnacci A, Bagni B, Zini A, Cannoletta M, Generali M, Volpe A, et al. Relation of folates, Vitamin B12 and homocysteine to vertebral bone mineral density change in postmenopausal women. A five-year longitudinal evaluation. Bone. 2008;42:314–20. doi: 10.1016/j.bone.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Tao X, Wu J. Association of homocysteine, Vitamin B12, and folate with bone mineral density in postmenopausal women: A meta-analysis. Arch Gynecol Obstet. 2014;289:1003–9. doi: 10.1007/s00404-013-3075-6. [DOI] [PubMed] [Google Scholar]
- 20.Cagnacci A, Baldassari F, Rivolta G, Arangino S, Volpe A. Relation of homocysteine, folate, and Vitamin B12 to bone mineral density of postmenopausal women. Bone. 2003;33:956–9. doi: 10.1016/j.bone.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Tucker KL, Hannan MT, Qiao N, Jacques PF, Selhub J, Cupples LA, et al. Low plasma vitamin B12 is associated with lower BMD: the Framingham Osteoporosis Study. J Bone Miner Res. 2005;20:152–158. doi: 10.1359/JBMR.041018. [DOI] [PubMed] [Google Scholar]
- 22.Kumar J, Garg G, Kumar A, Sundaramoorthy E, Sanapala KR, Ghosh S, et al. Single nucleotide polymorphisms in homocysteine metabolism pathway genes: Association of CHDH A119C and MTHFR C677T With hyperhomocysteinemia. Circ Cardiovasc Genet. 2009;2:599–606. doi: 10.1161/CIRCGENETICS.108.841411. [DOI] [PubMed] [Google Scholar]
- 23.Meier C, Meinhardt U, Greenfield JR, De Winter J, Nguyen TV, Dunstan CR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with paget's disease. Clin Lab. 2006;52:1–0. [PubMed] [Google Scholar]
- 24.Adolf D, Wex T, Jahn O, Riebau C, Halangk W, Klose S, et al. Serum cathepsin K levels are not suitable to differentiate women with chronic bone disorders such as osteopenia and osteoporosis from healthy pre- and postmenopausal women. Maturitas. 2012;71:169–72. doi: 10.1016/j.maturitas.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Garnero P. New biochemical markers of bone turnover. IBMS BoneKEy. 2008;5:84–102. [Google Scholar]