Abstract
BACKGROUND
Asthma exacerbations occur frequently despite the regular use of asthma-controller therapies, such as inhaled glucocorticoids. Clinicians commonly increase the doses of inhaled glucocorticoids at early signs of loss of asthma control. However, data on the safety and efficacy of this strategy in children are limited.
METHODS
We studied 254 children, 5 to 11 years of age, who had mild-to-moderate persistent asthma and had had at least one asthma exacerbation treated with systemic glucocorticoids in the previous year. Children were treated for 48 weeks with maintenance low-dose inhaled glucocorticoids (fluticasone propionate at a dose of 44 μg per inhalation, two inhalations twice daily) and were randomly assigned to either continue the same dose (low-dose group) or use a quintupled dose (high-dose group; fluticasone at a dose of 220 μg per inhalation, two inhalations twice daily) for 7 days at the early signs of loss of asthma control (“yellow zone”). Treatment was provided in a double-blind fashion. The primary outcome was the rate of severe asthma exacerbations treated with systemic glucocorticoids.
RESULTS
The rate of severe asthma exacerbations treated with systemic glucocorticoids did not differ significantly between groups (0.48 exacerbations per year in the high-dose group and 0.37 exacerbations per year in the low-dose group; relative rate, 1.3; 95% confidence interval, 0.8 to 2.1; P = 0.30). The time to the first exacerbation, the rate of treatment failure, symptom scores, and albuterol use during yellow-zone episodes did not differ significantly between groups. The total glucocorticoid exposure was 16% higher in the high-dose group than in the low-dose group. The difference in linear growth between the high-dose group and the low-dose group was −0.23 cm per year (P = 0.06).
CONCLUSIONS
In children with mild-to-moderate persistent asthma treated with daily inhaled glucocorticoids, quintupling the dose at the early signs of loss of asthma control did not reduce the rate of severe asthma exacerbations or improve other asthma outcomes and may be associated with diminished linear growth. (Funded by the National Heart, Lung, and Blood Institute; STICS ClinicalTrials.gov number, NCT02066129.)
Asthma exacerbations are common events, particularly in school-age children.1 Exacerbations are costly and are associated with considerable complications. In addition, asthma exacerbations may lead to progressive loss of lung function and greater asthma severity over time.2,3 Although conventional therapies, particularly the daily use of inhaled glucocorticoids, effectively control day-to-day asthma symptoms, they have only partial efficacy in preventing exacerbations.4 The identification of strategies to prevent asthma exacerbations remains an important unmet need.
Asthma guidelines recommend that patients be provided with a written action plan to guide the management of asthma at home.5,6 However, limited evidence is available to inform clinicians’ selection and implementation of strategies in the “yellow zone” (i.e., when there are signs of early loss of asthma control) to prevent these early symptoms from progressing to a full asthma exacerbation.7,8 The Global Initiative for Asthma strategy recommends short-term increases in the dose of inhaled glucocorticoids at the early signs of loss of asthma control in patients receiving daily inhaled glucocorticoids.5 However, a recent Cochrane review9 concluded that there was no evidence indicating that doubling the dose of inhaled glucocorticoids in response to increasing symptoms decreased the likelihood of asthma exacerbations among children or adults. Quadrupling the dose of inhaled glucocorticoids was identified in post hoc analyses of a single trial as a potentially efficacious intervention in adult patients,10 but data on the safety or efficacy of an intervention that uses more than a doubled dose of inhaled glucocorticoids are limited in children. Therefore, we performed the Step Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations (STICS) trial to assess the efficacy and safety of increasing the dose of inhaled glucocorticoids from a baseline daily low dose to five times the daily dose for 7 days in school-age children with mild-to-moderate persistent asthma who began to have short-term loss of asthma control.
METHODS
TRIAL PARTICIPANTS
We enrolled children 5 to 11 years of age who had doctor-diagnosed asthma and a history of at least one asthma exacerbation treated with systemic glucocorticoids in the previous year. Eligible participants were required to have one of the following: mild-to-moderate persistent asthma treated with step 2 therapy according to the National Asthma Education and Prevention Program Expert Panel Report (EPR) 3 (steps range from 1 to 6, with step 6 therapy being used in patients with the most severe disease)6; current symptoms or an exacerbation history that qualified the child for step 2 therapy; or current treatment with step 3 therapy according to the EPR 3 and a score on the Childhood Asthma Control Test (C-ACT) of more than 19 (on a scale from 0 to 27, with higher scores indicating greater asthma control; minimal clinically important difference, 2.0)11 at enrollment, no more than two prednisone-treated exacerbations in the past 6 months, a forced expiratory volume in 1 second before bronchodilator use that was 80% or more of the predicted value, and a willingness to step down therapy (from step 3 to step 2). Participants were excluded if asthma was too severe (>5 exacerbations in the previous year that had been treated with systemic glucocorticoids or a history of life-threatening asthma).
TRIAL PROTOCOL
This randomized, double-blind, parallel group trial was conducted at 17 trial sites in the United States until March 2017. The protocol is available, along with the statistical analysis plan, with the full text of this article at NEJM.org. Parents or legal guardians provided written informed consent, and children provided assent.
Participants were entered into a 4-week run-in period to establish adherence of more than 75% to the use of open-label trial medication (fluticasone propionate [Flovent, GlaxoSmithKline] at a dose of 44 μg per inhalation, two inhalations twice daily), daily completion of an electronic diary, and asthma control (C-ACT score >19) at the randomization visit. All the participants continued to receive open-label low-dose therapy as maintenance (“green zone”) therapy throughout the 52-week trial.
Participants were randomly assigned in a 1:1 ratio to receive blinded therapy either at the low dose or at the high dose (fluticasone at a dose of 220 μg per inhalation, two inhalations twice daily) for 7 days at the early signs of loss of asthma control. The green-zone low-dose inhaler was discontinued while the blinded yellow-zone inhaler was used; thus, the low-dose group continued to receive the same dose of inhaled glucocorticoids throughout the trial.
Yellow-zone episodes were identified by the occurrence of any of the following: the use of two doses (four inhalations) of rescue albuterol in 6 hours, the use of three doses (six inhalations) of rescue albuterol in 24 hours, or one night awakening that was due to asthma that was treated with albuterol. Symptoms and medication use were recorded once nightly by the participant or by the parent or guardian in an electronic diary (Spirotel, Medical International Research); there was no electronic link between the inhaler and the electronic diary.
To prevent a delay from the onset of a yellow-zone episode to the initiation of treatment, participants were provided with a written asthma action plan that instructed them not to wait for the yellow-zone alert from the electronic diary before starting the blinded yellow-zone inhaler. Peak expiratory flows were obtained once daily in the evening with the use of the electronic diary in a blinded fashion such that the participants did not see the results. Neither preemptive albuterol before exercise nor peak expiratory flows were included in the yellow-zone criteria.
OUTCOME MEASURES
The primary outcome was the rate of severe asthma exacerbations treated with systemic glucocorticoids during the blinded treatment period. Systemic glucocorticoids were started after consultation with a trial clinician according to previously published criteria12: the use of more than 6 inhalations of albuterol in 6 hours, the use of 12 or more inhalations of albuterol in 24 hours, night awakenings leading to albuterol use during 2 of 3 consecutive nights, or the use of 8 or more inhalations of albuterol during 2 of 3 consecutive days. Secondary outcome measures included the time to the first asthma exacerbation, treatment failure (defined as two asthma exacerbations in 6 months, three asthma exacerbations in 1 year, or six treated yellow-zone episodes), the area under the curve for symptom scores during yellow-zone episodes (as assessed from daily entries in the electronic diary),13 albuterol use during yellow-zone episodes, unscheduled emergency department or urgent care visits for asthma, hospitalizations for asthma, total glucocorticoid exposure (inhaled glucocorticoids plus systemic glucocorticoids), and linear growth. Exploratory outcomes included the peak expiratory flows and the number of days of asthma control, which were defined as full calendar days without symptoms, use of rescue medication, or unscheduled health care visits.
GROWTH AND CLINICAL ASSESSMENTS
Standing height measurements (in centimeters) were obtained at each trial visit while the participant was not wearing shoes. Measurements were made with the use of a Harpenden stadiometer (Seritex–Holtain) that was either wall-mounted (product number, 602VR) or portable (product number, 603VR).
Spirometry was performed according to American Thoracic Society–European Respiratory Society guidelines.14 Peripheral-blood eosinophil counts were determined by standard methods at each clinical site. The total serum IgE level and the levels of IgE specific to aeroallergens (see the Supplemental Methods section in the Supplementary Appendix, available at NEJM.org) were quantified by a commercial laboratory (Advanced Diagnostic Laboratories).
TRIAL OVERSIGHT
The trial was funded by the National Heart, Lung, and Blood Institute and approved by the AsthmaNet steering committee, protocol review committee, and data and safety monitoring board. Trial medications (fluticasone propionate with hydrofluoroalkane [HFA] propellant at doses of 44 μg per inhalation and 220 μg per inhalation) and rescue therapy with albuterol (90 μg per inhalation) were donated by GlaxoSmithKline. GlaxoSmithKline did not play a role in the trial design or the collection or interpretation of the data but was given an opportunity to read the manuscript draft and did not provide any comments. The authors are responsible for the trial design, data collection, data interpretation and analysis, manuscript preparation, and decision to submit the manuscript for publication. The authors vouch for the accuracy and completeness of the data, for the accuracy of the analyses, and for the fidelity of the trial to the protocol.
STATISTICAL ANALYSIS
The primary research question addressed the rate of severe asthma exacerbations with the use of a generalized linear model, with response following the negative binomial distribution and log-link function. The model incorporated the observed follow-up time so that the exacerbation rates (number of events per year) were estimated appropriately. Randomization was stratified according to clinical center, which was included as a covariate in the model. The primary intention-to-treat analysis compared the overall efficacy of each treatment strategy, regardless of whether any yellow-zone episodes actually occurred.
The treatment effect on prespecified secondary outcomes was also investigated. Discrete outcomes were analyzed with the use of the log-linear model framework described above. Outcomes in time-to-event analyses were summarized by Kaplan–Meier curves, and treatments were compared with the log-rank test. Linear mixed-effects models with participant as a random effect and treatment as a fixed effect were applied for outcomes that were measured over time on a continuous scale, such as the area under the curve for the symptom scores, albuterol use, and height. Transformations were applied for continuous outcomes that showed a skewed distribution. Additional details regarding the analyses of total exposure to glucocorticoids and growth are included in the Supplemental Methods section in the Supplementary Appendix. Exploratory analyses were conducted on the subset of treated (i.e., per-protocol) yellow-zone episodes. Adjustments for multiple tests were made for exploratory outcomes but not for prespecified primary and secondary outcomes. All the tests were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. All the analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
The target sample of 250 children (125 per treatment group) was chosen so that the trial would have power of at least 90%, with a two-sided type I error rate of 0.05, to detect a ratio of 0.6 for the exacerbation rate with the active treatment versus with the control treatment. This calculation assumed an exacerbation rate of 0.9 events per year for the inferior treatment and allowed for a rate of withdrawal or loss to follow-up of 15%. A prespecified interim feasibility analysis that was conducted when 50% of the children had completed 6 months of follow-up revealed that the exacerbation frequency was lower than expected and that the anticipated power for the same effect size would be approximately 80%. The options of prolonging the trial or increasing the sample size were discussed. However, the data and safety monitoring board and the AsthmaNet steering committee believed that the anticipated power of 80% was acceptable, and they chose to continue the trial as originally designed.
RESULTS
CHARACTERISTICS OF THE PARTICIPANTS
From August 2014 through March 2016, we enrolled 444 children, of whom 190 were excluded during the run-in period, most commonly because of inadequate adherence to the electronic diary. A total of 254 participants underwent randomization, with 127 participants assigned to each treatment group (Fig. 1). The characteristics of the patients are described in Table 1. A total of 44 participants withdrew from the trial early, and an additional 18 participants were withdrawn from the trial because of treatment failure. A total of 192 participants, including 94 participants in the high-dose group and 98 in the low-dose group, completed the final trial visit.
Figure 1. Trial Design and Enrollment.
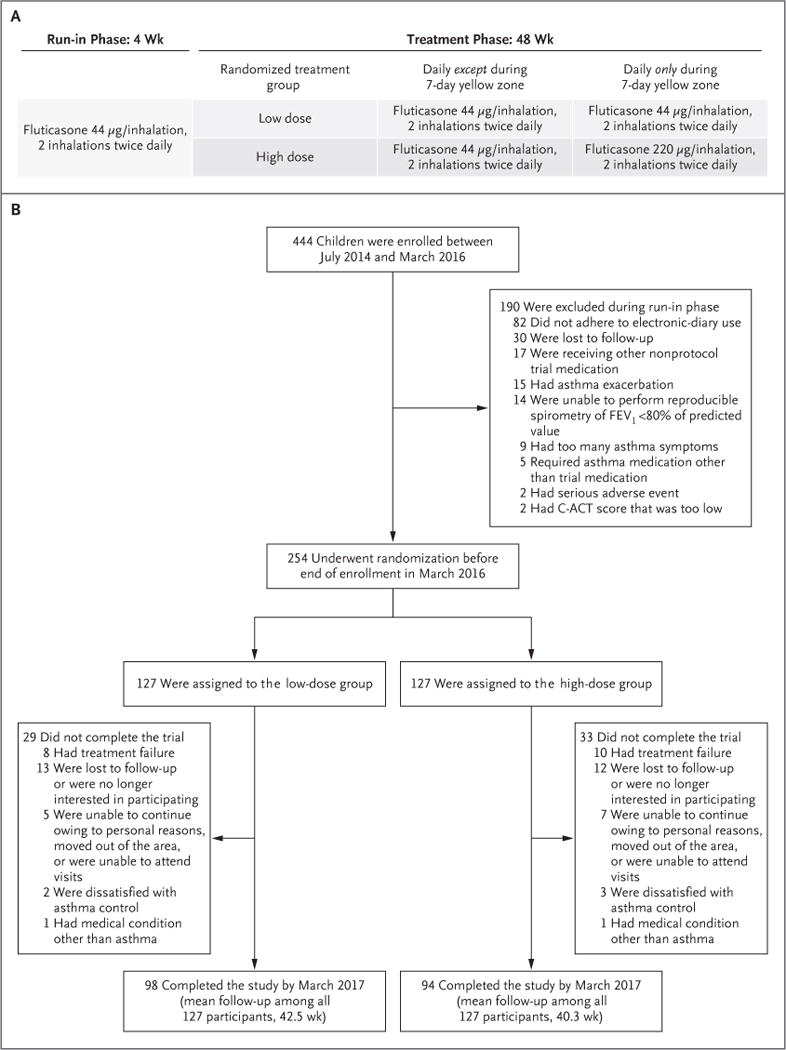
Panel A shows the trial design. All the children were treated for 48 weeks with maintenance low-dose inhaled glucocorticoids (fluticasone propionate at a dose of 44 μg per inhalation, two inhalations twice daily) and were randomly assigned either to continue the same dose (low-dose group) or to use a quintupled dose (high-dose group; fluticasone at a dose of 220 μg per inhalation, with two inhalations twice daily) for 7 days at the early signs of loss of asthma control (“yellow zone”). Panel B shows the number of participants who enrolled in the trial, underwent randomization, and completed the trial. Scores on the childhood Asthma Control Test (C-ACT) range from 0 to 27, with higher scores indicating greater control (minimally important difference, 2.0)11; among potential participants with a C-ACT score, a score of more than 19 was required for inclusion in the trial. FEV1 denotes forced expiratory volume in 1 second.
Table 1.
Characteristics of the Participants.*
| Characteristic | Total (N = 254) |
Low-Dose Group (N = 127) |
High-Dose Group (N = 127) |
|---|---|---|---|
| Age at enrollment — yr | 8.0±1.9 | 7.9±1.9 | 8.1±1.8 |
| BMI percentile —%† | 66.8±28.0 | 67.9±27.3 | 65.8±28.8 |
| Male sex — no. (%) | 163 (64.2) | 80 (63.0) | 83 (65.4) |
| Race — no. (%)‡ | |||
| White | 140 (55.1) | 64 (50.4) | 76 (59.8) |
| Black | 56 (22.0) | 29 (22.8) | 27 (21.3) |
| Other | 58 (22.8) | 34 (26.8) | 24 (18.9) |
| Hispanic ethnic group — no. (%)‡ | 75 (29.5) | 36 (28.3) | 39 (30.7) |
| Tobacco smoke exposure — no. (%)§ | 97 (38.2) | 46 (36.2) | 51 (40.2) |
| Controller therapy at enrollment — no. (%)¶ | |||
| Step 2 | 181 (71.3) | 96 (75.6) | 85 (66.9) |
| Step 3 | 43 (16.9) | 18 (14.2) | 25 (19.7) |
| No previous controller therapy | 30 (11.8) | 13 (10.2) | 17 (13.4) |
| No. of positive allergen-specific IgE tests, of 16 tests | 5.0±4.4 | 4.8±4.2 | 5.2±4.5 |
| ≥1 Positive test for aeroallergen — no./total no. (%) | 187/243 (77.0) | 88/118 (74.6) | 99/125 (79.2) |
| Blood eosinophil count — cells/mm3 | 346.4±268.2 | 367.4±299.8 | 326.6±234.3 |
| IgE — kU/liter | 401.3±586.4 | 418.3±655.9 | 385.4±515.1 |
| No. of systemic glucocorticoid courses in previous year | 1.7±0.9 | 1.8±0.9 | 1.7±0.9 |
| No. of urgent care or emergency department visits in previous year | 2.0±1.7 | 2.0±1.7 | 2.0±1.7 |
| Hospitalization in previous year — no. (%) | 31 (12.2) | 16 (12.6) | 15 (11.8) |
Plus-minus values are means ±SD. All the children were treated for 48 weeks with maintenance low-dose inhaled glucocorticoids (fluticasone propionate at a dose of 44 μg per inhalation, two inhalations twice daily) and were randomly assigned either to continue the same dose (low-dose group) or to use a quintupled dose (high-dose group; fluticasone at a dose of 220 μg per inhalation, with two inhalations twice daily) for 7 days at the early signs of loss of asthma control. There were no significant between-group differences at baseline. Percentages may not total 100 because of rounding.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. The BMI percentile was assessed as the value expected for age.
Race and ethnic group were reported by the participants or their parents or guardians.
Tobacco smoke exposure refers to the current or past use of tobacco by a parent or family member in the home.
Controller therapy was categorized according to the National Asthma Education and Prevention Program Expert Panel Report 3. Steps range from 1 to 6, with step 6 therapy being used in patients with the most severe disease. Step 2 indicates therapy for mild persistent asthma, and step 3 therapy for moderate persistent asthma.
During the course of the trial, the electronic diary was completed on 73% of the days in the high-dose group and on 72% of the days in the low-dose group. Adherence to the daily therapy with inhaled glucocorticoids was reported on 98% of the days that the electronic diary was completed in each treatment group.
A total of 395 yellow-zone episodes, including 192 episodes among 80 patients in the high-dose group and 203 episodes among 88 patients in the low-dose group, occurred during the trial, according to yellow-zone alerts on the electronic diary. The rate of yellow-zone episodes was similar in the high-dose group and the low-dose group (2.01 episodes per year and 1.96 episodes per year, respectively; P = 0.90) (Fig. 2A).
Figure 2. Yellow Zones, Exacerbations, and Treatment Failure.
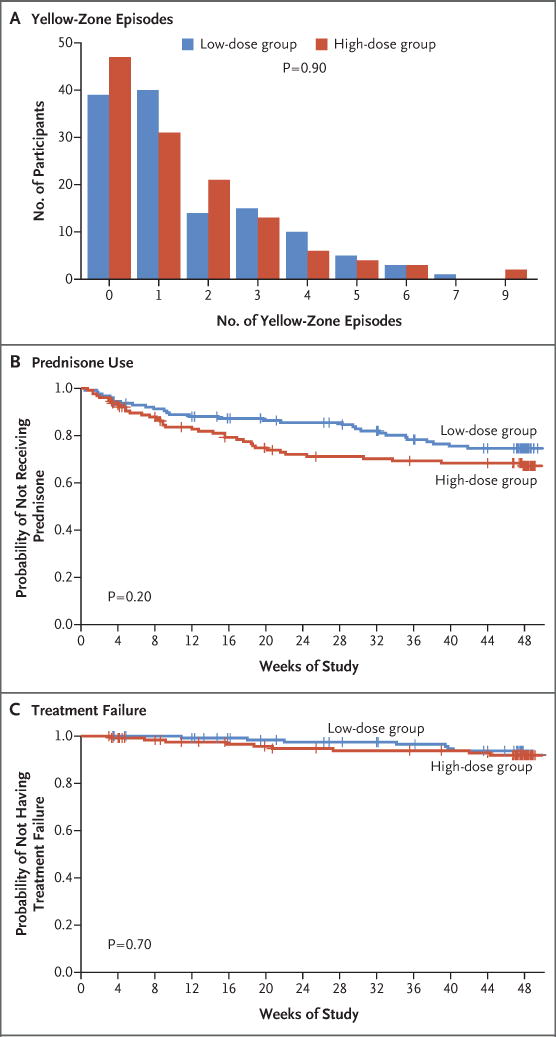
Panel A shows the frequency of yellow-zone episodes, according to dose group. Panel B shows the time to the first exacerbation that was treated with systemic glucocorticoids (prednisone). Tick marks indicate censored data. Panel C shows the time to treatment failure.
PRIMARY OUTCOME
A total of 38 participants in the high-dose group and 30 in the low-dose group had at least one severe asthma exacerbation that was treated with systemic glucocorticoids. The rate did not differ significantly between the two groups. The rate among participants who had been randomly assigned to the high-dose group was 0.48 exacerbations (95% confidence interval [CI], 0.33 to 0.70) per year, and the rate among those who had been randomly assigned to the low-dose group was 0.37 exacerbations (95% CI, 0.25 to 0.55) per year (relative rate, 1.3; 95% CI, 0.8 to 2.1; P = 0.30) (Table 2).
Table 2.
Outcomes.*
| Outcomes | Low-Dose Group (N = 127) |
High-Dose Group (N = 127) |
Treatment Effect (95% CI)† |
P Value |
|---|---|---|---|---|
| Primary outcome | ||||
| No. of exacerbations per year (95% CI) | 0.37 (0.25 to 0.55) | 0.48 (0.33 to 0.70) | 1.3 (0.8 to 2.1) | 0.30 |
| Secondary outcomes | ||||
| No. of emergency department or urgent care visits per year (95% CI) | 0.47 (0.31 to 0.72) | 0.64 (0.42 to 0.96) | 1.3 (0.8 to 2.4) | 0.30 |
| No. of hospitalizations | 0 | 4 | — | 0.12 |
| Equivalent of hydrocortisone exposure — g/yr (95% CI) | ||||
| Fluticasone only | 10.6 (10.4 to 10.9) | 12.2 (11.9 to 12.4) | 1.14 (1.10 to 1.19) | |
| Fluticasone and prednisone | 11.1 (10.6 to 11.4) | 12.8 (12.4 to 13.2) | 1.16 (1.10 to 1.22) | |
| Growth — cm/yr (95% CI) | ||||
| Mean | 5.65 (5.48 to 5.81) | 5.43 (5.26 to 5.60) | −0.23 (−0.47 to 0.01) | 0.06 |
| Effect per 7-day exposure to high-dose regimen | ||||
| Overall | — | −0.07 (−0.17 to 0.03) | −0.07 (−0.17 to 0.03) | 0.20 |
| According to age group‡ | ||||
| 5-7 yr | — | −0.12 (−0.22 to −0.02) | −0.12 (−0.22 to −0.02) | 0.02 |
| 8-11 yr | — | 0.02 (−0.21 to 0.26) | 0.02 (−0.21 to 0.26) | 0.80 |
The primary outcome was the rate of severe asthma exacerbations (number of events per year) treated with systemic glucocorticoids during the blinded treatment period.
The treatment effect is a relative rate for the primary outcome of the number of exacerbations per year and for the secondary outcomes of the number of emergency department or urgent care visits per year. The treatment effect is a relative difference for the secondary outcomes related to hydrocortisone exposure equivalents. The treatment effect is an absolute difference (measured in centimeters per year) for the secondary outcomes regarding growth.
A total of 126 participants were 5 to 7 years of age, and 128 were 8 to 11 years of age.
SECONDARY OUTCOMES
The time to the first severe asthma exacerbation treated with systemic glucocorticoids did not differ significantly between the high-dose group and the low-dose group (P = 0.20) (Fig. 2B). The rate of emergency department or urgent care visits for asthma, as assessed by the electronic diary, did not differ significantly between the high-dose group and the low-dose group (relative rate, 1.3; 95% CI, 0.8 to 2.4; P = 0.30) (Table 2). Similarly, there was no significant difference in the rate of treatment failure between the high-dose group and the low-dose group (relative rate, 1.3; P = 0.70) (Fig. 2C). There were four hospitalizations due to asthma during the trial, all of which occurred in the high-dose group; however, the between-group difference was not significant (P = 0.12) (Table 2).
We also assessed symptoms and albuterol use during yellow-zone episodes. There was no significant difference between the high-dose group and the low-dose group in the total symptom burden during yellow-zone episodes, as assessed according to the area under the curve for symptom scores (P = 0.30) (Fig. 3A). Albuterol use during yellow-zone episodes did not differ significantly between the high-dose group and the low-dose group (P = 0.30) (Fig. 3B).
Figure 3. Outcomes during Yellow-Zone Episodes.
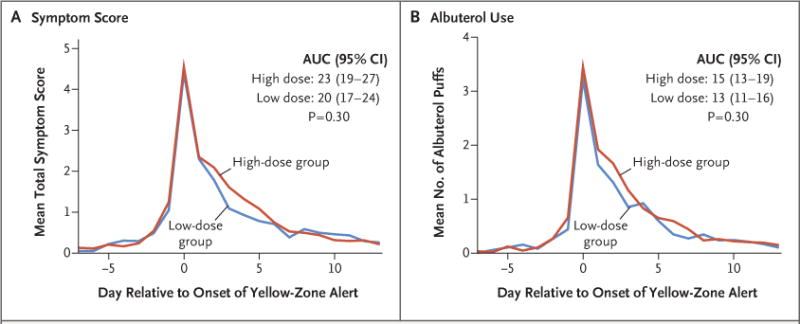
Panel A shows the mean symptom scores 7 days before and 14 days after the onset of yellow-zone alerts. The total symptom burden was assessed according to the area under the curve (AUC) for symptom scores. Panel B shows albuterol use, as assessed according to the number of inhalations per day during the same time period.
SAFETY
Children in the high-dose group had 14% greater exposure to inhaled glucocorticoids than those in the low-dose group, and they also had 16% greater total exposure to glucocorticoids than those in the low-dose group during the trial (Table 2). The growth rate among children who had been randomly assigned to the high-dose group (5.43 cm per year) was 0.23 cm per year less than the rate among children who had been randomly assigned to the low-dose group (5.65 cm per year) (P = 0.06) (Table 2). There was a dose– response relationship in children younger than 8 years of age in the high-dose group (0.12 cm per year lower growth per yellow-zone episode, P = 0.02 for the comparison with the low-dose group) (Table 2, and Fig. S1 in the Supplementary Appendix) but not in children 8 to 11 years of age. There were no significant between-group differences in the adverse events reported by the participants. There were no deaths among the trial participants.
EXPLORATORY OUTCOMES
With regard to symptom assessments, the mean percentage of days of asthma control over the entire follow-up period was 95% in the high-dose group and 96% in the low-dose group, a finding that was consistent with good overall day-to-day symptom control in each treatment group. We also examined the percentage of days of asthma control during yellow-zone episodes only, which did not differ significantly between the high-dose group and the low-dose group (72% and 74%, respectively; P = 0.90) (Fig. S2A in the Supplementary Appendix).
Peak expiratory flows were obtained daily during the course of the trial and revealed significant day-to-day variability that was not strongly associated with symptoms or albuterol use (correlations, <0.1). There were no significant differences in peak expiratory flows between the high-dose group and the low-dose group during yellow-zone episodes (Fig. S2B in the Supplementary Appendix).
OUTCOMES IN TREATED YELLOW-ZONE EPISODES
Because not all the children who underwent randomization had at least one yellow-zone episode during the trial (63% of the participants in the high-dose group and 69% of those in the low-dose group had at least one episode), we examined whether there were differences in outcomes among children who used blinded yellow-zone therapy. A total of 32% (37 of 114) of the treated yellow-zone episodes in the high-dose group led to an exacerbation that was treated with glucocorticoids, whereas 19% (25 of 134) of the treated yellow-zone episodes in the low-dose group led to an exacerbation that was treated with glucocorticoids. There were no significant between-group differences in symptom scores, albuterol use (mean number of puffs), the percentage of days of asthma control, and peak expiratory flows during episodes in which use of the blinded yellow-zone inhaler was initiated (Fig. S3A through S3D in the Supplementary Appendix).
DISCUSSION
In this trial, we found that in children 5 to 11 years of age with asthma who were treated with daily therapy with low-dose inhaled glucocorticoids, increasing the dose of inhaled glucocorticoids by a factor of 5 for 7 days at the early signs of loss of asthma control (yellow zone) did not reduce the rate of severe asthma exacerbations leading to treatment with systemic glucocorticoids. Furthermore, this treatment strategy did not prolong the time to the first asthma exacerbation, reduce symptom scores or albuterol use, or reduce the rate of treatment failure. Finally, this strategy resulted in a greater total exposure to glucocorticoids and a lower linear growth rate.
Early observational studies examining the potential benefit of increasing doses of inhaled glucocorticoids in the yellow zone were promising,15–17 but subsequent randomized trials examining increased doses of inhaled glucocorticoids in these contexts have been disappointing.9,10,18 One potential explanation for this apparent discrepancy is that, even without intervention beyond the use of a short-acting beta-agonist, a substantial proportion of yellow-zone episodes do not progress to severe exacerbations that lead to the use of systemic glucocorticoids.19 In our trial, 81% of the treated yellow-zone episodes in the low-dose group did not lead to treatment with systemic glucocorticoids. This degree of “success” in clinical practice probably underlies the perceived benefit, by clinicians and families, of increased doses of inhaled glucocorticoids.
Blinded peak expiratory flows were included as an exploratory variable in this trial to help determine whether these data provide useful information, as compared with a symptom-based asthma action plan. A minimal signal was observed during yellow-zone episodes beyond the day-to-day variability in the measurements observed throughout the trial. We also observed a variation in kinetics (<24 hours to several days) from early yellow-zone symptoms to the initiation of systemic glucocorticoids for asthma ex-acerbations. This finding highlights the considerable unmet need for individualized indicators of impending exacerbations that will allow for the earlier and more specific use of treatment strategies aimed at exacerbation prevention.
The association with slower growth in height that was observed in children who had been randomly assigned to the high-dose group was unexpected. Although the overall difference was relatively small, this finding was observed in children who, on average, had just greater than two treated yellow-zone episodes per year. The dose–response relationship that was observed in younger children (<8 years of age) (Fig. S1 in the Supplementary Appendix) arouses the concern that more frequent or prolonged use of this strategy, if the use of inhaled glucocorticoids was its cause, could lead to greater adverse effects.
A limitation of our trial is that we observed fewer yellow-zone episodes and 40% fewer exacerbations treated with systemic glucocorticoids than were anticipated, for unclear reasons. Although all the participants had a history of at least one exacerbation in the previous year, the requirement for adequate asthma control during the run-in period, with a C-ACT score of more than 19 at randomization along with good adherence to daily inhaled glucocorticoids, may have contributed to the lower-than-expected rate of exacerbations. This lower exacerbation rate reduced the power of the trial to detect a difference between the treatment groups. However, the 95% confidence interval for the primary outcome (0.8 to 2.1 exacerbations per year) allows for an effect ranging from a 20% lower rate to just more than a doubling of the risk of exacerbations with the quintupled-dose treatment than with the low-dose therapy. Given that more ex-acerbations occurred in the high-dose group, we speculate that it is unlikely that a clinically significant beneficial effect of treatment with a quintupled dose would have been observed in this trial even if we had enrolled more participants.
It is important to recognize that our findings are specific to school-age children with mild-to-moderate persistent asthma regularly treated with daily low-dose inhaled glucocorticoids (with good adherence). There is evidence that the intermittent use of high-dose inhaled glucocorticoids during yellow-zone episodes is an effective strategy to prevent exacerbations in preschool children and in adults with mild asthma that is not treated with daily inhaled glucocorticoids.19–21 Our findings are consistent with those of other groups that examined smaller dose increases at early signs of loss of asthma control in children regularly treated with inhaled glucocorticoids and that found no added benefit as compared with the standard daily dose.22,23 Only one randomized trial involving children has shown potential benefits of increasing doses of inhaled glucocorticoids during yellow-zone episodes with the use of budesonide–formoterol as a single inhaler for both maintenance therapy and reliever therapy.24 Whether this difference in efficacy is related to greater disease severity, synergistic effects of inhaled glucocorticoids and long-acting beta-agonists, or other factors is not clear.
In conclusion, in children with mild-to-moderate persistent asthma treated with daily inhaled glucocorticoids, quintupling the dose of inhaled glucocorticoids at the early signs of loss of asthma control did not result in a lower rate of exacerbations than continuation of the daily maintenance dose, did not improve other asthma outcomes, and may be associated with diminished linear growth.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute. Dr. Jackson reports receiving fees for serving on an advisory board from Vectura Group, Boehringer Ingelheim, and Glaxo-SmithKline and consulting fees from Novartis; Dr. Bacharier, receiving consulting fees and lecture fees from Aerocrine, GlaxoSmithKline, Genentech–Novartis, Teva Pharmaceuticals, and Boehringer Ingelheim, fees for serving on an advisory board and lecture fees from Merck, consulting fees from Cephalon, fees for serving on a data and safety monitoring board from DBV Technologies, lecture fees from AstraZeneca, honoraria for continuing medical education (CME) program development from WebMD–Medscape, and fees for serving on an advisory board from Sanofi, Vectura Group, and Circassia; Dr. Mauger, receiving drugs for trials from Merck and Boehringer Ingelheim; Dr. Chmiel, receiving honoraria from Nivalis Therapeutics, honoraria paid to his institution from Corbus Pharmaceuticals, and consulting fees from Verona Pharma, Catabasis, Albumedix, and Patara Pharma; Dr. Morgan, receiving fees for serving as co-chair of an epidemiologic study of cystic fibrosis for Genentech; Dr. Peters, receiving fees for serving on an advisory board from AstraZeneca, Boehringer Ingelheim, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and Regeneron Pharmaceuticals– Sanofi, fees for serving on a data and safety monitoring board from Gilead Sciences, Genentech, and Novartis, fees for clinical-trial adjudication from Quintiles, fees for CME WebEx from PRIME, and fees for a CME program from Haymarket Media Group; Dr. Cabana, receiving fees for serving on a speakers bureau from Merck and consulting fees from Thermo Fisher Scientific, Genentech, and Novartis; Dr. Martinez, receiving grant support from Johnson & Johnson and consulting fees from Compañía Agropecuaria Copeval and Commense; Dr. Pongracic, receiving study drugs from Boehringer Ingelheim, Glaxo Smith-Kline, Teva Pharmaceuticals, and Merck; Dr. Covar, receiving grant support from Roche and AstraZeneca; Dr. Gentile, receiving grant support and lecture fees from Stallergenes Greer; Dr. Israel, receiving consulting fees from AstraZeneca, Philips Respironics, Regeneron Pharmaceuticals, Bird Rock Bio, Nuvelution Pharmaceuticals, Vitaeris, and Entrinsic Health Solutions, receiving consulting fees and fees for serving on data and safety monitoring board from Novartis, serving as unpaid member of a data and safety monitoring board for a trial funded by Novartis, receiving travel support from Research in Real Life, receiving consulting fees, travel support, and study drugs from Teva Specialty Pharmaceuticals, receiving grant support from Genentech, receiving grant support and study drugs from Boehringer Ingelheim, receiving consulting fees and study drugs from GlaxoSmithKline and Merck, receiving study drugs from Sunovion, and receiving grant support and consulting fees from Sanofi; Dr. Ly, receiving lecture fees from ABcomm and fees for serving on an advisory board from Gilead Sciences; and Dr. Ross, receiving study drugs from Boehringer Ingelheim, Glaxo-SmithKline, and Merck and grant support and study drugs from Teva Pharmaceuticals.
APPENDIX
The authors’ full names and academic degrees are as follows: Daniel J. Jackson, M.D., Leonard B. Bacharier, M.D., David T. Mauger, Ph.D., Susan Boehmer, M.A., Avraham Beigelman, M.D., James F. Chmiel, M.D., Anne M. Fitzpatrick, Ph.D., Jonathan M. Gaffin, M.D., Wayne J. Morgan, M.D., Stephen P. Peters, M.D., Ph.D., Wanda Phipatanakul, M.D., William J. Sheehan, M.D., Michael D. Cabana, M.D., M.P.H., Fernando Holguin, M.D., Fernando D. Martinez, M.D., Jacqueline A. Pongracic, M.D., Sachin N. Baxi, M.D., Mindy Benson, M.S.N., P.N.P., Kathryn Blake, Pharm.D., Ronina Covar, M.D., Deborah A. Gentile, M.D., Elliot Israel, M.D., Jerry A. Krishnan, M.D., Ph.D., Harsha V. Kumar, M.D., Jason E. Lang, M.D., M.P.H., Stephen C. Lazarus, M.D., John J. Lima, Pharm.D., Dayna Long, M.D., Ngoc Ly, M.D., Jyothi Marbin, M.D., James N. Moy, M.D., Ross E. Myers, M.D., J. Tod Olin, M.D., Hengameh H. Raissy, Pharm.D., Rachel G. Robison, M.D., Kristie Ross, M.D., Christine A. Sorkness, Pharm.D., and Robert F. Lemanske, Jr., M.D.
The authors’ affiliations are as follows: the Department of Pediatrics, University of Wisconsin School of Medicine and Public Health (D.J.J., R.F.L.J.), and the University of Wisconsin–Madison (C.A.S.) — both in Madison; the Department of Pediatrics, Washington University in St. Louis School of Medicine and St. Louis Children’s Hospital, St. Louis (L.B.B., A.B.); the Department of Public Health Sciences, Penn State University, Hershey (D.T.M., S.B.), and the University of Pittsburgh Asthma Institute at University of Pittsburgh Medical Center–University of Pittsburgh School of Medicine (F.H.) and the Department of Pediatrics, Allegheny General Hospital (D.A.G.), Pittsburgh — all in Pennsylvania; the Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children’s Hospital, Cleveland (J.F.C., R.E.M., K.R.); the Department of Pediatrics, Emory University, Atlanta (A.M.F.); the Divisions of Respiratory Diseases (J.M.G.) and Allergy–Immunology, Boston Children’s Hospital (W.P., W.J.S., S.N.B.), Harvard Medical School, and Brigham and Women’s Hospital, Harvard Medical School (E.I.) — all in Boston; the Arizona Respiratory Center, University of Arizona, Tucson (W.J.M., F.D.M.); Wake Forest University School of Medicine, Winston-Salem, NC (S.P.P.); the Departments of Pediatrics (M.D.C., N.L.), Epidemiology (M.D.C.), Biostatistics (M.D.C.), and Medicine (S.C.L.), University of California, San Francisco (UCSF), and UCSF Benioff Children’s Hospital (M.D.C.) — both in San Francisco; Ann and Robert H. Lurie Children’s Hospital of Chicago (J.A.P., R.G.R.), University of Illinois at Chicago (J.A.K., H.V.K.), and the Department of Pediatrics, Stroger Hospital of Cook County, Rush University Medical Center (J.N.M.) — all in Chicago; UCSF Benioff Children’s Hospital Oakland, Oakland (M.B., D.L., J.M.); Nemours Children’s Health System, Jacksonville (K.B., J.J.L.), and Nemours Children’s Hospital, University of Central Florida College of Medicine, Orlando (J.E.L.) — both in Florida; the Department of Pediatrics, National Jewish Health, Denver (R.C., J.T.O.); and the Department of Pediatrics, University of New Mexico, Albuquerque (H.H.R.).
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 2.O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 3.O’Brian AL, Lemanske RF, Jr, Evans MD, Gangnon RE, Gern JE, Jackson DJ. Recurrent severe exacerbations in early life and reduced lung function at school age. J Allergy Clin Immunol. 2012;129:1162–4. doi: 10.1016/j.jaci.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorkness CA, Lemanske RF, Jr, Mauger DT, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. 2017 http://www.ginasthma.org/
- 6.Guidelines for the diagnosis and management of asthma: National Asthma Education and Prevention Program expert panel report 3. Bethesda, MD: National Heart, Lung, and Blood Institute; Oct, 2007. [Google Scholar]
- 7.Thomas A, Lemanske RF, Jr, Jackson DJ. Approaches to stepping up and stepping down care in asthmatic patients. J Allergy Clin Immunol. 2011;128:915–26. doi: 10.1016/j.jaci.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinakar C, Oppenheimer J, Portnoy J, et al. Management of acute loss of asthma control in the yellow zone: a practice parameter. Ann Allergy Asthma Immunol. 2014;113:143–59. doi: 10.1016/j.anai.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Kew KM, Quinn M, Quon BS, Du-charme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst Rev. 2016;(6):CD007524. doi: 10.1002/14651858.CD007524.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double-blind, placebo-controlled, parallel-group clinical trial. Am J Respir Crit Care Med. 2009;180:598–602. doi: 10.1164/rccm.200904-0616OC. [DOI] [PubMed] [Google Scholar]
- 11.Voorend-van Bergen S, Vaessen-Verberne AA, Landstra AM, et al. Monitoring childhood asthma: web-based diaries and the asthma control test. J Allergy Clin Immunol. 2014;133(6):1599–605.e2. doi: 10.1016/j.jaci.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacharier LB, Phillips BR, Zeiger RS, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122(6):1127–1135.e8. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.Wilson NM, Silverman M. Treatment of acute, episodic asthma in preschool children using intermittent high dose inhaled steroids at home. Arch Dis Child. 1990;65:407–10. doi: 10.1136/adc.65.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Arch Dis Child. 1993;68:85–7. doi: 10.1136/adc.68.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volovitz B, Nussinovitch M, Finkel-stein Y, Harel L, Varsano I. Effectiveness of inhaled corticosteroids in controlling acute asthma exacerbations in children at home. Clin Pediatr (Phila) 2001;40:79–86. doi: 10.1177/000992280104000203. [DOI] [PubMed] [Google Scholar]
- 18.Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet. 2004;363:271–5. doi: 10.1016/s0140-6736(03)15384-6. [DOI] [PubMed] [Google Scholar]
- 19.Zeiger RS, Mauger D, Bacharier LB, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365:1990–2001. doi: 10.1056/NEJMoa1104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducharme FM, Lemire C, Noya FJD, et al. Preemptive use of high-dose flutica-sone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–53. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 21.Boushey HA, Sorkness CA, King TS, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352:1519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 22.Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipro-pionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–7. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrett J, Williams S, Wong C, Hold-away D. Treatment of acute asthmatic exacerbations with an increased dose of inhaled steroid. Arch Dis Child. 1998;79:12–7. doi: 10.1136/adc.79.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisgaard H, Le Roux P, Bjåmer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest. 2006;130:1733–43. doi: 10.1378/chest.130.6.1733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


