Abstract
Subchronic administration of (R,S)-ketamine, (R,S)-Ket, is used in the treatment of neuropathic pain, in particular Complex Regional Pain Syndrome, but the effect of this protocol on the metabolism of (R,S)-Ket is unknown. In this study, daily administration of a low dose of (R,S)-Ket for 14-days to Wistar rats was conducted to determine the impact of sub-chronic dosing on the pharmacokinetics of (R,S)-Ket and its major metabolites. The data indicate that, relative to a single administration of (R,S)-Ket, subchronic administration resulted in increased clearance of (R,S)-Ket and the N-demethylated metabolite norketamine measured as elimination half-life (t1/2) and decreased plasma concentrations of these compounds. Subchronic administration produced a slight decrease in t1/2 and an increase in plasma concentration of the major metabolite, (2S,6S;2R,6R)-hydroxynorketamine, and produced significant increases in the plasma concentrations of the (2S,6R;2R,6S)-hydroxynorketamine and (2S,4R;2R,4S)-hydroxynorketamine metabolites. The metabolism of (R,S)-Ket predominately occurs via two microsomal enzyme-mediated pathways: (R,S)-Ket ⇒ (R,S)-norketamine ⇒ (2S,6S;2R,6R)-hydroxynorketamine and (2S,4R;2R,4S)-hydroxynorketamine and the (R,S)-Ket ⇒ (2S,6R;2R,6S)-hydroxyketamine ⇒ (2S,6R;2R,6S)-hydroxynorketamine and (2S,6S;2R,6R)-hydroxynorketamine. The results indicate that the activity of both metabolic pathways are increased by subchronic administration of (R,S)-Ket producing new metabolite patterns and potential differences in clinical effects.
Keywords: neuropathic pain; Complex Regional Pain Syndrome; (2S,6R)-hydroxynorketamine
1. Introduction
(R,S)-Ketamine ((R,S)-Ket) is a chiral phencyclidine derivative that was initially developed in the 1970’s as a short acting anesthetic agent [1,2]. The clinical use of (R,S)-Ket revealed that intra-operative administration of (R,S)-Ket reduced the post-operative need for opiate medications and that the drug provided excellent pain relief for opiate-dependent patients [3,4]. Recent studies have expanded the clinical use of (R,S)-Ket to the treatment of depression and neuropathic pain [1,2,5–8], which has resulted in a renewed interest in the compound’s pharmacokinetics and pharmacodynamics.
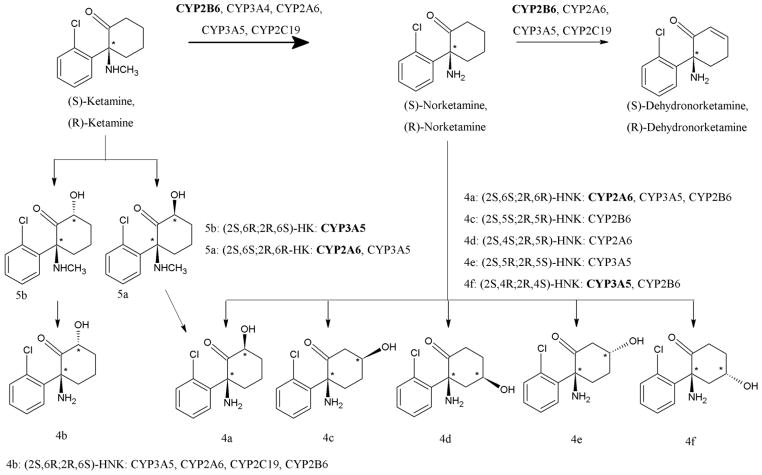
(R,S)-Ket is extensively metabolized by a variety of Phase I microsomal enzymes resulting in >20 enantiomeric and diastereomeric metabolites, Fig. 1, many of which are further glucuronidated and/or sulfated [9,10]. There are two major metabolic pathways, one involving initial N-demethylation to norketamine ((R,S)-norKet) which is subject to further transformation involving hydroxylation of the cyclohexanone ring to form a series of diastereomeric hydroxynorKet (HNK) metabolites or dehydration to (R,S)-dehydronorketamie (DHNK) [10]. The second pathway proceeds via initial hydroxylation of the cyclohexanone ring producing diastereomeric hydroxyketamines (HKet), which can be subsequently N-demethylated to the corresponding HNKs. We have recently demonstrated that high concentrations of most of these metabolites are present in the plasma of patients receiving (R,S)-Ket in the treatment of depression [11,12] and in the plasma and urine of patients receiving (R,S)-Ket for relief of neuropathic pain associated with the Complex Regional Pain Syndrome (CRPS) [10,13].
Figure 1.
Metabolism of (R,S)-ketamine [reproduced from ref 10].
In the treatment of depression, (R,S)-Ket is administered as a single 40-min intravenous infusion of a sub-anesthetic dose (50 mg/kg) and a significant clinical response is observed in most patients by 80 min after the initiation of the infusion [5,6]. The pharmacokinetics of (R,S)-Ket after a single administration has been well described, and although (R,S)-Ket is extensively and rapidly metabolized, the majority of these studies only describe the plasma clearance and disposition of (R,S)-Ket and (R,S)-norKet, and their respective enantiomers c.f. [14,15]. The scope of the earlier studies was limited due to the erroneous designation of the HNK and DHNK metabolites as “pharmacologically inactive” [16]. However, we have recently demonstrated that these metabolites are pharmacologically active [17–20] and contribute to the antidepressant effects produced by (R,S)-Ket [12]. We have reported the plasma concentrations and distributions of the major (R,S)-Ket metabolites in patients receiving (R,S)-Ket for treatment of depression [11,12]. In the Wistar rat, we determined the concentrations of (R,S)-Ket, (R,S)-norKet, and HNK metabolites in plasma and brain tissues after acute administration of (R,S)-Ket, (R)-Ket, (S)-Ket, (R,S)-norKet and (2S,6S)-HNK [19], as well as the pharmacokinetic parameters for (2S,6S)-HNK after i.v. and oral administration of (2S,6S)-HNK [20].
In the treatment of CRPS, (R,S)-Ket is administered as a daily or continuous infusion over 5 days with significant therapeutic response observed on Day 3 of the protocol [7,8]. Only one study has described the plasma disposition of (R,S)-Ket and (R,S)-norKet during a 5-day infusion to CRPS patients [7] and a second determined the enantioselective pharmacokinetics of (R)- and (S)-Ket and (R)- and (S)-norKet in CRPS patients [21]. Neither study addressed the fate of the other major metabolites. However, the effect of subchronic administration on the metabolism of (R,S)-Ket is an important clinical issue as repetitive administration of (R,S)-Ket induces the expression of the cytochrome P450 (CYP) enzymes involved in the hepatic metabolism of the drug, thereby altering its clearance and disposition [22,23]. The subchronic administration of (R,S)-Ket also produces different pharmacological effects relative to a single administration of the drug as a single administration of (R,S)-Ket produced a significant increase in the mRNA expression of serine racemase in the Wistar rat [24] while subchronic administration decreased the mRNA expression of serine racemase [25]. In addition, subchronic administration of (R,S)-Ket has been associated with cognitive impairment in the mouse and rat [26,27] and changes in hippocampal protein expression similar to those found in human schizophrenia in the rat [28].
We have recently determined that plasma samples obtained from CRPS patients on Day 3 of a 5-day continuous administration of (R,S)-Ket contained all of the major (R,S)-Ket metabolites and that the major circulating metabolites were (2S,6S;2R,6R)-HNK and (2S,6R;2R,6S)-HNK [10]. A key observation was that the concentration of (2S,6R;2R,6S)-HNK relative to (2S,6S;2R,6R)-HNK was increased when compared to the relationship observed after an acute administration of (R,S)-Ket [6,10]. In addition, the results from in vitro and in vivo studies have established that the (2S,6R;2S,6R)-HNK metabolite only arises from the N-demethylation of (2S,6R;2S,6R)-HK, and, therefore, this metabolite is a marker of the pathway [10,19,20]. Thus, the data obtained from the CRPS patients suggest that chronic administration of (R,S)-Ket induces the ring hydroxylation of (R,S)-Ket, which may be the source of the pharmacological effects observed after subchronic administration of the drug. The current study was designed to assess the effect of subchronic administration of (R,S)-Ket on the two major metabolic pathways associated with the drug’s metabolism as a precursor of future pharmacodynamic studies.
2. Materials and Methods
2.1. Ketamine and ketamine metabolites
(R,S)-Ket hydrochloride salt used in the study was purchased from Sigma-Aldrich (St. Louis, MO), and (R,S)-norKet), (R,S)-DHNK, (2S,6S;2R,6R)-HNK and (2S,6R;2R,6S)-HNK and the individual HNK enantiomers were prepared as previously described [13].
2.2. Animals
All procedures in this study were in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals (1996) and the Animal Welfare Standards incorporated in 9 CFR Part 3, 1991. Male Wistar rats were obtained from Harlan (Livermore, CA) were 9–10 weeks old, 271 to 354 g. They were housed 3 per cage, in polycarbonate hanging cages, 12 hr light/12 hr dark cycle, at 68–73°F, and 20–60% humidity. The rats were provided Harlan Teklad Certified Rodent Chow #2018C and water (Purified, Reverse Osmosis) ad libitum.
2.3. Study design
A single 40 mg/kg dose of (R,S)-Ket in saline (5 ml/kg) was administered by intraperitoneal (ip) injection in the acute dosing arm of the study and once per day for 14 days in the sub-chronic dosing arm of the study. At the end of dose administration in the acute arm and on Day 14 in the sub-chronic arm, blood samples were obtained from three rats per time point at 5, 10, 20, 40, 60 min, 2, 4, 8, 12, 24, 48, and 72 hr post-administration via the retro-orbial sinus under 60:40% CO2:O2 anesthesia. The blood was collected in tubes containing potassium EDTA, the tubes were placed on wet ice and processed to plasma within 15 min of collection by centrifugation at 2–8°C. The plasma samples were transferred to cryovials and stored frozen at −70°C ± 10°C until analysis. At the end of the study, the remaining animals were euthanized with an overdose of pentobarbital.
2.4. General Analytical Procedures
The plasma samples collected in this study were analyzed using a previously reported and validated achiral-chiral liquid chromatographic method utilizing mass spectrometric detection [19,20]. In brief, the separations of Ket, norKet, DHNK and HNK were accomplished using an Eclipse XDB-C18 guard column (4.6 mm × 12.5 mm) and an analytical column Varian Pursuit XRs 5 C18 (250×4.0 mm ID, 5 μm) purchased from Varian, Inc. The mobile phase consisted of ammonium acetate [5 mM, pH 7.6] as Component A and acetonitrile as component B. A linear gradient was run as follows: 0 min 20% B; 5 min 20% B; 15 min 80% B; 20 min 20% B at a flow rate of 0.4 ml/min. The total run time was 30 min per sample. A concentrated stock solution was prepared containing (R,S)-Ket (100 μg/ml), (R,S)-norKet (100 μg/ml), (R,S)-DHNK (100 μg/ml), (2R,6R;2S,6S)-HNK (100 μg/ml). The solution was prepared in ultra-pure water and stored in polypropylene tubes at −20°C. Serial dilutions of the stock solution were used to prepare the samples for the calibration curves and quality control standards (QC). The standards used in the calibration studies as QC standards the achiral analysis of or (R,S)-Ket, (R,S)-norKet and (2R,6R;S,6S)-HNK were a 0.5 serial dilution from 6,000 ng/ml to 5.85 ng/ml and 600 ng/ml to 0.58 ng/ml for (R,S)-DHNK.
The quantification of (R,S)-Ket, (R,S)-norKet, (R,S)-DHNK, (2R,6R;2S,6S)-HNK was accomplished using area ratios calculated using D4-(R,S)-Ket as the internal standard, where the concentration of the internal standard was set at 500 ng/ml. Quality control standards were prepared daily by adding 10 μl of the corresponding spiking standard solution and 10 μl of internal standard to 30 μl of drug free plasma purchased from Bioreclamation (East Meadow, NY, USA), for the plasma samples.
The extraction of (R,S)-Ket and its metabolites from Wistar rat plasma was carried out as previously described [19,20]. Briefly, the 1 ml solid phase extraction (SPE) cartridges (Oasis HLB, Waters Corp.) were preconditioned with 1 ml of methanol, followed by 1 ml of water and then 1 ml ammonium acetate [10 mM, pH 9.5]. Subsequently, plasma samples were added and washed with 1 ml of water and then the compounds were eluted with 1 ml of methanol, which was transferred to the autosampler vial for analysis.
2.5. Partitioning of (R,S)-Ket, (R,S)-norKet, (R,S)-DHNK and (2S,6S;2R,6R)-HNK between whole blood and plasma from the mouse
A 200 μl aliquot of whole blood and a 200 μl aliquot of blank plasma from the mouse were spiked with 10 μM, 1 μM, and 0.1 μM of (R,S)-Ket, (R,S) norKet, (R,S)-DHNK, (2S,6S;2R,6R)-HNK. The samples were shaken for 30 min at 37°C. The samples were then centrifuged at 21,000 × g for 15 min. A 30 μl aliquot of the resulting supernatant was removed and 10 μl of water and 10 μl of the 5 μg/ml of Ket-D4 were added. The whole blood samples and the plasma were then extracted as described above. Calibration curves and quality controls were carried out ranging in concentration from 5 μg/ml 19.5 ng/ml using a 0.5 serial dilution for the calibration curve and 2.5 μg/ml, 312.5 ng/ml and 39 ng/ml for QC1, QC2 and QC3.
2.6. Statistical Analysis
The pharmacokinetic parameters assessed in this study were maximum plasma concentration (Cmax), time point of maximum plasma concentration (Tmax), area under the plasma concentration–time curve from 0 to the last measurable concentration (AUC0 – t), area under the plasma concentration–time curve from 0 to infinity (AUC0 – ∞), and half-life of drug elimination during the terminal phase (t1/2). These parameters were estimated using non-compartmental analysis of WinNonlin Professional Software Version 5.2.1 (Pharsight Corporation, St. Louis, MO).
3. Results
3.1 Plasma pharmacokinetics
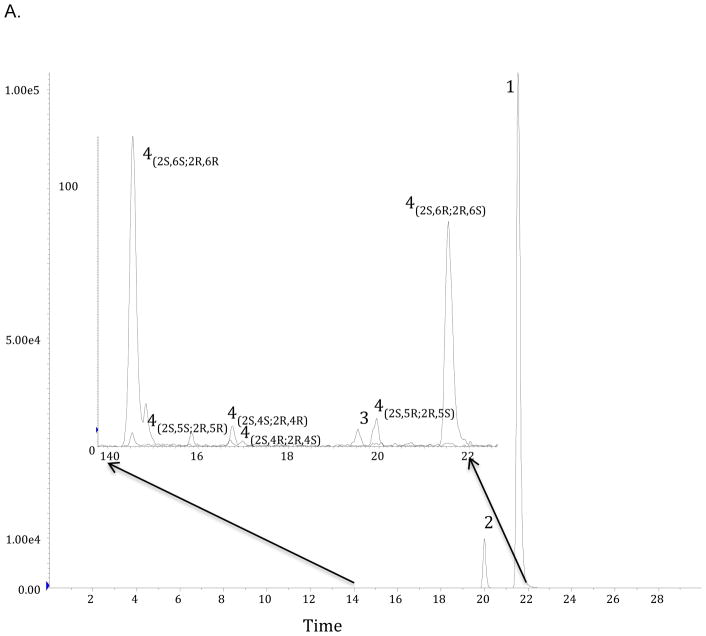
In the acute dosing arm of the study, the plasma samples obtained between 5 min and 60 min post-administration contained high (>1,000 ng/ml) concentrations of (R,S)-Ket, (R,S)-norKet and (2S,6S;2R,6R)-HNK, smaller (>50 ng/ml) concentrations of (2S,6R;2R,6S)-HNK, (2S,6S,2R,5R)-HNK, (2S,4S,2R,4R)-HNK and (2S,4R;2R,4S)-HNK (Fig. 2A, Supplemental Table S1). Quantifiable concentrations, ≥ 5.0 ng/ml, of (R,S)-Ket, (R,S)-norKet and (2S,6S;2R,6R)-HNK were present in the plasma samples in samples obtained 48 hr post administration and earlier, while the remaining metabolites were below quantitation by 4 hr post-dosing (Fig. 3A Supplemental Table S1). The plasma concentration data for (R,S)-Ket, (R,S)-norKet and (2S,6S;2R,6R)-HNK were used to calculate the apparent half-life of drug elimination during the terminal phase (t1/2), maximum plasma concentration (Cmax), time point of maximum plasma concentration (Tmax) and area under the plasma concentration-time curve from 0 to the last sampling point and from 0 to infinity (AUC0 – t and AUC0 – ∞) (Table 1). The Cmax values for (R,S)-norKet and (2S,6S;2R,6R)-HNK were 2562 ± 850 ng/ml and 4641 ± 2020 ng/ml, respectively, and the plasma concentrations of the other HNK metabolites at 10 min post-dosing were 252 ± 81 ng/ml ((2S,6R;2R,6S)-HNK), 257 ± 195 ng/ml ((2S,4R;2R,4S)-HNK), 196 ± 132 ng/ml ((2S,4S;2R,4R)-HNK), 181 ± 141 ng/ml ((2S,5S;2R,5R)-HNK).
Figure 2.
Chromatographic determination of the concentrations of (R,S)-ketamine and its major metabolites in plasma samples obtained from Wistar rats receiving a single intraperitoneal injection of 40 mg/kg (R,S)-Ket once per day for 14 days. Insert represents an expanded view of the hydroxynorketamines (14–22 min) See text for experimental details.
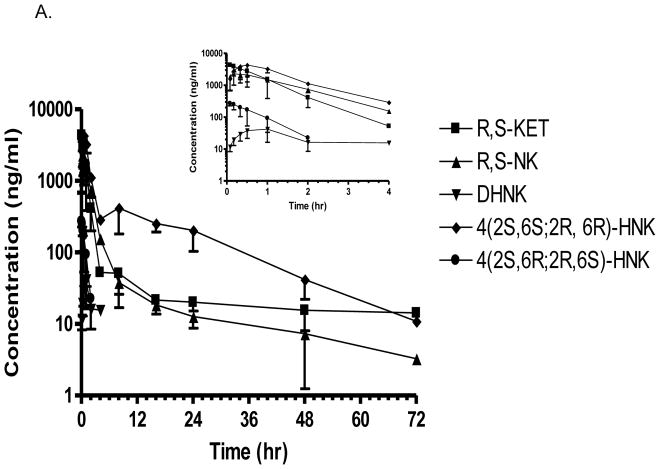
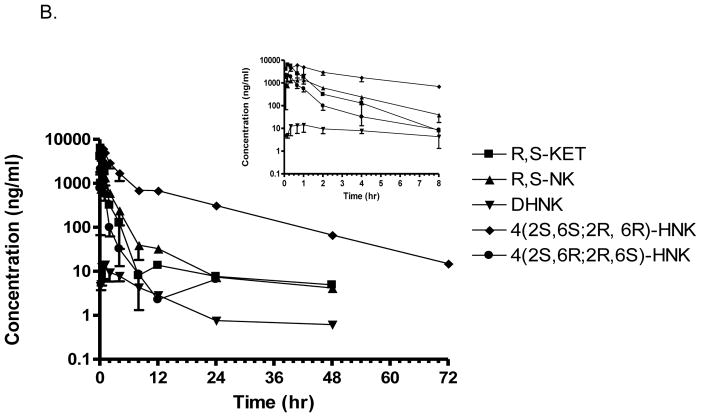
Figure 3.
Plasma pro le of ketamine, norketamine and (2S,6S;2R,6R) HNK and 4(2S,6R;2R,6S)-HNK obtained from Wistar rats receiving a single i.p. injection (A) and a single i.p. injection of 40 mg/kg (R,S)-Ket once per day for 14 days (B). Each data point represents the mean ± SD for n=3 rats. Time points were collected through 72 h.
Table 1.
Plasma pharmacokinetic parameters for (R,S)-Ket, (R,S)-norKet, (2S,6S;2R,6R)-HNK and (2S,6R;2R,6S)-HNK determined in Wistar rats after administration of a single intraperitoneal injection of 40 mg/kg (R,S)-Ket (Acute) and on Day 14 in Wistar rats after administration of a single intraperitoneal injection of 40 mg/kg (R,S)-Ket once per day for 14 days. See text for experimental details.
| Compound | t1/2 (hr) | Tmax (hr) | Cmax (ng/ml) | AUCinf (hr·ng/ml) |
|---|---|---|---|---|
| Acute | ||||
| (R,S)-Ket | 10.2 ± 5.2 | 0.11 ± 0.05 | 4738 ± 765 | 5382 ±2257 |
| (R,S)-norKet | 6.2 ± 3.1 | 0.44 ± 0.48 | 2562 ± 850 | 4863 ± 575 |
| (2S,6S;2R,6R)-HNK | 13.2 ± 3.7 | 0.61 ± 0.35 | 4641 ± 2020 | 16331 ±4209 |
| Sub-Chronic | ||||
| (R,S)-Ket | 2.8 ± 1.3 | 0.17 ± 0.0 | 6365 ± 820 | 5749 ± 513 |
| (R,S)-norKet | 4.4 ± 05.5 | 0.58 ± 0.46 | 2052 ± 678 | 3882 ± 866 |
| (2S,6S;2R,6R)-HNK | 11.1 ± 1.8 | 0.78 ± 0.19 | 6491 ± 3008 | 33177 ± 3720 |
The plasma samples obtained on Day 14 in the sub-chronic arm of the study contained (R,S)-Ket, (R,S)-norKet, (2S,6S;2R,6R)-HNK, (2S,6R;2R,6S)-HNK, (2S,6S,2R,5R)-HNK, (2S,4S,2R,4R)-HNK, (2S,4R;2R,4S)-HNK, and (2S,5R;2R,5S)-HNK (Fig. 2). Significant concentrations of all of the detected compounds were present in the plasma sample collected 5 min after (R,S)-Ket administration and quantifiable concentrations, i.e. ≥ 5.0 ng/ml, were present in the samples obtained 8 hr post administration (Fig. 3B Supplemental Table S1). The average maximum plasma concentration of (R,S)-norKet was 2052 ng/ml, (2S,6S;2R,6R)-HNK 6491 ng/ml, (2S,6R;2R,6S)-HNK 2101 ng/ml and (2S,4R;2R,4S)-HNK 610 ng/ml. Quantitative concentrations of the major metabolites were observed in the 48 hr samples, but only (2S,6S;2R,6R)-HNK, concentration 15 ± 7 ng/ml, was determined in the plasma samples collected at 72 hr post-administration (Supplementary Table S1). A pharmacokinetic analysis was performed using the plasma concentration data for (R,S)-Ket, (R,S)-norKet, (2S,6S;2R,6R)-HNK and the t1/2, Cmax, Tmax, AUC0 – t and AUC0 – ∞ values were determined (Table 1).
3.2 Blood-plasma partitioning of (R,S)-DHNK
Unlike the (R,S)-norKet and HNK metabolites, the plasma concentrations of (R,S)-DHNK did not exceed 10 ng/ml at any sampling time (Supplemental Table S1, Supplemental Table S2) and the pharmacokinetic parameters for (R,S)-DHNK were not calculated in this study. The low plasma concentrations of (R,S)-DHNK were unexpected as (R,S)-DHNK is a major circulating metabolite in patients receiving (R,S)-Ket for the treatment of CRPS [13] and depression [11,12]. The data raised the issue of the partitioning of (R,S)-DHNK into red blood cells in the mouse. The studies were conducted using (R,S)-Ket, (R,S)-norKet and (R,S)-DHNK. The results demonstrated that when (R,S)-Ket and (R,S)-norKet were incubated with whole blood, the majority, >90%, of the compounds were found in the plasma fraction. While when (R,S)-DHNK was incubated with whole blood, only a small percentage, <20%, of the spiked (R,S)-DHNK was present in the plasma fraction. Disruption of the collected red cell pellets did not result in the release of (R,S)-DHNK suggesting that there is an irreversible binding of the compound to red blood cell components (data not shown).
4. Discussion
An early study of the fate of (R,S)-Ket in the Wistar rat demonstrated that the administration of a single dose of (R,S)-Ket produced significant plasma and brain tissue concentrations of (R,S)-norKet and (2S,6S;2R,6R)-HNK and that the administration of (R,S)-norKet resulted in significant plasma and brain tissue concentrations of (2S,6S;2R,6R)-HNK, confirming a sequential metabolic transformation of (R,S)-Ket [16]. However, until this study, the effect of sub-chronic administration of (R,S)-Ket on the sequential conversion of the compound into the array of HNK and DHNK metabolites has not been studied. This was the result of data from the initial pharmacodynamic study of the anesthetic effects of (R,S)-Ket in the Wistar rat, which demonstrated that (R,S)-Ket and (R,S)-norKet produced the CNS activities associated with general anesthesia and increased spontaneous locomotor activity during the post-anesthetic recovery phase, while (2S,6S;2R,6R)-HNK was inactive in both of these tests [16]. As a result, (2S,6S;2R,6R)-HNK and the other HNK and DHNK metabolites, were labeled as “inactive” and have not been considered in subsequent pharmacokinetic, pharmacodynamic and clinical studies of (R,S)-Ket.
We have recently demonstrated that (2S,6S;2R,6R)-HNK, (2S,6R;2R,6S)-HNK and (R,S)-DHNK and their individual stereoisomers are pharmacologically active and that these compounds contribute to the clinical efficacy of (R,S)-Ket [17–19]. We have also recently reported the plasma disposition and clearance of (R,S)-Ket and its major metabolites in patients receiving a single sub-anesthetic dose of (R,S)-Ket in the treatment of depression and identified an association between the plasma concentrations of (R,S)-Ket metabolites and antidepressant response [11,12]. In addition, we demonstrated that high concentrations of HNK metabolites are present in the brain tissue of Wistar rats within 10 min of the administration of (R,S)-Ket, (R,S)-norKet and (2S,6S)-HNK and that the administration of (2S,6S)-HNK increases the activities of intracellular biological signaling pathways associated with the antidepressant effects produced by (R,S)-Ket [19].
In this study, we have examined the effect of sub-chronic dosing of (R,S)-Ket on the plasma disposition and clearance of the major metabolites of (R,S)-Ket in the Wistar rats. This study was prompted by the use of sub-chronic (R,S)-Ket dosing protocols in the treatment of patients with neuropathic pain suffering from CRPS [7,8] and by the observations that sub-chromic administration of (R,S)-Ket produces pharmacological and toxicological effects not observed after acute dosing of the drug [25–28]. The results of this study indicate that the daily administration of (R,S)-Ket over a 14 day period reduced the elimination half lives (t1/2) of (R,S)-Ket, (R,S)-norKet and (2S,6S;2R,6R)-HNK relative to the t1/2 observed after a single administration of (R,S)-Ket by 4.4-fold, 1.4-fold and 1.2-fold, respectively (Table 2). The apparent t1/2 values were calculated for (2S,6R;2R,6S)-HNK and (2S,4R;2R,4S)-HNK using the data in the subchronic arm of the study, but the plasma concentrations of were too low in the acute arm of the study to permit the estimation of the pharmacokinetic parameters. The relative plasma concentrations of (R,S)-Ket and its major metabolites in plasma samples drawn 30 min post dosing were compared between the two arms of the study (Table 2). This time point was chosen as it captured the maximum concentrations of the HNK metabolites. At this time point, there was no significant difference between the plasma concentrations of (R,S)-Ket in the two arms of the study (p=0.89), probably due to absorption of the drug occurring after intraperitoneal administration. Statistically significant differences between the two arms was observed at later time points for DHNK, (2S,6S;2R,6R)-HNK and (2S,6R;2R,6S)-HNK were observed in plasma samples collected at later time points (Fig 3). Daily dosing in the subchronic study reduced the relative plasma concentration of (R,S)-norKet by 1.6 fold while increasing the relative concentrations of (2S,6S;2R,6R)-HNK and (2S,4R;2R,4S)-HNK by 1.4-fold and 1.7-fold, respectively. The greatest effect was observed for (2S,6R;2R,6S)-HNK as subchronic dosing increased the relative plasma concentration by 4.5-fold (Table 2). The observed effects are consistent with data from the initial studies of (R,S)-Ket in the Wistar rat in which the sub-chronic administration of (R,S)-Ket reduced the plasma concentrations and increased the clearance of (R,S)-Ket and (R,S)-norKet [22,23].
Table 2.
Comparison of the plasma concentrations of (R,S)-Ket and major metabolites at 30 min after the intraperitioneal administration of (R,S)-Ket and their elimination half-lives (t1/2) in Wistar rats receiving a single administration of (R,S)-Ket (Acute Arm) and after receving a single injection of (R,S)-Ket for 14 days (Chronic Arm).
| (R,S)-Ket | (R,S)-norKet | (2S,6S,2R,6R)-HNK | (2S,6R,2R,6S)-HNK | (2S,4R,2R,4S)-HNK | |
|---|---|---|---|---|---|
| t1/2 (hr) | |||||
| Acute Arm | 10.2 | 6.2 | 13.2 | ND | ND |
| Chronic Arm | 2.8 | 4.4 | 11.1 | 1.3 | 5.0 |
| Ratio (A/C) | 4.4 | 1.4 | 1.2 | - | - |
| Plasma (ng/ml) | |||||
| Acute Arm | 2780 | 2128 | 4327 | 172 | 218 |
| Chronic Arm (40 min) | 2598 | 1336 | 6146 | 780 | 378 |
| Ratio (C/A) | 0.93 | 0.62 | 1.42 | 4.53 | 1.73 |
The data from this study are consistent with the induction of the expression and/or activity of multiple CYPs by the sub-chronic administration of (R,S)-Ket. The results also demonstrate that the induction may not be equal for all of the CYPs participating in the metabolic transformation of (R,S)-Ket. The principle CYP associated with the (R,S)-Ket ⇒ (R,S)-norKet transformation is CYP2B6 while (R,S)-nor-Ket ⇒ (2S,6S;2R,6R)-HNK and (2S,4R;2R,4S)-HNK are primarily mediated by CYP2A6 and CYP3A5, respectively (Figure 1) [10]. The principle CYP associated with the (R,S)-Ket ⇒ (2S,6R;2R,6S)-HK ⇒ (2S,6R;2R,6S)-HNK pathway is CYP3A5 (Figure 1) [10]. It is important to note that in vitro microsomal studies [10] and studies in the Wistar rat [19] have confirmed that (2S,6R;2R,6S)-HK is the sole precursor of (2S,4R;2R,4S)-HNK. Thus, it is reasonable to assume that the sub-chronic administration of (R,S)-Ket produces a greater effect on the expression/activity of CYP3A5 and CYP2A6 than CYP2B6 and that this difference coupled with inter-patient pharmacogenetic variations and potential metabolic drug interactions will produce vastly different HNK metabolite patterns. Indeed, different relative concentration patterns of (2S,6S;2R,6R)-HNK and (2S,6R;2R,6S)-HNK have been reported in the plasma of CRPS patients [10]. Since (2S,6S;2R,6R)-HNK and (2S,6R;2R,6S)-HNK and their enantiomers are pharmacologically active [29], it will be important to establish the pharmacodynamic consequences of different metabolic patterns in the treatment of CRPS and other diseases requiring chronic (R,S)-Ket administration. The necessary studies are in progress and the results will be reported elsewhere.
Highlights.
Blood-plasma partitioning of (R,S)-DHNK in rats
acute versus subchronic administration of Ket
subchronic administration of (R,S)-Ket results in a different metabolite pattern
Acknowledgments
This work was supported by funding from the Intramural Research Program of the National Institute on Aging/NIH and NIA Contract no. HHSN271201000008I.
Footnotes
This manuscript is submitted in honor of the priceless contributions of Roman Kaliszan to chromatographic and biological sciences. His work has provided important insights into the inter-relationships between chromatographic retention and pharmacological response and has been, and continues to be, an inspiration to all of us working to understand and to improve human life. Thank you. (Irv Wainer)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Domino EF, Chodoff P, Corssen G. Pharmacologic Effects of CI-581, a New Dissociative Anesthetic, in Man. Clin Pharmacol Ther. 1965;6:279–91. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- 2.Domino EF. Taming the ketamine tiger. Anesthesiology. 2010;113:678–86. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 3.Hirota K, Lambert DG. Ketamine. new uses for an old drug. Br J Anaesth. 2011;107:123–6. doi: 10.1093/bja/aer221. [DOI] [PubMed] [Google Scholar]
- 4.Li J-H, Vicknasingam B, Cheung Y-W, Zhou W, Nurhidayat AW, Des Jarlais DC, Schottenfeld R. To use or not to use: an update on licit and illicit ketamine use. Subst Abuse Rehab. 2011;2:11–20. doi: 10.2147/SAR.S15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant depression. Arch Gen Psychiat. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 6.LIbrahim L, DiazGranados N, Franco-Chaves J, Brutsche NE, Henter ID, Kronstein P, Moaddel R, Wainer IW, Luckenbaugh DA, Manji HK, Zarate CA., Jr Course of Improvement in Depressive Symptoms to a Single Intravenous Infusion of Ketamine vs. Add-on Riluzole: Results from a Four-Week, Double-Blind, Placebo-Controlled Study. Neuropsychopharmacology. 2012;37:1526–33. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Pharmacodynamic profiles of ketamine (R)- and (S)- with 5-day inpatient infusion for the treatment of complex regional pain syndrome. Pain Physician. 2010;13:379–87. [PMC free article] [PubMed] [Google Scholar]
- 8.Sabia M, Hirsh R, Torjman MC, Wainer IW, Cooper N, Domsky R, Goldberg ME. Advances in Translational Neuropathic Research: Example of Enantioselective Pharmacokinetic–Pharmacodynamic Modeling of Ketamine-induced Pain Relief in Complex Regional Pain Syndrome. Curr Pain Headache Rep. 2011;15:207–14. doi: 10.1007/s11916-011-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JD, Baille TA, Trevor AJ, Castagnoli N., Jr Studies on the biotransformation of ketamine: Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spec 1981. 1981;8:527–38. doi: 10.1002/bms.1200081103. [DOI] [PubMed] [Google Scholar]
- 10.Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SLV, Sanghvi M, Goldberg ME, Torjman MC, Wainer IW. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–87. doi: 10.3109/00498254.2012.685777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Venkats SLV, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA, Jr, Mager DE, Wainer IW. Simultaneous Population Pharmacokinetic Modeling of Ketamine and Three Major Metabolites in Patients with Treatment-Resistant Bipolar Depression. Br J Clin Pharmacol. 2012;74:304–14. doi: 10.1111/j.1365-2125.2012.04198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarate CA, Jr, Brutsche NE, Laje G, Luckenbaugh DA, Ramamoorthy A, Moaddel R, Wainer IW. Relationship of Ketamine’s Plasma Metabolites with Response and Diagnosis, and Side Effects in Major Depression. Biol Psychiatry. 2012;72:331–8. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moaddel R, Venkata SLV, Tanga MJ, Bupp JE, Green CE, LaIyer L, Furimsky A, Goldberg ME, Torjman MC, Wainer IW. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82:1892–1904. doi: 10.1016/j.talanta.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen ML, Chan S-L, Way WL, Trevor AJ. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;30:370–6. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Marietta MP, Way WL, Castagnoli N, Jr, Trevor AJ. On the pharmacology of the ketamine enantiomorphs in the rat. J Pharmacol Exp Ther. 1977;202:157–65. [PubMed] [Google Scholar]
- 16.Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29:2396–9. doi: 10.1021/jm00161a043. [DOI] [PubMed] [Google Scholar]
- 17.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, Jr, Wainer IW. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in a7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh NS, Paul RK, Ramamoorthy A, Torjman MC, Moaddel R, Bernier M, Wainer IW. Nicotinic acetylcholine receptor antagonists alter the function and expression of serine racemase in PC-12 and 1321N1 cells. Cellular Signalling. 2013;25:2634–45. doi: 10.1016/j.cellsig.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O’Loughlin K, Torjman MC, Bernier M, Wainer IW. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin (mTOR) function. Anesthesiology. 2014;121:149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moaddel R, Sanghvi S, Dossou KS, Ramamoorthy A, Green C, Bupp J, Swezey R, O’Loughlin K, Wainer IW. The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol Res Perspect. 2015;3:e00157. doi: 10.1002/prp2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Enantioselective pharmacokinetics of (R)- and (S)-ketamine after a 5-day infusion in patients with complex regional pain syndrome. Chirality. 2011;23:138–43. doi: 10.1002/chir.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marietta MP, White PF, Pudwell CR, Way WL, Trevor AJ. Biodisposition of ketamine in the rat: self-induction of metabolism. J Pharmacol Exp Ther. 1976;196:536–44. [PubMed] [Google Scholar]
- 23.Chan W-H, Sun W-Z, Ueng T-H. Induction of rat hepatic cytodhrome P-450 by ketamine and its toxicological implications. J Toxicol Environ Health A. 2005;68:1581–97. doi: 10.1080/15287390590967522. [DOI] [PubMed] [Google Scholar]
- 24.Takeyama K, Yoshikawa M, Oka T, Kawaguchi M, Suzuki T, Hashimoto A. Ketamine enchances the expression of serine racemase and D-amino acid oxidase mRNAs in rat brain. Eur J Pharmacol. 2006;540:82–6. doi: 10.1016/j.ejphar.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Yoshikawa M, Takeyama K, Hashimoto A, Kobayashi H, Suzuki T. Subchronic administration of ketamine decreases the mRNA expression of serine racemase in rat brain. Tokai J Exp Clin Med. 2010;35:137–43. [PubMed] [Google Scholar]
- 26.Featherstone RE, Liang Y, Saunders JA, Tatard-Leitman VM, Ehrlichman RE, Siegel SJ. Subchronic ketamine treatment leads to permanent changes in EEG, cognition and astrocytic glutamate transporter EAAT2 in mice. Neurobiol Disease. 2012;47:338–46. doi: 10.1016/j.nbd.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Venancio C, Magalhaes A, Antunes L, Summervielle T. Impaired spatial memory after ketamine administration in chronic low doses. Curr Neuropharmacol. 2011;9:251–5. doi: 10.2174/157015911795016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein H-G. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–8. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 29.Singh N, Rutkowsk E, Plazinsk A, Khadeer M, Moaddel R, Jozwiak K, Bernier M, Wainer IW. Ketamine metabolites enantioselectively decrease intracellular D-serine concentrations in PC-12 cells. PLOSone. doi: 10.1371/journal.pone.0149499. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]