Abstract
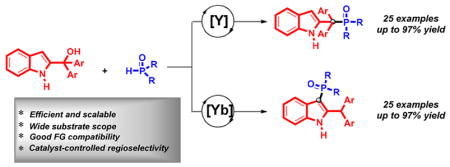
The Lewis acid-promoted phosphorylation of 2-indolylmethanols with diarylphosphine oxides is described. The regioselectivity of the reaction can be modulated by the choice of rare earth metal Lewis acid, offering a highly selective approach to structurally diverse indole derivatives in up to 97% yield for over 50 examples. This strategy features high selectivity, good functional group tolerance, and easy scalability. The utility of this method is further highlighted by facile modification of the products to access novel indole-based phosphine ligand.
Graphical Abstract
INTRODUCTION
The indole nucleus widely exists in natural products and pharmaceutical drugs.1–3 Specially, indole-based phosphines have attracted significant attention due to their unique synthetic applications and pharmacological properties.4 For example, 3-phosphoindoles represent novel second-generation NNRTIs (non-nucleoside reverse transcriptase inhibitors), which exhibit excellent potency against HIV-1 in vitro.5 Therefore, various methods have been developed to prepare indole-based phosphines.6 Very recently, the Lu group demonstrated a new approach to phosphorylate indoles, in which diaryl-((trifluoromethanesulfonyl)oxy) phosphines are generated in situ as electrophilic phosphination reagents from diarylphosphine oxides.7 Thereafter, the Li group reported carbonate-catalyzed direct phosphorylation of indoles in the presence of magnesium nitrate, affording both 2- and 3-phosphorylated indoles.8 However, these advances focus on the phosphorylation of the simple indole skeleton. The development of novel methods to generate highly functionalized indole-based phosphine oxides is still needed.
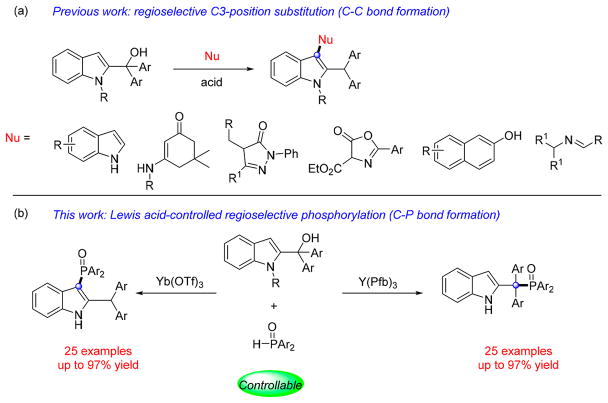
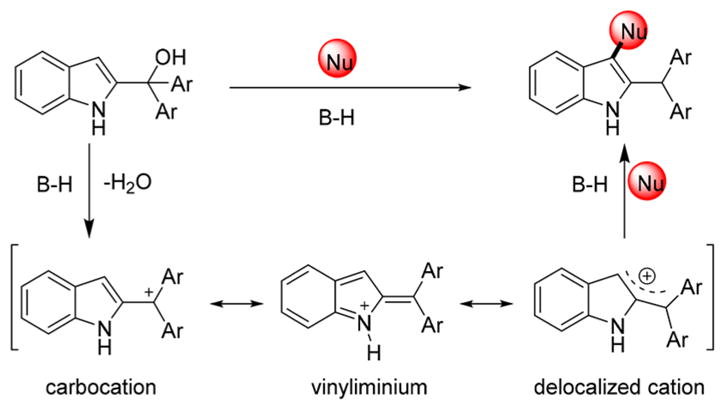
It is well established that direct activation of benzylic, propargylic, and allylic alcohols may be achieved through SN1 reactions by the use of Brønsted or Lewis acids,9 among which, indolylmethanols have attracted much attention as important and versatile reactants in organic synthesis.10 In particular, 2-indolylmethanols show great potential in the construction of indole derivatives.11 As shown in Scheme 1, in the presence of Brønsted acid (B–H), 2-indolylmethanols can be converted into their delocalized cation intermediates, which are stabilized by two Ar groups. Subsequently, the delocalized cation can be regioselectively attacked by nucleophiles at the C3-position because the two bulky Ar groups block the attack to the benzylic position.
Scheme 1.
C3-Functionalization of 2-Indolylmethanols with Nucleophile
On the basis of this strategy, the Shi group has made significant contributions to this research field. In their creative work, a series of nucleophiles including indoles, cyclic enaminones, pyrazol-5-ones, 2-naphthols et al. were successfully added to the C3-position of the indole moiety (Scheme 2a).12 Notably, phosphine nucleophiles have not been studied in this transformation, nor have any processes been reported involve selective functionalization at the benzylic site. On the basis of our research interest in new synthetic methods to generate organophosphorus compounds,13 we detail our findings herein on both of these aspects. In particular, it was discovered that C3 or benzyl functionalization could be induced selectively by use of different Lewis acid catalysts (Scheme 2b).
Scheme 2.
Regioselective Substitutions of 2-Indolylmethanols
RESULTS AND DISCUSSION
To begin, the reaction of 2-indolymethanol 1a and diphenylphosphine oxide 2a in the presence of Lewis acids was investigated (Table 1). In an initial experiment, the reaction was performed in acetonitrile at 100 °C catalyzed by FeCl3 (Table 1, entry 1). In contrast to a large number of reports with other nucleophiles,12 regioisomers of the phosphine oxide adducts were generated with FeCl3 affording 3aa in 54% yield as well as rearranged product 4aa in 15% yield. Further screening of Lewis acids including CuBr2, AlCl3, BF3·OEt2 and Brønsted acid TsOH (Table 1, entries 1–6) revealed that 3aa could be obtained with complete selectivity in 85% with AlCl3 (Table 1, entry 3). On the other hand, we discovered that TsOH afforded solely the rearranged product 4aa in 70% yield (Table 1, entry 4). Encouraged by these results, additional strong Lewis acids were examined including the rare earth triflates [RE(OTf)3] and rare earth pentafluorobenzoates [RE(Pfb)3] (Table 1, entries 7–15). Notably, the counterion (triflate, pentafluorobenzoate) of the lanthanide salt had significant impact on the regioselectivity of this transformation. RE(Pfb)3 led to predominant formation of 3aa while RE(OTf)3 furnished predominantly 4aa. Of the tested rare earth salts, Y(Pfb)3 afforded 3aa in the highest yield (87%, Table 1, entry 15) and Yb(OTf)3 afforded 4aa in the highest yield (76%, Table 1, entry 12). TfOH and pentafluorobenzoic acid, which might be generated from Yb(OTf)3 and Y(Pfb)3 with trace amounts of water, showed a poorer regioselectivity (Table 1, entries 16 and 17), indicating a key role of the rare earth cation. It should be noted that though AlCl3 is cheap and works well, we then tested AlCl3 in another two substituted examples (1b, 1e). Still, Y(Pfb)3 turned out to be the best. Variation of the amount of catalyst, solvent, temperature, and reaction time did not improve the yield of 3aa or 4aa further (see Supporting Information Table S1 for details). Also, it was found that the optimal reaction conditions employed in Moran’s work (TfOH (10 mol %), HFIP as solvent, 100 °C, 24 h) led to the ratio of 4aa/3aa is 69/19.14
Table 1.
Optimization of the Reaction Conditionsa
| |||||
|---|---|---|---|---|---|
| entry | catalyst | solvent | temp (°C) | time (h) | 3aa/4aa yield (%) |
| 1 | FeCl3 | CH3CN | 100 | 12 | 54/15 |
| 2 | CuBr2 | CH3CN | 100 | 12 | 48/0 |
| 3 | AlCl3 | CH3CN | 100 | 12 | 85/0 |
| 4 | TsOH | CH3CN | 100 | 12 | 0/70 |
| 5 | BF3·OEt2 | CH3CN | 100 | 12 | 18/26 |
| 6 | Cu(OTf)2 | CH3CN | 100 | 12 | 0/69 |
| 7 | La(OTf)3 | CH3CN | 100 | 12 | 28/31 |
| 8 | Sc(OTf)3 | CH3CN | 100 | 12 | 22/40 |
| 9 | Nd(Pfb)3 | CH3CN | 100 | 12 | 67/<5 |
| 10 | Sc(Pfb)3 | CH3CN | 100 | 12 | 31/<5 |
| 11 | Yb(Pfb)3 | CH3CN | 100 | 12 | 77/<5 |
| 12 | Yb(OTf)3 | CH3CN | 100 | 12 | <5/76 |
| 13 | Y(OTf)3 | CH3CN | 100 | 12 | 0/72 |
| 14 | Dy(OTf)3 | CH3CN | 100 | 12 | 12/53 |
| 15 | Y(Pfb)3 | CH3CN | 100 | 12 | 87/<5 |
| 16 | TfOH | CH3CN | 100 | 12 | 35/43 (21/57)c |
| 17 | PFBAb | CH3CN | 100 | 12 | 53/27 |
Reaction conditions: 1a (0.25 mmol), 2a (0.375 mmol), catalyst (20 mol %), CH3CN (2 mL), 100 °C, 12 h, under air.
PFBA: pentafluorobenzoic acid.
Run 48 h.
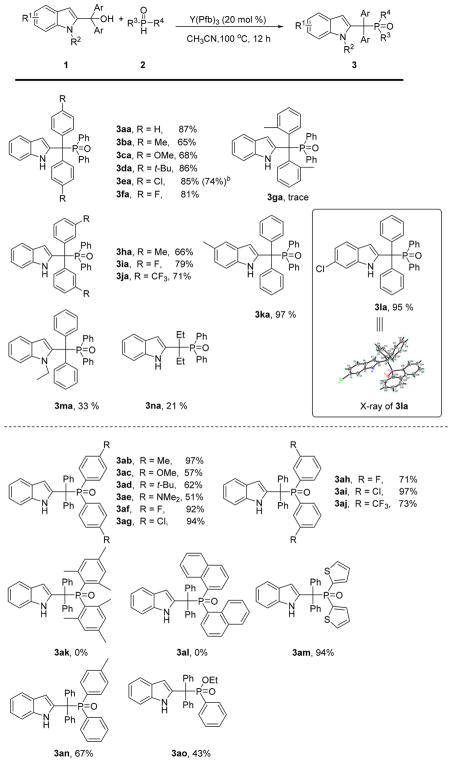
Y(Pfb)3, which was identified as the best catalyst for the benzylic phosphorylation of 2-indolylmethanols, was used to assess the scope of the reaction (Scheme 3). A number of 2-indolylmethanols 1 were subjected to the reactions with diphenylphosphine oxide 2a (top of Scheme 3). Both electron-rich and electron-deficient aryl 2-indolylmethanols reacted well and with very high to complete selectivity.
Scheme 3. Regioselective Y(Pfb)3-Catalyzed Benzylic Phosphorylation of 2-Indolylmethanolsa.
aReaction conditions: 1 (0.25 mmol), 2 (0.375 mmol), Y(Pfb)3 (20 mol %), CH3CN (2 mL) at 100 °C for 12 h. bConducted on a 5 mmol scale.
The reaction of 2-indolylmethanols 1a–f having substitution at the 4-position with methyl, methoxy, tert-butyl, chloro, and fluoro functional groups furnished the corresponding products 3aa–fa in 65–87% yields. Similar results were observed with the substrates 1h–j bearing substitution at the 3-position of the phenyl ring with methyl, fluoro, and trifluoromethyl groups, affording the corresponding products 3ha–ja in 66–79% yields. Only trace of product 3ga was observed for the 2-methyl substituted substrate 1g most likely due to steric hindrance. In addition, 2-indolylmethanols bearing C-5 methyl and C-6 chloro groups gave the corresponding products 3ka, 3la in excellent yields. The structure of 3la was confirmed by X-ray analysis.15 The N-ethyl-substrate 1m afforded the corresponding product 3ma in a low yield of 33%, and the N-methyl-protected substrate was also not effective with this protocol. Allylic strain interactions from the N-substituents are proposed to destabilize the planar carbocation intermediate (see below). In line with a carbocation intermediate, the transformation was less effective with ethyl (3na in 21% yield) vs the aryl substitution described above. Notably, the gram-scale synthesis of product 3ea proceeded with comparable catalytic efficacy.
The scope of the phosphine oxide component was next examined (bottom of Scheme 3). Diarylphosphine oxides bearing substituents at 3- or 4-positions were all amenable to the reaction, and the yields ranged from 51 to 97%. It should be noted that the reaction of 2-indolylmethanol 1a with 2c, 2e gave lower yields (51%, 57%) of corresponding products; the strong electron-donating group may limit the acidity of the phosphine oxide. Steric hindrance effect was found to be extremely detrimental, and no products were detected at all for 2-substituted aryl derivatives 2k and 2l. The reaction proceeded well with bisthiophenephosphine oxide 2m, and phenyl(p-tolyl)phosphine oxide 2n, furnishing the desired products in 67–94% yields. When one arene ring was replaced by ethoxy groups, the product 3ao was obtained in 43% yield. However, diethyl phosphonate failed to give the targeted product under the current reaction conditions.
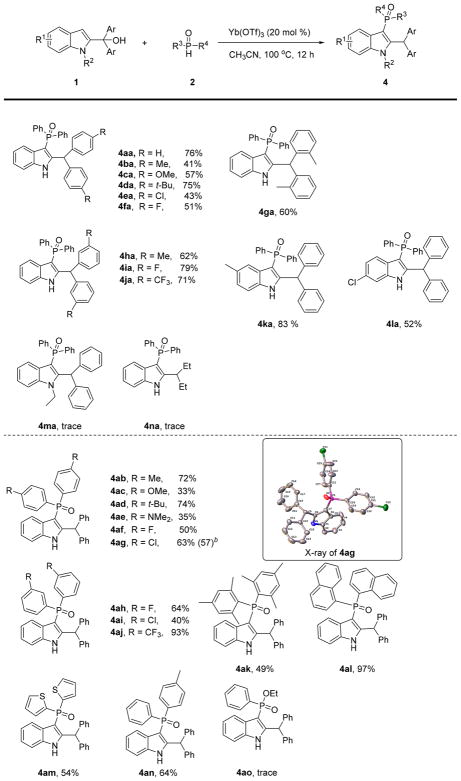
We next investigated the scope of Yb(OTf)3-catalyzed C3-phosphorylation of 2-indolylmethanols (top of Scheme 4). Both electron-donating and electron-withdrawing substituents on the phenyl ring of 2-indolylmethanols were well tolerated. The corresponding C3-phosphorylation products were isolated in 41–79% yields (4aa–4ja). Notably, 2-methyl substituted 1g, which was not active in Y(Pfb)3-mediated reaction, successfully takes part in C3-phosphorylation, providing 4ga in 60% yield. 2-Indolylmethanols bearing substituents on the indole ring were also well accommodated reaction (4ka, 4la). Again, little or no product was observed when the N-ethyl substrate (1m) and the diethyl methanol substrate group (1n) were utilized.
Scheme 4. Regioselective Yb(OTf)3-Catalyzed C3-Phosphorylation of 2-Indolylmethanolsa.
aReaction conditions: 1 (0.25 mmol), 2 (0.375 mmol), Yb(OTf)3 (20 mol %), CH3CN (2 mL) at 100 °C for 12 h. bConducted on a 5 mmol scale.
Diarylphosphine oxides with various substituents at the 3-and 4-positions were suitable substrates for this reaction (4ab–4aj, 4an). In contrast to the benzylic reaction, substrates with 2-substitution gave good reactivity for the C3-phosphorylation adduct. For example, corresponding products 4ak and 4al were obtained in 49% and 97% yields, respectively. We theorize that the steric hindrance of these substrates promotes rearrangement.
Bisthiophenephosphine oxide 2m was also well tolerated, giving the product 4am in 54% yield. Ethyl phenylphosphinate failed to yield the desired product (4ao). Notably, this Yb(OTf)3-catalyzed C3-phosphorylation was also executed successfully on gram-scale, delivering 4ag with a slightly decreased 57% yield. The structure of 4ag was secured by X-ray diffraction.15
To probe the requirements for reactivity, surrogates of the 2-indolylmethanols 1 and diarylphosphine oxides 2 were explored (Figure 1). Treatment of diphenylphosphine oxide 2a with benzhydrol or 2-indolylphenylmethanol yielded none of the adducts under the standard reaction conditions. These results show that the indolyl ring and two phenyl groups are all necessary, consistent with the intermediacy of a delocalized carbocation. Treatment of 2-indolylmethanol 1a with diphenylphosphine or diphenylphosphine sulfide under the standard reaction conditions resulted in no product formation.
Figure 1.
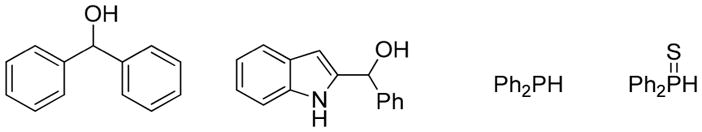
Limited substrates under Yb(OTf)3 or Y(Pfb)3-catalyzed conditions.
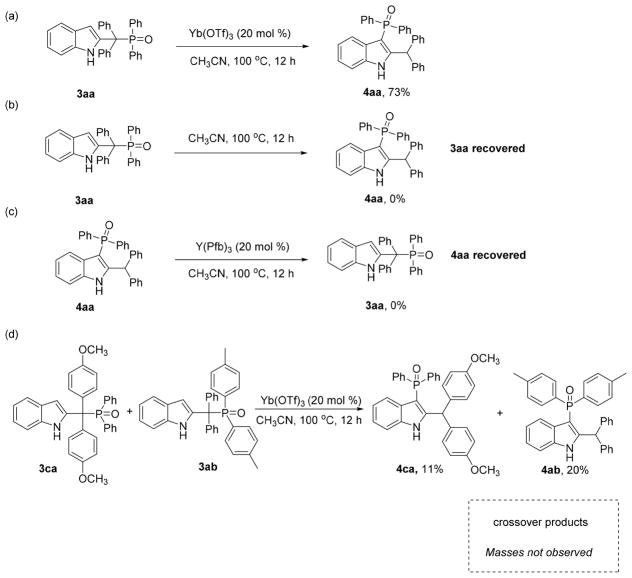
To obtain further insight into the mechanism, a series of control experiments were performed (Scheme 5). When benzylic adduct 3aa was treated with Yb(OTf)3, 73% yield of rearranged product 4aa was obtained (Scheme 5a). The same transformation did occur in the absence of Yb(OTf)3 (3aa was recovered in over 90% yield) indicating that a thermal rearrangement is not responsible for the C3 adducts (Scheme 5b). However, rearranged product 4aa was not converted to 3aa in the presence of Y(Pfb)3 (Scheme 5c). These results indicate the C3-phosphorylation of 2-indolylmethanols involves the benzylic phosphorylation product 3 as an intermediate. Finally, a crossover experiment was designed to distinguish whether the rearrangement was occurring via an intramolecular or intermolecular pathway (Scheme 5d). An equimolar mixture of 3ca and 3ab was treated with Yb(OTf)3 in CH3CN. Product 4ca was isolated in 11% yield, along with 20% of 4ab, while no crossover products were observed, thus indicating that the rearrangement product arises from an intramolecular process.
Scheme 5.
Mechanistic Probes
On the basis of the experimental results and previous reports,12 a reaction mechanism for the formation of 3 and 4 is proposed in Scheme 6. First, association of the hydroxyl group of 2-indolylmethanol 1 with the Lewis acid triggers ionization. The resultant carbocation is stabilized by resonance delocalization; allylic strain between the aryl groups and the R2 group explains the lack of the reactivity of the N-methyl and N-ethyl congeners (see above).
Scheme 6.
Proposed Mechanistic Profile
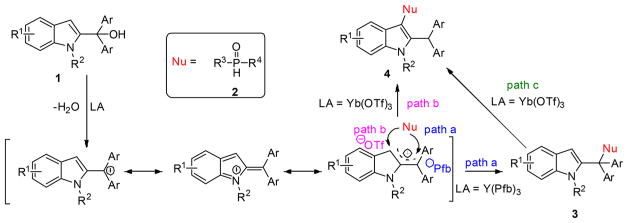
With Y(Pfb)3, we speculate that the perfluorobenzoate forms an ion pair such that the site with the greatest LUMO density is attacked (Figure 2). Thus, phosphine oxide 2 adds to the benzylic position cation (via path a) giving the kinetic product, benzylic adduct 3. With Yb(OTf)3, the more stable triflate anion16 will form a weaker ion pair, allowing reversible addition. Addition of nucleophile 2 to C3 (via path b) followed by tautomerization leads to a thermodynamic sink in the form of 4 due to the stronger vinyl-Nu bond. Resubjection of product 3 to Yb(OTf)3 yields rearrangement product 4 (path c), providing further evidence that 4 is a thermodynamic adduct. Notably, the putative ion pair must not dissociate readily as crossover experiments (Scheme 5d) support an intramolecular transfer.
Figure 2.
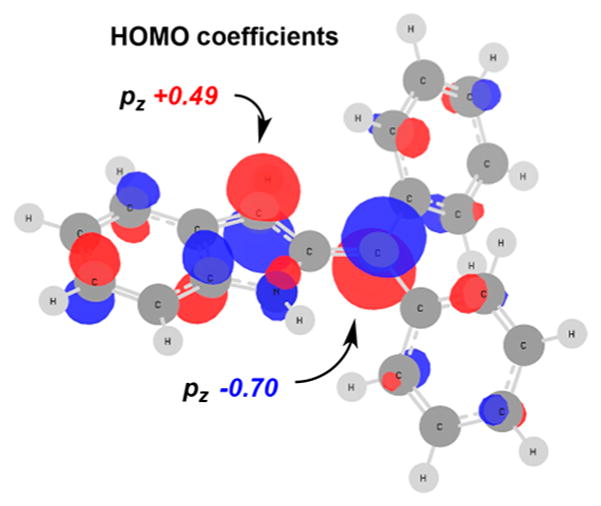
LUMO orbital of the cation from ionization of 1.
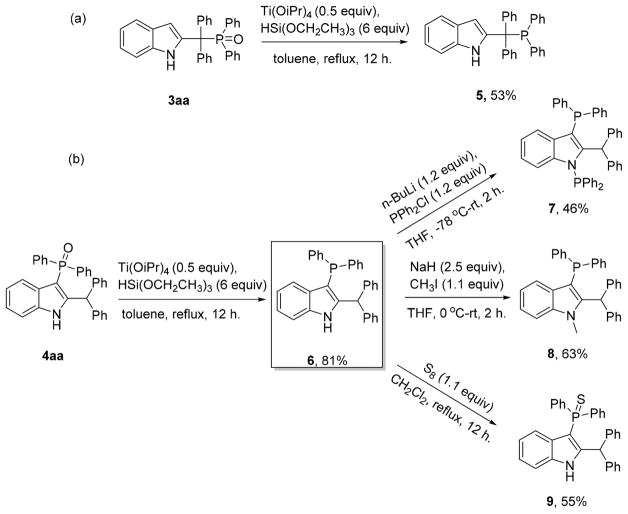
Indole-based phosphine ligands such as CMphos, amide CMPhos in combination with a palladium metal precursor have been found to be effective catalysts for Suzuki–Miyaura coupling of unactivated aryl chlorides17 and Buchwald–Hartwig amination.18 To show the utility of this method for generating useful building blocks, a number of further transformations were carried out on products obtained in the course of these studies (Scheme 7). Treatment of product 3aa with 6 equiv of triethoxysilane and a catalytic amount of Ti(OiPr)4 in refluxing toluene afforded corresponding phosphine 5 in 53% yield (Scheme 7a).19 Also, reduction of 4aa using the same reagents gave phosphine 6 in 81% yield. The straightforward deprotonation of 6 by n-BuLi and trapping of the lithiated intermediate with PPh2Cl afforded the indolyl bisphosphine 7 in 46% yield (Scheme 7b). N-Methylation of 6 using MeI gave corresponding phosphine 8 in 63% yield. Diphenyphosphine sulphides are alternative ligands that are stable to oxidation where the sulfur atom functions as a soft donor.20 For this system, phosphine sulphide 9 was easily generated in 55% yield by oxidation of 6 with elemental sulfur. Thus, this strategy gives rise to novel structures for developing new indole-based phosphine ligands.
Scheme 7.
Derivatization of Products
CONCLUDING REMARKS
In summary, we have developed a method for selective phosphorylation of 2-indolylmethanols with diarylphosphine oxides. The use of Y(Pfb)3 as a catalyst provides benzylic phosphorylation products in good yields (up to 97% yield). In contrast, C3-phosphorylation products are isolated as the major product with Yb(OTf)3 as the catalyst. A wide variety of 2-indolylmethanols and diarylphosphine oxides perform well in this process. This protocol represents the first catalyst-controlled regioselective phosphorylation of 2-indolylmethanols in a one-pot manner. The nature of the Lewis acid counterion appears to have the largest impact on the regiocontrol, where strong Lewis acids stabilize the cationic intermediate allowing formation of the thermodynamic C3-phosphorylation product. Weaker Lewis acids, cause phosphorylation at the site of greatest positive charge, leading to the benzylic phosphorylation adduct. A number of transformations carried out on the reaction products illustrate the value that this method holds for the creation of highly functionalized indole-based phosphine ligands. Further studies of these novel phosphorus-containing molecules are underway in our laboratory.
EXPERIMENTAL SECTION
General Information
1H NMR, 13C NMR, 19F NMR and 31P NMR spectra were recorded at 400 MHz, 100 MHz, 376 and 162 MHz respectively using tetramethylsilane as an internal reference. Chemical shifts (δ) and coupling constants (J) were expressed in parts per million and hertz, respectively. Melting points were uncorrected. High-resolution mass spectrometry (HRMS) was performed on an ESI-TOF spectrometer. 2-Indolylmethanols compound substrates were prepared according to the literature procedure.12 Substituted phenylphosphine oxides were synthesized according to the reported procedure.21 Chemicals were commercially available and used without purification. Chromatography: Column chromatography was performed with silica gel (200–300 mesh ASTM).
General Procedure A
2-Indolylmethanols 1 (0.25 mmol, 1 equiv), Diarylphosphine Oxides 2 (0.375 mmol, 1.5 equiv), Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv), dry CH3CN (2 mL) and a stir bar were added to a sealed tube. After being stirred at 100 °C for indicated time, the mixture was evaporated under vacuum. The corresponding product 3 was isolated by silica gel column chromatography with a petroleum ether/ethyl acetate mixture as eluent.
((1H-Indol-2-yl)diphenylmethyl)diphenylphosphine oxide (3aa)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3aa (105.17 mg) in 87% yield as a white solid (mp 232–234 °C): 1H NMR (400 MHz, DMSO-d6) δ 10.59 (s, 1H), 7.57–7.49 (m, 3H), 7.44 (d, J = 7.7 Hz, 1H), 7.38–7.30 (m, 6H), 7.26 (dd, J = 8.4, 6.8 Hz, 4H), 7.12–7.02 (m, 9H), 7.01–6.96 (m, 1H), 6.29 (t, J = 2.2 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 139.1, 136.3, 133.1 (d, JC–P = 8.4 Hz), 132.1 (d, JC–P = 2.8 Hz), 131.6, 131.0 (d, JC–P = 5.1 Hz), 130.7, 128.3 (d, JC–P = 11.4 Hz), 128.0, 127.7 (d, JC–P = 1.4 Hz), 127.1 (d, JC–P = 1.3 Hz), 122.2, 120.4, 119.7, 111.5, 105.8 (d, JC–P = 6.5 Hz), 61.2 (d, JC–P = 61.8 Hz). 31P NMR (162 MHz, DMSO-d6) δ 33.46. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H27NOP 484.1830, found 484.1834.
((1H-Indol-2-yl)di-p-tolylmethyl)diphenylphosphine oxide (3ba)
General procedure A was followed using 1b (81.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ba (127.90 mg) in 65% yield as a white solid (mp 240–242 °C): 1H NMR (400 MHz, CDCl3) δ 10.31 (s, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.46–7.41 (m, 3H), 7.29–7.24 (m, 4H), 7.22–7.16 (m, 5H), 7.09 (t, J = 7.5 Hz, 1H), 6.95 (d, J = 8.1 Hz, 4H), 6.87 (d, J = 8.0 Hz, 4H), 6.25 (d, J = 2.4 Hz, 1H), 2.30 (s, 6H). 13C{1H}NMR (100 MHz, CDCl3) δ 139.3, 137.4, 136.3, 136.1, 133.1 (d, JC–P = 8.4 Hz), 131.9 (m, JC–P = 2.7 Hz), 130.9, 130.8 (d, JC–P = 5.1 Hz), 128.6, 128.3 (d, JC–P = 11.4 Hz), 127.2, 122.1, 120.4, 119.6, 111.5, 105.7 (d, JC–P = 6.5 Hz), 60.6 (d, JC–P = 62.3 Hz), 21.0. 31P NMR (162 MHz, CDCl3) δ 36.62. HRMS (ESI-TOF) m/z [M + Na]+ Calcd for C35H30NNaOP 534.1963, found 534.1970.
((1H-Indol-2-yl)bis(4-methoxyphenyl)methyl)diphenylphosphine oxide (3ca)
General procedure A was followed using 1c (89.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ca (92.41 mg) in 68% yield as a white solid (mp 215–217 °C): 1H NMR (400 MHz, CDCl3) δ 10.32 (s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.47–7.42 (m, 3H), 7.29–7.24 (m, 4H), 7.23–7.17 (m, 5H), 7.09 (t, J = 7.4 Hz, 1H), 6.90 (d, J = 8.4 Hz, 4H), 6.71–6.65 (m, 4H), 6.23 (d, J = 2.5 Hz, 1H), 3.75 (s, 6H). 13C{1H}NMR (100 MHz, CDCl3) δ 158.8 (d, JC–P = 1.5 Hz), 139.6, 136.3, 133.1(d, JC–P = 8.5 Hz),132.0 (m, JC–P = 5.1 Hz), 131.8, 131.2, 130.9, 128.4 (d, JC–P = 11.3 Hz), 127.1, 122.1, 120.4, 119.6, 113.2, 111.5, 105.6 (d, JC–P = 6.5 Hz), 59.8 (d, JC–P = 62.6 Hz), 55.3. 31P NMR (CDCl3, 162 MHz) δ 36.49. HRMS (ESI-TOF) m/z [M + Na]+ Calcd for C35H30NNaO3P 566.1861, found 566.1871.
(Bis (4-(tert-butyl) phenyl) (1H-indol-2-yl) methyl)-diphenylphosphine oxide (3da)
General procedure A was followed using 1d (102.90 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3da (128.09 mg) in 86% yield as a white solid (mp 239–241 °C): 1H NMR (400 MHz, CDCl3) δ 10.15 (s, 1H), 7.52 (d, J = 7.9 Hz, 1H), 7.47–7.41 (m, 3H), 7.25 (d, J = 3.3 Hz, 1H), 7.24–7.19 (m, 4H), 7.16 (d, J = 8.3 Hz, 4H), 7.13–7.07 (m, 5H), 6.90 (d, J = 8.1 Hz, 4H), 6.33 (d, J = 2.6 Hz, 1H), 1.28 (s, 18H). 13C{1H}NMR (100 MHz, CDCl3) δ 150.6 (d, JC–P = 1.7 Hz), 139.2, 136.3, 136.0, 133.1 (d, JC–P = 8.5 Hz), 131.9 (m, JC–P = 3.1 Hz), 131.0, 130.5 (d, JC–P = 5.1 Hz), 128.3 (d, JC–P = 11.4 Hz), 127.2 (d, JC–P = 1.5 Hz), 124.9, 122.1, 120.4, 119.6, 111.5, 105.8, 60.5 (d, JC–P = 62.5 Hz), 34.5, 31.3. 31P NMR (162 MHz, CDCl3) δ 36.66. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C41H43NOP 596.3082, found 596.3079.
(Bis(4-chlorophenyl)(1H-indol-2-yl)methyl)diphenylphosphine oxide (3ea)
General procedure A was followed using 1e (92.06 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ea (117.39 mg) in 85% yield as a white solid (mp 264–266 °C): 1H NMR (400 MHz, CDCl3) δ 10.34 (s, 1H), 7.53–7.44 (m, 4H), 7.34–7.27 (m, 4H), 7.24–7.17 (m, 5H), 7.16–7.08 (m, 5H), 6.93 (d, J = 8.2 Hz, 4H), 6.18 (d, J = 2.4 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 137.4, 136.3, 133.9 (d, JC–P = 2.2 Hz), 133.0 (d, JC–P = 8.6 Hz), 132.5 (d, JC–P = 2.7 Hz), 132.3 (d, JC–P = 4.9 Hz), 131.0, 130.0, 128.6 (d, JC–P = 1.3 Hz), 128.2, 127.0, 122.6, 120.6, 120.0, 111.7, 105.8, 60.3 (d, JC–P = 61.2 Hz). 31P NMR (CDCl3, 162 MHz) δ 36.47. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H25Cl2NOP 552.1051, found 552.1046.
(Bis(4-fluorophenyl)(1H-indol-2-yl)methyl)diphenylphosphine oxide (3fa)
General procedure A was followed using 1f (83.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3fa (105.21 mg) in 81% yield as a white solid (mp 234–236 °C): 1H NMR (400 MHz, CDCl3) δ 10.30 (s, 1H), 7.55–7.43 (m, 4H), 7.33–7.26 (m, 4H), 7.25–7.16 (m, 5H), 7.12 (t, J = 7.5 Hz, 1H), 6.99–6.93 (m, 4H), 6.89–6.83 (m, 4H), 6.20 (d, J = 2.5 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 163.3 (d, JC–F = 247.3 Hz), 136.3, 134.8, 132.9 (d, JC–P = 8.4 Hz), 132.6 (q, JC–F = 5.1 Hz), 132.4 (d, JC–P = 2.7 Hz), 131.1, 130.2, 128.6 (d, JC–P = 11.4 Hz), 127.0 (d, JC–P = 1.6 Hz), 122.5, 120.5, 119.3, 115.1 (d, JC–F = 21.0 Hz), 111.6, 105.7, 60.0 (d, JC–P = 62.0 Hz). 31P NMR (162 MHz, CDCl3) δ 36.63. 19F NMR (376 MHz, CDCl3) δ −113.87. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23F2NOP 518.1485, found 518.1475.
((1H-Indol-2-yl)di-m-tolylmethyl)diphenylphosphine oxide (3ha)
General procedure A was followed using 1h (81.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ha (92.41 mg) in 66% yield as a white solid (mp 193–195 °C): 1H NMR (400 MHz, CDCl3) δ 10.34 (s, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.47–7.42 (m, 3H), 7.29–7.23 (m, 4H), 7.20–7.14 (m, 5H), 7.11 (d, J = 7.6 Hz, 1H), 7.07–7.01 (m, 4H), 6.79 (d, J = 9.1 Hz, 4H), 6.27 (d, J = 2.4 Hz, 1H), 2.10 (s, 6H). 13C{1H}NMR (100 MHz, CDCl3) δ 138.9, 137.2 (d, JC–P = 1.1 Hz), 136.3, 133.1 (d, JC–P = 7.9 Hz), 132.0 (d, JC–P = 2.8 Hz), 131.8 (d, JC–P = 5.1 Hz), 131.7, 130.8, 128.3(d, JC–P = 2.0 Hz), 128.2 (t, JC–P = 2.6 Hz), 127.9, 127.2, 122.1, 120.4, 119.6, 111.6, 105.7 (d, JC–P = 6.7 Hz), 61.1 (d, JC–P = 61.8 Hz), 29.8, 21.6. 31P NMR (162 MHz, CDCl3) δ 36.91. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NOP 512.2143, found 512.2146.
(Bis(3-fluorophenyl)(1H-indol-2-yl)methyl)diphenylphosphine oxide (3ia)
General procedure A was followed using 1i (83.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ia (102.61 mg) in 79% yield as a white solid (mp 240–242 °C): 1H NMR (400 MHz, CDCl3) δ 10.29 (s, 1H), 7.55–7.45 (m, 4H), 7.31 (td, J = 7.7, 3.3 Hz, 4H), 7.25 (s, 1H), 7.21 (d, J = 1.2 Hz, 1H), 7.19 (d, J = 2.8 Hz, 2H), 7.16 (dt, J = 4.6, 2.8 Hz, 2H), 7.14–7.09 (m, 2H), 6.99 (td, J = 8.3, 2.8 Hz, 2H), 6.80 (d, J = 8.0 Hz, 2H), 6.72–6.67 (m, 2H), 6.23 (d, J = 2.4 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 163.5 (d, JC–F = 244.5 Hz), 141.2, 136.3, 133.0 (d, JC–P = 8.6 Hz), 132.5 (d, JC–P = 2.8 Hz), 130.9, 130.0, 129.4 (d, JC–P = 7.5 Hz), 128.6 (d, JC–P = 11.5 Hz), 127.0 (d, JC–P = 1.5 Hz), 126.6 (q, JC–F = 2.9 Hz), 122.6, 120.6, 120.0, 118.3 (d, JC–P = 28.7 Hz), 115.0 (d, JC–F = 22.3 Hz), 111.6, 106.1 (d, JC–P = 6.3 Hz), 60.8 (d, JC–P = 59.6 Hz). 19F NMR (376 MHz, CDCl3) δ −111.75. 31P NMR (162 MHz, CDCl3) δ 36.68. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H25F2NOP 520.1642, found 520.1640.
((1H-Indol-2-yl)bis(3-(trifluoromethyl)phenyl)methyl)-diphenylphosphine oxide (3ja)
General procedure A was followed using 1j (108.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ja (92.41 mg) in 71% yield as a white solid (mp 224–226 °C): 1H NMR (400 MHz, CDCl3) δ 10.52 (s, 1H), 7.57–7.48 (m, 6H), 7.34 (d, J = 3.9 Hz, 4H), 7.33–7.29 (m, 4H), 7.27 (d, J = 7.9 Hz, 1H), 7.23–7.18 (m, 3H), 7.17–7.15 (m, 1H), 7.15–7.10 (m, 3H), 6.19 (d, J = 2.4 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 139.7, 136.4, 134.4 (d, JC–P = 4.3 Hz), 133.0 (d, JC–P = 8.6 Hz), 132.7 (d, JC–P = 2.7 Hz), 130.3, 130.1 (d, JC–P = 7.5 Hz), 129.2, 128.9, 128.8 (d, JC–P = 12.5 Hz), 127.5 (q, JC–F = 4.1 Hz), 127.0 (d, JC–P = 1.4 Hz), 125.0 (d, JC–F = 271.0 Hz), 124.8 (q, JC–F = 1.6 Hz), 122.8, 120.7, 120.1, 111.7, 105.8 (d, JC–P = 6.3 Hz), 61.0 (d, JC–P = 60.2 Hz). 19F NMR (376 MHz, CDCl3) δ −62.81. 31P NMR (162 MHz, CDCl3) δ 36.97. HRMS (ESI-TOF) m/z [M – H]− Calcd for C35H23F6NOP 618.1421, found 618.1421.
((5-Methyl-1H-indol-2-yl)diphenylmethyl)diphenylphosphine oxide (3ka)
General procedure A was followed using 1k (78.35 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ka (120.66 mg) in 97% yield as a white solid (mp 262–264 °C): 1H NMR (400 MHz, CDCl3) δ 10.17 (s, 1H), 7.47–7.42 (m, 2H), 7.34 (d, J = 8.3 Hz, 1H), 7.30–7.26 (m, 5H), 7.24–7.22 (m, 2H), 7.19–7.12 (m, 8H), 7.07–6.99 (m, 5H), 6.27–6.10 (m, 1H), 2.45 (s, 3H). 13C{1H}NMR (100 MHz, CDCl3) δ 139.2, 134.6, 133.1 (d, JC–P = 9.0 Hz), 132.0 (d, JC–P = 2.7 Hz), 131.7, 131.0 (d, JC–P = 5.1 Hz), 130.8, 128.8, 128.3 (d, JC–P = 11.4 Hz), 127.9 (d, JC–P = 0.5 Hz), 127.6 (d, JC–P = 1.6 Hz), 127.4, 123.9, 120.0, 111.2, 105.4 (d, JC–P = 6.6 Hz), 61.3 (d, JC–P = 61.9 Hz), 21.5. 31P NMR (162 MHz, CDCl3) δ 36.65. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C34H29NOP 498.1987, found 498.1990.
((6-Chloro-1H-indol-2-yl)diphenylmethyl)diphenylphosphine oxide (3la)
General procedure A was followed using 1l (83.45 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3la (123.02 mg) in 95% yield as a white solid (mp 249–251 °C): 1H NMR (400 MHz, CDCl3) δ 10.28 (s, 1H), 7.42–7.36 (m, 3H), 7.32 (d, J = 8.5 Hz, 1H), 7.23–7.16 (m, 6H), 7.10 (s, 2H), 7.09–7.04 (m, 6H), 6.99 (dd, J = 8.4, 1.9 Hz, 1H), 6.93–6.89 (m, 4H), 6.18 (d, J = 2.4 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 139.9, 138.8, 136.6, 133.0 (d, JC–P = 8.5 Hz), 132.2 (d, JC–P = 2.8 Hz), 131.4, 131.0 (d, JC–P = 5.1 Hz), 130.5, 128.4 (d, JC–P = 11.5 Hz), 128.0, 127.8 (d, JC–P = 1.5 Hz), 125.6 (d, JC–P = 1.7 Hz), 121.3, 120.5, 111.4, 105.7 (d, JC–P = 11.5 Hz), 61.2 (d, JC–P = 61.6 Hz). 31P NMR (162 MHz, CDCl3) δ 36.74. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H26ClNOP 518.1441, found 518.1440.
((1-Ethyl-1H-indol-2-yl)diphenylmethyl)diphenylphosphine oxide (3ma)
General procedure A was followed using 1m (81.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ma (42.21 mg) in 33% yield as a white solid (mp 247–249 °C): 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.8 Hz, 1H), 7.30–7.21 (m, 7H), 7.19–7.11 (m, 6H), 7.07 (td, J = 7.8, 3.2 Hz, 4H), 7.01 (d, J = 4.0 Hz, 2H), 6.97–6.90 (m, 1H), 6.87–6.79 (m, 4H), 3.36 (q, J = 7.1 Hz, 2H), 1.14 (s, 3H). 13C{1H}NMR (100 MHz, CDCl3) δ 138.1 (d, JC–P = 3.3 Hz), 136.4, 136.1, 133.7 (d, JC–P = 7.9 Hz), 131.9 (d, JC–P = 4.0 Hz), 131.6 (d, JC–P = 1.6 Hz), 131.0, 130.0, 128.0 (d, JC–P = 3.0 Hz), 127.7 (d, JC–P = 11.3 Hz), 127.4, 121.6, 121.1, 119.2, 109.5, 105.8 (d, JC–P = 4.7 Hz), 60.5 (d, JC–P = 59.8 Hz), 40.7, 12.1. 31P NMR (162 MHz, CDCl3) δ 33.81. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NOP 512.2143, found 512.2150.
(3-(1H-indol-2-yl)pentan-3-yl)diphenylphosphine oxide (3na)
General procedure A was followed using 1n (50.82 mg, 0.25 mmol), 2a(75.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3na (20.34 mg) in 21% yield as a white solid (mp 202–204 °C): 1H NMR (400 MHz, CDCl3) δ 11.28 (s, 1H), 7.98–7.89 (m, 2H), 7.88–7.81 (m, 2H), 7.58–7.42 (m, 8H), 7.15–7.08 (m, 1H), 7.06–6.99 (m, 1H), 6.25 (dt, J = 2.1, 1.0 Hz, 1H), 3.19–3.09 (m, 1H), 2.79–2.67 (m, 1H), 2.25–2.07 (m, 1H), 1.80–1.59 (m, 1H), 1.03–0.82 (m, 6H). 13C{1H}NMR (100 MHz, CDCl3) δ 139.3, 136.4, 132.2, 132.0 (d, JC–P = 9.4 Hz), 131.1 (d, JC–P = 8.5 Hz), 129.0 (d, JC–P = 3.5 Hz), 128.0, 120.9, 119.5, 118.9, 111.4, 100.1, 35.2 (d, JC–P = 75.1 Hz), 25.3, 12.6. 31P NMR (162 MHz, CDCl3) δ 39.30. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C25H27NOP 388.1830, found 388.1833.
((1H-Indol-2-yl)diphenylmethyl)di-p-tolylphosphine oxide (3ab)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2b (86.34 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ab (124.06 mg) in 97% yield as a white solid (mp 225–227 °C): 1H NMR (400 MHz, CDCl3) δ 10.35 (s, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.28–7.25 (m, 2H), 7.23–7.19 (m, 2H), 7.18–7.11 (m, 5H), 7.09–7.03 (m, 7H), 7.00 (d, J = 8.5 Hz, 4H), 6.28 (t, J = 2.4 Hz, 1H), 2.32 (s, 6H). 13C{1H}NMR (100 MHz, CDCl3) δ 142.5 (d, JC–P = 3.8 Hz), 139.3, 136.3, 133.1 (d, JC–P = 9.0 Hz), 131.1, 129.1 (d, JC–P = 11.8 Hz), 128.5, 127.9, 127.5 (d, JC–P = 1.3 Hz), 127.1 (d, JC–P = 7.7 Hz), 126.9, 122.1, 120.4, 119.6, 111.6, 105.7, 61.2 (d, JC–P = 61.9 Hz), 21.6. 31P NMR (162 MHz, CDCl3) δ 37.19. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NOP 512.2143, found 512.2147.
((1H-Indol-2-yl)diphenylmethyl)bis(4-methoxyphenyl)phosphine oxide (3ac)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2c (98.34 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ac (77.46 mg) in 57% yield as a white solid (mp 237–239 °C): 1H NMR (400 MHz, CDCl3) δ 10.37 (s, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.28–7.22 (m, 3H), 7.19–7.12 (m, 5H), 7.08–6.99 (m, 8H), 6.76 (dd, J = 8.9, 2.6 Hz, 4H), 6.29 (d, J = 2.4 Hz, 1H), 3.78 (s, 6H). 13C{1H}NMR (100 MHz, CDCl3) δ 162.4 (d, JC–P = 2.9 Hz), 139.5, 136.2, 134.9 (d, JC–P = 9.7 Hz), 131.0 (d, JC–P = 5.0 Hz), 127.9, 127.5, 127.2 (d, JC–P = 1.6 Hz), 122.9, 122.1, 121.9, 120.4, 119.6, 113.9 (d, JC–P = 12.4 Hz), 111.6, 105.6, 61.1 (d, JC–P = 62.8 Hz), 55.3. 31P NMR (162 MHz, CDCl3) δ 36.92. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NO3P 544.2042, found 544.2052.
((1H-Indol-2-yl)diphenylmethyl)bis(4-(tert-butyl)phenyl)-phosphine oxide (3ad)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2d (117.90 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ad (92.34 mg) in 62% yield as a white solid (mp 226–228 °C): 1H NMR (400 MHz, DMSO-d6) δ 10.65 (s, 1H), 7.54 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 7.9 Hz, 1H), 7.40–7.30 (m, 6H), 7.25 (t, J = 7.5 Hz, 5H), 7.12–7.03 (m, 5H), 7.02–6.95 (m, 4H), 6.29 (s, 1H), 1.23 (s, 18H). 13C{1H}NMR (100 MHz, DMSO-d6 with CDCl3) δ 138.8, 137.1 (d, JC–P = 1.1 Hz), 136.2, 133.0 (d, JC–P = 7.9 Hz), 132.0 (d, JC–P = 2.8 Hz), 131.7 (d, JC–P = 5.1 Hz), 130.8, 128.2 (d, JC–P = 2.0 Hz), 128.1 (d, JC–P = 2.6 Hz), 127.8, 127.1, 122.1, 120.3, 119.6, 111.5, 105.7 (d, JC–P = 6.7 Hz), 61.1 (d, JC–P = 61.8 Hz), 29.8, 21.5. 31P NMR (162 MHz, DMSO-d6) δ 33.59. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C41H43NOP 596.3082, found 596.3084.
((1H-Indol-2-yl)diphenylmethyl)bis(4-(dimethylamino)phenyl)-phosphine oxide (3ae)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2e (108.12 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ae (72.64 mg) in 51% yield as a white solid (mp 168–170 °C): 1H NMR (400 MHz, CDCl3) δ 10.61 (s, 1H), 7.52 (d, J = 7.7 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.24–7.19 (m, 3H), 7.17–7.10 (m, 5H), 7.08–7.01 (m, 4H), 6.97–6.89 (m, 4H), 6.47 (dd, J = 9.1, 2.6 Hz, 4H), 6.25 (t, J = 2.4 Hz, 1H), 2.94 (s, 12H). 13C{1H}NMR (100 MHz, CDCl3) δ 152.0 (d, JC–P = 2.4 Hz), 140.3, 136.1, 134.3 (d, JC–P = 9.8 Hz), 131.1 (d, JC–P = 6.0 Hz), 127.6, 127.3, 127.1, 121.7, 120.3, 119.3, 116.8, 115.8, 111.6, 111.0 (d, JC–P = 12.2 Hz), 105.2, 61.1 (d, JC–P = 62.8 Hz), 39.9. 31P NMR (162 MHz, CDCl3) δ 38.42. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C37H37N3OP 570.2674, found 570.2681.
((1H-Indol-2-yl)diphenylmethyl)bis(4-fluorophenyl)phosphine oxide (3af)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2f (89.32 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3af (119.49 mg) in 92% yield as a white solid (mp 290–292 °C): 1H NMR (400 MHz, CDCl3) δ 10.02 (s, 1H), 7.53 (d, J = 7.9 Hz, 1H), 7.42 (d, J = 8.1 Hz, 1H), 7.31–7.27 (m, 2H), 7.25–7.21 (m, 5H), 7.19 (t, J = 7.7 Hz, 4H), 7.15–7.10 (m, 1H), 7.14–7.08 (m, 8H), 6.31 (t, J = 2.4 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 163.1 (d, JC–F = 247.3 Hz), 138.7, 136.3, 134.8, 133.0 (d, JC–P = 8.4 Hz), 132.6 (q, JC–F = 5.0 Hz), 131.1, 130.2, 128.6 (d, JC–P = 11.5 Hz), 127.0 (d, JC–P = 1.6 Hz), 122.5, 120.5, 119.9, 115.1 (d, JC–F = 20.9 Hz), 111.6, 105.7, 60.0 (d, JC–P = 62.0 Hz). 19F NMR (376 MHz, CDCl3) δ −113.88. 31P NMR (162 MHz, CDCl3) δ 36.63. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23F2NOP 518.1485, found 518.1481.
((1H-Indol-2-yl)diphenylmethyl)bis(4-chlorophenyl)phosphine oxide (3ag)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2g (101.65 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ag (129.82 mg) in 94% yield as a white solid (mp 277–279 °C): 1H NMR (400 MHz, CDCl3) δ 10.03 (s, 1H), 7.53 (d, J = 7.9 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.32–7.23 (m, 7H), 7.19 (t, J = 7.7 Hz, 4H), 7.14–7.09 (m, 1H), 7.05–6.98 (m, 8H), 6.33 (t, J = 2.4 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 139.0 (d, JC–P = 3.5 Hz), 138.7, 136.2 (d, JC–P = 9.2 Hz), 134.4, 130.9 (d, JC–P = 6.3 Hz), 129.8, 128.9, 128.8 (d, JC–P = 12.0 Hz), 128.2, 128.0 (d, JC–P = 1.5 Hz), 127.0 (d, JC–P = 1.7 Hz), 122.5, 120.6, 120.0, 111.5, 106.1 (d, JC–P = 6.6 Hz), 61.3 (d, JC–P = 63.4 Hz). 31P NMR (162 MHz, CDCl3) δ 35.49. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23Cl2NOP 550.0894, found 550.0888.
((1H-Indol-2-yl)diphenylmethyl)bis(3-fluorophenyl)phosphine oxide (3ah)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2h (89.32 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ah (92.22 mg) in 71% yield as a white solid (mp 259–261 °C): 1H NMR (400 MHz, CDCl3) δ 10.01 (s, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.45 (d, J = 8.2 Hz, 1H), 7.34–7.27 (m, 3H), 7.24–7.16 (m, 8H), 7.12 (t, J = 7.4 Hz, 1H), 7.03 (d, J = 7.9 Hz, 4H), 6.99–6.91 (m, 2H), 6.82–6.74 (m, 2H), 6.33 (d, J = 2.6 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 163.4 (d, JC–F = 248.6 Hz), 138.5, 137.9, 136.3, 133.9, 130.9 (d, JC–P = 5.3 Hz), 130.4 (q, JC–F = 7.2 Hz), 128.8 (q, JC–F = 3.2 Hz), 128.2, 128.0 (d, JC–P = 1.5 Hz), 127.0 (d, JC–P = 1.7 Hz), 122.6, 120.5, 120.1 (d, JC–P = 9.6 Hz), 119.6 (d, JC–P = 2.5 Hz), 119.4 (d, JC–P = 2.5 Hz), 111.5, 106.1 (d, JC–P = 6.7 Hz), 61.4 (d, JC–P = 63.4 Hz). 31P NMR (162 MHz, CDCl3) δ 34.54. 19F NMR (376 MHz, CDCl3) δ −110.55. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23F2NOP 518.1485, found 518.1484.
((1H-Indol-2-yl)diphenylmethyl)bis(3-chlorophenyl)phosphine oxide (3ai)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2i (101.65 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3ai (133.97 mg) in 97% yield as a white solid (mp 216–218 °C):1H NMR (400 MHz, CDCl3) δ 9.89 (s, 1H), 7.53 (d, J = 7.9 Hz, 1H), 7.47–7.41 (m, 3H), 7.32 (t, J = 7.4 Hz, 2H), 7.27–7.21 (m, 6H), 7.20 (s, 1H), 7.12 (t, J = 7.5 Hz, 1H), 7.08–7.00 (m, 6H), 6.95 (dt, J = 10.7, 1.8 Hz, 2H), 6.37 (d, J = 2.6 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 138.4, 137.6, 136.3, 134.8 (d, JC–P = 14.8 Hz), 133.4, 132.9 (d, JC–P = 9.3 Hz), 132.5, 132.4 (d, JC–P = 2.7 Hz), 131.0 (t, JC–P = 8.7 Hz), 129.8 (d, JC–P = 12.4 Hz), 128.2, 128.1 (d, JC–P = 1.7 Hz), 127.0 (d, JC–P = 1.9 Hz), 122.6, 120.5, 120.0, 111.5, 106.2 (d, JC–P = 6.6 Hz), 61.5 (d, JC–P = 63.0 Hz). 31P NMR (162 MHz, CDCl3) δ 34.53. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23Cl2NOP 550.0894, found 550.0886.
((1H-Indol-2-yl)diphenylmethyl)bis(3-(trifluoromethyl)phenyl)-phosphine oxide (3aj)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2j (126.82 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3aj (113.07 mg) in 73% yield as a white solid (mp 220–222 °C): 1H NMR (400 MHz, CDCl3) δ 9.82 (s, 1H), 7.74 (dd, J = 5.3, 3.5 Hz, 2H), 7.52 (d, J = 7.9 Hz, 1H), 7.49–7.42 (m, 5H), 7.33 (td, J = 7.4, 1.3 Hz, 2H), 7.26–7.19 (m, 7H), 7.12 (t, J = 7.5 Hz, 1H), 7.02 (d, J = 7.8 Hz, 4H), 6.38 (t, J = 2.5 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 138.1, 136.3 (m), 132.3, 131.4, 131.0, 130.9, 130.8 (d, JC–P = 5.4 Hz), 130.6, 129.9 (q, JC–F = 3.8 Hz), 129.1 (q, JC–F = 11.3 Hz), 128.4 (d, JC–P = 0.6 Hz), 128.3 (d, JC–P = 1.6 Hz), 127.0 (d, JC–P = 1.9 Hz), 124.6 (q, JC–F = 271.4 Hz), 122.8, 120.5, 120.1, 111.4, 106.2 (d, JC–P = 7.0 Hz), 61.5 (d, JC–P = 63.6 Hz). 31P NMR (162 MHz, CDCl3) δ 34.01. 19F NMR (376 MHz, CDCl3) δ −63.12. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H25F6NOP 620.1578, found 620.1578.
((1H-Indol-2-yl)diphenylmethyl)di(thiophen-2-yl)phosphine oxide (3am)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2m (80.34 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3am (116.46 mg) in 94% yield as a white solid (mp 303–305 °C): 1H NMR (400 MHz, CDCl3) δ 10.08 (s, 1H), 7.63 (t, J = 4.5 Hz, 2H), 7.56 (d, J = 7.8 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.32–7.25 (m, 3H), 7.21 (t, J = 7.8 Hz, 4H), 7.17–7.12 (m, 1H), 7.11–6.99 (m, 6H), 6.89–6.81 (m, 2H), 6.30 (s, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 138.5, 138.1 (d, JC–P = 9.1 Hz), 136.3, 135.5 (d, JC–P = 4.3 Hz), 132.7, 131.6, 130.8 (d, JC–P = 5.3 Hz), 128.2 (m), 128.1, 128.0 (d, JC–P = 1.6 Hz), 127.4 (d, JC–P = 1.5 Hz), 122.5, 120.6, 120.0, 111.7, 106.5, 61.5 (d, JC–P = 72.0 Hz). 31P NMR (162 MHz, CDCl3) δ 29.18. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C29H23NOPS2 496.0959, found 496.0958.
((1H-Indol-2-yl)diphenylmethyl)(phenyl)(p-tolyl)phosphine oxide (3an)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2n (81.08 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexane) afforded 3an (83.34 mg) in 67% yield as a white solid (mp 164–166 °C): 1H NMR (400 MHz, CDCl3) δ 10.29 (s, 1H), 7.51 (d, J = 7.9 Hz, 1H), 7.49–7.39 (m, 2H), 7.30–7.23 (m, 2H), 7.26–7.19 (m, 3H), 7.15 (td, J = 8.0, 4.6 Hz, 7H), 7.09–6.96 (m, 8H), 6.27 (t, J = 2.4 Hz, 1H), 2.33 (s, 3H). 13C{1H}NMR (100 MHz, CDCl3) δ 142.6 (d, JC–P = 2.9 Hz), 139.3 (d, JC–P = 11.9 Hz), 136.3, 133.1, 133.0 (d, JC–P = 2.6 Hz), 131.9 (d, JC–P = 3.2 Hz), 131.1 (d, JC–P = 3.6 Hz), 131.0 (d, JC–P = 3.5 Hz), 129.2 (d, JC–P = 11.8 Hz), 128.3 (d, JC–P = 8.4 Hz), 128.0, 127.9 (d, JC–P = 1.7 Hz), 127.6, 127.3, 127.1 (d, JC–P = 1.6 Hz), 122.2, 120.4, 119.6, 111.5, 105.7 (d, JC–P = 4.8 Hz), 61.2 (d, JC–P = 64.9 Hz), 21.5. 31P NMR (162 MHz, CDCl3) δ 36.93. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C34H29NOP 498.1987, found 498.1992.
Ethyl ((1H-indol-2-yl)diphenylmethyl)(phenyl)phosphinate (3ao)
General procedure A was followed using 1a (74.84 mg, 0.25 mmol), 2o (63.81 mg, 0.375 mmol) and Y(Pfb)3 (36.10 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (20% EtOAc/hexanes) afforded 3ao (48.54 mg) in 43% yield as a white solid (mp 200–202 °C): 1H NMR (400 MHz, CDCl3) δ 9.91 (s, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.49–7.45 (dt, J = 8.0, 1.6 Hz, 2H), 7.45–7.41 (m, 1H), 7.42–7.38 (m, 1H), 7.30–7.26 (m, 2H), 7.25–7.19 (m, 6H), 7.19–7.15 (m, 5H), 7.10–7.04 (m, 1H), 6.30 (dt, J = 2.8, 1.4 Hz, 1H), 4.18–3.85 (m, 2H), 1.20 (t, J = 7.0 Hz, 3H). 13C{1H}NMR (100 MHz, CDCl3) δ 140.0 (d, JC–P = 4.4 Hz), 138.7, 136.1 (d, JC–P = 7.2 Hz), 133.6 (d, JC–P = 14.2 Hz), 132.5 (d, JC–P = 2.7 Hz), 130.9 (d, JC–P = 5.2 Hz), 130.0, 128.8, 128.1 (d, JC–P = 12.5 Hz), 127.8, 127.4 (d, JC–P = 1.8 Hz), 121.9, 120.4, 119.6, 111.2, 104.8 (d, JC–P = 7.2 Hz), 62.2 (d, JC–P = 7.2 Hz), 60.2 (d, JC–P = 89.9 Hz), 16.4. 31P NMR (162 MHz, CDCl3) δ 42.47. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C29H27NO2P 452.1779, found 452.1779.
General Procedure B
2-Indolylmethanols 1 (0.25 mmol, 1 equiv), Diarylphosphine Oxides 2 (0.375 mmol, 1.5 equiv), Yb(OTf)3 (31.01 mg, 0.05 mmol), dry CH3CN (2 mL) and a stir bar were added to a sealed tube. After being stirred at 100 °C for indicated time, the mixture was evaporated under vacuum. The corresponding product 4 was isolated by silica gel column chromatography with a dichloro-methane/ethyl acetate mixture as eluent.
(2-Benzhydryl-1H-indol-3-yl)diphenylphosphine oxide (4aa)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4aa (91.87 mg) in 76% yield as a white solid (mp 212–214 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.70 (s, 1H), 7.63–7.55 (m, 7H), 7.49 (dd, J = 7.5, 2.6 Hz, 4H), 7.41 (d, J = 8.3 Hz, 1H), 7.31–7.26 (m, 4H), 7.23 (d, J = 7.1 Hz, 1H), 7.16–7.11 (m, 4H), 7.08–7.04 (m, 1H), 6.84–6.78 (m, 1H), 6.75 (s, 1H), 6.54 (d, J = 8.1 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.0 (d, JC–P = 16.8 Hz), 148.6, 138.8, 136.9 (d, JC–P = 6.5 Hz), 135.3, 134.2, 131.6 (d, JC–P = 2.4 Hz), 131.2 (d, JC–P = 10.1 Hz), 128.5 (d, JC–P = 11.8 Hz), 128.3, 125.0, 121.6, 120.1, 119.5, 111.9, 100.6 (d, JC–P = 124.8 Hz), 54.8. 31P NMR (162 MHz, DMSO-d6) δ 21.95. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H27NOP 484.1830, found 484.1831.
2-(Di-p-tolylmethyl)-1H-indol-3-yl)diphenylphosphine oxide (4ba)
General procedure B was followed using 1b (81.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ba (52.43 mg) in 41% yield as a white solid (mp 244–246 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.60 (d, J = 2.9 Hz, 1H), 7.62–7.59 (m, J = 1.2 Hz, 1H), 7.59–7.54 (m, J = 8.3 Hz, 5H), 7.49–7.43 (m, J = 7.4 Hz, 4H), 7.42–7.38 (m, 1H), 7.10–7.03 (m, 5H), 6.99 (d, J = 8.1 Hz, 4H), 6.82–6.77 (m, J = 8.1 Hz, 1H), 6.61 (s, 1H), 6.55 (d, J = 8.1 Hz, 1H), 2.25 (s, 6H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.2 (d, JC–P = 17.0 Hz), 138.8, 136.9 (d, JC–P = 11.4 Hz), 135.5, 135.1, 134.0, 131.7 (d, JC–P = 2.8 Hz), 131.2 (d, JC–P = 10.2 Hz), 128.8, 128.5 (t, JC–P = 7.5 Hz), 128.2 (d, JC–P = 12.0 Hz), 121.7, 120.2, 119.6, 112.0, 100.5 (d, JC–P = 125.1 Hz), 46.3, 20.5. 31P NMR (DMSO-d6, 162 MHz) δ 22.25. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NOP 512.2143, found 512.2140.
(2-(Bis (4-methoxyphenyl) methyl)-1H-indol-3-yl)-diphenylphosphine oxide (4ca)
General procedure B was followed using 1c (89.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ca (77.46 mg) in 57% yield as a white solid (mp 177–179 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.55 (d, J = 2.9 Hz, 1H), 7.54–7.47 (m, 7H), 7.41–7.37 (m, 4H), 7.34 (dd, J = 8.1, 1.6 Hz, 1H), 6.98–6.92 (m, 5H), 6.78–6.71 (m, 4H), 6.47 (d, J = 8.1 Hz, 1H), 6.42 (s, 1H), 3.63 (s, 6H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 157.7, 136.9 (d, JC–P = 11.5 Hz), 135.1, 134.1, 133.9, 131.7 (d, JC–P = 2.3 Hz), 131.2 (d, JC–P = 10.1 Hz), 130.3 (d, JC–P = 11.4 Hz), 129.6, 129.0 (d, JC–P = 12.4 Hz), 128.6 (d, JC–P = 47.8 Hz), 121.7, 120.2 (d, JC–P = 61.1 Hz), 113.7, 112.0, 100.3 (d, JC–P = 125.1 Hz), 55.0, 45.5. 31P NMR (DMSO-d6, 162 MHz) δ 22.05. HRMS (ESI-TOF) m/z [M + Na]+ Calcd for C35H30NNaO3P 566.1861, found 566.1869.
(2-(Bis (4-(tert-butyl) phenyl) methyl)-1H-indol-3-yl)-diphenylphosphine oxide (4da)
General procedure B was followed using 1d (102.90 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4da (117.71 mg) in 75% yield as a white solid (mp 247–249 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.69 (d, J = 2.9 Hz, 1H), 7.62–7.55 (m, 6H), 7.49–7.44 (m, 4H), 7.43–7.39 (m, 1H), 7.31–7.26 (m, 4H), 7.10–7.03 (m, 5H), 6.82–6.77 (m, 1H), 6.66 (s, 1H), 6.55 (d, J = 8.1 Hz, 1H), 1.24 (s, 18H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.3 (d, JC–P = 16.9 Hz), 148.6, 138.9, 137.0 (d, JC–P = 11.5 Hz), 135.3, 134.3, 131.6, 131.2 (d, JC–P = 10.2 Hz), 128.6 (d, JC–P = 11.8 Hz), 128.3, 125.0, 121.6, 120.1, 119.6, 112.0, 100.7 (d, JC–P = 124.8 Hz), 54.9, 34.1, 31.1. 31P NMR (162 MHz, DMSO-d6) δ 22.01. HRMS (ESI-TOF) m/z [M + H]+Calcd for C41H43NOP 596.3082, found 596.3092.
(2-(Bis(4-chlorophenyl)methyl)-1H-indol-3-yl)diphenylphosphine oxide (4ea)
General procedure B was followed using 1e (92.06 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ea (59.39 mg) in 43% yield as a white solid (mp 244–246 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.73 (d, J = 2.9 Hz, 1H), 7.60–7.53 (m, 6H), 7.49–7.44 (m, 4H), 7.43–7.39 (m, 1H), 7.37–7.32 (m, 4H), 7.14–7.05 (m, 5H), 6.84–6.79 (m, 1H), 6.76 (s, 1H), 6.49 (d, J = 8.1 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 148.8 (d, JC–P = 16.8 Hz), 140.1, 136.9 (d, JC–P = 11.3 Hz), 134.7, 133.7, 131.8, 131.4, 131.1 (d, JC–P = 10.3 Hz), 130.5, 128.6 (d, JC–P = 12.6 Hz), 128.4, 122.0, 120.5, 119.5, 112.1, 101.1 (d, JC–P = 124.3 Hz), 45.9. 31P NMR (162 MHz, DMSO-d6) δ 22.40. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23Cl2NOP 550.0894, found 550.0908.
(2-(Bis(4-fluorophenyl)methyl)-1H-indol-3-yl)diphenylphosphine oxide (4fa)
General procedure B was followed using 1f (83.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4fa (66.24 mg) in 51% yield as a white solid (mp 272–274 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.76 (d, J = 2.8 Hz, 1H), 7.56–7.48 (m, 6H), 7.44–7.38 (m, 4H), 7.37–7.34 (m, 1H), 7.32–7.23 (m, 2H), 7.05–6.98 (m, 3H), 6.95–6.90 (m, 2H), 6.85–6.78 (m, 3H), 6.79–6.72 (m, 1H), 6.42 (d, J = 8.1 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 163.3 (d, JC–F = 242.3 Hz), 143.7 (d, JC–P = 6.8 Hz), 134.9, 133.8, 131.8, 131.2 (d, JC–P = 10.2 Hz), 130.5 (d, JC–P = 8.3 Hz), 128.7 (d, JC–P = 11.8 Hz), 124.8, 122.1, 120.5, 119.6, 115.4 (d, JC–F = 21.9 Hz), 113.8 (d, JC–F = 20.7 Hz), 112.1, 101.5 (d, JC–P = 123.5 Hz), 46.5. 19F NMR (376 MHz, DMSO-d6) δ −112.76. 31P NMR (162 MHz, DMSO-d6) δ 22.13. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23F2NOP 518.1485, found 518.1479.
(2-(Di-o-tolylmethyl)-1H-indol-3-yl)diphenylphosphine oxide (4ga)
General procedure B was followed using 1g (81.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ga (76.74 mg) in 60% yield as a white solid (mp 193–195 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.11 (d, J = 2.9 Hz, 1H), 7.53–7.44 (m, 6H), 7.43 (d, J = 1.6 Hz, 1H), 7.41–7.34 (m, 4H), 7.15–7.11 (m, 4H), 7.08–7.01 (m, 3H), 6.99 (s, 1H), 6.85–6.79 (m, 1H), 6.72 (d, J = 7.5 Hz, 2H), 6.59 (d, J = 8.0 Hz, 1H), 2.09 (s, 6H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 170.3, 149.2 (d, JC–P = 16.7 Hz), 140.1, 136.5 (d, JC–P = 11.4 Hz), 136.3, 135.0, 134.0, 131.4 (d, JC–P = 2.4 Hz), 131.0 (d, JC–P = 10.1 Hz), 130.1, 128.4 (m), 126.6, 125.8, 121.4, 120.3, 119.5, 112.3, 99.5 (d, JC–P = 124.8 Hz), 42.5, 19.1. 31P NMR (162 MHz, DMSO-d6) δ 21.82. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NOP 512.2143, found 512.2133.
(2-(Di-m-tolylmethyl)-1H-indol-3-yl)diphenylphosphine oxide (4ha)
General procedure B was followed using 1h (81.86 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ha (79.30 mg) in 62% yield as a white solid (mp 275–277 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.57 (d, J = 3.0 Hz, 1H), 7.55–7.48 (m, 6H), 7.44–7.38 (m, 4H), 7.34 (d, J = 8.2 Hz, 1H), 7.10 (t, J = 7.6 Hz, 2H), 7.01–6.93 (m, 3H), 6.88 (d, J = 7.8 Hz, 2H), 6.81 (s, 2H), 6.73 (t, J = 7.6 Hz, 1H), 6.58 (s, 1H), 6.45 (d, J = 8.2 Hz, 1H), 2.12 (s, 6H). 13C{1H}NMR (100 MHz, DMSO-d6 with CDCl3) δ 149.9 (d, JC–P = 16.7 Hz), 141.6, 137.1, 136.9 (d, JC–P = 11.8 Hz), 135.1, 134.0, 131.4, 131.2 (d, JC–P = 10.2 Hz), 129.4, 128.2 (d, JC–P = 11.9 Hz), 127.9, 126.9, 125.7, 121.4, 120.0, 119.6, 111.9, 99.2 (d, JC–P = 125.5 Hz), 47.0, 21.1. 31P NMR (162 MHz, DMSO-d6) δ 22.10. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NOP 512.2143, found 512.2142.
(2-(Bis(3-fluorophenyl)methyl)-1H-indol-3-yl)diphenylphosphine oxide (4ia)
General procedure B was followed using 1i (83.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ia (102.61 mg) in 79% yield as a white solid (mp 329–331 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.82 (s, 1H), 7.59 (dd, J = 12.5, 7.3 Hz, 7H), 7.53–7.46 (m, 4H), 7.43 (d, J = 8.1 Hz, 1H), 7.36 (q, J = 7.5 Hz, 2H), 7.09 (t, J = 7.9 Hz, 3H), 7.00 (d, J = 7.8 Hz, 2H), 6.93–6.81 (m, 3H), 6.50 (d, J = 8.1 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 163.3 (d, JC–F = 242.3 Hz), 148.2 (d, JC–P = 16.6 Hz), 143.7 (d, JC–P = 6.5 Hz), 137.0 (d, JC–P = 12.6 Hz), 134.9, 133.8, 131.8 (q, JC–F = 5.5 Hz), 131.2 (d, JC–P = 10.2 Hz), 130.5 (d, JC–P = 8.3 Hz), 128.7 (d, JC–P = 11.6 Hz), 127.9, 124.8, 122.1, 120.5, 119.6, 115.4 (d, JC–F = 21.8 Hz), 113.8 (d, JC–F = 17.6 Hz), 112.1, 101.5 (d, JC–P = 123.4 Hz). 19F NMR (376 MHz, DMSO-d6) δ −108.01. 31P NMR (162 MHz, DMSO-d6) δ 22.12. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H25F2NOP 520.1642, found 520.1641.
(2-(Bis(3-(trifluoromethyl)phenyl)methyl)-1H-indol-3-yl)-diphenylphosphine oxide (4ja)
General procedure B was followed using 1j (108.84 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ja (109.97 mg) in 71% yield as a white solid (mp 275–277 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.63 (d, J = 2.9 Hz, 1H), 7.54 (d, J = 1.3 Hz, 1H), 7.52–7.47 (m, 5H), 7.43–7.36 (m, 4H), 7.35–7.32 (m, 1H), 7.23–7.19 (m, 4H), 7.02–6.97 (m, 5H), 6.76–6.67 (m, 1H), 6.58 (s, 1H), 6.46 (d, J = 8.0 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.3 (d, JC–P = 1.8 Hz), 150.1, 148.6, 138.9, 136.9, 136.8, 135.2, 134.2, 131.7 (q, JC–F = 1.9 Hz), 131.2 (d, JC–P = 10.2 Hz), 128.6 (d, JC–P = 11.8 Hz), 128.3, 128.0 (d, JC–F = 2.5 Hz), 125.1, 121.7 (d, JC–F = 210.1 Hz), 120.2, 119.6, 112.0, 100.6 (d, JC–P = 124.9 Hz), 45.1. 31P NMR (162 MHz, DMSO-d6) δ 22.10. 19F NMR (376 MHz, DMSO-d6) δ −62.66. HRMS (ESI-TOF) m/z [M – H]− Calcd for C35H23F6NOP 618.1421, found 618.1431.
(2-Benzhydryl-5-methyl-1H-indol-3-yl)diphenylphosphine oxide (4ka)
General procedure B was followed using 1k (78.35 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ka (103.25 mg) in 83% yield as a white solid (mp 276–278 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.61 (s, 1H), 7.65–7.52 (m, 6H), 7.52–7.43 (m, 4H), 7.34–7.17 (m, 7H), 7.12 (d, J = 7.5 Hz, 4H), 6.94–6.85 (m, 1H), 6.64 (s, 1H), 6.31 (s, 1H), 2.07 (s, 3H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 149.4 (d, JC–P = 17.0 Hz), 141.7, 135.2 (d, JC–P = 1.5 Hz), 135.1, 134.2, 131.6 (d, JC–P = 1.8 Hz), 131.2 (d, JC–P = 10.1 Hz), 128.7, 128.6, 128.5, 128.3, 126.5, 123.2, 119.4, 111.7, 100.5 (d, JC–P = 94.9 Hz), 47.1, 21.3. 31P NMR (162 MHz, DMSO-d6) δ 22.17. HRMS (ESI-TOF) m/z [M – H]− Calcd for C34H27NOP 496.1830, found 496.1825.
(2-Benzhydryl-6-chloro-1H-indol-3-yl)diphenylphosphine oxide (4la)
General procedure B was followed using 1l (83.45 mg, 0.25 mmol), 2a (75.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4la (67.34 mg) in 52% yield as a white solid (mp 287–289 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.82 (d, J = 2.9 Hz, 1H), 7.62–7.55 (m, 6H), 7.51–7.44 (m, 4H), 7.43 (d, J = 2.0 Hz, 1H), 7.31–7.25 (m, 4H), 7.25–7.20 (m, 2H), 7.14–7.09 (m, 4H), 6.87 (dd, J = 8.6, 2.1 Hz, 1H), 6.67 (s, 1H), 6.55 (d, J = 8.7 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.6 (d, JC–P = 16.5 Hz), 141.3, 137.4 (d, JC–P = 11.4 Hz), 134.8, 133.7, 131.8 (d, JC–P = 2.4 Hz), 131.2 (d, JC–P = 10.2 Hz), 128.7 (d, JC–P = 11.8 Hz), 128.6, 128.4, 127.0 (d, JC–P = 11.8 Hz), 126.6, 121.0, 120.6, 111.6, 101.5 (d, JC–P = 123.3 Hz), 47.0. 31P NMR (162 MHz, DMSO-d6) δ 21.55. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H26ClNOP 518.1441, found 518.1440.
(2-Benzhydryl-1H-indol-3-yl)di-p-tolylphosphine oxide (4ab)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2b (86.34 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ab (92.09 mg) in 72% yield as a white solid (mp 225–227 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.66 (d, J = 2.9 Hz, 1H), 7.50–7.43 (m, 4H), 7.42–7.39 (m, 1H), 7.30 (t, J = 1.4 Hz, 1H), 7.29–7.26 (m, 7H), 7.24 (d, J = 1.4 Hz, 1H), 7.22 (s, 1H), 7.17–7.12 (m, 4H), 7.07–7.01 (m, 1H), 6.84–6.79 (m, 2H), 6.62 (d, J = 8.1 Hz, 1H), 2.34 (s, 6H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 149.5 (d, JC–P = 16.7 Hz), 141.8, 141.5 (d, JC–P = 2.7 Hz), 136.9 (d, JC–P = 10.8 Hz), 132.3, 131.2 (d, JC–P = 10.5 Hz), 129.1 (d, JC–P = 12.1 Hz), 128.7, 128.3, 128.1, 126.4, 121.7 (d, JC–P = 11.9 Hz), 120.1, 119.8, 111.9, 101.2 (d, JC–P = 124.0 Hz), 46.9, 21.1. 31P NMR (162 MHz, DMSO-d6) δ 22.03. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NOP 512.2143, found 512.2142.
(2-Benzhydryl-1H-indol-3-yl)bis(4-methoxyphenyl)phosphine oxide (4ac)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2c (98.34 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ac (44.85 mg) in 33% yield as a white solid (mp 299–301 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.64 (d, J = 2.8 Hz, 1H), 7.53–7.45 (m, 4H), 7.43–7.38 (m, 1H), 7.32–7.26 (m, 4H), 7.25–7.19 (m, 2H), 7.17–7.13 (m, 4H), 7.06–6.98 (m, 5H), 6.86–6.80 (m, 2H), 6.62 (d, J = 8.1 Hz, 1H), 3.78 (s, 6H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 161.6 (d, JC–P = 2.6 Hz), 149.3 (d, JC–P = 16.3 Hz), 141.9, 136.9 (d, JC–P = 11.5 Hz), 133.0 (d, JC–P = 11.6 Hz), 128.7 (d, JC–P = 38.2 Hz), 128.1 (d, JC–P = 11.6 Hz), 126.9, 126.4, 125.8, 121.6, 120.1, 119.8, 114.1 (d, JC–P = 12.8 Hz), 111.9, 101.7 (d, JC–P = 124.6 Hz), 55.3, 46.8. 31P NMR (162 MHz, DMSO-d6) δ 21.23. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H31NO3P 544.2042, found 544.2043.
(2-Benzhydryl-1H-indol-3-yl)bis(4-(tert-butyl)phenyl)phosphine oxide (4ad)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2d (117.90 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ad (110.22 mg) in 74% yield as a white solid (mp 260–262 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.67 (d, J = 2.8 Hz, 1H), 7.57–7.50 (m, 4H), 7.49–7.44 (m, 4H), 7.43–7.39 (m, 1H), 7.29–7.24 (m, 4H), 7.23–7.18 (m, 2H), 7.15–7.11 (m, 4H), 7.06–7.01 (m, 1H), 6.81–6.75 (m, 1H), 6.73 (s, 1H), 6.65 (d, J = 8.1 Hz, 1H), 1.26 (s, 18H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 154.3 (d, JC–P = 2.5 Hz), 149.3 (d, JC–P = 16.8 Hz), 141.8, 137.0 (d, JC–P = 11.4 Hz), 132.3, 131.1 (t, JC–P = 6.4 Hz), 128.7, 128.3, 128.1 (d, JC–P = 11.9 Hz), 126.4, 125.4 (d, JC–P = 11.9 Hz), 121.7, 120.1, 119.8, 111.9, 101.5 (d, JC–P = 124.0 Hz), 46.9, 34.6, 30.8. 31P NMR (162 MHz, DMSO-d6) δ 21.48. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C41H43NOP 596.3082, found 596.3091.
(2-Benzhydryl-1H-indol-3-yl)bis(4-(dimethylamino)phenyl)-phosphine oxide (4ae)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2e (108.12 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ae (49.85 mg) in 35% yield as a white solid (mp 303–305 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.50 (s, 1H), 7.40–7.26 (m, 9H), 7.24–7.14 (m, 6H), 7.07–6.95 (m, 2H), 6.81 (t, J = 7.6 Hz, 1H), 6.77–6.65 (m, 5H), 2.93 (s, 12H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.1 (d, JC–P = 16.3 Hz), 148.6, 138.6, 136.7 (d, JC–P = 6.5 Hz), 135.3, 134.2, 131.6 (d, JC–P = 2.5 Hz), 131.0 (d, JC–P = 10.0 Hz), 128.3 (d, JC–P = 11.9 Hz), 128.1, 125.0, 121.4, 120.1, 119.3, 111.9, 100.3 (d, JC–P = 124.8 Hz), 54.9, 46,2. 31P NMR (162 MHz, DMSO-d6) δ 21.92. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C37H37N3OP 570.2674, found 570.2674.
(2-Benzhydryl-1H-indol-3-yl)bis(4-fluorophenyl)phosphine oxide (4af)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2f (89.32 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4af (64.94 mg) in 50% yield as a white solid (mp 304–306 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.79–11.75 (m, 1H), 7.69–7.58 (m, 4H), 7.47–7.41 (d, J = 8.2 Hz, 1H), 7.38–7.26 (m, 8H), 7.24 (d, J = 7.0 Hz, 2H), 7.14 (d, J = 7.5 Hz, 4H), 7.08 (t, J = 7.7 Hz, 1H), 6.86 (t, J = 7.6 Hz, 1H), 6.77 (s, 1H), 6.52 (d, J = 8.1 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) 165.3 (d, JC–F = 247.4 Hz), 150.1 (d, JC–P = 17.2 Hz), 141.5, 137.0 (d, JC–P = 11.7 Hz), 133.9 (q, JC–F = 19.2 Hz), 131.4, 130.4, 128.7 (d, JC–P = 29.1 Hz), 127.9 (d, JC–P = 12.2 Hz), 126.5, 121.9, 120.5, 119.3, 115.9 (d, JC–F = 13.0 Hz), 112.2, 100.4 (d, JC–P = 126.1 Hz), 47.1. 19F NMR (376 MHz, DMSO-d6) δ −102.83, −102.84. 31P NMR (162 MHz, DMSO-d6) δ 20.50. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23F2NOP 518.1485, found 518.1480.
(2-Benzhydryl-1H-indol-3-yl)bis(4-chlorophenyl)phosphine oxide (4ag)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2g (101.65 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ag (87.01 mg) in 63% yield as a white solid (mp 281–283 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.78 (d, J = 2.9 Hz, 1H), 7.61–7.55 (m, 4H), 7.55–7.52 (m, 4H), 7.46–7.42 (m, 1H), 7.31–7.26 (m, 4H), 7.24–7.20 (m, 2H), 7.16–7.12 (m, 4H), 7.11–7.05 (m, 1H), 6.89–6.84 (m, 1H), 6.76 (s, 1H), 6.55 (d, J = 8.1 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.3 (d, JC–P = 17.0 Hz), 141.5, 137.0 (d, JC–P = 2.8 Hz), 136.9, 133.7, 133.0 (d, JC–P = 11.3 Hz), 132.7, 128.9 (d, JC–P = 12.5 Hz), 128.7 (d, JC–P = 31.1 Hz), 127.8 (d, JC–P = 12.2 Hz), 126.5, 121.9, 120.6, 119.3, 112.2, 99.8 (d, JC–P = 126.9 Hz), 78.9 (t, JC–P = 33.0 Hz). 31P NMR (162 MHz, DMSO-d6) δ 20.87. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23Cl2NOP 550.0894, found 550.0900.
(2-Benzhydryl-1H-indol-3-yl)bis(3-fluorophenyl)phosphine oxide (4ah)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2h (89.32 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ah (83.13 mg) in 64% yield as a white solid (mp 270–272 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.80 (d, J = 3.0 Hz, 1H), 7.59–7.52 (m, 2H), 7.48–7.41 (m, 5H), 7.34–7.27 (m, 6H), 7.26–7.22 (m, 2H), 7.14–7.07 (m, 5H), 6.90–6.35 (m, 1H), 6.62 (s, 1H), 6.55 (d, J = 8.1 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 163.1 (d, JC–F = 263.1 Hz), 150.3 (d, JC–P = 17.5 Hz), 141.3, 137.4 (d, JC–P = 5.1 Hz), 137.0 (d, JC–P = 1.9 Hz), 136.4 (d, JC–P = 5.3 Hz), 131.4 (q, JC–F = 7.5 Hz), 128.6 (d, JC–P = 25.0 Hz), 127.7, 127.4 (d, JC–P = 3.0 Hz), 126.6, 122.0, 120.6, 119.3 (d, JC–P = 7.0 Hz), 119.0, 117.7 (q, JC–F = 11.2 Hz), 112.3, 99.6 (d, JC–P = 127.5 Hz), 47.3. 19F NMR (376 MHz, DMSO-d6) δ −111.31, −111.32. 31P NMR (162 MHz, DMSO-d6) δ 19.89. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23F2NOP 518.1485, found 518.1480.
(2-Benzhydryl-1H-indol-3-yl)bis(3-chlorophenyl)phosphine oxide (4ai)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2i (101.65 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ai (55.24 mg) in 40% yield as a white solid (mp 229–231 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.83 (d, J = 3.0 Hz, 1H), 7.70–7.62 (m, 2H), 7.59–7.50 (m, 6H), 7.48–7.44 (m, 1H), 7.32–7.26 (m, 4H), 7.26–7.20 (m, 2H), 7.13–7.08 (m, 5H), 6.93–6.86 (m, 1H), 6.62–6.57 (m, 2H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.4 (d, JC–P = 17.4 Hz), 141.2, 137.1, 137.0 (d, JC–P = 12.0 Hz), 136.1, 133.8 (d, JC–P = 15.6 Hz), 132.0 (d, JC–P = 2.2 Hz), 131.0 (d, JC–P = 13.3 Hz), 130.5 (d, JC–P = 11.0 Hz), 129.8 (d, JC–P = 9.9 Hz), 128.6 (d, JC–P = 23.0 Hz), 127.8 (d, JC–P = 12.2 Hz), 126.7, 122.1, 120.7, 119.2, 112.4, 99.4 (d, JC–P = 127.6 Hz), 47.3. 31P NMR (162 MHz, DMSO-d6) δ 20.03. HRMS (ESI-TOF) m/z [M – H]− Calcd for C33H23Cl2NOP 550.0894, found 550.0892.
(2-Benzhydryl-1H-indol-3-yl)bis(3-(trifluoromethyl)phenyl)-phosphine oxide (4aj)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2j (126.82 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4aj (144.04 mg) in 93% yield as a white solid (mp 249–251 °C): 1H NMR (400 MHz, CDCl3) δ 8.83 (d, J = 3.0 Hz, 1H), 7.87 (d, J = 12.4 Hz, 2H), 7.75–7.62 (m, 4H), 7.47–7.41 (m, 2H), 7.30 (dd, J = 8.3, 1.9 Hz, 1H), 7.19–7.09 (m, 7H), 7.07–7.00 (m, 4H), 6.93 (t, J = 7.6 Hz, 1H), 6.68 (d, J = 8.1 Hz, 1H), 6.61 (s, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 150.9 (d, JC–P = 17.6 Hz), 140.9, 135.8 (d, JC–P = 11.4 Hz), 135.5, 134.9 (d, JC–P = 10.6 Hz), 134.4, 131.2 (q, JC–F = 12.5 Hz), 129.1 (q, JC–F = 12.7 Hz), 128.9, 128.6, 128.5 (d, JC–P = 4.8 Hz), 127.1, 124.9 (q, JC–F = 270.6 Hz), 122.7, 121.6, 120.0, 119.5, 111.7, 99.7 (d, JC–P = 128.9 Hz), 48.5. 19F NMR (376 MHz, CDCl3) δ −62.67. 31P NMR (162 MHz, CDCl3) δ 21.52. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C35H25F6NOP 620.1578, found 620.1576.
((1H-Indol-2-yl)diphenylmethyl)dimesitylphosphine oxide (4ak)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2k (107.38 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4ak (69.54 mg) in 49% yield as a white solid (mp 219–221 °C): 1H NMR (400 MHz, CDCl3) δ 8.37 (s, 1H), 7.23 (d, J = 8.3 Hz, 1H), 7.18–7.13 (m, 7H), 7.10–7.05 (m, 4H), 6.84–6.79 (m, 1H), 6.72 (d, J = 3.7 Hz, 5H), 6.43 (d, J = 8.2 Hz, 1H), 2.24 (s, 6H), 2.13 (s, 12H). 13C{1H}NMR (100 MHz, CDCl3) δ 149.2 (d, JC–P = 15.3 Hz), 142.6 (d, JC–P = 10.1 Hz), 141.3, 140.6 (d, JC–P = 2.4 Hz), 135.7 (d, JC–P = 11.0 Hz), 131.8, 131.1 (d, JC–P = 10.6 Hz), 129.1, 128.3 (d, JC–P = 12.1 Hz), 126.9, 126.4, 121.9, 120.9, 119.8, 111.1, 107.9 (d, JC–P = 119.7 Hz), 58.4, 23.5, 21.0. 31P NMR (162 MHz, CDCl3) δ 20.88. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C39H39NOP 568.2769, found 568.2768.
((1H-Indol-2-yl)diphenylmethyl)di(naphthalen-1-yl)phosphine oxide (4al)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2l (113.37 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4al (141.54 mg) in 97% yield as a white solid (mp 213–215 °C): 1H NMR (400 MHz, CDCl3) δ 8.90 (s, 1H), 8.70 (s, 1H), 8.51 (d, J = 3.0 Hz, 1H), 7.84 (d, J = 43.9 Hz, 4H), 7.55–7.27 (m, 6H), 7.24–7.21 (m, 1H), 7.21–7.10 (m, 7H), 7.08–6.94 (m, 6H), 6.85–6.76 (m, 2H), 6.53 (s, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 148.8 (d, JC–P = 12.8 Hz), 141.5, 137.8, 135.9, 135.8, 134.1, 133.9, 132.9, 129.7, 129.6, 129.1, 128.6, 127.7, 127.2, 126.9, 126.8, 126.4, 124.4, 122.4, 121.2 (d, JC–P = 5.4 Hz), 111.1, 102.1 (d, JC–P = 125.9 Hz), 48.0. 31P NMR (162 MHz, CDCl3) δ 31.01. HRMS (ESI-TOF) m/z [M – H]− Calcd for C41H29NOP 582.1987, found 582.1992.
(2-Benzhydryl-1H-indol-3-yl)di(thiophen-2-yl)phosphine oxide (4am)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2m (80.34 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4am (66.91 mg) in 54% yield as a white solid (mp 303–305 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.80 (d, J = 3.4 Hz, 1H), 8.06–7.98 (m, 2H), 7.47–7.40 (m, 3H), 7.36–7.29 (m, 4H), 7.27–7.18 (m, 8H), 7.12–7.05 (m, 1H), 6.96 (s, 1H), 6.93–6.86 (m, 1H), 6.77 (d, J = 8.0 Hz, 1H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 150.0 (d, JC–P = 19.0 Hz), 141.8, 137.5, 136.9 (d, JC–P = 12.3 Hz), 136.3, 135.7 (d, JC–P = 11.2 Hz), 134.4 (d, JC–P = 5.2 Hz), 128.7, 128.4 (d, JC–P = 5.6 Hz), 127.6 (d, JC–P = 13.2 Hz), 126.6, 122.0, 120.4, 119.7, 112.0, 101.5 (d, JC–P = 138.1 Hz), 46.7. 31P NMR (162 MHz, DMSO-d6) δ 5.73. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C29H23NOPS2 496.0959, found 496.0950.
(2-Benzhydryl-1H-indol-3-yl)(phenyl)(p-tolyl)phosphine oxide (4an)
General procedure B was followed using 1a (74.84 mg, 0.25 mmol), 2n (81.08 mg, 0.375 mmol) and Yb(OTf)3 (31.01 mg, 0.05 mmol, 0.2 equiv) in CH3CN (2 mL) at 100 °C for 12 h. Chromatography (10% EtOAc/dichloromethane) afforded 4an (79.61 mg) in 64% yield as a white solid (mp 290–292 °C): 1H NMR (400 MHz, DMSO-d6) δ 11.71 (d, J = 2.9 Hz, 1H), 7.63–7.54 (m, 3H), 7.52–7.44 (m, 4H), 7.41 (d, J = 8.2 Hz, 1H), 7.33–7.27 (m, 6H), 7.24–7.19 (m, 2H), 7.18–7.13 (m, 4H), 7.09–7.02 (m, 1H), 6.91–6.73 (m, 2H), 6.59 (d, J = 8.1 Hz, 1H), 2.34 (s, 3H). 13C{1H}NMR (100 MHz, DMSO-d6) δ 149.6 (d, JC–P = 16.8 Hz), 141.8 (d, JC–P = 2.7 Hz), 141.7 (d, JC–P = 3.7 Hz), 137.0 (d, JC–P = 11.5 Hz), 135.5, 134.4, 132.0, 131.6, 131.3 (d, JC–P = 10.9 Hz), 130.9, 129.2 (d, JC–P = 12.3 Hz), 128.7 (d, JC–P = 13.0 Hz), 128.4 (d, JC–P = 11.1 Hz), 128.1 (d, JC–P = 12.0 Hz), 126.5, 121.7, 120.2, 119.7, 112.0, 101.1 (d, JC–P = 124.4 Hz), 46.9, 21.1. 31P NMR (162 MHz, DMSO-d6) δ 22.01. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C34H29NOP 498.1987, found 498.1983.
General Procedure C
5 was made by using the known procedure.22 To a mixture of 3aa (174.078 mg, 0.36 mmol, 1 equiv), Ti(OiPr)4 (51.16 mg, 0.18 mmol, 0.5 equiv) and HSi-(OCH2CH3)3 (354.82 mg, 2.16 mmol, 6 equiv) was added 5 mL of anhydrous toluene, under Ar atmosphere. The pale brown slurry was heated to reflux, the solid dissolved and the solution turned gray-green 12 h later. The reaction was stopped, cooled to r.t. and the solvent was removed in vacuo to afford a brown solid. The corresponding product 5 was isolated by silica gel column chromatography with a petroleum ether/ethyl acetate (50/1) mixture as eluent to afford 5 (89.21 mg) in 53% yield.
2-((Diphenylphosphanyl)diphenylmethyl)-1H-indole (5)
General procedure C was followed using 3aa (174.078 mg, 0.36 mmol, 1 equiv), Ti(OiPr)4 (51.16 mg, 0.18 mmol, 0.5 equiv) and HSi-(OCH2CH3)3 (354.82 mg, 2.16 mmol, 6 equiv) in toluene (5 mL) at 120 °C for 12 h. Chromatography (2% EtOAc/petroleum ether) afforded 5 (89.21 mg) in 53% yield as a white solid (mp 90–92 °C): 1H NMR (400 MHz, CDCl3) δ 10.27 (s, 1H), 7.52 (d, J = 7.9 Hz, 1H), 7.49–7.42 (m, 3H), 7.30–7.23 (m, 7H), 7.21–7.10 (m, 9H), 7.01 (d, J = 7.9 Hz, 4H), 6.28 (d, J = 2.3 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 137.4, 136.3, 133.9 (d, JC–P = 2.2 Hz), 133.0 (d, JC–P = 8.6 Hz), 132.5 (d, JC–P = 2.7 Hz), 132.3 (d, JC–P = 4.9 Hz), 131.0, 130.0, 128.6 (d, JC–P = 11.5 Hz), 128.2, 127.0 (d, JC–P = 1.5 Hz), 122.6, 120.6, 120.0, 111.7, 105.8 (d, JC–P = 6.1 Hz), 60.3 (d, JC–P = 61.3 Hz). 31P NMR (162 MHz, CDCl3) δ −16.13. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H27NP 468.1881, found 468.1880.
2-Benzhydryl-3-(diphenylphosphanyl)-1H-indole (6)
General procedure C was followed using 4aa (174.078 mg, 0.36 mmol, 1 equiv), Ti(OiPr)4 (51.16 mg, 0.18 mmol, 0.5 equiv) and HSi(OCH2CH3)3 (354.82 mg, 2.16 mmol, 6 equiv) in toluene (5 mL) at 120 °C for 12 h. Chromatography (2% EtOAc/petroleum ether) afforded 6 (136.34 mg) in 81% yield as a white solid (mp 102–104 °C): 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 3.1 Hz, 1H), 7.24–7.22 (m, 4H), 7.21–7.17 (m, 3H), 7.16 (d, J = 4.9 Hz, 3H), 7.12 (dd, J = 5.8, 2.3 Hz, 7H), 7.09–7.04 (m, 4H), 6.98 (t, J = 7.5 Hz, 1H), 6.91 (d, J = 8.0 Hz, 1H), 6.76 (t, J = 7.6 Hz, 1H), 6.46 (d, J = 5.3 Hz, 1H). 13C{1H}NMR (100 MHz, CDCl3) δ 149.0 (d, JC–P = 43.4 Hz), 142.1, 137.2 (d, JC–P = 6.1 Hz), 136.7 (d, JC–P = 2.9 Hz), 132.6 (d, JC–P = 18.0 Hz), 130.1 (d, JC–P = 4.8 Hz), 129.1, 128.7, 128.2 (d, JC–P = 6.3 Hz), 127.7, 126.9, 121.9, 121.8, 120.2, 111.1, 103.8 (d, JC–P = 123.1 Hz), 49.1 (d, JC–P = 15.8 Hz). 31P NMR (162 MHz, CDCl3) δ −31.62. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H27NP 468.1881, found 468.1886.
General Procedure D
To a stirred solution of 6 (935.10 mg, 2 mmol, 1 equiv) in THF (7 mL) under Ar atmosphere, was added n-BuLi (0.96 mL, 2.4 mmol, 1.2 equiv, 2.5 M) dropwise at −78 °C. The reaction was then slowly warmed to r.t. and then stirred for a further 2 h at room temperature. After the mixture cooled to −78 °C, PPh2Cl (529.12 mg, 2.4 mmol, 1.2 equiv) in THF (5 mL) was added dropwise. The mixture was then warmed to rt and stirred for a further 2 h. It was then quenched with NH4Cl solution at 0 °C. Chromatography (petroleum ether/ethyl acetate = 50/1) afforded 7 (599.59 mg) in 46% yield as a white solid.17
2-Benzhydryl-1,3-bis(diphenylphosphanyl)-1H-indole (7)
General procedure D was followed using 6 (935.10 mg, 2 mmol, 1 equiv), n- BuLi (0.96 mL, 2.4 mmol, 1.2 equiv, 2.5 M) and PPh2Cl (529.12 mg, 2.4 mmol, 1.2 equiv) in THF (12 mL) at −78 °C for 2 h. Chromatography (2% EtOAc/petroleum ether) afforded 7 (599.59 mg) in 46% yield as a white solid (mp 121–123 °C): 1H NMR (400 MHz, CDCl3) δ 7.31 (dd, J = 7.5, 1.7 Hz, 4H), 7.29–7.21 (m, 5H), 7.19–7.11 (m, 12H), 7.05–6.93 (m, 10H), 6.84 (dd, J = 7.3, 1.9 Hz, 1H), 6.74 (dd, J = 7.4, 1.7 Hz, 1H), 6.66–6.57 (m, 2H). 13C{1H}NMR (100 MHz, CDCl3) δ 156.7 (d, JC–P = 63.5 Hz), 141.2, 141.2, 140.6 (d, JC–P = 9.3 Hz), 137.2 (d, JC–P = 9.1 Hz), 134.1 (d, JC–P = 17.0 Hz), 132.6 (d, JC–P = 18.3 Hz), 131.2 (d, JC–P = 20.1 Hz), 130.0 (t, JC–P = 2.9 Hz), 128.9, 128.5 (d, JC–P = 5.6 Hz), 128.2 (d, JC–P = 5.5 Hz), 128.0, 127.7, 126.3, 122.4, 121.6, 120.8, 115.4, 109.4 (d, JC–P = 8.2 Hz), 60.5. 31P NMR (162 MHz, CDCl3) δ 36.24 (d, J = 3.7 Hz), −29.02 (d, J = 2.7 Hz). HRMS (ESI-TOF) m/z [M + H]+ Calcd for C45H36NP2 652.2323, found 652.2330.
General Procedure E
To a THF (5 mL) solution of 6 (935.10 mg, 2 mmol, 1 equiv) was added NaH (120.00 mg, 5 mmol, 2.5 equiv) at 0 °C. The reaction mixture was stirred at the same temperature for 30 min. Then the corresponding iodide (312.268 mg, 2.2 mmol, 1.1 equiv) was added. The reaction mixture was stirred at room temperature until complete consumption of stirred material (detected by TLC). The reaction mixture was quenched with water. Chromatography (petroleum ether/ethyl acetate = 50/1) afforded 8 (606.79 mg) in 63% yield as a white solid.17
2-Benzhydryl-3-(diphenylphosphanyl)-1-methyl-1H-indole (8)
General procedure E was followed using 6 (935.10 mg, 2 mmol, 1 equiv), NaH (120.00 mg, 5 mmol, 2.5 equiv) and iodide (312.268 mg, 2.2 mmol, 1.1 equiv) in THF (12 mL) at −78 °C for 2 h. Chromatography (2% EtOAc/petroleum ether) afforded 8 (606.79 mg) in 63% yield as a white solid (mp 101–103 °C): 1H NMR (400 MHz, CDCl3) δ 7.35–7.27 (m, 3H), 7.21–7.10 (m, 18H), 7.10–7.05 (m, 1H), 7.03 (d, J = 10.9 Hz, 1H), 6.92 (d, J = 8.0 Hz, 1H), 6.82–6.77 (m, 1H), 3.33 (s, 3H). 13C{1H}NMR (100 MHz, CDCl3) δ 149.0 (d, JC–P = 40.4 Hz), 139.5, 138.0 (d, JC–P = 2.8 Hz), 136.4 (d, JC–P = 6.6 Hz), 131.4, 131.2, 128.1, 127.4, 127.1 (d, JC–P = 6.2 Hz), 126.5, 125.5, 120.9, 120.7, 119.0, 108.2, 103.4, 46.7, 30.9. 31P NMR (162 MHz, CDCl3) δ −29.83. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C34H29NP 482.2038, found 482.2035.
General Procedure F
6 (467.55 mg, 1 mmol, 1 equiv), S8 (32.00 mg, 0.125 mmol, 0.125 equiv), dry CH2Cl2 (2 mL) and a stir bar were added to a sealed tube. After being stirred at 80 °C for 12 h, the mixture was evaporated under vacuum. The corresponding product 9 (274.79 mg, 55% yield) was isolated by silica gel column chromatography with petroleum ether/ethyl acetate (50/1) mixture as eluent.23
(2-Benzhydryl-1H-indol-3-yl)diphenylphosphine sulfide (9)
General procedure F was followed using 6 (467.55 mg, 1 mmol, 1 equiv), S8 (32.00 mg, 0.125 mmol, 0.125 equiv) in CH2Cl2 (2 mL) at 80 °C for 12 h. Chromatography (2% EtOAc/petroleum ether) afforded 9 (274.79 mg) in 55% yield as a white solid (mp 175–177 °C): 1H NMR (400 MHz, CDCl3) δ 8.23 (s, 1H), 7.78–7.67 (m, 4H), 7.45–7.35 (m, 2H), 7.33–7.27 (m, 5H), 7.20–7.14 (m, 6H), 7.14–7.09 (m, 1H), 6.98–6.92 (m, 4H), 6.88–6.82 (m, 1H), 6.60–6.54 (m, 2H). 13C{1H}NMR (100 MHz, CDCl3) δ 147.9 (d, JC–P = 18.9 Hz), 141.1, 135.4 (d, JC–P = 11.2 Hz), 134.2, 133.3, 132.1 (d, JC–P = 11.0 Hz), 131.2 (d, JC–P = 3.0 Hz), 129.3 (d, JC–P = 9.9 Hz), 129.1, 128.5, 128.4 (d, JC–P = 12.6 Hz), 126.9, 122.5, 121.1, 111.1, 101.9 (d, JC–P = 105.8 Hz), 47.7. 31P NMR (162 MHz, CDCl3) δ 30.55. HRMS (ESI-TOF) m/z [M + H]+ Calcd for C33H27NPS 500.1602, found 500.1609.
Supplementary Material
Acknowledgments
This research was financially supported by the National Nature Science Foundation of China (21772039, 21272069) and the Fundamental Research Funds for the Central Universities and Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and we thank the Chinese Scholarship Council for financial support. We are grateful to Sergei Tcyrulnikov for molecular orbital calculations and XSEDE (TG-CHE120052) for computational resources.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.8b00541.
Reaction condition optimization, NMR and LC–MS spectra (PDF)
Crystallographic data for compound 3la (CIF)
Crystallographic data for compound 4ag (CIF)
References
- 1.(a) Chen F-E, Huang J. Reserpine: A Challenge for Total Synthesis of Natural Products. Chem Rev. 2005;105:4671–4706. doi: 10.1021/cr050521a. [DOI] [PubMed] [Google Scholar]; (b) Atta-ur-Rahman BA. Indole Alkaloids. Harwood Academic; Chichester: 1998. [Google Scholar]
- 2.(a) Li YM, Yang SD. New Strategies for Transition-Metal-Catalyzed C–P Bond Formation. Synlett. 2013;24:1739–1744. [Google Scholar]; (b) Hu J, Zhao N, Yang B, Wang G, Guo N, Liang YM, Yang SD. Copper-Catalyzed C–P Coupling through Decarboxylation. Chem - Eur J. 2011;17:5516–5521. doi: 10.1002/chem.201003561. [DOI] [PubMed] [Google Scholar]; (c) Li YM, Sun M, Wang HL, Tian QP, Yang SD. Direct Annulations toward Phosphorylated Oxindoles: Silver-Catalyzed Carbon-Phosphorus Functionalization of Alkenes. Angew Chem. 2013;125:4064–4068. doi: 10.1002/anie.201209475. [DOI] [PubMed] [Google Scholar]
- 3.Sechi M, Derudas M, Dallocchio R, Dessi A, Bacchi A, Sannia L, Carta F, Palomba M, Ragab O, Chan C, Shoemaker R, Sei S, Dayam R, Neamati N. Design and Synthesis of Novel Indole β-Diketo Acid Derivatives as HIV-1 Integrase Inhibitors. J Med Chem. 2004;47:5298–5310. doi: 10.1021/jm049944f. [DOI] [PubMed] [Google Scholar]
- 4.(a) Regina GL, Coluccia A, Silvestri R. Looking for An Active Conformation of the Future HIV Type-1 Non-Nucleoside Reverse Transcriptase Inhibitors. Antiviral Chem Chemother. 2010;20:213–237. doi: 10.3851/IMP1607. [DOI] [PubMed] [Google Scholar]; (b) Jia T, Bellomo A, Baina KEL, Dreher SD, Walsh PJ. Palladium-Catalyzed Direct Arylation of Methyl Sulfoxides with Aryl Halides. J Am Chem Soc. 2013;135:3740–3743. doi: 10.1021/ja4009776. [DOI] [PubMed] [Google Scholar]; (c) So CM, Kwong FY. Palladium-Catalyzed Cross-Coupling Reactions of Arylmesylates. Chem Soc Rev. 2011;40:4963–4972. doi: 10.1039/c1cs15114b. [DOI] [PubMed] [Google Scholar]
- 5.(a) Zhou X-J, Pietropaolo K, Damphousse D, Belanger B, Chen J, Sullivan-Bólyai J, Mayers D. Antimicrob. Single-Dose Escalation and Multiple-Dose Safety, Tolerability, and Pharmacokinetics of IDX899, a Candidate Human Immuno Deficiency Virus Type 1 Nonnucleoside Reverse Transcriptase Inhibitor, in Healthy Subjects. Antimicrob Agents Chemother. 2009;53:1739–1746. doi: 10.1128/AAC.01479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) La Regina G, Coluccia A, Silvestri R. Looking for an Active Conformation of the Future HIV Type-1 Non-Nucleoside Reverse Transcriptase Inhibitors. Antiviral Chem Chemother. 2010;20:213–237. doi: 10.3851/IMP1607. [DOI] [PubMed] [Google Scholar]; (c) Zhou XJ, Garner RC, Nicholson S, Kissling CJ, Mayers DJ. Microdose Pharmacokinetics of IDX899 and IDX989, Candidate HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors, Following Oral and Intravenous Administration in Healthy Male Subjects. J Clin Pharmacol. 2009;49:1408–1416. doi: 10.1177/0091270009343698. [DOI] [PubMed] [Google Scholar]
- 6.Selected examples: Zhou AX, Mao LL, Wang GW, Yang SD. A Unique Copper-Catalyzed Cross-Coupling Reaction by Hydrogen (H2) Removal for the Stereoselective Synthesis of 3-Phosphoindoles. Chem Commun. 2014;50:8529–8532. doi: 10.1039/c4cc01815j.Sun WB, Xue JF, Zhang GY, Zeng RS, An LT, Zhang PZ, Zou JP. Silver-Catalyzed Direct Csp2-H Phosphorylation of Indoles Leading to Phosphoindoles. Adv Synth Catal. 2016;358:1753–1758.Kondoh A, Yorimitsu H, Oshima K. Synthesis of 2-Indolylphosphines by Palladium-Catalyzed Annulation of 1-Alkynyl-phosphine Sulfides with 2-Iodoanilines. Org Lett. 2010;12:1476–1479. doi: 10.1021/ol1001544.Gao Y, Lu G, Zhang P, Zhang L, Tang G, Zhao Y. A Cascade Phosphinoylation/Cyclization/Desulfonylation Process for the Synthesis of 3-Phosphinoylindoles. Org Lett. 2016;18:1242–1245. doi: 10.1021/acs.orglett.6b00056.Thielges S, Meddah E, Bisseret P, Eustache J. New Synthesis of Benzo[b]furan and Indole Derivatives from 1,1-Dibromo-1-Alkenes Using a Tandem Pd-Assisted Cyclization-Coupling Reaction. Tetrahedron Lett. 2004;45:907–910.
- 7.Yuan T, Huang S, Chun C, Lu GP. Metal-Free Electrophilic Phosphination of Electron-Rich Arenes, Arenols and Aromatic Thiols with Diarylphosphine Oxides. Org Biomol Chem. 2018;16:30–33. doi: 10.1039/c7ob02620j. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Li S, Wang BQ, Li B. The Regioselective Synthesis of 2-Phosphinoylindoles via Rh(III)-Catalyzed C–H Activation. Org Chem Front. 2018;5:88–91. [Google Scholar]
- 9.(a) Emer E, Sinisi R, Capdevila MG, Petruzziello D, Vincentiis FD, Cozzi PG. Direct Nucleophilic SN1-Type Reactions of Alcohols. Eur J Org Chem. 2011;2011:647–666. [Google Scholar]; (b) Dryzhakov M, Richmond E, Moran J. Recent Advances in Direct Catalytic Dehydrative Substitution of Alcohols. Synthesis. 2016;48:935–959. [Google Scholar]
- 10.(a) Mei GJ, Shi F. Indolylmethanols as Reactants in Catalytic Asymmetric Reaction. J Org Chem. 2017;82:7695–7707. doi: 10.1021/acs.joc.7b01458. [DOI] [PubMed] [Google Scholar]; (b) Palmieri A, Petrini M, Shaikh RR. Synthesis of 3-Aubstituted Indoles via Reactive Alkylideneindolenine Intermediates. Org Biomol Chem. 2010;8:1259–1270. doi: 10.1039/b919891a. [DOI] [PubMed] [Google Scholar]; (c) Wang L, Chen Y, Xiao J. Alkylideneindoleninium Ions and Alkylideneindolenines: Key Intermediates for the Asymmetric Synthesis of 3-Indolyl Derivatives. Asian J Org Chem. 2014;3:1036–1052. [Google Scholar]
- 11.Selected examples: Liu CY, Han FS. Organocatalytic Asymmetric Reaction of Indol-2-yl Carbinols with Enamides: Synthesis of Chiral 2-Indole-Substituted 1,1-Diarylalkanes. Chem Commun. 2015;51:11844–11847. doi: 10.1039/c5cc03345d.Qi S, Liu CY, Ding JY, Han FS. Chiral Phosphoramide-Catalyzed Enantioselective Synthesis of 2,3′-Diindolylarylmethanes from Indol-2-yl Carbinols and Indoles. Chem Commun. 2014;50:8605–8608. doi: 10.1039/c4cc03605k.Granger BA, Jewett IT, Butler JD, Hua B, Knezevic CE, Parkinson EI, Hergenrother PJ, Martin SF. Synthesis of (±)-Actinophyllic Acid and Analogs: Applications of Cascade Reactions and Diverted Total Synthesis. J Am Chem Soc. 2013;135:12984–12986. doi: 10.1021/ja4070206.Zhong X, Li Y, Zhang J, Han FS. Synthetic Study toward the Misassigned (±)-Tronoharin. Org Lett. 2015;17:720–723. doi: 10.1021/ol503734x.Cao KS, Bian HX, Zheng WH. Mild Arylboronic Acid Catalyzed Selective [4 + 3] Cycloadditions: Access to Cyclohepta[b]-benzofurans and Cyclohepta[b]indoles. Org Biomol Chem. 2015;13:6449–6452. doi: 10.1039/c5ob00653h.
- 12.(a) Zhang HH, Wang CS, Li C, Mei GJ, Li Y, Shi F. Design and Enantioselective Construction of Axially Chiral Naphthyl-Indole Skeletons. Angew Chem, Int Ed. 2017;56:116–121. doi: 10.1002/anie.201608150. [DOI] [PubMed] [Google Scholar]; (b) Zhu ZQ, Shen Y, Sun XX, Tao JY, Liu JX, Shi F. Catalytic Asymmetric [3 + 2] Cycloadditions of C-3 Unsubstituted 2-Indolylmethanols: Regio-, Diastereo- and Enantioselective Construction of the Cyclopenta[b]indole Framework. Adv Synth Catal. 2016;358:3797–3808. [Google Scholar]; (c) Sun XX, Zhang HH, Li GH, He YY, Shi F. Catalytic Enantioselective and Regioselective [3 + 3] Cycloadditions Using 2-Indolylmethanols as 3 C Building Blocks. Chem - Eur J. 2016;22:17526–17532. doi: 10.1002/chem.201603049. [DOI] [PubMed] [Google Scholar]; (d) He YY, Sun XX, Li GH, Mei GJ, Shi F. Substrate-Controlled Regioselective Arylations of 2-Indolylmethanols with Indoles: Synthesis of Bis-(indolyl)methane and 3,3′-Bisindole Derivatives. J Org Chem. 2017;82:2462–2471. doi: 10.1021/acs.joc.6b02850. [DOI] [PubMed] [Google Scholar]; (e) Li C, Zhang HH, Fan T, Shen Y, Wu Q, Shi F. Brϕnsted Acid-Catalyzed Regioselective Reactions of 2-Indolylmethanols with Cyclic Enaminone and Anhydride Leading to C3-Functionalized Indole Derivatives. Org Biomol Chem. 2016;14:6932–6936. doi: 10.1039/c6ob01282e. [DOI] [PubMed] [Google Scholar]; (f) Zhu ZQ, Shen Y, Liu JX, Tao JY, Shi F. Enantioselective Direct α-Arylation of Pyrazol-5-ones with 2-Indolylmethanols via Organo-Metal Cooperative Catalysis. Org Lett. 2017;19:1542–1545. doi: 10.1021/acs.orglett.7b00351. [DOI] [PubMed] [Google Scholar]; (g) Shen Y, Zhu ZQ, Liu JX, Yu L, Du BX, Mei GJ, Shi F. Brϕnsted Acid Catalyzed C3-Alkylation of 2-Indolylmethanols with Azlactones via an Umpolung Strategy. Synthesis. 2017;49:4025–4034. [Google Scholar]; (h) Wan Y, Wang H, Xu M, Mei G, Shi F. Direct C3-Arylations of 2-Indolylmethanols with Tryptamines and Tryptophols via an Umpolung Strategy. Org Biomol Chem. 2018;16:1536–1542. doi: 10.1039/c7ob03182c. [DOI] [PubMed] [Google Scholar]
- 13.(a) Hong G, Mao D, Wu SY, Wang LM. Palladium-Catalyzed Direct Regioselective ortho-Phosphonation of Aromatic Azo Compounds with Dialkyl Phosphites. J Org Chem. 2014;79:10629–10635. doi: 10.1021/jo501928x. [DOI] [PubMed] [Google Scholar]; (b) Hong G, Zhu XY, Hu C, Aruma AN, Wu S, Wang L. Base-Catalyzed Hydrophosphination of Azobenzenes with Diarylphosphine Oxides: A Precise Construction of N-N-P Unit. J Org Chem. 2016;81:6867–6874. doi: 10.1021/acs.joc.6b01210. [DOI] [PubMed] [Google Scholar]
- 14.Vuković VD, Richmond E, Wolf E, Moran J. Catalytic Friedel–Crafts Reactions of Highly Electronically Deactivated Benzylic Alcohols. Angew Chem, Int Ed. 2017;56:3085–3089. doi: 10.1002/anie.201612573. [DOI] [PubMed] [Google Scholar]
- 15.CCDC 1824762 for 3la and CCDC 1824763 for 4ag; see SI for details.
- 16.(a) Kobayashi S, Busujima T, Nagayama S. A Novel Classification of Lewis Acids on the Basis of Activity and Selectivity. Chem - Eur J. 2000;6:3491–3494. doi: 10.1002/1521-3765(20001002)6:19<3491::aid-chem3491>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]; (b) Hostalek Z, Mundil R, Cisarova I, Trhlikova O, Grau E, Peruch F, Cramail H, Merna J. Salphen-Co(III) Complexes Catalyzed Copolymerization of Epoxides with CO2. Polymer. 2015;63:52–61. [Google Scholar]; (c) Pike SJ, Hutchinson JJ, Hunter CA. H-Bond Acceptor Parameters for Anions. J Am Chem Soc. 2017;139:6700–6706. doi: 10.1021/jacs.7b02008. [DOI] [PubMed] [Google Scholar]
- 17.(a) So CM, Lau CP, Kwong FY. Easily Accessible and Highly Tunable Indolyl Phosphine Ligands for Suzuki–Miyaura Coupling of Aryl Chlorides. Org Lett. 2007;9:2795–2798. doi: 10.1021/ol070898y. [DOI] [PubMed] [Google Scholar]; (b) Saha D, Ghosh R, Sarkar A. 3-Indolylphosphines as Ligand for Palladium in Suzuki–Miyaura Coupling Reaction of Chloroarenes: Substituent Effects. Tetrahedron. 2013;69:3951–3960. [Google Scholar]; (c) Ghosh R, Adarsh NN, Sarkar A. A Novel, Air-Stable Phosphine Ligand for the Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling Reaction of Chloro Arenes. J Org Chem. 2010;75:5320–5322. doi: 10.1021/jo100643j. [DOI] [PubMed] [Google Scholar]
- 18.So CM, Zhou Z, Lau CP, Kwong FY. Palladium-Catalyzed Amination of Aryl Mesylates. Angew Chem, Int Ed. 2008;47:6402–6406. doi: 10.1002/anie.200802157. [DOI] [PubMed] [Google Scholar]
- 19.Berk SC, Buchwald SL. An Air-Stable Catalyst System for the Conversion of Esters to Alcohols. J Org Chem. 1992;57:3751–3753. [Google Scholar]
- 20.(a) Sivasankar C, Bera JK, Nethaji M, Samuelson AG. Reactions of Cu3(dppm)3(μ3-OH)(ClO4)2 (dppm = bis-(diphenylphosphino) methane) with Soft Heterocumulenes. J Organomet Chem. 2004;689:2726–2732. [Google Scholar]; (b) Ýriþli S, Yanar S. Synthesis and Crystallographic Characterization of Cationic Pd(II) Complexes Containing Bis(diphenylthiophosphinyl)methane. Polyhedron. 2006;25:1333–1336. [Google Scholar]; (c) Li B, Yang J, Xu H, Song HB, Wang BQ. Rhodium(III)-Catalyzed C–H Activation and Annulation with 1-Alkynylphosphine Sulfides: A Mild and Regioselective Access for the Synthesis of Bulky Phosphine Ligands. J Org Chem. 2015;80:12397–12409. doi: 10.1021/acs.joc.5b02265. [DOI] [PubMed] [Google Scholar]
- 21.Hlasta DJ, Luttinger D, Perrone MH, Silbernagel MJ, Ward SJ, Haubrich DR. α2-Adrenergic Agonists/Antagonists: The Synthesis and Structure-Activity Relationships of a Series of Indolin-2-yl and Tetrahydroquinolin-2-yl Imidazolines. J Med Chem. 1987;30:1555–1562. doi: 10.1021/jm00392a005. [DOI] [PubMed] [Google Scholar]
- 22.Allen A, Jr, Ma L, Lin W. Facile Synthesis of Chelating Bisphosphine Oxides and Bisphosphines via Palladium-Catalyzed Bishydrophosphinylation Reactions. Tetrahedron Lett. 2002;43:3707–3710. [Google Scholar]
- 23.Hu G, Zhang Y, Su J, Li Z, Gao Y, Zhao Y. Ag-Mediated Cascade Decarboxylative Coupling and Annulation: A Convenient Route to 2-Phosphinobenzo[B]Phosphole Oxides. Org Biomol Chem. 2015;13:8221–8231. doi: 10.1039/c5ob00959f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.