Abstract
Plasma gamma-aminobutyric acid (GABA) levels were measured in 14 subjects with Prader-Willi syndrome, 9 subjects with Angelman syndrome, and matched control subjects. Mean levels in both patient groups were 2 to 3 times higher than in nonretarded moderately obese or retarded nonobese control subjects. Levels in each patient group differed significantly from both control groups. Neither the two patient groups nor the two control groups differed. GABA levels seemed unrelated to genetic status (chromosome 15 deletion or disomy). These preliminary findings of elevated plasma GABA levels possibly represent a compensatory increase in presynaptic GABA release in response to hyposensitivity of a subset of GABA receptors and could produce increased postsynaptic activation of other normal GABA receptor subtypes, resulting in complex alterations of GABAergic function throughout the brain.
Prader-Willi syndrome (PWS) was first identified by Prader et al.,1 and subsequently more than 700 subjects have been reported.2,3 PWS is characterized by infantile hypotonia, obesity in early childhood, mental deficiency, small hands and feet, hypogonadism, and characteristic minor abnormalities.2,4,5 A chromosomal deletion (15q11q13) of paternal origin is found in 60% of PWS subjects. Ten to 15 percent of PWS patients have submicroscopic deletions, and 20% to 25% have maternal uniparental chromosome 15 disomy (that is, both members of the chromosome pair 15 come from the mother).2,3,5–7 In addition, 5% to 10% of subjects exhibiting PWS characteristics have biparental inheritance of normal-appearing chromosome 15’s,6 although the clinically typical PWS patient has either a deletion or maternal disomy of chromosome 15.8 A similar deletion on chromosome 15 (15q11q13) of maternal origin produces an entirely different clinical condition, Angelman syndrome (AS), which is characterized by severe mental retardation, absent speech, brachycephaly, ataxic gait, large mouth, and bouts of inappropriate laughter. The 15q11q13 deletion of maternal origin is observed in 70% of AS patients.3,7,9–11 Paternal uniparental disomy of chromosome 15 is seen in 2% to 3% of AS patients, and normal-appearing chromosomes are seen in the remaining AS patients. PWS and AS represent the first examples of genetic imprinting in humans; that is, the differential expression of genetic material from the mother versus the father.12 Recurrence of PWS rarely occurs in families, but 20% of AS families have more than one affected family member.
Although considerable effort has been devoted to a further understanding of the precise genetic deficits that underlie these syndromes, comparatively little is known about genetically induced alterations in neurochemical and physiological function involved in mediating the various signs and symptoms of these disorders. A large number of gamma-aminobutyric acid (GABA) receptor subunit genes have been identified,13,14 and various combinations of these subunits constitute the functional GABA-A receptor. However, the precise subunit composition and stoichiometry of GABA-A receptors in vivo and the relationship of these different constructs to function is not well understood. Similarly, although the GABA-B receptor has been isolated, the primary structure and functional significance of the GABA-B receptor in brain remains to be clarified.14 Wagstaff et al.15 first reported that the GABA-A beta 3 receptor subunit gene was located on the proximal long arm of chromosome 15. Since then, the alpha 5 and gamma 3 GABA receptor subunit genes have also been localized to the 15q11q13 region.16 These GABA receptor genes are usually deleted in PWS and AS patients, the observed chromosome deletion being identified by cytogenetic or karyotypic analysis. Thus, alterations in GABAergic function resulting from deletions or imprinting of these genes, or other as yet unidentified GABAergic receptor subunit genes localized on chromosome 15, may be involved in aspects of PWS and/or AS.3,15,17
There is substantial evidence supporting a relationship between plasma GABA levels and CNS GABAergic function. Cerebrospinal fluid (CSF) GABA originates in the brain, it is indicative of central GABAergic function,18 and a significant positive correlation exists between CSF and plasma GABA levels in normal humans.19 Pharmacological manipulations of GABA levels in the brain result in similar alterations in plasma GABA levels,20–23 whereas significant elevations of plasma GABA in fulminating hepatic failure in humans24 or portocaval shunting in rats25 failed to alter brain GABA levels. In more recent studies, both Rundfeldt and Loscher26 and Petty et al.27 concluded that plasma GABA levels could be used as a peripheral marker for central GABAergic function. To test whether central GABAergic function is altered, plasma GABA levels were measured in PWS and/or AS patients and in control subjects of similar age, weight, and cognitive ability.
METHODS
Plasma GABA Analysis
Peripheral blood was collected in ethylenediamine-tetraacetic acid (EDTA) tubes from each subject in the course of routine clinic visits to the Vanderbilt University Medical Center. The plasma was separated by centrifugation and stored at −70°C until analysis. Plasma GABA levels were measured by a modification of the high-performance liquid chromatography-electrochemical method previously reported by Donzanti and Yamamoto28 using the ESA Coulometric Electrode Array System (ESA Inc., Chelmsford, MI). Briefly, a 200–400 μl aliquot of plasma was mixed with an equal volume of 0.4 mol perchloric acid and centrifuged to remove precipitated proteins. The supernatant was then filtered by centrifugation through a Microcon-10 filter (Amicon Inc., Beverly, MA) to remove remaining high-molecular-weight compounds. The orthophthalaldehyde (OPT) derivative of GABA was prepared immediately prior to sample injection by the autoinjector, using OPT and thioethanol reagents as described by Donzanti and Yamamoto.28 Quantitation was accomplished by comparing the peak heights obtained from similar analysis of authentic GABA to the peak heights obtained from the filtered plasma. The system was calibrated with the GABA standard at the beginning of the analysis and after each five plasma samples.
Subjects
Fourteen PWS subjects (8 females and 6 males, age range 2–21 years) and 9 AS subjects (2 females and 7 males, age range 2–17 years) were studied. Diagnostic criteria for PWS included neonatal hypotonia, early childhood onset of obesity, hypogonadism, mental deficiency, and small hands and feet. AS diagnostic criteria included mental retardation, seizures, ataxia, inappropriate laughter, and a particular facial appearance.2,3,5 Informed consent was obtained from all subjects (or in the case of children, from the parent or legal guardian) by use of a consent form containing a complete description of the procedure that had been approved by the institutional internal review board. Six PWS and 4 AS subjects showed the 15q11q13 deletion with high-resolution chromosome analysis. The deletion was confirmed with quantitative Southern hybridization of proximal 15q DNA probes (for example, 3–21, 34,189–1, 1 IR-39, 43R, and/or IR-10) by using H2-26 or H2-42 as internal control probes or polymerase chain reaction (PCR) amplification of short-tandem DNA repeats from 15q11q13 (for example, D15S128, D15S122, D15S210, D15S113, GABRB3, GABRA5, D15S11, D15S541, D15S542, and/or MN-1) following established protocols.6,12,29–33 The remaining PWS patients showed either maternal uniparental disomy 15 (5 patients) or normal chromosome 15’s with no evidence of submicroscopic deletions and biparental inheritance (3 patients). The remaining 5 AS patients showed normal chromosome 15’s with no evidence of submicroscopic deletions and biparental inheritance. Seven non-mentally retarded moderately obese control subjects (5 males and 2 females, age range 2–20 years) were studied for comparison with the PWS and AS patients. In addition, 5 subjects without obesity, but with mental retardation (4 males and 1 female, age range 3–17 years) were also studied. Obesity, a cardinal feature in PWS subjects, was present in most PWS patients in this study with the exception of younger subjects (females ages 2, 3, and 6 years old) and 1 diet-controlled 16-year-old female. The range of percentage of ideal body weight (expressed as [subject’s weight divided by average weight expected for age and sex] multiplied by 100) was 90% to 380% for the PWS patients and 100% to 210% for the non–mentally retarded moderately obese control subjects. Relevant clinical and genetic data for all subjects are given in Table 1.
TABLE 1.
Clinical and genetic data on subjects
| Subject Numbera | Age (Yrs) | Sex | %IBW | Cognitive Status | Chromosome 15 Status |
|---|---|---|---|---|---|
| PWS-1 | 10 | M | 150 | Mildly retarded | Maternal disomy |
| PWS-2 | 2 | F | 100 | Mildly retarded | Maternal disomy [t(15;15)] |
| PWS-3 | 16 | F | 100 | Mildly retarded | Deletion 15q |
| PWS-4 | 20 | M | 120 | Borderline normal | Deletion 15q |
| PWS-5 | 14 | F | 160 | Mildly retarded | Maternal disomy |
| PWS-6 | 3 | F | 90 | Mildly retarded | Maternal disomy |
| PWS-7 | 11 | F | 160 | Moderately retarded | Normal |
| PWS-8 | 14 | M | 120 | Borderline normal | Deletion 15q |
| PWS-9 | 6 | F | 90 | Mildly retarded | Deletion 15q |
| PWS-10 | 3 | F | 150 | Mildly retarded | Deletion 15q |
| PWS-11 | 21 | F | 160 | Mildly retarded | Normal |
| PWS-12 | 20 | M | 130 | Borderline normal | Deletion 15q |
| PWS-13 | 8 | M | 380 | Mildly retarded | Normal |
| PWS-14 | 7 | M | 180 | Mildly retarded | Maternal disomy |
| Obese-1 | 23 | M | 140 | Normal | Normal |
| Obese-2 | 12 | M | 210 | Normal | Normal |
| Obese-3 | 2 | M | 100 | Borderline normal | Normal |
| Obese-4 | 20 | F | 150 | Normal | Normal |
| Obese-5 | 8 | M | 170 | Borderline normal | Normal |
| Obese-6 | 14 | M | 200 | Normal | Normal |
| Obese-7 | 10 | F | 110 | Normal | Normal |
| AS-1 | 8 | M | 100 | Severely retarded | Normal |
| AS-2 | 3 | F | 110 | Moderately retarded | Deletion 15q |
| AS-3 | 4 | M | 100 | Severely retarded | Normal |
| AS-4 | 2 | F | 110 | Moderately retarded | Deletion 15q |
| AS-5 | 17 | M | 90 | Severely retarded | Normal |
| AS-6 | 14 | M | 60 | Severely retarded | Normal |
| AS-7 | 3 | M | 90 | Moderately retarded | Deletion 15q |
| AS-8 | 14 | M | 50 | Moderately retarded | Normal |
| AS-9 | 11 | M | 90 | Severely retarded | Deletion 15q |
| MR-1 | 3 | M | 120 | Moderately retarded | Normal |
| MR-2 | 15 | M | 80 | Mildly retarded | Normal |
| MR-3 | 4 | M | 90 | Mildly retarded | Normal |
| MR-4 | 11 | M | 110 | Moderately retarded | Normal |
| MR-5 | 17 | F | 120 | Mildly retarded | Normal |
Note: IBW = ideal body weight; PWS = Prader-Willi syndrome; Obese = non–mentally retarded, moderately obese; AS = Angelman syndrome; MR = mentally retarded, nonobese.
Subject numbers correspond to subject numbers in Figure 1.
RESULTS
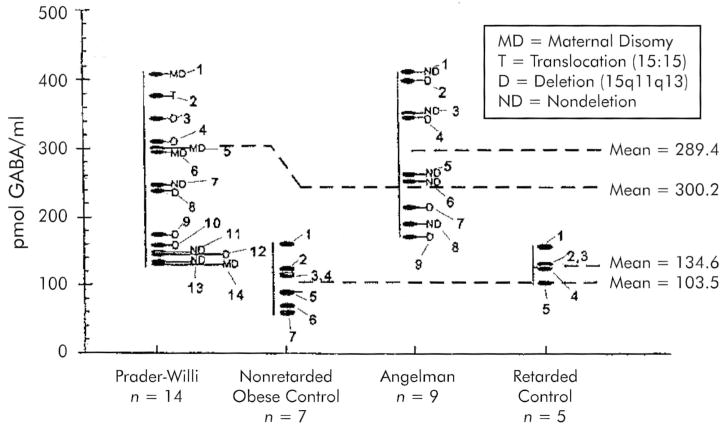
The individual plasma GABA values obtained from the PWS, AS, and control subjects are presented in Figure 1. The mean plasma GABA levels in both PWS and AS subjects are approximately 2 to 3 times higher than in the obese and mentally retarded control groups, and some PWS and AS subjects had GABA values in excess of fourfold higher than the mean seen in the control subjects. Significant differences were found in plasma GABA levels between PWS and non–mentally retarded moderately obese subjects (Mann-Whitney U-test, P < 0.001), PWS and mentally retarded nonobese individuals (Mann-Whitney U-test; P = 0.018), AS and mentally retarded nonobese subjects (Mann-Whitney U-test; P < 0.001), and AS and nonretarded obese subjects (Mann-Whitney U-test; P < 0.001). However, plasma GABA levels did not differ significantly when comparing PWS and AS subjects (independent t-test; P = 0.26) or between the mentally retarded nonobese and nonretarded moderately obese control subjects (independent t-test; P = 0.11). The plasma GABA levels in these latter groups, which serve as our controls, are in the range of plasma GABA levels reported in normative human controls (100–130 pmol/ml) by other investigators.19,34
FIGURE 1.
Plasma γ-aminobutyric acid (GABA) levels in Prader-Willi syndrome (PWS), Angelman syndrome (AS), and control subjects. Individual data and mean values of plasma GABA levels (pmol/ml plasma) as determined by high-performance liquid chromatographic (HPLC) analysis are presented. The mean values of both the PWS and the AS subjects were significantly different from those of the controls (mentally retarded nonobese and non–mentally retarded moderately obese subjects), but no difference was found between non–mentally retarded moderately obese and mentally retarded nonobese subjects. Subject numbers correspond to the subject numbers in Table 1.
There was no obvious relationship between GABA levels and the genetic status (deletion, disomy) of the patients; no such relationship would be expected if one or more GABA receptor subunit genes were deleted in the 15q deletion patients or imprinted or functionally inactivated in disomy chromosome 15 patients.
DISCUSSION
If one or more GABA receptor subunit genes are not being expressed in the brain of PWS and AS subjects because of genetic alterations, it is likely that the functional status of one or more subsets of postsynaptic GABA receptors will be altered. A decrease in the ability of these altered receptors to bind to GABA and/or to initiate the normal postsynaptic signal might result in a homeostatic feedback-induced increase in presynaptic GABA release to overcome postsynaptic subsensitivity and thus might result in the elevated GABA levels. However, it is unlikely that all of the multiple GABA receptor subtypes in the CNS would be adversely affected by the absence of one or two receptor subunit genes. The elevated GABA release in response to a subset of hyposensitive GABA receptors could result in increased postsynaptic activation of normal GABA receptor subtypes. This would produce a complex mosaic of altered function at GABAergic receptors throughout the brain, with equally complex physiological and behavioral consequences. Ninety-nine percent of the total GABA and 95% of its synthesizing enzyme, glutamic acid decarboxylase, occur in the brain and spinal cord.35 GABA is the primary inhibitory transmitter in the CNS, it is present in virtually every brain region, and it is active at approximately 20% to 40% of brain synapses.36 GABA is known to be involved in the regulation of a wide variety of behaviors, including regulation of eating and satiety, aggressive behavior, motor function, and seizures. The distribution of GABA receptors and the physiological and behavioral systems thought to involve GABAergic mechanisms have been reviewed by Matsumoto.37
There were no obvious differences between GABA levels in PWS patients with a deletion of chromosome 15, in which one or more receptor subunit genes are likely to be missing, and in subjects with maternal uniparental disomy of chromosome 15. Such a difference would not be expected if GABA receptor function in disomy subjects was also altered owing to nonexpression of one or more GABA receptor subunit genes because of imprinting. The GABRB3 gene, which is localized to the 15q11q13 region, may be paternally imprinted in humans38 and thus may play a role in the pathophysiology of AS. Additional GABA receptor genes have also been identified in this chromosome region, specifically GABRA5, and GABRG3.16 The order of the three recognized GABA receptor genes in the 15q11q13 region from proximal (centromere) to distal (telomere) is GABRB3, GABRA5, and GABRG3, respectively. It seems reasonable to speculate that additional GABA receptor genes maybe grouped in this region and that one or more may be either maternally or paternally imprinted in humans. Studies have shown that most PWS and AS patients with a recognizable cytogenetic deletion of 15q11q13 region show loss of GABRB3 and GABRA5 genes16,39 The PWS and AS subjects in this study with a recognizable 15q11q13 deletion also showed a deletion of the GABRB3 and GABRA5 subunit genes (data not presented). There also was no relationship between weight and plasma GABA levels in PWS subjects. This, along with normal GABA levels in obese control subjects, suggests that elevated plasma GABA levels are not directly related to presence of obesity.
Treatment of individuals with PWS is mostly limited to diet restriction and increased physical activity to control weight gain, and treatment of AS subjects is currently limited to control of seizures. Early diagnosis and intervention (such as physical therapy and infant stimulation programs) can be useful to avoid the marked, potentially life-threatening obesity frequently seen in PWS subjects. Clearly, a better understanding of the role of CNS neurotransmitters such as GABA in mediating the physical and behavioral problems of PWS and AS will be essential in order to devise more rational and effective uses of psychopharmacological agents to treat these devastating disorders.
In summary, we propose that the elevation of plasma GABA levels indicates that a significant alteration in central GABAergic function is present in PWS and AS subjects and that these alterations may be involved in mediating aspects of the pathophysiology of these genetic disorders. We recognize that our sample sizes are comparatively small and these results should thus be considered preliminary, especially in view of the 5 PWS subjects who had GABA levels within the range observed in the nonretarded obese subjects. Studies in additional patient and control groups are currently under way.
Acknowledgments
This work was partially supported by Grant PO-1 H030329-01 A2 (National Institute of Child Health and Human Development).
References
- 1.Prader A, Labhart A, Willi H. Ein Syndrome von Adipoitas, Kleinwuchs, Kryptochismus und Oligophrenie nach myatonieartigem Zustand in Neugeborenenalter [Syndrome of adiposity, short stature, cryptorchidism, and mental retardation after hypotonia in the newborn period] Schweiz Med Wochenschr. 1956;86:1260–1261. [Google Scholar]
- 2.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MG. Prader-Willi and Angelman syndromes: examples of genetic imprinting in man. In: Seth PK, Seth S, editors. Human Genetics. New Delhi, India: Omega Scientific; 1993. pp. 185–200. [Google Scholar]
- 4.Cassidy SB. Prader-Willi syndrome. Curr Probl Pediatr. 1984;14:1–55. doi: 10.1016/0045-9380(84)90043-4. [DOI] [PubMed] [Google Scholar]
- 5.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23:793–809. doi: 10.1002/ajmg.1320230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascari MJ, Ladda RL, Gottlieb W, et al. Molecular diagnosis in Prader-Willi syndrome identifies a common occurrence of maternal uniparental disomy. New Engl J Med. 1992;326:1599–1607. doi: 10.1056/NEJM199206113262404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoll JHM, Wagstaff J, Lalande M. Cytogenetic and molecular studies in the Prader-Willi and Angelman syndromes: an overview. Am J Med Genet. 1993;46:2–6. doi: 10.1002/ajmg.1320460103. [DOI] [PubMed] [Google Scholar]
- 8.Lai LW, Erickson RP, Cassidy SB. Clinical correlates of chromosome 15 deletions and maternal disomy in Prader-Willi syndrome. Am J Dis Child. 1993;147:1217–1223. doi: 10.1001/archpedi.1993.02160350091014. [DOI] [PubMed] [Google Scholar]
- 9.Angelman H. Puppet children: a report on three cases. Dev Med Child Neurol. 1965;7:681–688. doi: 10.1111/j.1469-8749.2008.03035.x. [DOI] [PubMed] [Google Scholar]
- 10.Williams CA, Gray BA, Hendrickson JE, et al. Incidence of 15q deletions in the Angelman syndrome: a survey of twelve affected persons. Am J Med Genet. 1989;32:339–345. doi: 10.1002/ajmg.1320320313. [DOI] [PubMed] [Google Scholar]
- 11.Yamada KA, Volpe JJ. Angelman syndrome in infancy. Dev Med Child Neurol. 1990;32:1005–1021. doi: 10.1111/j.1469-8749.1990.tb08124.x. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls RD, Knoll JHM, Butler MG, et al. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989;342:281–285. doi: 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen RW, Tobin AJ. Molecular biology of GABA-A receptors. FASEB J. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- 14.Kuriyama K, Hirouchi M, Nakayasu H. Structure and function of cerebral GABA-A and GABA-B receptors. Neurosd Res. 1994;17:91–99. doi: 10.1016/0168-0102(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 15.Wagstaff J, Knoll JHM, Fleming J, et al. Localization of the gene encoding the GABA-A receptor β3 subunit to the Angelman/Prader-Willi region of human chromosome 15. Am J Hum Genet. 1991;49:330–337. [PMC free article] [PubMed] [Google Scholar]
- 16.Lalande M, Sinnett D, Glatt K, et al. Fine mapping of the GABA-A receptor subunit gene cluster in chromosome 15q11q13 and assignment of GABRG3 to this region: report of the Second International Workshop of Human Chromosome 15 Mapping. Cytogenetic Cell Genet. 1994;67:17. [Google Scholar]
- 17.Dahir GA, Butler MG. Is GABA-A receptor β3 subunit abnormality responsible for obesity in persons with Prader-Willi syndrome? Dys-morphology and Clinical Genetics. 1991;5:112–113. [Google Scholar]
- 18.Schechter PJ, Sjoerdsma A. Clinical relevance of measuring GABA concentrations in cerebrospinal fluid. Neurochem Res. 1990;15:419–423. doi: 10.1007/BF00969927. [DOI] [PubMed] [Google Scholar]
- 19.Uhlhaas S, Lange H, Wappenschmidt J, et al. Free and conjugated CSF and plasma GABA in Huntington’s chorea. Acta Neurol Scand. 1986;74:261–265. doi: 10.1111/j.1600-0404.1986.tb03511.x. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W. GABA in plasma and cerebrospinal fluid of different species: effects of gamma-acetylenic GABA, gamma-vinyl GABA and sodium valproate. J Neurochem. 1979;32:342–351. doi: 10.1111/j.1471-4159.1979.tb11104.x. [DOI] [PubMed] [Google Scholar]
- 21.Loscher W, Frey HH. Transport of GABA at the blood-CSF interface. J Neurochem. 1982;38:1072–1079. doi: 10.1111/j.1471-4159.1982.tb05350.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferkany JW, Butler IJ, Enna SJ. Effect of drugs on rat brain, cerebrospinal and blood GABA content. J Neurochem. 1979;33:29–33. doi: 10.1111/j.1471-4159.1979.tb11702.x. [DOI] [PubMed] [Google Scholar]
- 23.Bohlen P, Huot S, Palfreyman MG. The relationship between GABA concentrations in brain and cerebrospinal fluid. Brain Res. 1979;167:297–305. doi: 10.1016/0006-8993(79)90824-2. [DOI] [PubMed] [Google Scholar]
- 24.Record CO. Plasma and brain amino acids in fulminating hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976;6:387–394. doi: 10.1111/j.1365-2362.1976.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 25.Mans AM, Kukulka KM, McAvoy KJ, et al. Regional distribution and kinetics of three sites on the GABA receptor: lack of effect of portocaval shunting. J Cereb Blood Flow Metab. 1992;12:334–346. doi: 10.1038/jcbfm.1992.46. [DOI] [PubMed] [Google Scholar]
- 26.Rundfeldt C, Loscher W. Development of tolerance to the anticonvulsant effect of vigabatrin in amygdaloid kindled rats. Eur J Pharmacol. 1992;213:351–366. doi: 10.1016/0014-2999(92)90624-d. [DOI] [PubMed] [Google Scholar]
- 27.Petty F, Kramer GL, Fulton M, et al. Low plasma GABA is a trait-like marker for bipolar illness. Neuropsychopharmacology. 1993;9:125–132. doi: 10.1038/npp.1993.51. [DOI] [PubMed] [Google Scholar]
- 28.Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino neurotransmitters from brain issue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- 29.Tantravahi U, Nicholls RD, Stroh H, et al. Quantitative calibration and use of DNA probes for investigating chromosome abnormalities in the Prader-Willi syndrome. Am J Med Genet. 1989;33:78–87. doi: 10.1002/ajmg.1320330110. [DOI] [PubMed] [Google Scholar]
- 30.Nicholls RD, Knoll JHM, Glatt K, et al. Restriction fragment length polymorphisms within proximal 15q and their use in molecular cytogenetics and the Prader-Willi syndrome. Am J Med Genet. 1989;33:66–77. doi: 10.1002/ajmg.1320330109. [DOI] [PubMed] [Google Scholar]
- 31.Butler MG, Dahir GA, Schwartz HS. Molecular analysis of transforming growth factor beta in giant cell tumor of bone. Cancer Genet Cytogenet. 1993;66:108–112. doi: 10.1016/0165-4608(93)90237-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutiranguri A, Jayakumar A, Sutcliffe JS, et al. A complete YAC contig of the Prader-Willi/Angelman chromosome region (15q11q13) and refined localization of the SNRPN gene. Genomics. 1993;18:546–552. doi: 10.1016/s0888-7543(11)80011-x. [DOI] [PubMed] [Google Scholar]
- 33.Mutiranguri A, Greenberg F, Butier MG, et al. Muitplex PCR of three dinucleotide repeats in the Prader-Willi/Angelman critical region (15q11q13): molecular diagnosis and mechanism of uniparental disomy. Hum Mol Genet. 1993;2:143–151. doi: 10.1093/hmg/2.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhlhass VS, Olek K. Konzentration von frier und gebunderner 4-aminobuttersaure immenschlichen serum [Concentration of free and bound 4-aminobutyric acid in human serum] J Clin Chem Clin Biochem. 1985;23:525–528. [PubMed] [Google Scholar]
- 35.Zachmann M, Tocci P, Nyhan WL. The occurrence of γ-aminobutyric acid in human tissues other than brain. Journal of Biological Chemistry. 1966;241:1355–1358. [PubMed] [Google Scholar]
- 36.Bloom F, Iverson L. Localizing GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography. Nature (London) 1971;229:628–630. doi: 10.1038/229628a0. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto RR. GABA receptors: are cellular differences reflected in function? Brain Res Rev. 1989;14:203–225. doi: 10.1016/0165-0173(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 38.Kubota T, Niikawa N, Jinno Y, et al. GABA receptor beta-3 subunit gene is possibly paternally imprinted in humans. Am J Med Genet. 1994;49:452–453. doi: 10.1002/ajmg.1320490422. [DOI] [PubMed] [Google Scholar]
- 39.Butler MG, Forrest K, Hedges LK. Cytogenetic and molecular characterization of 57 individuals with Prader-Willi syndrome (abstract) Am J Hum Genet. 1994;55:A100. [Google Scholar]