Abstract
Modulating deep regions of the brain with noninvasive technology has challenged researchers for decades. In a new study, Grossman et al. leverage the emergence of a slowly oscillating “beat” from intersecting high-frequency electric fields to stimulate deep brain regions, opening a frontier in the biophysics and technology of brain stimulation.
As tools to treat neuropsychiatric disorders and to probe cognition, noninvasive techniques for stimulating the brain are an area of intense development. Electromagnetic approaches such as transcranial magnetic stimulation and transcranial direct current stimulation are at the forefront. One seemingly unavoidable limitation of these and other electromagnetic techniques is the inability to target deep brain regions. With noninvasive stimulation, the intensity of electromagnetic fields drops off with distance from the surface of the head, meaning that superficial brain areas are activated first. In this issue of Cell, Grossman et al. (2017) exploit a long-standing acoustical phenomenon to propose a form of noninvasive electrical brain stimulation capable of stimulating deep brain areas in a selective manner: “temporal interference” (TI) stimulation.
When two tones of similar frequency are simultaneously emitted, the envelope of the net signal oscillates at a low frequency equal to the difference of the two tones, known as the “beat frequency.” The authors adapt this concept to electrical stimulation, using two pairs of surface electrodes to concurrently apply 2 kHz and 2.01 kHz sinusoidal stimulation. This creates three classes of brain regions—two superficial regions exposed to an electric field at either 2 or 2.01 kHz with a fixed amplitude (flat envelope) and an intermediate brain region with a net electric field whose envelope oscillates at 10 Hz, the beat frequency (Figure 1). The underlying assumption of TI stimulation is that neurons are unresponsive to high-frequency stimulation with a flat envelope, while neurons will respond to the “beating” high-frequency stimulation, leading to selective stimulation of neurons in the intermediate (i.e., deep) brain region.
Figure 1. Temporal Interference for Noninvasive Electrical Stimulation of Deep Brain Regions.
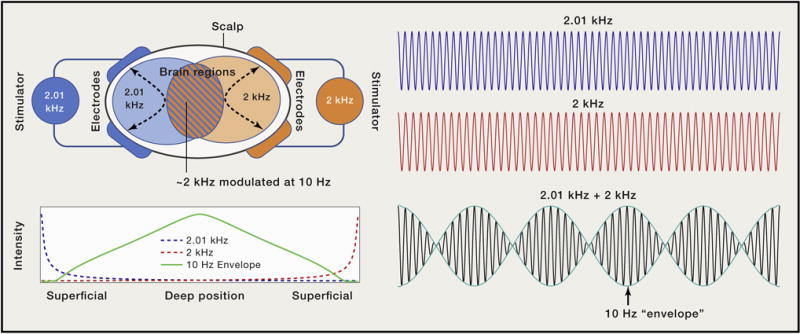
Two pairs of electrodes on the surface of the head concurrently deliver electric currents oscillating at high but slightly different frequencies (e.g., 2 kHz, blue; 2.01 kHz, orange). In the brain regions near each pair, high-frequency electric fields of corresponding frequency and fixed amplitude are generated. Where the two frequencies overlap, the superposition of the two sinusoids produces a slowly “beating” envelope at a frequency equal to the difference of the two sinusoids (e.g., 10 Hz, blue/orange stripes). While the intensity of the individual high-frequency electric fields is maximal at superficial regions, the intensity of the slow beat frequency peaks in the region marked by equal contribution of both signals, which coincides in this case with the middle of the brain.
Validation is not trivial: the same principle that renders high frequency ineffective, namely the low-pass properties of neuronal membranes (Bikson et al., 2004; Deans et al., 2007), would a priori be predicted to equally attenuate a beating high-frequency waveform.
Grossman et al. (2017) first applied TI stimulation to the skulls of anesthetized mice, reporting that stimulation with 2 and 2.01 kHz evokes firing at a rate of 10 Hz (matching the difference of the two applied frequencies) in the somatosensory cortex and hippocampus, as measured by patch clamp electro-physiology. In separate experiments, the authors used c-fos labeling to show that TI stimulation preferentially activates hippocampal over cortical neurons. In each case, control experiments with only 2 kHz stimulation produced no neuronal response, while direct 10 Hz stimulation produced diffuse activation. Supporting safety, there was no evidence for pathophysiological activity, brain temperature increase, or markers of brain injury.
The authors address the ability to “steerably” target various brain regions by changing electrode position or the applied current intensity. Finite element simulations of the electric field in a phantom and mouse head as well as in vivo experiments in mouse all suggest that spatial targeting may be achieved, at least with coarse resolution (i.e., lateralized cortical activation). The cellular biophysics explaining responsiveness to a beating high-frequency electric field remains an open question, limiting predictions of the focality that may be achieved with TI stimulation.
A key translational challenge will be demonstrating proof of concept in human. To extend the application of TI stimulation to the clinic, it will be essential to assess the role of human head anatomy in shaping both the magnitude and spatial distribution of TI-induced fields in the brain. Stimulation of human subjects through scalp electrodes is far less forgiving than epicranial in rodents (Bernabei et al., 2014; Bikson et al., 2016), with surface currents shunted by the skin, attenuated by the thick cranium, and further dispersed by the cerebrospinal fluid (Datta et al., 2009; Opitz et al., 2016; Huang et al., 2017). Moreover, validating the targeting of deep regions in humans will be challenging, as the tools available to image deep regions are limited: encephalography has poor spatial resolution, while the blood-oxygenation level dependent signal (BOLD) in functional MRI is difficult to relate to neural signaling. Realistic modeling of TI stimulation will support translational efforts, including determining the required current intensities and number of electrodes, and address the importance of customizing TI stimulation to individual anatomy (Datta et al., 2012).
To maximize the impact of the proposed technology, the limits of TI stimulation can be assessed with optimization techniques including, as mentioned by the authors, extension to arrays of electrodes. Whereas Grossman et al. (2017) experimented with manual adjustment of parameters such as inter-electrode distance, the development of automated algorithms (Dmochowski et al., 2011) will efficiently identify the optimal combination of electrode locations and applied intensities that steer the envelope of the beating electric field to the targeted brain region. As a first step, Grossman and colleagues suggest the feasibility of functionally meaningful noninvasive deep brain stimulation—a breakthrough if translated to clinical interventions as well as efforts to map human brain circuits.
Acknowledgments
Our research program is supported by grants fromthe NIH (grants 1R01NS101362-01, 1R01MH111896-01, 1R01NS095123-01, 1R01MH109289-01).
References
- Bernabei JM, Lee WH, Peterchev AV. Conf Proc IEEE Eng Med Biol Soc. 2014:406–409. doi: 10.1109/EMBC.2014.6943614. [DOI] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. J Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, et al. Brain Stimulat. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Brain Stimulat. 2009;2:201–207. 207.e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Truong D, Minhas P, Parra LC, Bikson M. Front Psychiatry. 2012;3:91. doi: 10.3389/fpsyt.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans JK, Powell AD, Jefferys JG. J Physiol. 2007;583:555–565. doi: 10.1113/jphysiol.2007.137711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. J Neural Eng. 2011;8:046011. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- Grossman N, Bono D, Dedic N, Kodandara-maiah SB, Rudenko A, Suk HJ, Cassara AM, Neufeld E, Kuster N, Tsai LH, et al. Cell. 2017;169:1029–1041. doi: 10.1016/j.cell.2017.05.024. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, Bikson M, Doyle WK, De-vinsky O, Parra LC. eLife. 2017;6:e18834. doi: 10.7554/eLife.18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz A, Falchier A, Yan CG, Yeagle EM, Linn GS, Megevand P, Thielscher A, Deborah A R, Milham MP, Mehta AD, Schroeder CE. Sci Rep. 2016;6:31236. doi: 10.1038/srep31236. [DOI] [PMC free article] [PubMed] [Google Scholar]


