Abstract
This article comments on:
Abu-Abied M, Belausov E, Hagay S, Peremyslov V, Dolja V, Sadot E. 2018. Myosin XI-K is involved in root organogenesis, polar auxin transport and cell division. Journal of Experimental Botany 69, 2869–2881.
Keywords: Arabidopsis, cell division, microtubules, MyoB, myosin XI, polar auxin transport, root organogenesis
Plant myosin XI motors are renowned for their function in cytoplasmic streaming. But the cell biology of myosin XI has taken an unexpected turn as a study by Abu-Abied et al. (2018) suggests a role of the motor in auxin transport and cell division plane alignment.
Only two types of myosins are present in green algae and land plants, myosin VIII and myosin XI, but in land plants each type is represented by a small gene family (Nebenfuhr and Dixit, 2018). Strictly speaking, there is one more myosin-like sequence present in the genomes of plants, the chimeric motor KCBP, which sports an N-terminal MyTH4-FERM domain with significant BLAST homology to myosin VII. However, the motor domain of KCBP is a 14-type kinesin (Reddy and Day, 2000). While a function of myosin VIII in cell division has been reported before, myosin XI is probably best known for its impact on cytoplasmic streaming (Tominaga et al., 2013). Indeed, some myosin XI motors are the fastest known cytoskeletal motors, and these are responsible for the remarkably vigorous cyclosis observed in Chara and related algae (Higashi-Fujime et al., 1995).
Recent research has revealed details of myosin XI-mediated motility in plants (Sparkes et al., 2008; Avisar et al., 2009), and it was found that specific myosin receptors, so-called MyoB proteins, are involved in myosin attachment to certain cargo (Peremyslov et al., 2013). Previous research has also used mutants to pinpoint the molecular functions of myosin XI. Knocking out myosin XI in Arabidopsis (frequently using a ‘3KO’ triple mutant of a group of closely related myosin XI genes) produced several phenotypes: trichomes, root hairs and pavement cells all showed defects in cellular differentiation (Peremyslov et al., 2010; Ojangu et al., 2012; Duan and Tominaga, 2018).
Myosin XI 3KO mutants have auxin-related and cell division phenotypes
The new paper by Abu-Abied et al. (2018) goes much further, reporting on analyses of the root growth of myosin 3KO plants in detail. Surprisingly, they found that the plants show several defects that point to the phytohormone auxin: myosin 3KO plants have surplus lateral roots, they mislocalize the PIN1 auxin exporters of stele cells and they show an aberrant auxin gradient in the root. But the authors found additional phenotypes: myosin 3KO plants show striking division-plane orientation defects in the stele, milder defects of the same type in endodermal/epidermal cells of the root meristem, and an increase in the time cells spend in mitosis and cytokinesis. There had been little evidence that myosin XI is involved in auxin signalling and cell division, and so the paper contributes a significant step forward in our understanding of myosin XI function in vascular plants.
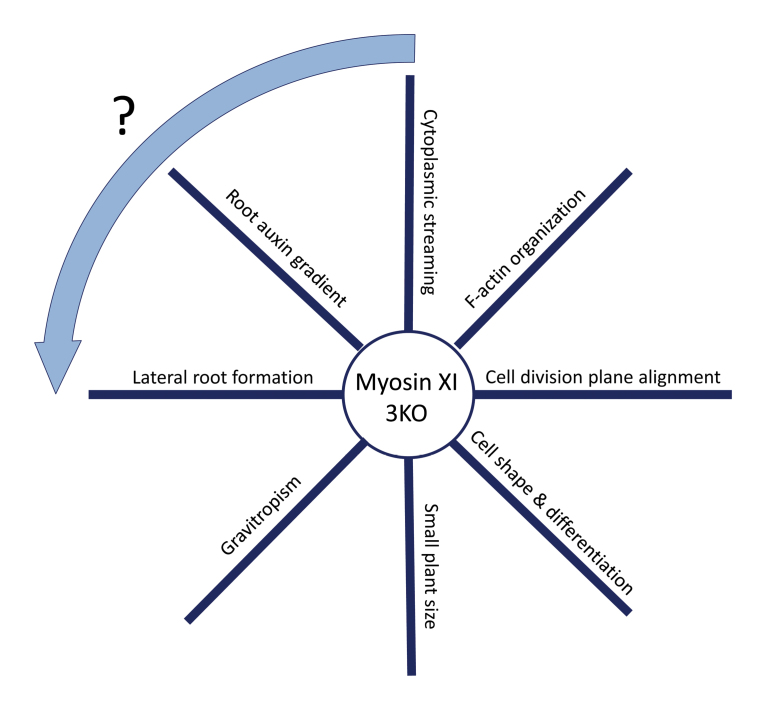
As exciting as these novel phenotypes may be, it is not that easy to put them into a simple coherent model. Are these phenotypes interrelated, and if yes, what is cause and what is effect? As humans we tend to infer causal relationships where sometimes there are just correlations. When speaking to my students I ask them initially to view the different phenotypes presented by a given mutant as entirely independent. I suggest comparing a set of phenotypes with the spokes of a wheel (see Box 1): a priori we know only about one causal relationship, which is the lack of a protein as the basis for all the separate phenotypes observed (here: the myosin XI motor). The establishment of further causal relationships needs careful experimental testing. Coming back to the myosin 3KO phenotypes presented by Abu-Abied and colleagues: could it be that the auxin effects are the basis for the cell division defects? Or is it rather the other way around?
Box 1. Phenotypic wheel for the Arabidopsis myosin 3KO mutant
The myosin 3KO mutant has many described phenotypes, as indicated on the spokes of the wheel (though not all phenotypes are depicted). It is tempting to speculate about causal relationships between these phenotypes, though this depiction emphasizes that we should initially view the different phenotypes presented by a given mutant as independent. However, one speculative interaction is indicated by the arrow.
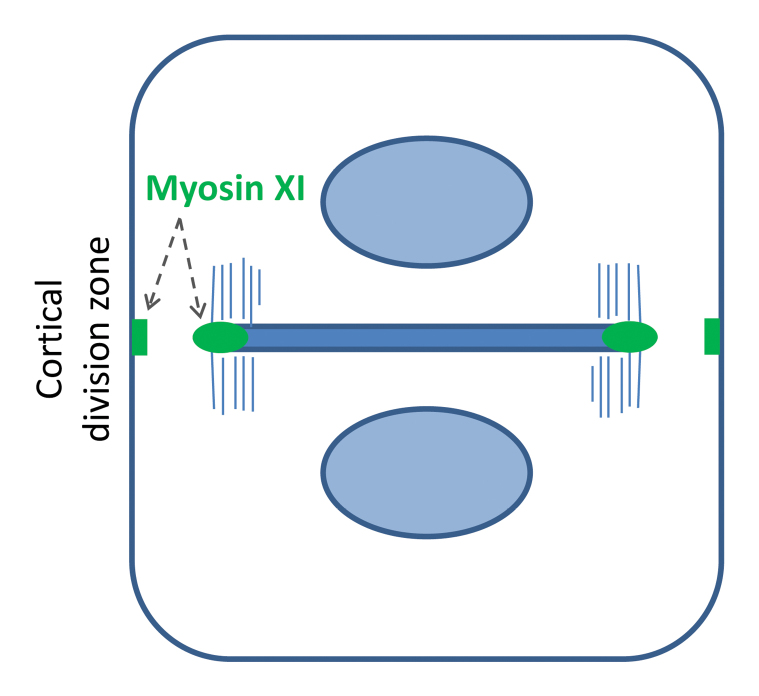
While this is a tricky question, Abu-Abied et al. provide some evidence that myosin XI has a genuine function in cell division, suggesting that the cell division phenotypes observed may be independent from the defect in auxin signalling. Plant cell division involves the succession of three microtubule arrays, the preprophase band, the mitotic spindle and the cytokinetic phragmoplast. The phragmoplast expands centrifugally and assembles in its midline the cell plate, the nascent division wall. In wild-type cells, the phragmoplast and cell plate grow back to the position on the parental membrane that was occupied and marked by the preprophase band before mitosis (Lloyd and Buschmann, 2007). By following a complemented 3KO mutant expressing YFP coupled to a myosin XI isotype through cell division, Abu-Abied et al. found that the motor associates with early cell plates and with the rim of the expanding cell plate during late cytokinesis. Interestingly, myosin XI of Arabidopsis is also found at the parental membrane for some time during pro-metaphase and then again during cytokinesis (Box 2). This membrane region is also known as the cortical division zone and is occupied by the preprophase band before nuclear envelope breakdown (Smertenko et al., 2017). Given that the myosin 3KO mutant has defects in division plane orientation, the localization pattern described may be taken as support for the idea that myosin XI has a function in cell plate guidance. In another recent paper, Sun et al. (2018) observed a similar localization of a myosin XI paralogue to cell plates of the moss Physcomitrella.
Box 2. Dividing plant cell and myosin XI localization
The diagram shows a cell at cytokinesis with the phragmoplast microtubules and cell plate highlighted. Myosin XI (green) localizes to the parental plasma membrane (the cortical division zone) and to the edge of the cell plate.
Have you seen the bridge?
Research on the mechanism of cell division plane orientation and, specifically, cell plate guidance has produced a set of novel molecular players in recent years. Most of the proteins discovered point directly towards involvement of the microtubule cytoskeleton, perhaps because the respective plant mutants have strong phenotypes. However, imaging and inhibitor studies have previously shown that the actin cytoskeleton must have an important role in the process of cell plate guidance (Traas et al., 1987; Sano et al., 2005). It is therefore safe to assume that the ‘bridge’ connecting the phragmoplast and cell plate with the plasma membrane area of the cortical division zone should be made of both F-actin and microtubules.
But the puzzle is incomplete as we know of only a handful of proteins involved and the mechanisms suggested to explain cell plate guidance are diverse (Lipka et al., 2014; Wu and Bezanilla, 2014; Buschmann et al., 2015). Importantly, recent reports suggest that myosins and a myosin-like sequence are involved. Myosin VIII, which was found to be present in the cortical division zone and on the phragmoplast of Physcomitrella was shown to interact with both F-actin and microtubules. Knockout mutants of myosin VIII showed division-plane orientation defects in the protonema of Physcomitrella (Wu and Bezanilla, 2014). Interestingly, the chimeric motor KCBP, which contains the myosin-like MyTH4-FERM domain in its N-terminus, is also found in the cortical division zone of dividing tobacco and Arabidopsis cells (Buschmann et al., 2015). The paper by Abu-Abied et al. now shows that myosin XI of Arabidopsis associates with the cortical division zone and with the expanding cell plate. Because the 3KO mutants of myosin XI in Arabidopsis show skewed division planes in the stele the analyses provide important molecular genetic evidence that myosin is involved in the division plane alignment of higher plants.
On new tracks
Together, the new results show that the cortical division zone of plants contains a group of myosins and myosin-like sequences. This alone is curious, as the cortical division zone itself is thought be F-actin deficient (Cleary, 1995; Sano et al., 2005). Non-plant eukaryotes divide by constriction using a contractile ring containing actin and myosin, while plants divide centrifugally using a phragmoplast with associated cell plate (Rasmussen et al., 2011; Cheffings et al., 2016). Perhaps the enigmatic cortical division zone of plants is simply a modified actomyosin ring without actin. The recent discovery of ROP (RHO of plants) signalling components localizing to the cortical division zone seems to support this notion (Zuo et al., 2014; Stöckle et al., 2016). However, the question arises as to what myosin is doing in the absence of actin in the cortical division zone. In the case of myosin VIII an interaction with microtubules of the phragmoplast periphery seems to be important. Given that myosin XI does not seem to interact with MyoB1 or MyoB2 receptors during cell division it is conceivable that this motor too has a microtubule-related function. One great challenge for plant cytokinesis research is to disentangle the contributions that actin and microtubule networks provide for cell plate guidance.
The paper by Abu-Abied (2018) shows convincing evidence that the 3KO myosin mutants have both auxin-related and cell division plane orientation defects. This in itself is remarkable as auxin gradients and division plane alignment are two major patterning devices employed by multicellular land plants (Buschmann and Zachgo, 2016; Du and Scheres, 2018). But it is perfectly possible that these are fully independent capacities of myosin XI: the auxin-related defects seen in myosin 3KO plants may result from dampened cytoplasmic streaming and concurrent effects on PIN1 transporter trafficking while the cytokinetic phenotype of myosin 3KO plants may point towards a direct role of myosin XI in cell plate guidance (as discussed above). Future research can clarify whether there is cross-talk between auxin transport and cell division plane orientation in the stele cells of Arabidopsis.
References
- Abu-Abied M,Belausov E,Hagay S,Peremyslov V,Dolja V,Sadot E.. 2018Myosin XI-K is involved in root organogenesis, polar auxin transport and cell division.Journal of Experimental Botany 69,2869–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avisar D,Abu-Abied M,Belausov E,Sadot E,Hawes C,Sparkes IA.. 2009A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles.Plant Physiology 150,700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann H,Dols J,Kopischke S,Peña EJ,Andrade-Navarro MA,Heinlein M,Szymanski DB,Zachgo S,Doonan JH,Lloyd CW.. 2015Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain.Journal of Cell Science 128,2033–2046. [DOI] [PubMed] [Google Scholar]
- Buschmann H,Zachgo S.. 2016The evolution of cell division: from streptophyte algae to land plants.Trends in Plant Science 21,872–883. [DOI] [PubMed] [Google Scholar]
- Cheffings TH,Burroughs NJ,Balasubramanian MK.. 2016Actomyosin ring formation and tension generation in eukaryotic cytokinesis.Current Biology 26,R719–R737. [DOI] [PubMed] [Google Scholar]
- Cleary AL. 1995F-actin redistributions at the division site in living Tradescantia stomatal complexes as revealed by microinjection of rhodamine-phalloidin.Protoplasma 185,152–165. [Google Scholar]
- Du Y,Scheres B.. 2018Lateral root formation and the multiple roles of auxin.Journal of Experimental Botany 69,155–167. [DOI] [PubMed] [Google Scholar]
- Duan Z,Tominaga M.. 2018Actin-myosin XI: an intracellular control network in plants.Biochemical and Biophysical Research Communicationsdoi: 10.1016/j.bbrc.2017.12.169. [DOI] [PubMed] [Google Scholar]
- Higashi-Fujime S,Ishikawa R,Iwasawa H,Kagami O,Kurimoto E,Kohama K,Hozumi T.. 1995The fastest actin-based motor protein from the green algae, Chara, and its distinct mode of interaction with actin.FEBS Letters 375,151–154. [DOI] [PubMed] [Google Scholar]
- Lipka E,Gadeyne A,Stöckle D,Zimmermann S,De Jaeger G,Ehrhardt DW,Kirik V,Van Damme D,Müller S.. 2014The phragmoplast-orienting kinesin-12 class proteins translate the positional information of the preprophase band to establish the cortical division zone in Arabidopsis thaliana.The Plant Cell 26,2617–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C,Buschmann H.. 2007Plant division: remembering where to build the wall.Current Biology 17,R1053–R1055. [DOI] [PubMed] [Google Scholar]
- Nebenfuhr A,Dixit R.. 2018Kinesins and myosins: molecular motors that coordinate cellular functions in plants.Annual Review of Plant Biologydoi: 10.1146/annurev-arplant-042817-040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojangu EL,Tanner K,Pata P,Jarve K,Holweg CL,Truve E,Paves H.. 2012Myosins XI-K, XI-1, and XI-2 are required for development of pavement cells, trichomes, and stigmatic papillae in Arabidopsis.BMC Plant Biologydoi: 10.1186/1471-2229-12-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV,Morgun EA,Kurth EG,Makarova KS,Koonin EV,Dolja VV.. 2013Identification of myosin XI receptors in Arabidopsis defines a distinct class of transport vesicles.The Plant Cell 25,3022–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV,Prokhnevsky AI,Dolja VV.. 2010Class XI myosins are required for development, cell expansion, and F-Actin organization in Arabidopsis.The Plant Cell 22,1883–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen CG,Humphries JA,Smith LG.. 2011Determination of symmetric and asymmetric division planes in plant cells.Annual Review of Plant Biology 62,387–409. [DOI] [PubMed] [Google Scholar]
- Reddy AS,Day IS.. 2000The role of the cytoskeleton and a molecular motor in trichome morphogenesis.Trends in Plant Science 5,503–505. [DOI] [PubMed] [Google Scholar]
- Sano T,Higaki T,Oda Y,Hayashi T,Hasezawa S.. 2005Appearance of actin microfilament ‘twin peaks’ in mitosis and their function in cell plate formation, as visualized in tobacco BY-2 cells expressing GFP-fimbrin.The Plant Journal 44,595–605. [DOI] [PubMed] [Google Scholar]
- Smertenko A,Assaad F,Baluška F, et al. . 2017Plant cytokinesis: terminology for structures and processes.Trends in Cell Biology 27,885–894. [DOI] [PubMed] [Google Scholar]
- Sparkes IA,Teanby NA,Hawes C.. 2008Truncated myosin XI tail fusions inhibit peroxisome, Golgi, and mitochondrial movement in tobacco leaf epidermal cells: a genetic tool for the next generation.Journal of Experimental Botany 59,2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckle D,Herrmann A,Lipka E,Lauster T,Gavidia R,Zimmermann S,Müller S.. 2016Putative RopGAPs impact division plane selection and interact with kinesin-12 POK1.Nature Plants 2,16120. [DOI] [PubMed] [Google Scholar]
- Sun H,Furt F,Vidali L.. 2018Myosin XI localizes at the mitotic spindle and along the cell plate during plant cell division in Physcomitrella patens.Biochemical and Biophysical Research Communicationsdoi: 10.1016/j.bbrc.2018.01.082. [DOI] [PubMed] [Google Scholar]
- Tominaga M,Kimura A,Yokota E,Haraguchi T,Shimmen T,Yamamoto K,Nakano A,Ito K.. 2013Cytoplasmic streaming velocity as a plant size determinant.Developmental Cell 27,345–352. [DOI] [PubMed] [Google Scholar]
- Traas JA,Doonan JH,Rawlins DJ,Shaw PJ,Watts J,Lloyd CW.. 1987An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus.The Journal of Cell Biology 105,387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SZ,Bezanilla M.. 2014Myosin VIII associates with microtubule ends and together with actin plays a role in guiding plant cell division.eLife 3,e03498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y,Oh W,Frost JA.. 2014Controlling the switches: Rho GTPase regulation during animal cell mitosis.Cellular Signalling 26,2998–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]