Abstract
Background and Aims
Low-dose naltrexone [LDN] is a controversial off-label treatment used by many Crohn’s disease [CD] and ulcerative colitis [UC] patients. A small number of preliminary studies indicate that LDN might be beneficial in CD, but evidence is too scarce to demonstrate efficacy. We sought to examine whether initiation of LDN therapy by patients with inflammatory bowel disease [IBD] was followed by changes in dispensing of relevant medication.
Methods
We performed a quasi-experimental before-and-after study following a sudden increase in LDN use in the Norwegian population in 2013. IBD patients were identified from among all the patients who had at least one LDN prescription recorded in the Norwegian Prescription Database [NorPD] in 2013. Drug dispensing 2 years before and after the first LDN prescription was compared.
Results
We identified 582 IBD patients who had received LDN. Of the 256 patients who became persistent LDN users, there were reductions in the number of users for [i] all examined drugs [–12%], [ii] intestinal anti-inflammatory agents [–17%], [iii] other immunosuppressants [–29%], [iv] intestinal corticosteroids [–32%] and [v] aminosalicylates [–17%]. In subgroups of identified CD and UC patients, there were significant reductions in the number of users of intestinal corticosteroids [CD: –44%, UC: –53%] and systemic corticosteroids [UC: –24%]. No significant differences in cumulative defined daily doses were observed.
Conclusions
Our findings imply that the initiation of LDN in IBD is followed by reduced dispensing of several drugs considered essential in the treatment of CD and UC.
Keywords: Low, dose naltrexone, Crohn’s disease, ulcerative colitis
1. Introduction
In the past few decades, a growing number of patients and doctors have been advocating off-label use of the opioid antagonist naltrexone in low doses [typically <5mg/day] in a number of conditions, mostly autoimmune. The only approved indications for naltrexone are opioid and alcohol addiction.1,2 A limited commercial potential has probably prevented the initiation of robust clinical studies in other conditions.
Low-dose naltrexone [LDN] is used in inflammatory bowel disease [IBD], and in the internet community CureTogether in January 2018, LDN was rated by patients as the most effective among 48 Crohn’s disease [CD] treatments, and ulcerative colitis [UC] patients reported LDN as being almost as effective as Remicade.3,4 Despite some small promising studies and case reports,5,6 a 2014 meta-analysis found that the evidence is insufficient to conclude whether LDN is safe and efficacious in the treatment of active Crohn’s disease.7
In Norway, the number of incident LDN users increased from almost none to more than 0.3 percent of the population [>15 000 patients] within few months following a TV documentary on its alleged effects.8,9 This sudden and unprecedented increase in LDN could be considered a natural experiment that enables pharmacoepidemiological studies on the entire Norwegian population.
1.1. Objective
If there are beneficial effects of LDN, initiation of LDN therapy could be reflected in detectable changes in prescription patterns of drugs used to treat CD and UC. We investigated whether there were changes in the dispensing of IBD-relevant medication related to LDN use.
We sought to answer the following questions: Was initiation of LDN therapy followed by changes in the dispensing of medication used to treat IBD? If so, was the magnitude of change associated with the level of LDN exposure?
2. Materials and Methods
2.1. Study design, setting, resources and data source for the study
We performed a quasi-experimental study with before-and-after comparisons of the dispensing of medications used in the treatment of CD and UC. The Norwegian Prescription Database [NorPD] was our data source for identification and inclusion of patients, and for outcomes. This register contains information on all prescriptions dispensed to the entire Norwegian population living outside hospitals and nursing homes. NorPD is described in detail elsewhere.10 Encrypted patient and prescriber identity numbers allows identification of prescription patterns because patients and prescribers can be followed over time. Reimbursed prescriptions have ICD-10 or ICPC-2 diagnosis codes. For non-reimbursed drugs, indication for use is not recorded. Over-the-counter [OTC] drugs, medications distributed within hospitals and nursing homes, and products without a product identifying number [e.g. pharmacy-produced specialty products] are not recorded in NorPD. The database is hosted by the Norwegian Institute of Public Health. For a fee and after an application according to data access procedures, NorPD provided us with a data file of all prescriptions from January 1, 2009 to December 31, 2015 dispensed to Norwegian patients who had collected at least one LDN prescription [product identification code 361181] in 2013.11
2.2. Study subjects
The majority of the medications dispensed and recorded in NorPD are based on prescriptions from general practitioners, whose ICPC-2 reimbursement codes do not distinguish between different IBDs. For the primary outcomes, we included patients with ICPC-2 reimbursement codes for IBD [D94], and ICD-10 reimbursement codes for CD [ICD-10 K50] or UC [ICD-10 K51.*] in NorPD in 2009 or 2010. In addition, we included patients who received prescriptions for medicines that are almost exclusively used by IBD patients [mesalazine, olsalazine, balsalazine, and intestinal corticosteroids]. We did not use include patients who received prescriptions for other therapies such as TNF-α inhibitors, since they have several other non-IBD indications. At least two prescriptions fulfilling the criteria in Supplementary data 1 were required in order to increase the specificity of diagnosis. For the secondary analyses of CD and UC, we identified patients who within the observation period collected at least one prescription with a reimbursement code for CD [ICD-10 K50] or UC [ICD-10 K51.*]. Patients who died before 2013 and individuals using naltrexone before 2013 were excluded.
The patients included were stratified into three groups based on LDN exposure: LDN ×1 [one LDN prescription dispensed], LDN ×2–3 [two or three LDN prescriptions dispensed] and LDN ×4+ [four or more LDN prescriptions dispensed].
2.3. Outcome variables
We used the following NorPD variables: Encrypted person identifier for patient, birth year and sex, reimbursement code, Anatomical Therapeutic Chemical Classification [ATC] code, product identifying number, date of dispensing, and dispensed volume in Defined Daily Doses [DDDs], the World Health Organization’s official assumed average maintenance dose per day for a drug used for its main indication in adults. We defined the date of dispensing the first prescription for LDN to each included patient in 2013 as the index date.
The outcomes were the differences in dispensing recorded in the 2 years before the index date compared with that recorded in the 2 years after the index date, expressed as average cumulative DDDs and number of users in LDN exposure each group.
Three primary outcomes were defined and calculated in the identified IBD patients:
[i] Sum of all drugs being studied [systemic immunosuppressants + intestinal anti-inflammatory agents]
[ii] Systemic immunosuppressants: (TNF-α inhibitors + systemic corticosteroids + other systemic immunosuppressants [methotrexate, azathioprine, mercaptopurine, ciclosporin, and tacrolimus])
[iii] Intestinal anti-inflammatory agents [aminosalicylates + intestinal corticosteroids].
Secondary outcomes were the differences in DDDs and in the number of users of the individual outcome drugs, and all equivalent outcomes calculated in the study subjects identified as CD and UC patients, respectively.
In order to compare changes in dispensing to our study population with trends in dispensing to the general population, we collected data on the drugs being studied from the publicly available NorPD website.12 We calculated relative changes [as percentages] in the population-adjusted total use in DDDs of the outcome drug in the entire Norwegian population from 2011 + 2012 to 2013 + 2014, so that there were 2 years comparable with the before and after LDN observation periods of 2 years. Relative changes in the number of users of the drugs being studied from 2012 to 2014 were also calculated.
2.4. Measurement
For each included study subject, we summarized the number of collected DDDs and the number of users for each drug being studied two full years [730 days] before the index date and 2 years after the index date [index date + 729 days]. The total observation time was 4 years for all included patients. The first observation date pre LDN was theoretically January 1, 2011, and the last observation date post LDN was December 31, 2015.
2.5. Bias
We expected newly diagnosed patients to have larger changes in medication compared with patients who were followed throughout the entire observation period. Inclusion was therefore based on NorPD data from the 2 years [2009 and 2010] preceding the observation period [2011–2015]. To increase the specificity of CD and UC diagnoses, these were based on reimbursement codes in the observation period, rather than in the inclusion period.
2.6. Statistical considerations
The number of patients fulfilling our inclusion criteria in NorPD determined the study size. We prepared data for analyses in SPSS 23 and Excel 2013. Data on DDDs were handled on an individual, non-aggregated level. A pairwise t-test was used to determine the significance of mean changes in the sum of the DDDs per patient in each group for all examined drugs, and 95% confidence intervals for difference of means were calculated. Change in the number of users was expressed as the proportion of each cohort, together with the 95% confidence interval for difference of proportion.13 Daily dispensing data was recorded, and this was used to construct curves to illustrate the timing of dispensing throughout the observation period.
2.7. Ethical statement
The Regional Committee for Medical and Health Research Ethics of Northern Norway reviewed the study protocol. The committee concluded that disclosure was not mandatory, since the data were encrypted. The project was approved by the local privacy ombudsman for research at the University Hospital of Northern Norway. Consent from individual patients is by law not required for research based on NorPD.
3. Results
3.1. Participants
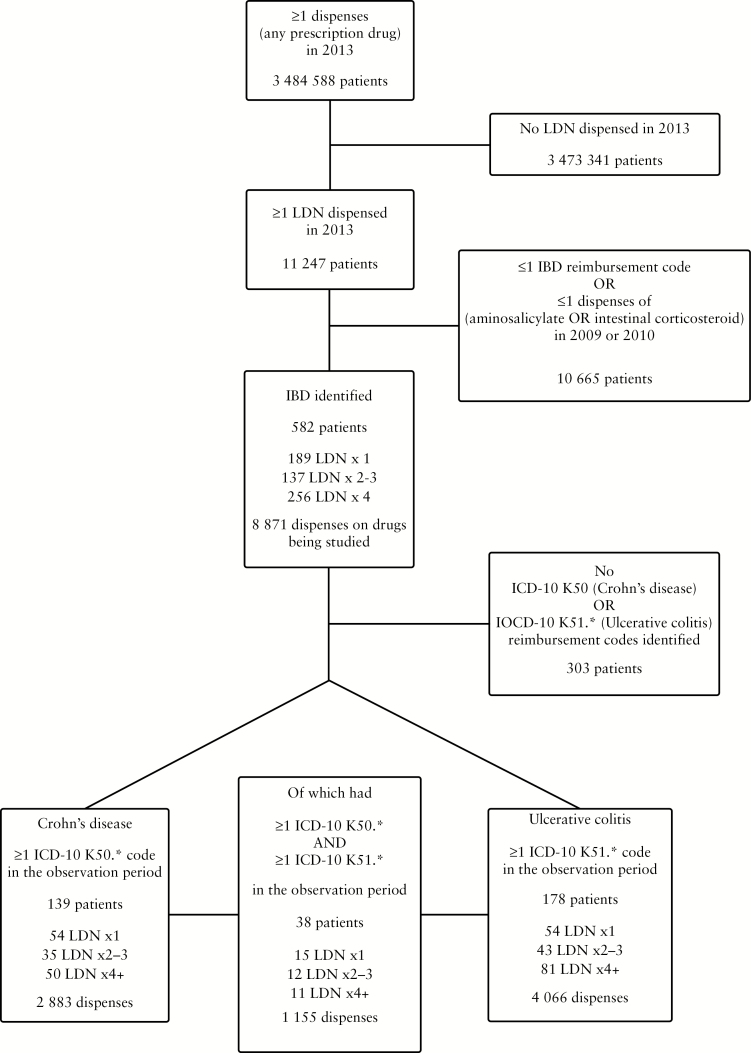
The patient inclusion logic is summarized in Figure 1. Of all recorded LDN users in Norway in 2013, 6.6% met our IBD inclusion criteria. All prescriptions collected by the included patients 2 years before and after their first LDN prescription were available for the analyses, totalling 27 936 patient months. There were 4 686 prescriptions dispensed before and 4 185 prescriptions dispensed after the index dates. We identified at least one ICD-10 CD [K50] or UC [K51.*] reimbursement code in 48% of the included IBD patients. In 6.5% of IBD patients, both K50 and K51.* occurred.
Figure 1.
Flowchart showing the inclusion of study subjects. Outcomes were analyzed separately in identified inflammatory bowel disease [IBD], Crohn’s disease and ulcerative colitis patients.
The baseline data for the three LDN exposure groups of IBD, CD and UC patients are presented in Table 1. The proportion of females was lower in the LDN ×1 groups, and the proportion of users of relevant medication was higher in the LDN ×4+ groups. If subject to hypothesis testing, none of the differences in the baseline data between LDN groups within each study subpopulation would be significant. In UC patients, there were more users of aminosalicylates and fewer users of intestinal corticosteroids, TNF-α inhibitors and other systemic immunosuppressants than in the CD patients.
Table 1.
Baseline data.
| LDN ×1 | LDN ×2–3 | LDN ×4+ | ||
|---|---|---|---|---|
| N [%] | IBD [N = 582] | 189 [32.5] | 137 [23.5] | 256 [44.0] |
| Crohn’s disease [N = 139] | 54 [38.8] | 35 [25.2] | 50 [36.0] | |
| Ulcerative colitis [N = 178] | 54 [30.3] | 43 [24.2] | 81 [45.5] | |
| Age in 2013 [± SD] | IBD | 48.7 [± 16.0] | 48.7 [± 13.5] | 50.0 [± 13.7] |
| Crohn’s disease | 42.4 [± 14.6] | 45.7 [± 14.3] | 41.7 [± 13.3] | |
| Ulcerative colitis | 44.7 [± 15.2] | 45.5 [± 14.3] | 48.7 [± 13.7] | |
| Females [%] | IBD | 152 [60.3] | 119 [70.8] | 210 [67.5] |
| Crohn’s disease | 32 [59.3] | 30 [85.7] | 31 [62.0] | |
| Ulcerative colitis | 24 [44.4] | 26 [60.5] | 48 [59.3] | |
| Users [2 years before first LDN prescription] of [%] | ||||
| All drugs being studied | IBD | 139 [73.5] | 109 [79.6] | 216 [84.4] |
| Crohn’s disease | 50 [92.6] | 30 [85.7] | 47 [94.0] | |
| Ulcerative colitis | 49 [90.7] | 37 [86.0] | 79 [97.5] | |
| Systemic glucocorticoids | IBD | 71 [37.6] | 60 [43.8] | 107 [41.8] |
| Crohn’s disease | 25 [46.3] | 19 [54.3] | 26 [52.0] | |
| Ulcerative colitis | 30 [55.6] | 24 [55.8] | 42 [51.9] | |
| TNF α inhibitors | IBD | 32 [16.9] | 14 [10.2] | 27 [10.5] |
| Crohn’s disease | 20 [37.0] | 8 [22.9]] | 10 [20.0] | |
| Ulcerative colitis | 11 [20.4] | 5 [11.6] | 10 [12.3] | |
| Other systemic immunosuppressants | IBD | 29 [20.6] | 23 [16.8] | 45 [17.6] |
| Crohn’s disease | 22 [40.7] | 10 [28.6] | 21 [42.0] | |
| Ulcerative colitis | 14 [25.9] | 16 [37.2] | 14 [17.3] | |
| Aminosalicylates | IBD | 89 [47.1] | 58 [42.3] | 139 [54.3] |
| Crohn’s disease | 22 [40.7] | 12 [34.3] | 22 [44.0] | |
| Ulcerative colitis | 46 [85.2] | 35 [81.4] | 71 [87.7] | |
| Intestinal corticosteroids | IBD | 42 [22.2] | 40 [29.2] | 53 [20.7] |
| Crohn’s disease | 21 [38.9] | 17 [48.6] | 25 [50.0] | |
| Ulcerative colitis | 16 [29.6] | 19 [44.2] | 21 [25.9] | |
Changes in the use of the outcome drugs in the IBD patients identified are shown in Table 2 [DDDs] and Table 3 [number of users]. The results for CD are presented in tables in Supplementary data 2 [DDDs] and Supplementary data 3 [number of users]. The results for UC are given in Tables in Supplementary data 4 [DDDs] and Supplementary data 5. In Table 4, the changes in the various LDN groups within the IBD patients are compared with changes in the dispensing of the same drugs in corresponding periods of time for the entire Norwegian population.
Table 2.
Average cumulative dispensing of agents used in the treatment of inflammatory bowel disease in disease-modifying medication in defined daily dose [DDD] in Norwegian patients with ulcerative colitis 2 years before and after first dispense of low-dose naltrexone [LDN]. Three groups based on number of LDN dispenses: LDN ×1 [N = 189] collected LDN once, LDN ×2–3 [N = 137] two or three times and LDN ×4+ [N = 256] four or more times. Other systemic immunosuppressants include azathioprine, mercaptopurine, methotrexate cyclosporine and tacrolimus.
| Average DDD per patient | Difference of mean | |||||
|---|---|---|---|---|---|---|
| Before LDN | After LDN | DDD | [95% CI] | p | ||
| All drugs being studied | LDN ×1 | 525.4 | 536.6 | 11.2 | [–73.0 to 95.4] | 0.793 |
| LDN ×2–3 | 487.9 | 469.4 | –18.5 | [–81.0 to 44.1] | 0.560 | |
| LDN ×4+ | 589.7 | 538.0 | –51.8 | [–111.4 to7.9] | 0.089 | |
| Systemic immunosuppressants | LDN ×1 | 222.5 | 238.0 | 15.4 | [–32.5 to 63.4] | 0.526 |
| LDN ×2–3 | 177.8 | 188.9 | 11.1 | [–28.5 to 50.7] | 0.581 | |
| LDN ×4+ | 221.9 | 198.7 | –23.2 | [–55.5 to 9.0] | 0.158 | |
| Systemic corticosteroids | LDN ×1 | 104.4 | 93.7 | –10.7 | [–36.0 to 14.6] | 0.404 |
| LDN ×2–3 | 82.0 | 95.9 | 14.0 | [–15.7 to 26.8] | 0.354 | |
| LDN ×4+ | 126.5 | 107.3 | –19.3 | [–46.0 to 7.5] | 0.157 | |
| TNF-α inhibitors | LDN ×1 | 61.4 | 88.3 | 26.9 | [–7.6 to 61.3] | 0.126 |
| LDN ×2–3 | 50.9 | 54.6 | 3.7 | [–26.8 to 34.1] | 0.812 | |
| LDN ×4+ | 47.1 | 41.1 | –5.9 | [–21.4 to 9.6] | 0.451 | |
| Other systemic immunosuppressants | LDN ×1 | 56.7 | 56.0 | –0.7 | [–19.3 to 18.0] | 0.943 |
| LDN ×2–3 | 44.9 | 38.4 | –6.5 | [–20.3 to 7.3] | 0.351 | |
| LDN ×4+ | 48.3.8 | 50.3 | 2.0 | [–13.3 to 17.2] | 0.799 | |
| Intestinal anti-inflammatory agents | LDN ×1 | 302.9 | 298.6 | –4.2 | [–60.7 to 52.3] | 0.883 |
| LDN ×2–3 | 310.1 | 280.5 | –29.5 | [–71.5 to 12.4] | 0.166 | |
| LDN ×4+ | 367.8 | 339.3 | –28.5 | [–77.7 to 20.0] | 0.248 | |
| Intestinal corticosteroids | LDN ×1 | 30.6 | 32.8 | 2.2 | [–9.7 to 14.1] | 0.716 |
| LDN ×2–3 | 40.4 | 33.9 | –6.6 | [–20.1 to 7.0] | 0.339 | |
| LDN ×4+ | 33.7 | 21.7 | –12.0 | [–25.4 to 1.4] | 0.079 | |
| Aminosalicylates | LDN ×1 | 272.3 | 265.8 | –6.4 | [–60.9 to 48.0] | 0.816 |
| LDN ×2–3 | 269.6 | 246.7 | –23.0 | [–65.0 to 19.0] | 0.281 | |
| LDN ×4+ | 334.1 | 317.5 | –16.5 | [–59.6 to 26.5] | 0.449 | |
Table 3.
Number of prevalent users of disease modifying medication in Norwegian patients with inflammatory bowel disease two years before and after first dispense of low dose naltrexone (LDN). Three groups based on number of LDN dispenses: LDN x 1 (N = 189) collected LDN once, LDN x 2-3 (N = 137) two or three times and LDN x 4+ (N = 256) four or more times. Other systemic immunosuppressants include azathioprine, methotrexate, mercaptopurine, cyclosporine, and tacrolimus.
| Users before LDN | Users after LDN | % points change in number of users | p | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | 95% CI | ||||
| All outcome drugs | LDN x1 | 139 | 73.5 | 141 | 74.6 | 1.1 | (-6.8 to 9.0) | 0.793 |
| LDN x 2-3 | 109 | 79.6 | 98 | 71.5 | -8.0 | (-15.6 to -0.4) | 0.043 | |
| LDN x 4+ | 216 | 84.4 | 191 | 74.6 | -9.8 | (-15.3 to -4.2) | 0.001 | |
| Systemic immunosuppressants | LDN x1 | 93 | 49.2 | 95 | 50.3 | 1.1 | (-6.6 to 8.7) | 0.786 |
| LDN x 2-3 | 73 | 53.3 | 69 | 50.4 | -2.9 | (-11.0 to 5.2) | 0.481 | |
| LDN x 4+ | 137 | 53.5 | 121 | 47.3 | -6.3 | (-12.4 to -0.1) | 0.050 | |
| Systemic corticosteroids | LDN x1 | 71 | 37.6 | 71 | 37.6 | 0.0 | (-8.3 to 8.3) | 1.000 |
| LDN x 2-3 | 60 | 43.8 | 54 | 39.4 | -4.4 | (-12.9 to 4.2) | 0.319 | |
| LDN x 4+ | 107 | 41.8 | 92 | 35.9 | -5.9 | (-12.4 to 0.6) | 0.080 | |
| TNF-α inhibitors | LDN x1 | 32 | 16.9 | 30 | 15.9 | -1.1 | (-6.5 to 4.4) | 0.706 |
| LDN x 2-3 | 14 | 10.2 | 15 | 10.9 | 0.7 | (-4.8 to 6.3) | 0.797 | |
| LDN x 4+ | 27 | 10.5 | 25 | 9.8 | -0.8 | (-4.3 to 2.0) | 0.468 | |
| Other systemic immunosuppressants | LDN x1 | 39 | 20.6 | 33 | 17.5 | -3.2 | (-8.4 to 2.1) | 0.241 |
| LDN x 2-3 | 23 | 16.8 | 28 | 20.4 | 3.6 | (-3.2 to 10.5) | 0.299 | |
| LDN x 4+ | 45 | 17.6 | 32 | 12.5 | -5.1 | (-8.8 to -1.3) | 0.010 | |
| Intestinal anti-inflammatory agents | LDN x1 | 109 | 57.7 | 102 | 54.0 | -3.7 | (-11.8 to 4.4) | 0.371 |
| LDN x 2-3 | 82 | 59.9 | 72 | 52.6 | -7.3 | (-15.8 to 1.2) | 0.098 | |
| LDN x 4+ | 168 | 65.4 | 140 | 54.5 | -10.9 | (-17.0 to -4.7) | 0.001 | |
| Intestinal corticosteroids | LDN x1 | 42 | 22.2 | 46 | 24.3 | 2.1 | (-4.4 to 8.7) | 0.528 |
| LDN x 2-3 | 40 | 29.2 | 36 | 26.3 | -2.9 | (-11.7 to 5.9) | 0.518 | |
| LDN x 4+ | 53 | 20.7 | 36 | 14.1 | -6.6 | (-11.7 to -1.6) | 0.012 | |
| Aminosalicylates | LDN x1 | 89 | 47.1 | 78 | 41.3 | -5.8 | (-12.7 to 1.1) | 0.103 |
| LDN x 2-3 | 58 | 42.3 | 50 | 36.5 | -5.8 | (-11.8 to 0.2) | 0.061 | |
| LDN x 4+ | 139 | 54.3 | 116 | 45.3 | -9.0 | (-14.3 to -3.6) | 0.001 | |
Table 4.
Relative differences [%] in the dispensing of medication used in the treatment of inflammatory bowel disease [IBD] in 2011 and 2012 compared with 2013 and 2014 in defined daily doses [DDDs] per inhabitant in the entire Norwegian population. This is compared with corresponding relative changes 2 years before and after the first dispense of low-dose naltrexone to Norwegian patients with IBD. Relative differences in proportions of users in the population [N, %] from 2012 to 2014 compared with difference in number of users 2 years before and after LDN among IBD patients. Three groups based on number of LDN dispenses: LDN ×1 [N = 189] collected LDN once, LDN ×2–3 [N = 137] two or three times and LDN ×4+ [N = 256] four or more times. *p <0.05
| IBD patients [N = 582] | |||||
|---|---|---|---|---|---|
| Entire population | LDN ×1 | LDN ×2–3 | LDN ×4+ | ||
| All drugs being studied | DDD | 9.4 | 2.1 | –3.8 | –8.8 |
| N | 4.8 | 1.4 | –10.1* | –11.6* | |
| Systemic immunosuppressants | DDD | 4.7 | 7.0 | 6.2 | –10.5 |
| N | 8.1 | 2.2 | –5.5 | –11.7 | |
| Systemic corticosteroids | DDD | 3.6 | –10.2 | 17.0 | –15.2 |
| N | 3.2 | 0.0 | –10.0 | –14.0 | |
| TNF-α inhibitors | DDD | 25.7 | 43.8 | 7.3 | –12.7 |
| N | 17.2 | –6.3 | 7.1 | –7.4 | |
| Other systemic immunosuppressants | DDD | 16.8 | –1.2 | –14.5 | 4,1 |
| N | 11.1 | –15.4 | 21.7 | –28.9* | |
| Intestinal anti-inflammatory agents | DDD | 6.1 | –1.4 | –9.5 | –7.8 |
| N | 15.0 | –6.4 | –12.2 | –16.7* | |
| Intestinal corticosteroids | DDD | 10.7 | 7.2 | –16.1 | –35.5 |
| N | 9.4 | 9.5 | –10.0 | –32.1* | |
| Aminosalicylates | DDD | 15.3 | –2.4 | –8.5 | –5.0 |
| N | 5.2 | –12.4* | –13.8* | –16.5* | |
3.2. All drugs being studied
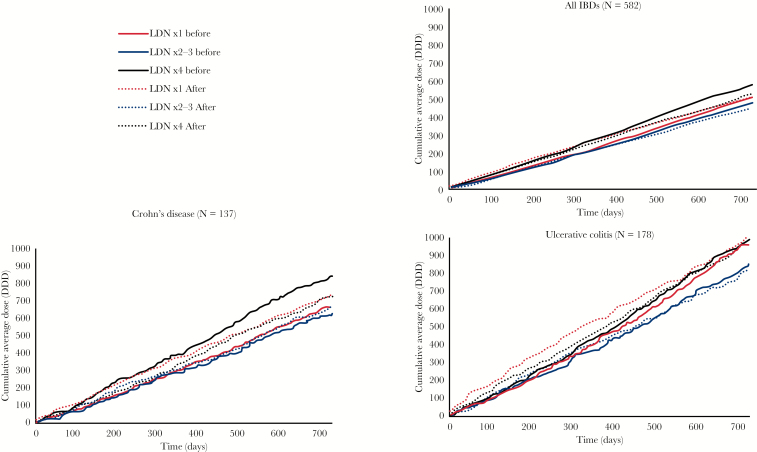
Among the identified IBD patients, there was a significant reduction in the number of users in the LDN ×2–3 [10%] and of number of users in the LDN ×4+ [12%] groups. In terms of DDDs, there were no significant before and after differences in any group. Analysis of data for patients associated with the CD reimbursement codes did not reveal significant differences in any of the drugs being studied. In UC, there was a significant 14% reduction in the number of users of any of the drugs being studied in the LDN ×2–3 group. As seen in Figure 2, the use of all drugs being studied among identified CD patients was consistently higher throughout the 2 years before index date compared with after in patients who became persistent users of LDN [LDN ×4+]. In contrast to the significantly fewer users of the drugs being studied in the LDN ×2–3 and LDN ×4+ groups, there was a 4.8% population-adjusted increase in users of the same drugs in the general Norwegian population [Table 4].
Figure 2.
Cumulative average defined daily doses [DDDs] of all drugs being studied by time before and after the first low-dose naltrexone [LDN] prescription. Solid lines show cumulative consumption for the 730 days preceding the first LDN dose, and dashed lines after.
3.3. Systemic immunosuppressants
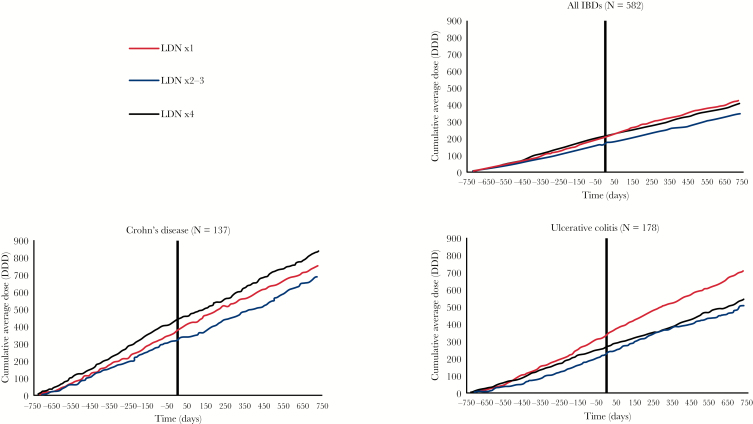
We found no significant differences in the dispensing of systemic immunosuppressants when total systemic immunosuppressants were aggregated for analysis. There was a 29% relative reduction in users of other immunosuppressants in the LDN ×4+ group, but there also was a [non-significant] 15% reduction in the LDN ×1 group. Among UC patients, the use of systemic corticosteroids was reduced by 24% in terms of number of users. In CD patients, no significant differences were observed.
In contrast to the findings for the LDN ×4 group, there was an increase in the use of all subgroups of immunosuppressants in the general population in the corresponding period [Table 4]. The cumulative dispensing of systemic immunosuppressants throughout the observation period is shown in Figure 3. For all IBD patients, there is a reduction in the pace of dispensing that coincides with the index date. In the figure in Supplementary data 6, we present the dispensing of the subgroups of systemic immunosuppressants. There was a striking 111% relative increase in the dispensing of TNF-α inhibitors in the UC LDN ×1 group that coincided with the initiation of LDN [see figures in Supplementary data 6 and 7], but this did not reach statistical significance. The increase in the number of TNF-α inhibitor users was similar in all UC groups.
Figure 3.
Cumulative average defined daily doses [DDDs] of systemic immunosuppressants throughout the entire 4-year observation period. The first LDN prescription was dispensed at time = 0. The sum of systemic corticosteroids, tumor necrosis factor α, mercaptopurine, methotrexate, azathioprine, cyclosporine and tacrolimus is displayed.
3.4. Intestinal anti-inflammatory agents
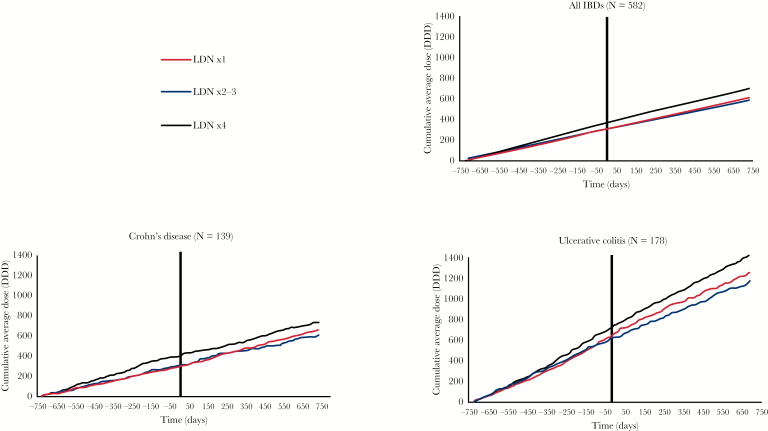
Significant changes in DDDs of intestinal anti-inflammatory agents before and after the first LDN prescription were not observed among the combined IBDs patient group, but there was a significant 17% relative reduction in the number of users of intestinal anti-inflammatory agents in the LDN ×4+ group. The LDN ×4+ group distinguished itself from the others, with a 32% relative reduction in the number of intestinal corticosteroid users. The number of aminosalicylate users was reduced in all groups, although this was not statistically significant in the LDN ×1 group.
The dispensing of intestinal anti-inflammatory agents was higher in UC than in CD patients [see Figure 3]. In identified UC patients, there was a 25% reduction in the number of users of these drugs in the LDN ×2–3 group. The number of users of intestinal corticosteroids in the LDN 4+ group was reduced by 44% in CD patients and 53% in UC patients. The dispensing of intestinal anti-inflammatory agents is shown in Figure 4 and of intestinal corticosteroids and aminosalicylates in the Figure in Supplementary data 7.
Figure 4.
Cumulative average defined daily doses [DDDs] of intestinal anti-inflammatory agents throughout the entire 4-year observation period. The first LDN prescription was dispensed at time = 0. The sum of aminosalicylates and intestinal corticosteroids is displayed.
4. Discussion
4.1. Main findings
This quasi-experimental before-and-after study, covering the entire Norwegian population over 4 years, is the first pharmacoepidemiological study on LDN in IBD. The initiation of LDN in patients with IBD was followed by reductions in the use of several agents that are considered essential in the treatment of Crohn’s disease and ulcerative colitis. For all drugs being studied, and for the examined systemic immunosuppressants, the reductions in number of users were larger in persistent LDN users than in IBD patients who collected LDN three times or less. Similar dose–response relationships with LDN exposure were seen for various drugs. These reductions were in contrast to increasing use of the same drugs in the general population. Some of the findings were confirmed in both the CD and UC subpopulations.
The results of this study show that the initiation of LDN was followed by reductions in dispensing of drugs with different mechanisms of action, and on therapy acting both systemically and locally in the gastrointestinal tract. Although no statistically significant differences in cumulative doses were observed, there was a trend towards increasing reduction of DDDs with increasing LDN exposure for all primary outcomes. There was a striking magnitude of change for some of the observed therapeutic groups. For example, in persistent LDN users, there was a reduction of DDDs for all outcome drugs by almost one-tenth, and the number of UC patients using intestinal corticosteroids was more than halved. We consider the observed changes in dispensing as clinically relevant. No significant difference-in-differences between groups were seen.
4.2. Strengths and limitations
The low commercial potential and the off-label status of LDN as a CD treatment are among the numerous obstacles to be overcome before implementation of randomized controlled trials. The sudden increase in LDN usage in Norway is probably the first opportunity to study this controversial therapy at the population level. The included patients not only served as their own controls, but three groups with different LDN exposure were compared. A major strength is that we used NorPD, a complete and reliable data source. In a study with a similar design, we found no association between the initiation of LDN and prescription patterns of relevant medication in multiple sclerosis.14
Before-and-after studies are often used to examine whether interventions implemented in a non-random manner cause change. The advantages are that such studies are relatively simple to perform, and that ethical challenges linked to randomized controlled trials are avoided. However, there are important limitations, and before-and-after studies are often considered to be the weakest quasi-experimental design.15 Three aspects require special attention:
[i] Bias in the identification of study subjects. In this study, we included only IBD patients who had collected LDN prescriptions. It could be argued that they were not representative IBD patients, and that their openness to new and alternative treatments may have led to selection bias. Although the baseline data show little difference between groups, it is possible that bias occurred in the grouping of the study subjects. This limitation could have been reduced by using a control group that was not exposed to LDN at all, but individuals naïve to LDN were not included in the available NorPD data, and our ethical committee approval would not cover such analyses. The baseline data indicate that there may have been differences in disease activity related to LDN exposure. On the other hand, with a before-and-after design, the study participants served as their own controls.
[ii] Temporal changes. The total observation time was 4 years for all included study subjects, and it is possible that our findings could be explained by the natural course of disease. A non-exposed control group would have shed light on this potential bias. We believe the inclusion of patients based on data 1–2 years before the observation period reduced this potential bias, among other things by excluding newly diagnosed [incident] IBD patients in the observation period. The differences between the groups in the dispensing patterns shown in the figures indicate that course of disease is an unlikely explanation for our findings.
[iii] Regression to a mean is a problem in before-and-after studies. As seen in Figure 2, the drop in DDDs for all drugs being studied in the LDN × 4+ group could be interpreted as regression to a mean. However, for intestinal corticosteroids in the LDN ×4+ group, this was not the case. Here, the number of users dropped more than in the LDN ×1 group, even though the LDN ×4+ group initially had the lowest number of users.
Correct information from the prescribing doctors is important for reliable inclusion of patients and analysis. The data suggest some imprecision in NorPD. One example is that only 52% of the included IBD patients were given a CD- or UC-specific reimbursement code, and 14% of these were given ICD-10 codes for both diseases on different prescriptions. However, the observed prescription patterns indicate that a large majority of the study subjects actually had IBD. For example, approximately 90% of the identified CD and UC patients used any of the drugs being studied before starting LDN. Possibly, patients with ICD-10 reimbursement codes experienced a more active phase of disease than did the patients who were handled solely by primary care. Codes used in primary care [ICPC-2] do not distinguish between CD and UC. Therefore, a selection bias towards patients with more severe disease is possible, since they received specific ICD-10 codes by a specialist in secondary care. This is reflected in the higher consumption of several drugs being studied. On the other hand, medication given directly to inpatients by hospitals is not recorded, which means that medication administered in the most severe flares may not have been captured.
LDN was first included in NorPD in 2013, so use before this was not recorded. Bias due to misclassification of exposure is possible, but we believe this would be negligible due to there being very few LDN-using patients in Norway before the TV documentary in February 2013.
We used NorPD data to identify study participants. The number of included patients was limited by the prevalence of LDN-using IBD patients in the entire Norwegian population. Previous research based on NorPD data has confirmed optimal and unbiased case finding in myasthenia gravis,16 and the database has been used to calculate reliable incidence rates of diabetes mellitus.17 Lower statistical power was achieved in the stratified analyses of CD and UC patients than for all IBD patients identified. Even though the study population was larger than in previous studies on LDN use in CD and UC, the results indicate that the number of included study subjects was insufficient to detect differences that could be clinically relevant. Similar, but not statistically significant, patterns in the changes in medication were seen in CD and UC as were seen in many of the findings in the total IBD analysis. We could have used less strict inclusion criteria, but this would have reduced the external validity.
We have not identified other pharmacoepidemiological studies on LDN apart from our own research. In a previous study with a similar design we documented a 46% reduction in opioid use among among persistent LDN users, whereas one-time users had no change in their opioid use.18
A few small clinical studies on LDN in CD have been promising. In an open-label study without a control group, in which 17 patients received 4.5 mg naltrexone daily for 12 weeks, 67% experienced remission.5 There were significant improvements in Crohn’s disease activity index [CDAI], quality of life measures, C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]. In a subsequent double-blind placebo-controlled trial including 40 patients with active CD,19 there was a significantly higher response rate among patients receiving LDN compared with the placebo, both in terms of remission defined by CDAI [88% vs 40%], by endoscopic response [78% vs 28%], and by histologic assessment of inflammation. Adverse effects were minimal in both of these studies. In a pilot study designed to evaluate safety and tolerability of LDN in 14 pediatric CD patients, significant clinical improvements were observed.20 Several patients with treatment-resistant IBD who responded on LDN have also been reported.6 The large number of study subjects and the long observation time are major strengths of our study compared with these clinical studies. A controlled study using a rat model of indomethacin-induced CD showed significant improvements following LDN treatment.21
There are several proposed mechanisms of action for the alleged effects in IBD. The gastrointestinal tract has a large number of opioid receptors, and antagonism of these could have therapeutic effects in the inflamed mucosa, both through direct effects on bowel motility and through the postulated opioid receptor–modulated immunologic effects. Interference with the regulation of endogenous opioids has also been suggested.22,23
The most important, though controversial, possible explanation of our findings, is that the patients who started LDN therapy experienced clinical improvement, and that the need for medication was decreased. It is important to emphasize that, at best, the observed changes in drug use may serve only as a proxy for efficacy. Our data do not provide any direct clinical information. It is plausible that clinical improvements are linked to reductions in drug dispensing in IBD, but there is insufficient evidence to conclude that this is a prudent efficacy measure in CD or UC.
Nevertheless, the observed reductions in drug use following LDN use should be interesting for patients, clinicians and for those who pay for health care. They could be an indication that LDN may play a role in reducing polypharmacy in this patient group, thereby reducing costs and risks of adverse effects. If actually proven efficacious in future studies, LDN could partially replace more expensive standard treatments. We believe the results of this study reinforce the need for more research on LDN use in IBD.
5. Conclusion
Our findings imply that the initiation of LDN in IBD is followed by reduced dispensing of several drugs considered essential in the treatment of CD and UC.
Funding
This work was supported by the institutions involved: Regional Medicines Information and Pharmacovigilance Centre [RELIS], University Hospital of North Norway, Tromsø, Norway and UiT – The Arctic University of Norway, Tromsø, Norway.
Conflict of Interest
All authors declare they have no conflicts of interest to disclose.
Author Contributions
GR contributed to the conception of the study, designed the study, performed statistical analyses and drafted the manuscript. PS contributed to analyses. LS contributed to the design of the study and to statistical analyses. All authors participated in revising the manuscript and approving the final version.
Supplementary Material
Acknowledgments
We would like to thank Frode Skjold for preparing the data files and Trude Giverhaug for encouragement, valuable cooperation and useful input to the manuscript.
Glossary
Abbreviations:
- LDN
low-dose naltrexone
- DDD
defined daily dose
- IBD
inflammatory bowel disease
- CD
Crohn’s disease
- UC
ulcerative colitis
- NorPD
Norwegian Prescription Database
- ATC
anatomical therapeutic chemical classification.
References
- 1. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2011; 4:CD001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev 2010;12:CD001867. [DOI] [PubMed] [Google Scholar]
- 3. CureTogether. 47 Crohn’s Disease Treatments Compared (Accessed January 2, 2018, at http://curetogether.com/crohns-disease/ig/treatment- effectiveness-vs-popularity).
- 4. CureTogether. 44 Ulcerative Colitis Treatments Compared (Accessed January, 2018, at http://curetogether.com/ulcerative-colitis/ig/treatment- effectiveness-vs-popularity).
- 5. Smith JP, Stock H, Bingaman S, Mauger D, Rogosnitzky M, Zagon IS. Low-Dose Naltrexone Therapy Improves Active Crohn’s Disease. Am J Gastroenterol 2007;102:820–8. [DOI] [PubMed] [Google Scholar]
- 6. Lie M, Fuhler G, de Lima A, van der Ent C, van der Woude CJ. P418 Low dose naltrexone in therapy resistant IBD, a case series. J Crohns Colitis 2014; 8:S240. [Google Scholar]
- 7. Segal D, MacDonald JK, Chande N, van der Ent C, van der Woude CJ. Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2014;2:CD010410. [DOI] [PubMed] [Google Scholar]
- 8. Raknes G, Småbrekke L. A sudden and unprecedented increase in low dose naltrexone (LDN) prescribing in Norway. Patient and prescriber characteristics, and dispense patterns. A drug utilization cohort study. Pharmacoepidemiol Drug Saf 2017;26:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skard K. Unknown medicine LDN gives hope to thousands of patients, 2013. (Accessed January 2, 2018, at https://www.youtube.com/watch?v=rBd2gv8UGU0).
- 10. Furu K. Establishment of the nationwide Norwegian Prescription Database (NorPD)–new opportunities for research in pharmacoepidemiology in Norway. Nor J Epidemiol 2008;18:129–36. [Google Scholar]
- 11. The Norwegian Institute of Public Health. Norwegian Prescription Database 2018. (Accessed January 2, 2018, at https://www.fhi.no/en/hn/health-registries/norpd/)
- 12. The Norwegian Institute of Public Health. Welcome to the Norwegian Prescription Database http://www.norpd.no/ Accessed January 2, 2018.
- 13. Altman DG. Comparing groups - categorical data. In: Altman DG, ed. Practical Statistics for Medical Research, 1edn. London: Chapman & Hall, 1991:234–41. [Google Scholar]
- 14. Raknes G, Småbrekke L. Low dose naltrexone in multiple sclerosis: Effects on medication use. A quasi-experimental study. PLoSONE 2017; 12(11): e0187423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torgerson DJ, Torgerson CJ. The limitations of before and after designs. In: Torgerson DJ, Torgerson CJ. Designing Randomised Trials in Health, Education and the Social Sciences. London: Palgrave Macmillan UK, 2008:9–16. [Google Scholar]
- 16. Andersen JB, Heldal AT, Engeland A, Gilhus NE. Myasthenia gravis epidemiology in a national cohort; combining multiple disease registries. Acta Neurol Scand Suppl. 2014;198: 26–31. [DOI] [PubMed] [Google Scholar]
- 17. Berg C, Strøm H. The Norwegian Prescription Database (NorPD) as a data source for diabetes research. Nor J Epidemiol 2013; 23:109–10.23269126 [Google Scholar]
- 18. Raknes G, Smabrekke L. Low dose naltrexone and opioid consumption. A drug utilization cohort study based on data from the Norwegian prescription database. Pharmacoepidemiol Drug Saf 2017;26:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith JP, Bingaman SI, Ruggiero F, Smith JP, Stock H, Bingaman S. Therapy with the Opioid Antagonist Naltrexone Promotes Mucosal Healing in Active Crohn’s Disease: A Randomized Placebo-Controlled Trial. Dig Dis Sci 2011;56:2088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith JP, Field D, Bingaman S, Evans R, Mauger DT. Safety and tolerability of low dose naltrexone therapy in children with moderate to severe crohn’s disease: a pilot study. J Clin Gastroenterol 2013; 47:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tawfik DI, Osman AS, Tolba HM, Khattab A, Abdel-Salam LO, Kamel MM. Evaluation of therapeutic effect of low dose naltrexone in experimentally-induced Crohn’s disease in rats. Neuropeptides 2016;59:39–45. [DOI] [PubMed] [Google Scholar]
- 22. Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses 2009;72:333–7. [DOI] [PubMed] [Google Scholar]
- 23. Zagon IS, McLaughlin PJ. Targeting opioid signaling in Crohn’s disease: new therapeutic pathways. Expert Rev Gastroenterol Hepatol. 2011;5:555–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.