This review is an assessment of the various factors that contribute to disparities in lung cancer between European Americans and African Americans and key knowledge gaps that remain.
Abstract
Compared with all other racial and ethnic groups in the United States, African Americans are disproportionally affected by lung cancer, both in terms of incidence and survival. It is likely that smoking, as the main etiological factor associated with lung cancer, contributes to these disparities, but the precise mechanism is still unclear. This paper seeks to explore the history of lung cancer disparities and review to the literature regarding the various factors that contribute to them.
Introduction
Compared with all other racial and ethnic groups in the United States, African Americans (AAs) are disproportionally affected by lung cancer, both in terms of incidence and survival (1–3). These differences were first formally noted in 1972 (4,5) and have been continuously observed since Surveillance Epidemiology and End Results (SEER) began data collection in 1973 (3). Currently, the age-adjusted lung cancer incidence rate is ~32% higher in AAs compared with European Americans (EAs), with disparities most predominant among men. On average, AAs are diagnosed with lung cancer 3 years earlier than EAs (6).
Approximately 156 000 people die from lung cancer every year, more than the next three most incident cancers combined. Since 1987, more women have died from lung cancer than breast cancer, while lung cancer in never smokers is the 7th leading cause of cancer-related death. There are several histological subtypes. Non-small cell lung cancer accounts for ~85% of new diagnoses, while small cell carcinoma accounts for the remaining 15%. Non-small cell lung cancer is heterogeneous: adenocarcinoma, squamous cell carcinoma and large cell carcinoma account for 40, 25 and 10%, respectively, with bronchalveolar carcinoma and carcinoid tumors largely accounting for the rest. The age-adjusted lung cancer incidence rate in AAs and EAs varies by histology. The disparity holds for adenocarcinoma, squamous cell carcinoma and large cell, though to differing degrees: In a cohort study of over 126 000 individuals in the Kaiser Permanente Medical Program in California, Tran and colleagues found that AAs had a 30% increased hazard of adenocarcinoma diagnosis, and a 70% increased hazard of squamous cell carcinoma diagnosis compared with EAs (2). The incidence of small cell carcinoma tends to be lower in AA. While these differences are not always statistically significant (2), the trend is opposite to what is seen for other histological subtypes.
This review seeks to explore the history of lung cancer disparities between EAs and AAs and assess the literature regarding the various factors that contribute to them. As it is not possible to cover all articles on this topic, the author expresses regret for any key papers that are not covered herein. Various terms are used to describe populations of African or European descent, including AA, non-Hispanic (NH) Black, EAs and NH white, among others. While acknowledging the various circumstances where variations in terminology are used (7), for the purposes of this review the author uses the terms ‘EA’ and ‘AA’.
Although this review focuses on EAs and AAs, it is important to note that disparities exist across many populations in the United States. In men, the order of increasing incidence is: Hispanics (lowest), Asian/Pacific Islander, American Indian/Alaska Native, NH white and NH black, (highest). In women, the order is Hispanics (lowest), Asian/Pacific Islander, NH black, American Indian/Alaska Native and NH white (highest). Data from the Multiethnic Cohort Study (MEC) (8) has offered additional insight on how smoking contributes to these differences. As the majority of the literature to date focuses on EAs and AAs, this will be the primary subject of this review. However, I acknowledge that additional work is needed to focus on these other minority populations.
Etiological factors that contribute to disparities in lung cancer incidence
Smoking
Smoking is the strongest risk factor for lung cancer development. Heterogeneity in tobacco exposure across populations is a plausible root of disparities. However, measuring smoking exposure is complicated (9,10). In addition to status (i.e. current, former and never), dose (cigarettes per day, CPD), duration, age at initiation, time to first cigarette and daily versus non-daily use are key aspects of smoking relevant to its relationship with cancer. Moreover, tar content, which has a linear relationship with cancer risk (11), varies widely between cigarette brands (12).
Broadly speaking, the following observations argue against smoking being the main driver of disparities in lung cancer incidence: AAs diagnosed with lung cancer are significantly more likely to be intermittent or light smokers compared with EAs (8,13), they are more likely to start smoking later in life (14,15), and, when one compares smoking rates across racial groups matched for equal levels of smoking, AAs still experience a higher burden of lung cancer (8), suggesting that AAs could be more susceptible to lung cancer at lower doses of tobacco (8). Moreover, lung cancer disparities persist in never smokers (16).
However, there is an approximate 30-year lag between smoking initiation and the onset of lung cancer. Thus, to understand current disparities in cancer incidence, examining historical trends in smoking prevalence is prudent. As yet, there is not a clear picture that explains the relationship between disparities and lung cancer. However, the points discussed below try to shed some light on the key points and observations.
Prevalence of smoking
Holford and colleagues recently published a comprehensive compilation of smoking history by birth cohort in the USA from 1890 to 1990 (13). It shows that racial and ethnic differences in smoking have evolved over time and that the comparison of smoking prevalence across populations is complicated by the age at which one measures it. For example, EA men are more likely to be current smokers until around age 50, after which smoking prevalence is higher among AA men—interestingly, this crossover occurs at an earlier age in more recent birth cohorts (1900 cohort crossover age ~55, 1960 cohort crossover age ~25).
The likelihood of smoking initiation has also varied throughout generations. In EA men born from 1930 onwards, initiation rates were higher up to ~30 years of age, after which the initiation rate was higher in AAs, reflecting the observation that AA men tend to start smoking later in life. Since the 1970s, there has been a decrease in smoking initiation, especially among AAs, where the steeper decline has narrowed the gap in lung cancer incidence rates between AA and EA men under the age of 40 (17,18). The decline in the proportion of people smoking slowed after 1990, but the amount of tobacco that people consume is decreasing—in part due to public health policies on the use of tobacco in public places (13). Cumulative tobacco exposure is longer in AAs (13,19), possibly reflecting lower quit rates (13). Thus, smoking duration is one of the key smoking-related factors that is consistent with the trend of disparities.
While smoking prevalence and age at initiation patterns have changed throughout the life course, both historically, and currently, AAs have consistently consumed fewer cigarettes (13,20). For every cohort examined in the Holford Study, the mean consumption of CPD was significantly lower in AA men and women (13), which would seem to contradict racial differences in lung cancer rates. However, the relative importance of smoking intensity versus smoking duration is not equivalent (9,21–24). The effect of smoking duration on lung cancer risk is stronger than smoking dose (9,21–24) and lung cancer risk does not linearly increase with CPD—excess relative risk diminishes beyond 20 CPD (10,24), an observation that is sometimes called the ‘wasted dose’ effect. In essence, Lubin and colleagues argue that the excess odds ratio associated with each increasing pack-year is higher among smokers with high CPD and short duration compared with smokers with low CPD and longer duration, especially in the 15–20 CPD range (10,25). However, AAs are more likely to be low intensity smokers with a longer duration. Adding to the complexity of lung cancer disparities is evidence that at 10 CPD, controlled for smoking duration, AAs still experience a higher burden of lung cancer compared with EAs (8).
Burden of smoking
One key difference in the smoking habits of EAs and AAs is the type of cigarette used; AAs preferentially use mentholated cigarettes, which is regular tobacco flavored with the compound menthol. Due to its ‘cooling’ properties, menthol counters the irritant effect of toxicants found in tobacco (26,27). They were first developed in 1924 by Lloyd ‘Spud’ Hughes and quickly gained a significant market share in the 1930s. Historically, tobacco companies directly advertised mentholated tobacco products to AAs, in part due to genetic differences in an individual’s ability to perceive bitter taste—Asians and AAs are more likely to be ‘super-tasters’ and thus less likely to accept bitter taste (26,28,29). Today, ~70–85% of AAs use menthol cigarettes compared with 20–30% of EAs (30). Mentholation can affect smoking behavior, and thereby, cancer risk (26,31–36). Indeed some studies have linked mentholated tobacco with reduced odds of quitting (34–36), which could contribute to the lower quit rates among AAs overall (26) and is among the foundation arguments of recent calls to ban mentholation of tobacco (26). This is important, as lung cancer risk begins to decrease within 5 years of smoking cessation and reduces linearly over 20 years where upon it stabilizes at approximately three-times the risk of a never smoker (37–39).
However, multiple studies do not support the hypothesis that menthol cigarettes are associated with a greater risk of lung cancer compared with other tobacco types (40–43)—incongruent with the finding that menthol smoking status is associated with greater daily nicotine exposure (44). In fact, the relative risk of lung cancer compared with non-menthol cigarettes is lower. One possible reason is the inhibition of CYP2A6 activity by menthol (45,46)—CYP2A6 is the primary enzyme that metabolizes nicotine. Some (45,47–49), but not all (40,50), population-based studies support slower nicotine metabolism and clearance in menthol cigarette users. Moreover, there is evidence for ethnic differences in this relationship (50). Another mechanism by which menthol could reduce lung cancer risk is through its effect on the metabolism of NNK—also known as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and one of the key tobacco-specific nitrosamines involved in carcinogenesis. CYP2A6 activates NNK (51), therefore inhibition of CYP2A6 by menthol could decrease the bioactivation of this carcinogenic compound. However, the prevalence of menthol cigarette use among AAs, and the apparent (relative) lower risk of lung cancer associated with menthol cigarette use, contradicts cancer registry data of racial disparities in lung cancer risk as previously described.
Most mentholated cigarettes were filtered from an early time. Cigarette ventilation, which modifies the delivery of carcinogenic constituents (52), was invented by Boris Aivaz in 1925. Filtered cigarettes initially gained popularity in the 1950’s due to the perception that the filter made cigarettes ‘safer’ during a time in which a bolus of studies began to describe the carcinogenicity of tobacco. In 1956, R.J. Reynolds Tobacco Company launched the first filter-tip menthol cigarettes but it was not until the 1970s that the adoption of filters into non-menthol cigarettes became popular. By the end of that decade, filter-tipped cigarettes dominated more than 90% of the tobacco market (53).
The ventilation of cigarettes did not make them safer, but it did change the histological profiles of lung cancer. In fact, the rising incidence of adenocarcinoma over the last few decades parallels the emergence of filtered cigarettes (54). A recent paper by Song et al., elegantly outlines how cigarette filter ventilation contributed to increases in adenocarcinoma (54). They include an increase in the production of toxicants such as NNK and increased mutagenicity of tar. As AAs started using filtered cigarettes decades before EAs (by smoking menthol brands), one would expect to see an increased prevalence of adenocarcinoma in AAs compared with EAs, which isn’t the case (2,3). However, an analysis of histologic trends of lung cancer between 1973–1978 shows that lung adenocarcinoma rates increased faster in AAs compared with EAs during this time (APC = 13.5 and 7.2, respectively) (55). During the same period, lung squamous cell carcinoma decreased at a greater pace in AAs compared with EAs (APC = −3.8 and −1.3, respectively). In light of these trends, and given that the magnitude of the disparity is highest for the SCC histological subtype, these data suggest that smoking could explain the disparities in lung cancer between EAs and AAs.
Concluding comment
If the carcinogenicity of tobacco per cigarette smoked is equal across both EAs and AAs then one could expect lower incidence of lung cancer in AAs. But a paradox persists: AAs start smoking later in life and smoke fewer CPD. AAs primarily smoke menthol cigarettes—associated with reduced cancer risk compared with non-menthol. But, they have significantly fewer successful quit rates and thus have a longer cumulative duration of smoking and are also diagnosed at an earlier age. Indeed, as smoking is the main etiological factor associated with lung cancer, it may be wise to consider that racial disparities in lung cancer incidence are in some way tied to tobacco.
Genetics and metabolism
Genetics
Family, twin and genome-wide association studies, which together have explained ~20% of lung cancer heritability (56), confirm an underlying association between genetics and lung cancer risk (57,58). Recent reports indicate that the impact of genetic susceptibility is strongest for adenocarcinoma (59). Genetic susceptibility loci on chromosome 8 have been linked with the excess prostate cancer incidence among AA men (60–66), but similar results have not yet been described for AAs with lung cancer. For example, the recently completed (and only) GWAS of lung cancer in AA found that the two key loci associated with lung cancer risk in European Populations, 15q25 and 5p13, were also associated with lung cancer risk in AAs (67,68). No other loci met genome wide-significance in the analysis. On the one hand, this suggests that genetic susceptibility does not drive the excess incidence of lung cancer in AAs. However, the most recent GWAS of lung cancer in European populations included 30 000 cases and 56 000 controls (59). This study was extremely well powered and discovered new loci. It is not clear if these loci are also associated with lung cancer in AA, nor is it known whether novel AA-associated loci would be discovered if a higher-powered study was performed in AA. Supporting this possibility, the original lung cancer GWAS studies included a similar number of participants to that recent lung cancer GWAS of AA, and there, only 15q25 and 5p13 were discovered. This implies that additional perhaps novel, AA-specific genetic susceptibility loci remain undiscovered.
Cote et al. demonstrated that first-degree relatives of AAs with early-onset lung cancer have a greater risk of lung cancer compared with EAs (58,69) though only if the relative was the individual’s mother (OR 5.23, 95% CI 1.46–18.60). While this suggests that genetics might contribute to disparities, other studies of European populations in early-onset lung cancer also show a strong relationship between first-degree relatives with lung cancer risk (70,71). Also, recent data show that although African-born black men and women had an approximately 65% lower frequency of lung cancer compared with US-born NH blacks, cancer incidence varied by region of birth in Africa. For example, lung cancer in West African-born blacks was less common than in East-African-born blacks (72). Therefore, additional studies comparing this relationship in AA, EA and populations of African descent are needed.
Metabolism
Overall, the role of metabolism in the excess cancer incidence observed among AAs compared with EAs has not been clarified (73–78). Many studies have shown that AAs have higher circulating levels of urinary (33,79,80) and blood (79,81,82) cotinine concentration, even after controlling for CPD. Cotinine is the primary proximate metabolite of nicotine (83), more than 70% of nicotine is converted to cotinine by CYP2A6. A more complete measure of nicotine uptake computes a ‘total nicotine equivalent’ or TNE sum, that represents total nicotine, total cotinine, total 3-HCOT and nicotine N-oxide (and their glucuronide conjugates) (73,84,85). These studies have also found that AAs have higher TNE levels, compared with EAs, even after adjustment for CPD. Indeed, higher cotinine levels are observed among AA children exposed to secondhand tobacco smoke, compared with EA children (86,87).
Nicotine clearance is modulated by genetic variants in CYP2A6. Indeed, this metabolism can modulate smoking behavior and patterns. For example, fast metabolizers tend to smoke more to maintain a higher level of nicotine in their bloodstream. Indeed, genetic variants of CYP2A6 that slow nicotine metabolism and clearance are associated with lower nicotine intake and reduced lung cancer risk (84,88), indicating that retention of high residual nicotine can also lower tobacco consumption. From a population genetics perspective, AAs are thought to be slow metabolizers, but a correlation between this slower metabolism with higher TNE and cotinine remains puzzling (31,73–75,78,80,89). Moreover, in AAs, while slow metabolizing variants are associated with reduced lung cancer risk (especially AA men), there is limited evidence that this is linked with reduced tobacco intake, as measured by CPD (77). However, measurement of tobacco exposure via self-report is difficult in light smokers (77) and results can vary depending on whether daily or intermittent smokers are considered (32).
Interestingly, recent work has established a biological measure of CYP2A6 activity, a global metric that captures >75% of CYP2A6 activity and is not restricted to a few specific genetic variants (note that there is significant population diversity in the allele frequency of CYP2A6 variants (78)). Using this measure, [a ratio of urinary total trans-3′-hydroxycotinine (3-HCOT) to cotinine], AAs and EAs actually had similar levels of CYP2A6 activity (78). Even more interesting, AAs had a higher level of TNE, even after adjusting for CYP2A6 activity, suggesting that factors other than CYP2A6 control smoking behavior. Of, note, it is thought that TNE is a better predictor of nicotine and carcinogen exposure than CPD, especially in AA (85).
In addition to genetics, menthol (45,46), hormones (90) and smoking topography, i.e. puff volume, depth of inhalation, puff velocity and inter-puff interval, retention time of smoke in the lungs (44,80,85), can influence nicotine metabolism and TNE. For example, socioeconomic status (SES) is a modifier of tobacco use efficiency (91); individuals with low SES tend to extract more tobacco and nicotine per cigarette than high SES smokers. Typical smoking topographical patterns indicate that AAs smoke for positive reinforcement from nicotine with a ‘peak-seeking’ pattern (i.e. smoking individual cigarettes more intensively with greater intake of nicotine and tobacco smoke toxins), while EAs adopt a ‘trough-maintaining’ pattern (avoiding withdrawal by maintaining more consistent nicotine levels throughout the day by means of a more regular smoking pattern) (32,44). This is consistent with data showing AAs are more likely to smoke a cigarette within 5 min of waking (80,92)—time to first cigarette is independently associated with lung cancer risk.
It is important to note that while nicotine-derived metabolites, such as NNK, are considered carcinogenic, nicotine itself is not considered to be a direct risk factor for lung cancer. Thus, any relationship between nicotine metabolism and cancer disparities is likely to be mediated by how this metabolism affects smoking behavior and/or residual unexplained exposure related to other carcinogens. In addition to finding increased TNEs among AAs, increased NNK metabolites (79,93), NNAL (73) and thiocyanate (79) are higher in AAs compared with EAs. What this suggests, is that AAs extract more nicotine and carcinogens per cigarette that EAs so that even though they smoke less overall, their internal carcinogen load is higher.
It is possible that polymorphisms in genes other than CYP2A6, such as UGT2B10, UGT2B17, FMO3, NAT1 and OCT2, contribute to nicotine pharmacokinetics; however, recent work suggests that these genes represent minor sources of variation and they have been deemed insufficient to alter smoking habits in AAs (94). Thus, further work on genetic factors that regulate the metabolism, activation and clearance of the other carcinogens in tobacco is needed. For example, menthol is a significant inhibitor of CYP2A13 (95), an enzyme with high similarity to CYP2A6, that is also involved in NNK activation, and UGT2B17, an enzyme involved in cotinine glucuronidation and whose activity is slower in AAs (85).
We do not fully understand how nicotine and carcinogen metabolism contribute to lung cancer disparities. However, as nicotine is the key addictive component in cigarettes and how it is metabolized modulates smoking patterns and cancer risk, it is possible that the FDA’s recent recommendations to reduce nicotine content to that of non-addictive levels could both influence overall lung cancer risk as well as racial disparities of this malignancy.
Environment
While smoking is the main etiological factor associated with lung cancer, others such as alcohol consumption, body mass index, geography, radon, pollution and alternative/unidentified environmental exposures, could also contribute. Work by Tran and colleagues using the Kaiser Permanente Medical Care Cohort of 130 000 individuals (including ~35 000 AAs) found that while drinking more than three glasses of alcohol a day was associated with increased risk of lung cancer in EAs, it was not associated with increased risk in AAs (2). Increasing body mass index is associated with risk of many cancers, but it is inversely associated with lung cancer risk, something that is possibly due to reverse causality and the occurrence of weight loss among individuals with preclinical or undiagnosed lung cancer. As body mass index is a measure of weight adjusted for height, recent studies have assessed waist-to-hip ratio as an alternative measure of adiposity. This measure compares visceral to gluteofemoral adiposity—the former is thought to be more metabolically active. Indeed, a positive relationship between WHR and lung cancer risk has been observed (96). In AAs, however, increasing adiposity—as measured by waist-to-hip ratio—is not associated with increased cancer risk (97), suggesting that adiposity does not contribute to disparities in lung cancer incidence.
Geographical location also seems to be important mediator of lung cancer risk. Lung cancer rates are lower at higher altitude (98), and national studies have shown that the incidence of lung cancer varies by state (99). Our recent work analyzing disparities at the county level shows that while disparities exist across the USA, they are higher in rural counties (100). Many sources of pollution, including nitric oxides, industrialization, urbanization and radon exposure (101–104) are often located in, or near, poor working-class communities and disadvantaged groups (105–109), which may result in AA populations being at increased risk for exposure to environmental hazards (108–112). Residential proximity to industrial sources of pollution and chemical carcinogens is associated with increased lung cancer mortality, but the effects seem to be stronger in AAs. Interestingly, this mirrors the relationship between smoking and lung cancer in EAs and AAs, again suggesting that AAs could be more susceptible to the carcinogenic effects of chemical exposure (113).
Socioeconomic status is considered a surrogate for other unknown factors that may contribute to cancer disparities. Lung cancer risk rises with decreasing SES status and smoking prevalence is higher among individuals with low SES. AAs are almost three times as likely to live in poverty as EAs. However, studies have shown that at each level of SES, AAs have a higher incidence of lung cancer, compared with EAs (114–116). A recent study used a public health exposome model leveraging geographic information systems to model lung cancer disparities. This intriguing study found that, in additional to several social determinants, PM2.5—a tiny air pollutant particle—was associated with lung cancer incidence and disparities (117).
Early detection
Lung cancer screening
The US Preventive Services Task Force (USPSTF) recommends annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 55–80 years who have a 30-pack-year smoking history, and currently smoke or have quit within the past 15 years. In 2015, the Centers for Medicare and Medicaid Services (CMS) approved reimbursement for annual LDCT screening among individuals aged between 55 and 77 years, who have a 30-pack-year smoking history or have quit within the last 15 years. These guidelines were largely based on the findings of the National Lung Screening Trial (NLST), which documented a 20% reduction in lung cancer mortality among those screened with LDCT compared with chest X-ray (118). Approximately 9 million individuals in the USA are potentially eligible for screening, although the number of eligible smokers is decreasing in the USA, reflecting progress in tobacco control measures (119,120).
The expansion of LDCT screening has significant potential to reduce lung cancer deaths. It is also possible that the implementation of LDCT screening in its current form may lead to a widening of the disparity in cancer mortality among minorities. Several studies have shown that AAs are typically diagnosed with lung cancer at earlier ages compared with EAs (121,122). Analysis of SEER 17 shows that AAs under 50 are twice as likely to develop lung cancer compared with EAs in the same age group. Schwartz and colleagues also reported that AA men aged between 40 and 54 years of age are 2- to 4-times more likely to develop lung cancer compared with EA men, even after adjusting for smoking (123). Worryingly, research shows that lung cancer diagnosed in individuals less than 55 years of age is more likely to be at an advanced stage and therefore, less amenable to curative care (124). As mentioned, AAs have lower overall tobacco exposure (8,121). As age at diagnosis and smoking exposure define two of the main eligibility criteria for LDCT screening, it is possible that, with current guidelines, AAs would be more likely considered as screening ineligible, further increasing the disparity. A recent analysis of lung cancer cases diagnosed between 1998 and 2014 we found that, regardless of the screening criteria considered, AAs were more likely to fall within the screening ineligible subgroup, compared with EAs (125), suggesting that applying the same screening eligibility criteria across racial groups could unintentionally miss a proportion of high-risk individuals and exacerbate racial disparities in lung cancer survival. Interestingly, an individualized lung cancer risk model that includes additional demographic, clinical and smoking variables to identify and remove the lowest risk individuals from the USPSTF guidelines, and replace them with individuals with the highest risks, increased the inclusion of AAs from 7.7 to 12.8% and could potentially prevent 20% more lung-cancer deaths than current recommendations (126).
To determine the effect of lung cancer screening on disparities going forward it will be important to assess the uptake of screening recommendations in the population. Unfortunately, evidence already suggests that the uptake of lung cancer screening is slow. Data from the 2010 National Health Interview Survey indicate that just 3.3% of high-risk smokers underwent LDCT screening. Jemal et al. (119) recently investigated whether the introduction of LDCT screening guidelines increased uptake. Remarkably, only 3.9% of eligible individuals underwent screening in 2015. Possible reasons for the slow uptake include lack of access to care, lack of awareness of lung cancer screening, as well as a lack of knowledge among physicians regarding screening recommendations and reimbursement (119,127). In 2010, the analysis found that LDCT screening was higher in non-white compared with white populations (119). In 2015 however, the rate dropped and was almost half of that observed in white populations. Thus, uptake is still low, highlighting the need for increased awareness among both patients and clinicians about the efficacy of LDCT. These trends should be closely monitored so that interventions, if needed, are timely. For example, our recent data show that rates of adenocarcinoma are rising fastest in rural counties and that those increases are occurring at a higher rate in AAs (100). These could be areas where targeted screening interventions could be needed. Moreover, anti-smoking efforts are a key part of screening programs. Given the lower successful quit rates among AAs, compared with EAs (77), LDCT screening programs have a key opportunity to identify reasons for this racial difference and enact strategies to ensure cessation efforts have maximal efficacy in all populations.
Biomarkers
Until recently, there were no validated biomarkers for lung cancer early detection, however, Spira and colleagues have developed a genomic biomarker in the nasal passage that can predict the likelihood that a ‘positive CT scan’ is indicative of a malignant tumor and has been approved for clinical use (128). A key question is whether biomarkers will perform equally and optimally across populations. Our recent work suggests that racial differences in lung tumor biology exist (129). As circulating biomarkers frequently reflect the biology within a tumor, these data suggest that the key biomarkers selected for risk assessment or diagnosis may not be equally efficacious in all populations. The detection of biomarkers in a minimally/non-invasive biospecimen, such as blood, a nasal swab or urine, has significant potential for risk assessment and early diagnosis. For example, biomarkers that portend the existence of an emerging cancer could be very useful in risk stratification and the prioritization of patients for lung cancer screening—it is evident that the healthcare system will struggle to screen all eligible individuals. In addition, as up to 96% of nodules detected by LDCT in the NLST were benign, biomarkers, such as the genomic biomarker described above, that distinguish between benign and malignant nodules detected by LDCT would be very useful (118,130).
Recent work examining the relationship between inflammatory proteins and lung cancer diagnosis supports that hypothesis. For example, while IL-6 and IL-8 were associated with an increased odds of lung cancer diagnosis in both EAs and AAs, only increased levels of IL-1β, IL-10 and TNFα levels were associated with lung cancer among AAs. Moreover, levels of several cytokines were differentially expressed in EA compared with AA controls (131). These data suggest that circulating cytokine levels vary by race and might contribute to lung cancer differently in AAs and EAs. Future work examining risk prediction models of lung cancer need to include characterization of peripheral biomarkers across racial groups.
Treatment and survival
Survival
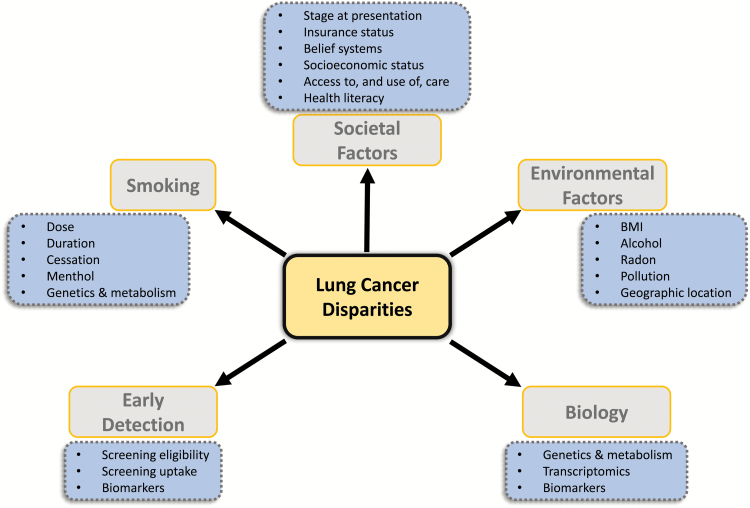
AAs have the highest lung cancer mortality of all racial/ethnic groups in the USA and the worst survival (1,132,133). It is likely a multi-factorial problem to which many issues may contribute, including access to (good quality) health care, neighborhood/residential factors, patient and provider factors, SES, insurance status, belief systems, aspects of the physician–patient relationship, education and tobacco use (Figure 1). Somewhat encouragingly, while survival rates are uniformly worse among AAs, studies also show that when access to care is controlled for—such as within the US military healthcare system—survival times are more equitable (134–141). Also, recent data suggest that declines in lung cancer mortality, a consequence of decreasing smoking exposure, are greater in AA populations (132). As with disparities in lung cancer incidence, understanding what factors contribute to racial/ethnic differences in lung cancer survival will hopefully provide clarity on how to diminish them.
Figure 1.
Schematic presentation of the multitude of factors that contribute to lung cancer health disparities.
For all stages of lung cancer combined, the 5-year lung cancer survival rates are among the worst of all cancer types, typically 15%. One of the major contributing factors to this dismal survival rate is the late stage at which most lung cancers are diagnosed. Unfortunately, greater than 50% of lung cancer is diagnosed at stages III or IV, a time when both local and systemic treatments are unlikely to be curative. However, for cancers diagnosed at stage I, where the standard of care is surgery alone, the survival rate is ~70%.
This is one of the key drivers in survival disparities between EAs and AAs: AAs are more likely to present with more advanced disease (1). A fundamental factor is access to care. As mentioned, AAs are three times more likely to live in poverty than EAs and low SES is linked with presentation of late-stage disease and a decreased likelihood to receive surgical interventions (140,142). Insurance status matters as survival improves among lung cancer patients with private insurance (143). Also, lung cancer patients without private health insurance receive surgical treatment less often than those with private health insurance (142).
However, even when equal access to care has been demonstrated, AAs are less likely to receive radiation and systemic therapy and undergo surgical resection (144–147), highlighting that access to care does not necessarily mean access to good quality health care. Aspects of the physician–patient relationship could also impact utility of health care resources (91). Further, patient belief systems regarding illness and a lack of trust in the health care system in general could determine whether or not a patient seeks medical care (148,149). The degree to which patients use spirituality in decision-making is also different among populations (91,150). However, belief systems are complex and dissection of their origin and impact on treatment decisions warrants further study.
Access to care following a lung cancer diagnosis can also affect outcomes. Following treatment for advanced disease, some studies show that the probability of 1-year survival is less in AAs than EAs (137). Bach and colleagues noted that physicians treating AA patients tend to have less clinical training and access to key clinical resources (151). It is also possible that the presence of co-morbidities at the time of diagnosis could complicate a patient’s treatment (146,152). Current smoking is associated with decreased lung cancer survival (153). Given that AAs are less likely to quit tobacco than EAs, this is also a factor that could contribute to outcomes. Programs that promote smoking cessation should continue among those with a cancer diagnosis.
Collectively, these data highlight the impact of societal determinants of health on lung cancer disparities. Moreover, the emergence of LDCT screening will require social and implementation research to disentangle and overcome barriers to high-quality health care in the USA for minority populations.
Biology and access to precision medicine
For centuries, disease has been diagnosed, classified and treated based on physical symptoms and relatively simple blood and radiographic tests. A deficiency of these diagnostic methods is the inability to determine how an individual patient should be treated or how they will respond to therapy. With the revolutionary advances in molecular biosciences over the past decades, we are entering a new age of medicine in which diagnosis and therapy decisions for each cancer patient will be based on detailed molecular and chemical fingerprints (154). The systematic molecular characterization of a patient’s disease should lead to more accurate diagnosis and more precise treatment. This movement is called Precision Medicine (154,155). In its purest sense, the Precision Medicine approach to health care and improving health outcomes will match each patient to an accurate diagnosis and the most effective, least toxic therapy.
The detection of tumor subtypes, key driver genes and critical pathways associated with outcome is a core tenet of Precision Medicine (156). Increasingly, sophisticated analyses that include multiple layers of taxonomic classification, including copy number variation, somatic mutations, coding and non-coding gene expression, have revealed a more refined and rigorous view of lung cancer biology, highlighting important pathways and networks with clinical significance. Already, the power of this approach has been demonstrated. In lung cancer, the discovery and subsequent targeting, of EGFR mutations has greatly improved response to treatment and lengthened progression-free survival. In breast cancer, gene expression analyses have led to the development of so called ‘recurrence scores’ that predict whether a patient is likely to experience a recurrence (157). Based on these molecular data, physicians can make informed individualized treatment decisions for their patients.
Given that lung cancer survival is mostly equivalent among lung cancer patients in an equal access to care setting, evidence to support a biologic or genetic contribution to survival differences between racial or ethnic groups is weak. However, we do not have data yet comparing outcomes between populations in clinical trials with targeted therapies and many clinical trials have too few individuals from minority populations for subgroup analyses. Also, the 5-year survival rate for advanced NSCLC is possibly too low (<5%) to tease apart differences in outcomes based on tumor biology.
To date, there have been limited Precision Medicine studies in minority populations. In lung cancer, initial studies focusing on EGFR mutations were conflicting: Some reported fewer mutations in AAs relative to EAs, while others found no relationship with race (158–167). One study has provided evidence for a lower overall mutation burden in AAs relative to EAs (167), but a majority of studies have not detected any striking differences in the somatic mutation burden between these racial groups (165–167).
Several studies have provided evidence for differences in tumor biology between EA and AA cancer cases (168–178), including lung cancer, where transcriptomic differences have been described (129). We found that key genes previously observed to be upregulated in AAs in several tissue types, i.e. PSPHL, CRYBB2 and AMFR (168,169,179–181), were also significantly upregulated in lung. Also, we noted a region on Chr17q21 where several genes had significantly higher expression in EAs, including KANSL1, LRRC37A3 and ARL17A. Interestingly, this region has a segmental duplication that is primarily found in populations of European descent (182,183). A key unanswered question is whether these population stratification genes are related to cancer. We also found that stem cell pathways were more predominant in lung cancer from AAs relative to EAs. Interestingly, this is consistent with observations in breast, prostate and colon cancer (173,180,184–188) where stem cell signatures also dominate in populations of African descent.
As the field moves towards a Precision Medicine model of cancer management, profiling genomic differences in lung cancer will be necessary in all populations so that everyone can benefit. Tied to this, there is a strong need to recruit minority patient populations into clinical trials. Though challenging, it is essential for the assessment of response and efficacy of new treatment modalities. Also, effort will be needed to aid the diffusion of EGFR and ALK mutation testing to all patients (189,190). Such testing is not always within reach for many underserved communities (189–191).
Perspectives
Despite an overall lower tobacco exposure, AAs have the highest burden of lung cancer in the USA of any racial/ethnic group. The causal factors—genetic, tobacco-related, environmental or otherwise—that lead to a greater incidence of lung cancer in AAs are not precisely defined and further research will be needed to do so.
As mentioned earlier, the effect of smoking duration on lung cancer risk is stronger than smoking dose (9,21–24) and lung cancer risk does not linearly increase with CPD—excess relative risk diminishes beyond 20 CPD (10,24) and smoking duration is one of the key smoking exposure variables where the pattern follows the trend of observed lung cancer disparities. The benefit of lower daily cigarette consumption among AAs may be offset by more intensive smoking resulting in greater carcinogen exposure than would be predicted based on CPD—interesting work from Lubin and colleagues suggests that for equal pack years of smoking, the level of lung cancer risk is more influenced by intensity in those that inhale deeply (32). Also, the benefit of a later age of regular smoking initiation among AAs may be offset by fewer successful quit rates compared with EAs (77). However, empirical epidemiological studies are needed to disentangle the relative importance of smoking duration versus smoking dose and its interaction with smoking behavior. If, as suggested by Haiman and colleagues, AAs are more susceptible to lower doses of tobacco, a key basic mechanistic question that needs to be answered is how. A study by Harris and colleagues found that a less efficient G2M checkpoint is significantly associated with lung cancer risk in AA, but not EA, women (192), offering, perhaps, a clue.
Further work is also needed in the field of carcinogen metabolism. As we currently understand it, metabolism kinetics have not provided a clear framework within which to understand lung cancer disparities, but they are likely to be important. While studies on racial differences in nicotine metabolism have helped inform how nicotine metabolism can affect smoking behavior among racial and ethnic groups, a deeper metabolomics analysis of group 1 tobacco carcinogens (including from menthol and non-menthol cigarettes) for potential racial differences is needed and could uncover population differences in tobacco carcinogen susceptibility. Integrated with this, an analysis of gene variants beyond CYP2A6 could help reveal key gene-environment interactions. Similarly, a larger GWAS of lung cancer in AAs, though difficult from a power perspective, may be able to identify new and/or novel susceptibility loci in this population. Further, integrated behavioral and laboratory studies are needed to understand why AAs have higher nicotine exposure at the same level of CPD and CYP2A6 activity. Moreover, if and how menthol modulates metabolism in the context of TNE and CYP2A6 activity needs to be empirically studied.
The lung cancer burden, and possibly disparities, are also determined by the tar content of the blend, type of cigarette smoked and the way in which a cigarette is smoked. Studies on the patterns and implications of smoking topology are needed. These are somewhat difficult studies to conduct, but they have already provided important insights and should be extended.
While these studies may help us to understand why current disparities exist, efforts are also needed to prevent an exacerbation in disparities in the coming years. Firstly, there needs to be a timely assessment of lung cancer screening practices and their impact. Data are currently being assembled within the National Lung Cancer Screening Registry, which, along with other surveys, will be a key resource to monitor screening trends and their impact. It will help determine whether current screening eligibility criteria need to be adapted for the specific age and smoking profile at which the majority of AAs present with lung cancer. Secondly, we need more extensive knowledge of tumor biology among AAs and additional basic science studies that will help us understand underlying mechanisms of disparities. We have access to technological advances that are enabling a Precision Medicine approach to cancer management. A priori emphasis should be placed on race/ethnicity and ancestral background as a factor in Precision Medicine. Power in these studies is always a concern, but it can be overcome through collaborative networks and pooling of resources among scientists. Moreover, efforts to ensure comparable enrollment of minority populations in clinical trials will be important to ensure timely assessment of potential racial differences in response to targeted therapies.
As precision medicine becomes standard of care, there is a possibility that cancer treatment outcomes could worsen for under-represented populations, even as treatments improve for the general population (189). Thus, increasing access to high-quality cancer care—including screening programs—and community-based education programs will enhance informed evidence-based decision making for patients and hopefully diminish the public health burden of lung cancer further. Integral to these research programs will be efforts to research and understand what approaches can increase access to high-level care and improve outcomes for all cancer patients (189).
Efforts to eliminate cancer health disparities require an eye to the past to understand how they arose in the first place; an eye on the present to understand how we can diminish their impact on health outcomes; and an eye to the future to ensure that all possible steps can be taken to prevent new disparities emerging. An integrative approach, involving epidemiology, behavioral research, basic research and clinical studies, is likely to be our best hope to eliminate lung cancer disparities and at the same time, improve health outcomes for all populations.
Funding
This work was supported by the intramural research program of the Center for Cancer Research, NCI.
Conflict of Interest Statement: None declared.
Abbreviations
- AAs
African Americans
- CPD
cigarettes per day
- EAs
European Americans
- LDCT
low-dose computed tomography
- NH
non-Hispanic
- SES
socioeconomic status
References
- 1. Siegel R.L., et al. (2017)Cancer Statistics, 2017. CA. Cancer J. Clin., 67, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Tran H.N., et al. (2013)Predictors of lung cancer: noteworthy cell type differences. Perm. J., 17, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis W.D., et al. (1996)United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer, 77, 2464–2470. [DOI] [PubMed] [Google Scholar]
- 4. Burbank F., et al. (1972)U.S. cancer mortality: nonwhite predominance. J. Natl. Cancer Inst., 49, 649–659. [PubMed] [Google Scholar]
- 5. Schneiderman M.A., et al. (1972)Trends in lung cancer. Mortality, incidence, diagnosis, treatment, smoking, and urbanization. Cancer, 30, 1320–1325. [DOI] [PubMed] [Google Scholar]
- 6. Robbins H.A., et al. (2015)Excess cancers among HIV-infected people in the United States. J. Natl. Cancer Inst., 107, dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takezawa Y., et al. (2014)Human genetic research, race, ethnicity and the labeling of populations: recommendations based on an interdisciplinary workshop in Japan. BMC Med. Ethics, 15, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haiman C.A., et al. (2006)Ethnic and racial differences in the smoking-related risk of lung cancer. N. Engl. J. Med., 354, 333–342. [DOI] [PubMed] [Google Scholar]
- 9. Doll R., et al. (1978)Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J. Epidemiol. Commun. Health, 32, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lubin J.H., et al. (2006)Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol. Biomarkers Prev., 15, 517–523. [DOI] [PubMed] [Google Scholar]
- 11. Stellman S.D., et al. (1989)Lung cancer risk is proportional to cigarette tar yield: evidence from a prospective study. Prev. Med., 18, 518–525. [DOI] [PubMed] [Google Scholar]
- 12. Calafat A.M., et al. (2004)Determination of tar, nicotine, and carbon monoxide yields in the mainstream smoke of selected international cigarettes. Tob. Control, 13, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holford T.R., et al. (2016)Comparison of smoking history patterns among African American and white cohorts in the United States born 1890 to 1990. Nicotine Tob. Res., 18 (Suppl 1), S16–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White H.R., et al. (2007)Stages and sequences of initiation and regular substance use in a longitudinal cohort of black and white male adolescents. J. Stud. Alcohol Drugs, 68, 173–181. [DOI] [PubMed] [Google Scholar]
- 15. Finkenauer R., et al. (2009)Race differences in factors relating to smoking initiation. Addict. Behav., 34, 1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thun M.J., et al. (2008)Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med., 5, e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jemal A., et al. (2009)The convergence of lung cancer rates between blacks and whites under the age of 40, United States. Cancer Epidemiol. Biomarkers Prev., 18, 3349–3352. [DOI] [PubMed] [Google Scholar]
- 18. O’Keefe E.B., et al. (2015)Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health, 3, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones M.R., et al. (2018)Racial/ethnic differences in duration of smoking among former smokers in the National Health and Nutrition Examination Surveys (NHANES). Nicotine Tob. Res. 20, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. (CDC), C.f.D.C.a.P. (1993)Cigarette smoking among adults--United States, 1991. MMWR Morb. Mortal Wkly Rep., 42, 230–3. [PubMed] [Google Scholar]
- 21. Flanders W.D., et al. (2003)Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res., 63, 6556–6562. [PubMed] [Google Scholar]
- 22. Lubin J.H., et al. (2013)Misunderstandings in the misconception on the use of pack-years in analysis of smoking. Br. J. Cancer, 108, 1218–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lubin J.H., et al. (2007)The association of a tobacco-specific biomarker and cigarette consumption and its dependence on host characteristics. Cancer Epidemiol. Biomarkers Prev., 16, 1852–1857. [DOI] [PubMed] [Google Scholar]
- 24. Samet J.M., et al. (2007)Models of smoking and lung cancer risk: a means to an end. Epidemiology, 18, 649–651. [DOI] [PubMed] [Google Scholar]
- 25. Lubin J.H., et al. (2007)Cigarette smoking and lung cancer: modeling effect modification of total exposure and intensity. Epidemiology, 18, 639–648. [DOI] [PubMed] [Google Scholar]
- 26. Alexander L.A., et al. (2016)Why we must continue to investigate menthol’s role in the African American smoking paradox. Nicotine Tob. Res., 18 (Suppl 1), S91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamatou G.P., et al. (2013)Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry, 96, 15–25. [DOI] [PubMed] [Google Scholar]
- 28. Ooi W.L., et al. (1986)Increased familial risk for lung cancer. J. Natl. Cancer Inst., 76, 217–222. [PubMed] [Google Scholar]
- 29. Ooi S.X., et al. (2010)Bitter receptor gene (TAS2R38) P49A genotypes and their associations with aversion to vegetables and sweet/fat foods in Malaysian subjects. Asia Pac. J. Clin. Nutr., 19, 491–498. [PubMed] [Google Scholar]
- 30. Giovino G.A., et al. (2015)Differential trends in cigarette smoking in the USA: is menthol slowing progress?Tob. Control, 24, 28–37. [DOI] [PubMed] [Google Scholar]
- 31. Patel Y.M., et al. (2015)The contribution of common genetic variation to nicotine and cotinine glucuronidation in multiple ethnic/racial populations. Cancer Epidemiol. Biomarkers Prev., 24, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiffman S., et al. (2014)A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol. Biomarkers Prev., 23, 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caraballo R.S., et al. (1998)Racial and ethnic differences in serum cotinine levels of cigarette smokers: third National Health And Nutrition Examination Survey, 1988-1991. JAMA, 280, 135–139. [DOI] [PubMed] [Google Scholar]
- 34. Nonnemaker J., et al. (2013)Initiation with menthol cigarettes and youth smoking uptake. Addiction, 108, 171–178. [DOI] [PubMed] [Google Scholar]
- 35. FDA Tobacco Products Scientific Advisory Committee. (2011)Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. www.fda.govIdownloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientific AdvisoryCommittee/UCM269697.pdf (21 July 2011, date last accessed). [Google Scholar]
- 36. Food and Drug Administration. (2013)Preliminary scientific evaluation of the possible public health effects of menthol versus nonmenthol cigarettes. https://www.fda.gov/downloads/scienceresearch/specialtopics/peerreviewofscientificinformationandassessments/ucm361598.pdf. [Google Scholar]
- 37. United States. Public Health Service. Office of the Surgeon General, et al. (2004)The health consequences of smoking: a report of the Surgeon General. U.S. Dept. of Health and Human Services, Public Health Service, Rockville, MD. [Google Scholar]
- 38. United States. Public Health Service. Office of the Surgeon General, et al. (1989)Reducing the health consequences of smoking: 25 years of progress: a report of the Surgeon General: 1989 executive summary. U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health, Rockville, MD. [Google Scholar]
- 39. United States. Public Health Service. Office of the Surgeon General, et al. (1979)Healthy people: the Surgeon General’s report on health promotion and disease prevention, 1979. U. S. Dept. of Health, Education, and Welfare, Public Health Service, Office of the Assistant Secretary for Health and Surgeon General; Washington: for sale by the Supt. of Docs., U. S. Govt. Print. Off, Rockville, MD. [Google Scholar]
- 40. Signorello L.B., et al. (2009)Racial differences in serum cotinine levels of smokers. Dis. Markers, 27, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blot W.J., et al. (2011)Lung cancer risk among smokers of menthol cigarettes. J. Natl. Cancer Inst., 103, 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stellman S.D., et al. (2003)Lung cancer risk in white and black Americans. Ann. Epidemiol., 13, 294–302. [DOI] [PubMed] [Google Scholar]
- 43. Carpenter C.L., et al. (1999)Mentholated cigarette smoking and lung-cancer risk. Ann. Epidemiol., 9, 114–120. [DOI] [PubMed] [Google Scholar]
- 44. Ross K.C., et al. (2016)The influence of puff characteristics, nicotine dependence, and rate of nicotine metabolism on daily nicotine exposure in African American smokers. Cancer Epidemiol. Biomarkers Prev., 25, 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benowitz N.L., et al. (2004)Mentholated cigarette smoking inhibits nicotine metabolism. J. Pharmacol. Exp. Ther., 310, 1208–1215. [DOI] [PubMed] [Google Scholar]
- 46. MacDougall J.M., et al. (2003)Inhibition of human liver microsomal (S)-nicotine oxidation by (-)-menthol and analogues. Chem. Res. Toxicol., 16, 988–993. [DOI] [PubMed] [Google Scholar]
- 47. Ho M.K., et al. (2009)Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin. Pharmacol. Ther., 85, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clark P.I., et al. (1996)Effect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest, 110, 1194–1198. [DOI] [PubMed] [Google Scholar]
- 49. Hoffman A.C. (2011)The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tob. Induc. Dis., 9 (Suppl 1), S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fagan P., et al. (2016)Nicotine metabolism in young adult daily menthol and nonmenthol smokers. Nicotine Tob. Res., 18, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jalas J.R., et al. (2005)Cytochrome P450 enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco specific carcinogen. Chem. Res. Toxicol., 18, 95–110. [DOI] [PubMed] [Google Scholar]
- 52. Hoffmann D., et al. (1997)The changing cigarette, 1950-1995. J. Toxicol. Environ. Health, 50, 307–364. [DOI] [PubMed] [Google Scholar]
- 53. Centers for Disease, C., et al. (1997)Filter ventilation levels in selected U.S. cigarettes, 1997. MMWR Morb. Mortal Wkly Rep., 46, 1043–7. [PubMed] [Google Scholar]
- 54. Song M.A., et al. (2017)Cigarette filter ventilation and its relationship to increasing rates of lung adenocarcinoma. J. Natl. Cancer Inst., 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meza R., et al. (2015)Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One, 10, e0121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sampson J.N., et al. (2015)Analysis of heritability and shared heritability based on genome-wide association studies for thirteen cancer types. J. Natl. Cancer Inst., 107, djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Musolf A.M., et al. (2017)Familial lung cancer: a brief history from the earliest work to the most recent studies. Genes, 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Coté M.L., et al. (2012)Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur. J. Cancer, 48, 1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McKay J.D., et al. ; SpiroMeta Consortium (2017)Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet., 49, 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haiman C.A., et al. (2011)Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat. Genet., 43, 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han Y., et al. (2016)Prostate cancer susceptibility in men of African ancestry at 8q24. J. Natl. Cancer Inst., 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Freedman M.L., et al. (2006)Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl. Acad. Sci. USA, 103, 14068–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bock C.H., et al. (2009)Results from a prostate cancer admixture mapping study in African-American men. Hum. Genet., 126, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robbins C., et al. (2007)Confirmation study of prostate cancer risk variants at 8q24 in African Americans identifies a novel risk locus. Genome Res., 17, 1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang B.L., et al. (2011)Validation of genome-wide prostate cancer associations in men of African descent. Cancer Epidemiol. Biomarkers Prev., 20, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hooker S., et al. (2010)Replication of prostate cancer risk loci on 8q24, 11q13, 17q12, 19q33, and Xp11 in African Americans. Prostate, 70, 270–275. [DOI] [PubMed] [Google Scholar]
- 67. Schwartz A.G., et al. (2011)Admixture mapping of lung cancer in 1812 African-Americans. Carcinogenesis, 32, 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zanetti K.A., et al. (2016)Genome-wide association study confirms lung cancer susceptibility loci on chromosomes 5p15 and 15q25 in an African-American population. Lung Cancer, 98, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coté M.L., et al. (2005)Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA, 293, 3036–3042. [DOI] [PubMed] [Google Scholar]
- 70. Bromen K., et al. (2000)Aggregation of lung cancer in families: results from a population-based case-control study in Germany. Am. J. Epidemiol., 152, 497–505. [DOI] [PubMed] [Google Scholar]
- 71. Schwartz A.G., et al. (1996)Familial risk of lung cancer among nonsmokers and their relatives. Am. J. Epidemiol., 144, 554–562. [DOI] [PubMed] [Google Scholar]
- 72. Medhanie G.A., et al. (2017)Cancer incidence profile in sub-Saharan African-born blacks in the United States: similarities and differences with US-born non-Hispanic blacks. Cancer, 123, 3116–3124. [DOI] [PubMed] [Google Scholar]
- 73. Derby K.S., et al. (2008)Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol. Biomarkers Prev., 17, 3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Murphy S.E., et al. (2014)Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis, 35, 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Patel Y.M., et al. (2016)Novel association of genetic markers affecting CYP2A6 activity and lung cancer risk. Cancer Res., 76, 5768–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang H., et al. (2015)Associations between genetic ancestries and nicotine metabolism biomarkers in the multiethnic cohort study. Am. J. Epidemiol., 182, 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wassenaar C.A., et al. (2015)CYP2A6 reduced activity gene variants confer reduction in lung cancer risk in African American smokers–findings from two independent populations. Carcinogenesis, 36, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park S.L., et al. (2016)Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risks of lung cancer and effect on their smoking intensity. Carcinogenesis, 37, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muscat J.E., et al. (2005)Racial differences in exposure and glucuronidation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cancer, 103, 1420–1426. [DOI] [PubMed] [Google Scholar]
- 80. Pérez-Stable E.J., et al. (1998)Nicotine metabolism and intake in black and white smokers. JAMA, 280, 152–156. [DOI] [PubMed] [Google Scholar]
- 81. Ahijevych K., et al. (1997)Nicotine dependence and smoking topography among black and white women. Res. Nurs. Health, 20, 505–514. [DOI] [PubMed] [Google Scholar]
- 82. English P.B., et al. (1994)Black-white differences in serum cotinine levels among pregnant women and subsequent effects on infant birthweight. Am. J. Public Health, 84, 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Benowitz N.L., et al. (1994)Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin. Pharmacol. Ther., 56, 483–493. [DOI] [PubMed] [Google Scholar]
- 84. Park S.L., et al. (2017)Association of CYP2A6 activity with lung cancer incidence in smokers: the multiethnic cohort study. PLoS One, 12, e0178435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Benowitz N.L., et al. (2011)Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob. Res., 13, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pattishall E.N., et al. (1985)Serum cotinine as a measure of tobacco smoke exposure in children. Am. J. Dis. Child., 139, 1101–1104. [DOI] [PubMed] [Google Scholar]
- 87. Dempsey D.A., et al. (2012)Determination of tobacco smoke exposure by plasma cotinine levels in infants and children attending urban public hospital clinics. Arch. Pediatr. Adolesc. Med., 166, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yuan J.M., et al. (2016)Genetic determinants of cytochrome P450 2A6 activity and biomarkers of tobacco smoke exposure in relation to risk of lung cancer development in the Shanghai cohort study. Int. J. Cancer, 138, 2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Piliguian M., et al. (2014)Novel CYP2A6 variants identified in African Americans are associated with slow nicotine metabolism in vitro and in vivo. Pharmacogenet. Genomics, 24, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Benowitz N.L., et al. (2006)Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther., 79, 480–488. [DOI] [PubMed] [Google Scholar]
- 91. Berger M., et al. (2007)Racial disparities in lung cancer. Curr. Probl. Cancer, 31, 202–210. [DOI] [PubMed] [Google Scholar]
- 92. Branstetter S.A., et al. (2015)Predictors of the nicotine dependence behavior time to the first cigarette in a multiracial cohort. Nicotine Tob. Res., 17, 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Richie J.P. Jr, et al. (1997)Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol. Biomarkers Prev., 6, 783–790. [PubMed] [Google Scholar]
- 94. Taghavi T., et al. (2017)Effect of UGT2B10, UGT2B17, FMO3, and OCT2 genetic variation on nicotine and cotinine pharmacokinetics and smoking in African Americans. Pharmacogenet. Genomics, 27, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kramlinger V.M., et al. (2015)Cytochrome P450 3A enzymes catalyze the O6-demethylation of thebaine, a key step in endogenous mammalian morphine biosynthesis. J. Biol. Chem., 290, 20200–20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dewi N.U., et al. (2016)Anthropometry and the risk of lung cancer in EPIC. Am. J. Epidemiol., 184, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bethea T.N., et al. (2013)Obesity in relation to lung cancer incidence in African American women. Cancer Causes Control, 24, 1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Weinberg C.R., et al. (1987)Altitude, radiation, and mortality from cancer and heart disease. Radiat. Res., 112, 381–390. [PubMed] [Google Scholar]
- 99. Houston K.A., et al. (2014)Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004-2009. Lung Cancer, 86, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Houston K.A., et al. (2018)Histologic lung cancer incidence rates and trends vary by race/ethnicity and residential county. J. Thorac Oncol, 13, 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pope C.A. 3rd, et al. (2002)Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA, 287, 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vineis P., et al. (2006)Air pollution and risk of lung cancer in a prospective study in Europe. Int. J. Cancer, 119, 169–174. [DOI] [PubMed] [Google Scholar]
- 103. Samet J.M., et al. (2009)Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin. Cancer Res., 15, 5626–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Samet J.M., et al. (1989)Indoor radon and lung cancer. N. Engl. J. Med., 320, 591–594. [DOI] [PubMed] [Google Scholar]
- 105. Perlin S.A., et al. (1995)Distribution of industrial air emissions by income and race in the United States: an approach using the toxic release inventory. Environ. Sci. Technol., 29, 69–80. [DOI] [PubMed] [Google Scholar]
- 106. Caldwell J.C., et al. (1998)Application of health information to hazardous air pollutants modeled in EPA’s Cumulative Exposure Project. Toxicol. Ind. Health, 14, 429–454. [DOI] [PubMed] [Google Scholar]
- 107. Apelberg B.J., et al. (2005)Socioeconomic and racial disparities in cancer risk from air toxics in Maryland. Environ. Health Perspect., 113, 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Boffetta P., et al. (2003)Contribution of environmental factors to cancer risk. Br. Med. Bull., 68, 71–94. [DOI] [PubMed] [Google Scholar]
- 109. Benedetti M., et al. (2001)Cancer risk associated with residential proximity to industrial sites: a review. Arch. Environ. Health, 56, 342–349. [DOI] [PubMed] [Google Scholar]
- 110. Hendryx M., et al. (2008)Lung cancer mortality is elevated in coal-mining areas of Appalachia. Lung Cancer, 62, 1–7. [DOI] [PubMed] [Google Scholar]
- 111. Vineis P., et al. (2007)Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ. Health, 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dockery D.W., et al. (1993)An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med., 329, 1753–1759. [DOI] [PubMed] [Google Scholar]
- 113. Luo J., et al. (2011)Environmental carcinogen releases and lung cancer mortality in rural-urban areas of the United States. J. Rural Health, 27, 342–349. [DOI] [PubMed] [Google Scholar]
- 114. Aizer A.A., et al. (2014)Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer, 120, 1532–1539. [DOI] [PubMed] [Google Scholar]
- 115. Wong M.L., et al. (2013)Incidence of non-small-cell lung cancer among California Hispanics according to neighborhood socioeconomic status. J. Thorac. Oncol., 8, 287–294. [DOI] [PubMed] [Google Scholar]
- 116. Yu M., et al. (2014)Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control, 25, 81–92. [DOI] [PubMed] [Google Scholar]
- 117. Juarez P.D., et al. (2017)A novel approach to analyzing lung cancer mortality disparities: using the exposome and a graph-theoretical toolchain. Environ. Dis., 2, 33–44. [PMC free article] [PubMed] [Google Scholar]
- 118. National Lung Screening Trial Research, T, et al. (2011)Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med., 365, 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jemal A., et al. (2017)Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol, 3, 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ma J., et al. (2013)Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer, 119, 1381–1385. [DOI] [PubMed] [Google Scholar]
- 121. Dela Cruz C.S., et al. (2011)Lung cancer: epidemiology, etiology, and prevention. Clin. Chest Med., 32, 605–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Robbins H.A., et al. (2015)Age at cancer diagnosis for blacks compared with whites in the United States. J. Natl. Cancer Inst., 107, dju489. https://doi.org/10.1093/jnci/dju489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Schwartz A.G., et al. (1997)Lung carcinoma in African Americans and whites. A population-based study in metropolitan Detroit, Michigan. Cancer, 79, 45–52. [DOI] [PubMed] [Google Scholar]
- 124. O’Rourke M.A., et al. (1987)Age trends of lung cancer stage at diagnosis. Implications for lung cancer screening in the elderly. JAMA, 258, 921–926. [PubMed] [Google Scholar]
- 125. Ryan B.M. (2016)Differential eligibility of African American and European American lung cancer cases using LDCT screening guidelines. BMJ Open Respir. Res., 3, e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Katki H.A., et al. (2016)Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA, 315, 2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ersek J.L., et al. (2016)Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer, 122, 2324–2331. [DOI] [PubMed] [Google Scholar]
- 128. Perez-Rogers J.F., et al. (2017)Shared gene expression alterations in nasal and bronchial epithelium for lung cancer detection. J. Natl. Cancer Inst, 109, djw327. https://doi.org/10.1093/jnci/djw327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mitchell K.A., et al. (2017)Comparative transcriptome profiling reveals coding and noncoding RNA differences in NSCLC from African Americans and European Americans. Clin. Cancer Res., 23, 7412–7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Patz E.F. Jr, et al. ; NLST Overdiagnosis Manuscript Writing Team (2014)Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern. Med., 174, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pine S.R., et al. (2016)Differential serum cytokine levels and risk of lung cancer between African and European Americans. Cancer Epidemiol. Biomarkers Prev., 25, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. DeSantis C.E., et al. (2016)Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA. Cancer J. Clin., 66, 290–308. [DOI] [PubMed] [Google Scholar]
- 133. Ryerson A.B., et al. (2016)Annual report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer, 122, 1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zheng L., et al. (2012)Lung cancer survival among black and white patients in an equal access health system. Cancer Epidemiol. Biomarkers Prev., 21, 1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Brzezniak C., et al. (2015)Survival and racial differences of non-small cell lung cancer in the United States Military. J. Gen. Intern. Med., 30, 1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mulligan C.R., et al. (2006)Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol. Biomarkers Prev., 15, 25–31. [DOI] [PubMed] [Google Scholar]
- 137. Blackstock A.W., et al. (2002)Outcomes among African-American/non-African-American patients with advanced non-small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J. Natl. Cancer Inst., 94, 284–290. [DOI] [PubMed] [Google Scholar]
- 138. Brawley O.W. (2006)Lung cancer and race: equal treatment yields equal outcome among equal patients, but there is no equal treatment. J. Clin. Oncol., 24, 332–333. [DOI] [PubMed] [Google Scholar]
- 139. Akerley W.L. 3rd, et al. (1993)Racial comparison of outcomes of male Department of Veterans Affairs patients with lung and colon cancer. Arch. Intern. Med., 153, 1681–1688. [PubMed] [Google Scholar]
- 140. Bach P.B., et al. (1999)Racial differences in the treatment of early-stage lung cancer. N. Engl. J. Med., 341, 1198–1205. [DOI] [PubMed] [Google Scholar]
- 141. Bach P.B., et al. (2002)Survival of blacks and whites after a cancer diagnosis. JAMA, 287, 2106–2113. [DOI] [PubMed] [Google Scholar]
- 142. Greenwald H.P., et al. (1998)Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am. J. Public Health, 88, 1681–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Niu X., et al. (2013)Cancer survival disparities by health insurance status. Cancer Med., 2, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lathan C.S., et al. (2006)The effect of race on invasive staging and surgery in non-small-cell lung cancer. J. Clin. Oncol., 24, 413–418. [DOI] [PubMed] [Google Scholar]
- 145. Clegg L.X., et al. (2009)Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: national Longitudinal Mortality Study. Cancer Causes Control, 20, 417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Soneji S., et al. (2017)Racial and ethnic disparities in early-stage lung cancer survival. Chest, 152, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Grenade C., et al. (2014)Race and ethnicity in cancer therapy: what have we learned?Clin. Pharmacol. Ther., 95, 403–412. [DOI] [PubMed] [Google Scholar]
- 148. Niu X., et al. (2010)Cancer survival disparities by race/ethnicity and socioeconomic status in New Jersey. J. Health Care Poor Underserved, 21, 144–160. [DOI] [PubMed] [Google Scholar]
- 149. Onega T., et al. (2010)Race versus place of service in mortality among medicare beneficiaries with cancer. Cancer, 116, 2698–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lathan C.S., et al. (2010)Racial differences in the perception of lung cancer: the 2005 Health Information National Trends Survey. Cancer, 116, 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bach P.B., et al. (2004)Primary care physicians who treat blacks and whites. N. Engl. J. Med., 351, 575–584. [DOI] [PubMed] [Google Scholar]
- 152. Blackstock A.W., et al. ; Cancer and Leukemia Group B (2006)Similar outcomes between African American and non-African American patients with extensive-stage small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J. Clin. Oncol., 24, 407–412. [DOI] [PubMed] [Google Scholar]
- 153. Tanner N.T., et al. (2015)The association between smoking abstinence and mortality in the National Lung Screening Trial. Am. J. Respir. Crit Care Med. [DOI] [PubMed] [Google Scholar]
- 154. National Research Council. (2011) Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: The National Academies Press; https://doi.org/10.17226/13284. [PubMed] [Google Scholar]
- 155. Vargas A.J., et al. (2016)Biomarker development in the precision medicine era: lung cancer as a case study. Nat. Rev. Cancer, 16, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Collins F., et al. (2015)A new initiative on precision medicine. N. Engl. J. Med., 372, 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Parker J.S., et al. (2009)Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol., 27, 1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bauml J., et al. (2013)Frequency of EGFR and KRAS mutations in patients with non small cell lung cancer by racial background: do disparities exist?Lung Cancer, 81, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cote M.L., et al. (2011)Frequency and type of epidermal growth factor receptor mutations in African Americans with non-small cell lung cancer. J. Thorac. Oncol., 6, 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Krishnaswamy S., et al. (2009)Ethnic differences and functional analysis of MET mutations in lung cancer. Clin. Cancer Res., 15, 5714–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Leidner R.S., et al. (2009)Genetic abnormalities of the EGFR pathway in African American Patients with non-small-cell lung cancer. J. Clin. Oncol., 27, 5620–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Phelps M.A., et al. (2014)Erlotinib in African Americans with advanced non-small cell lung cancer: a prospective randomized study with genetic and pharmacokinetic analyses. Clin. Pharmacol. Ther., 96, 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Reinersman J.M., et al. (2011)Frequency of EGFR and KRAS mutations in lung adenocarcinomas in African Americans. J. Thorac. Oncol., 6, 28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Yang S.H., et al. (2005)Mutations in the tyrosine kinase domain of the epidermal growth factor receptor in non-small cell lung cancer. Clin. Cancer Res., 11, 2106–2110. [DOI] [PubMed] [Google Scholar]
- 165. Bollig-Fischer A., et al. (2015)Racial diversity of actionable mutations in non-small cell lung cancer. J. Thorac. Oncol., 10, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Campbell J.D., et al. (2017)Comparison of prevalence and types of mutations in lung cancers among Black and White populations. JAMA Oncol., 3, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Araujo L.H., et al. (2015)Somatic mutation spectrum of non-small-cell lung cancer in African Americans: a pooled analysis. J. Thorac. Oncol., 10, 1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wallace T.A., et al. (2008)Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res., 68, 927–936. [DOI] [PubMed] [Google Scholar]
- 169. Martin D.N., et al. (2009)Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One, 4, e4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Powell I.J., et al. (2013)Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol. Biomarkers Prev., 22, 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ambs S., et al. (2008)Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res., 68, 6162–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Byron S.A., et al. (2016)Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat. Rev. Genet., 17, 257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Terunuma A., et al. (2014)MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J. Clin. Invest., 124, 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Theodore S.C., et al. (2010)MiRNA 26a expression in a novel panel of African American prostate cancer cell lines. Ethn. Dis., 20(1 Suppl 1), S1–96. [PMC free article] [PubMed] [Google Scholar]
- 175. Reams R.R., et al. (2011)Detecting gene-gene interactions in prostate disease in African American men. Infect. Agent. Cancer, 6 (Suppl 2), S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jones J., et al. (2013)MicroRNAs that affect prostate cancer: emphasis on prostate cancer in African Americans. Biotech. Histochem., 88, 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Theodore S.C., et al. (2014)MicroRNA profiling of novel African American and Caucasian prostate cancer cell lines reveals a reciprocal regulatory relationship of miR-152 and DNA methyltranferase 1. Oncotarget, 5, 3512–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Reams R.R., et al. (2015)Immunohistological analysis of ABCD3 expression in Caucasian and African American prostate tumors. Biomed Res. Int., 2015, 132981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Allard J.E., et al. (2012)Analysis of PSPHL as a candidate gene influencing the racial disparity in endometrial cancer. Front. Oncol., 2, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Field L.A., et al. (2012)Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer, 118, 1334–1344. [DOI] [PubMed] [Google Scholar]
- 181. Rummel S., et al. (2014)PSPHL and breast cancer in African American women: causative gene or population stratification?BMC Genet., 15, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zollino M., et al. (2015)Intragenic KANSL1 mutations and chromosome 17q21.31 deletions: broadening the clinical spectrum and genotype-phenotype correlations in a large cohort of patients. J. Med. Genet., 52, 804–814. [DOI] [PubMed] [Google Scholar]
- 183. Steinberg K.M., et al. (2012)Structural diversity and African origin of the 17q21.31 inversion polymorphism. Nat. Genet., 44, 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Nakshatri H., et al. (2015)Ethnicity-dependent and -independent heterogeneity in healthy normal breast hierarchy impacts tumor characterization. Sci. Rep., 5, 13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Weige C.C., et al. (2014)Transcriptomes and shRNA suppressors in a TP53 allele-specific model of early-onset colon cancer in African Americans. Mol. Cancer Res., 12, 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Farhana L., et al. (2016)Role of cancer stem cells in racial disparity in colorectal cancer. Cancer Med., 5, 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Leavell B.J., et al. (2012)Associations between markers of colorectal cancer stem cells and adenomas among ethnic groups. Dig. Dis. Sci., 57, 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kinseth M.A., et al. (2014)Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int. J. Cancer, 134, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lathan C.S. (2015)Lung cancer care: the impact of facilities and area measures. Transl. Lung Cancer Res., 4, 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lathan C.S., et al. (2015)Perspectives of African Americans on lung cancer: a qualitative analysis. Oncologist, 20, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lathan C.S., et al. (2016)Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J. Clin. Oncol., 34, 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zheng Y.L., et al. (2010)Elevated lung cancer risk is associated with deficiencies in cell cycle checkpoints: genotype and phenotype analyses from a case-control study. Int. J. Cancer, 126, 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]