ABSTRACT
Background
Evidence linking maternal diet quality during pregnancy with infant birth outcomes is limited in Asia.
Objective
We investigated the association of maternal diet quality with the risk of preterm birth, offspring birth size, and adiposity in a multiethnic Asian birth cohort.
Design
Dietary intakes of 1051 pregnant women were ascertained at 26–28 wk of gestation with the use of 24-h recalls and 3-d food diaries, from which diet quality (score range: 0–100) was measured by the Healthy Eating Index for pregnant women in Singapore (HEI-SGP). Gestational age was established by first-trimester ultrasound dating scan. Neonatal weight and length were measured at birth. Body composition was assessed by air displacement plethysmography in a subset of infants (n = 313) within 72 h after birth, and abdominal adiposity was assessed by MRI (n = 316) within the first 2 wk of life. Associations were assessed by multivariable linear regression for continuous outcomes and logistic regression for preterm birth.
Results
The mean ± SD maternal HEI-SGP score was 52.1 ± 13.6. Maternal diet quality during pregnancy was not associated with preterm birth or birth weight. Greater adherence to the HEI-SGP (per 10-point increment in HEI-SGP score) was associated with longer birth length [β (95% CI): 0.14 (0.03, 0.24 cm)], lower body mass index (in kg/m2) at birth [−0.07 (−0.13, −0.01)], lower sum of triceps and subscapular skinfold thickness [−0.15 (−0.26, −0.05 mm)], lower percentage body fat [−0.52% (−0.84%, −0.20%)], lower fat mass [−17.23 (−29.52, −4.94 g)], lower percentage abdominal superficial subcutaneous adipose tissue [−0.16% (−0.30%, −0.01%)], and lower percentage deep subcutaneous adipose tissue [−0.06% (−0.10%, −0.01%)].
Conclusions
Higher maternal diet quality during pregnancy was associated with longer birth length and lower neonatal adiposity but not with birth weight and preterm birth. These findings warrant further investigation in independent studies. This trial was registered at clinicaltrials.gov as NCT01174875.
Keywords: maternal diet, diet quality, preterm birth, birth weight, adiposity
INTRODUCTION
Globally, 11% of infants are born preterm (<37 wk of gestation) (1). Preterm birth and extreme birth weights are associated with higher risks of infant mortality and morbidity and of noncommunicable diseases in adulthood (1–3). Because infants with similar size can have marked variability in adiposity, increasing attention has focused on investigation of infant body composition in order to understand the relation between early-life development and later health outcomes (4). Neonatal adiposity may be associated with childhood obesity (5), and given the globally increasing trend of childhood obesity and consequent risk of chronic disease, examinations of infant body composition and adiposity are valuable and critical (6).
A substantial body of evidence has shown that maternal nutrition during pregnancy can influence birth outcomes (7) and subsequent chronic disease risk in the offspring (8). There has been growing interest in examining overall maternal diet quality (9–21) by using index scores such as the Healthy Eating Index (HEI) (7–10), the Mediterranean diet score (10–16), the New Nordic diet score (17, 18), and the Dietary Approaches to Stop Hypertension (DASH) score (19). This is because these take into account the multiplex interactions among nutrients and foods (22), an approach congruent with recommendations of the 2015 Dietary Guidelines Advisory Committee (23). Although each dietary index captures adherence to slightly different dietary guidelines, in general, these indexes indicate a good-quality diet as one that is high in vegetables, fruit, fish, and unsaturated fats and low in red and processed meat and saturated fats (9–21).
Although greater adherence to the DASH (21) and Mediterranean (18) diets has been associated with a lower incidence of preterm birth, several studies observed no association (13, 15, 16, 19). For birth size, a better-quality diet during pregnancy has generally been associated with higher birth weight (10, 14, 17) and a lower risk of fetal growth restriction (10, 14, 20), but other studies have reported no association (9, 11–14). To our knowledge, only one study has explored infant body composition; this study observed a lower HEI score (i.e., poorer diet quality) to be associated with increased infant adiposity (9).
Existing studies have been conducted in white subjects (9–21) and no study, to our knowledge, has examined the association between maternal diet quality and infant birth outcomes in Asians. Furthermore, only one study examined the association between maternal diet quality and infant body composition by using air displacement plethysmography (9); and to our knowledge, no study to date has explored abdominal adiposity. It is unclear whether similar associations are present in multiethnic Asian populations, particularly because important differences in body composition exist between Asian and white infants (24, 25). In this study (clinicaltrials.gov; NCT01174875), we investigated the association of maternal diet quality during pregnancy with the risk of preterm birth and offspring birth size and adiposity in a multiethnic Asian mother-offspring cohort study.
METHODS
Study population
We used data from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study, a prospective mother-offspring cohort designed to investigate early developmental pathways to noncommunicable diseases (26). Women in the first trimester of pregnancy (n = 1247), aged 18–50 y, were recruited from 2 major public maternity units in Singapore, National University Hospital, and KK Women's and Children's Hospital, between June 2009 and September 2010. Women were ineligible if they had type 1 diabetes, were receiving chemotherapy or psychotropic drugs, or whose parents and spouses’ parents had a different ethnicity (e.g., Chinese, Malay, or Indian descent). The study was approved by the National Health Care Group Domain Specific Review Board (reference D/09/021) and the SingHealth Centralized Institutional Review Board (reference 2009/280/D). All of the participants gave informed written consent upon recruitment.
Diet quality assessment
Maternal dietary intakes were ascertained at 26–28 wk of gestation with the use of 24-h recalls (n = 1127) and 3-d food diaries (n = 260) (27). Clinic staff trained by an experienced dietician administered the 24-h recall with a 5-stage multiple-pass interviewing technique (28). Standardized household measuring utensils and food pictures of various portion sizes were provided to assist women in quantifying their dietary intake. The clinic staff also guided the participants on completing food diaries at home for the following week. Nutrient analysis of the dietary records was performed by using nutrient analysis software (Dietplan; Forestfield Software Ltd.) containing a food-composition database of locally available foods (29), with modifications made for inaccuracies found.
Diet quality was measured by the Healthy Eating Index for pregnant women in Singapore (HEI-SGP) (30). The HEI-SGP has been validated to examine diet quality of pregnant women in Singapore (30) and, to date, has been associated with health outcomes such as maternal retinal microvasculature abnormalities (31) and gestational diabetes (in preparation). In brief, the HEI-SGP has 11 components with a maximum possible raw score of 90. The selection and weighting of each component are in accordance with the national (local) dietary guidelines for pregnant women as well as substantiated by other dietary quality indexes in which content validity has been established previously (e.g., HEI and Alternate HEI) (11, 32–35). Accordingly, the food groups were first classified into the following 3 categories, and under each category the score was then divided to reflect dietary adequacy and quality:
Fruit and vegetables (0–20 points)—adequacy components: total fruit (0–5 points), total vegetables (0–5 points); quality components: whole fruit (0–5 points), dark-green leafy and orange vegetables (0–5 points)
Grains (0–20 points)—adequacy component: total rice and alternatives (0–10 points); quality component: whole grains (0–10 points)
Meat and others (0–20 points)—adequacy component: total protein foods (0–10 points); quality component: dairy (0–10 points)
There are 2 nutrient-based moderation components that reflect compliance with recommended total fat (0–10 points) and total saturated fat (0–10 points); and last, the use of antenatal supplements containing iron, folate, and calcium (0–10 points). Scores from individual components were adjusted for energy intake by using the energy density method (36), summed and scaled up to 100 to get the total HEI-SGP score for each pregnant woman, with higher scores reflecting greater adherence to dietary guidelines, indicative of better diet quality.
Infant birth outcomes
Gestational age (GA) was determined by a dating ultrasound scan in the first trimester and preterm birth defined as delivery of a live birth <37 completed weeks of gestation. Birth weight was measured shortly after birth to the nearest 1 g (SECA model 334; SECA Corp.), whereas recumbent length was measured to the nearest 0.5 cm from the top of the head to the soles of the feet (SECA model 210). BMI at birth was calculated as birth weight divided by the square of recumbent length (kg/m2). We derived birth weight–for-GA and birth length–for-GA z scores by using references from our cohort (37). Weight-for-length z scores were calculated with the use of an established equation (38) on the basis of z scores for weight and length. Within 72 h after delivery, head, abdominal, and midupper arm circumferences were measured to the nearest 0.1 cm (SECA model 212), and triceps and subscapular skinfold thicknesses were measured in triplicate to the nearest 0.2 mm on the right side of the body and summed (Holtain Skinfold Caliper; Holtain Ltd.).
Anthropometric training and standardization sessions were conducted quarterly to ensure that each anthropometrist developed and maintained their techniques for accurate and precise measurements. The high intraobserver intraclass correlation coefficient values (between 0.994 and 0.997) (39) as well as the low interobserver technical error of measurement (between 0.03 and 0.04 mm) and CVs (between 2.05% and 2.19%) affirmed that the measurements were highly reliable (40).
Infant fat mass, fat-free mass, and percentage of body fat were assessed by using an air displacement plethysmography device (Pea Pod infant body-composition system, version 3.1.0; Cosmed) within 3 d after delivery (41). Neonatal abdominal adiposity was assessed by using MRI (GE Signa HDxt 1.5 Tesla MR scanner) approximately within the first 2 wk of life (42). Subsets of 313 infants and 316 infants in this study had Pea Pod and MRI data, respectively. Superficial subcutaneous adipose tissue (sSAT), deep subcutaneous adipose tissue (dSAT), and internal adipose tissue (IAT) were quantified and expressed as percentages of abdominal adipose tissue compartment volumes. Percentages of abdominal adipose tissue compartment volumes were derived from the ratio of each compartment volume and the total abdominal volume (42). Pearson's correlation coefficients for the sum of subscapular and triceps skinfold thicknesses, total body composition measured by Pea Pod, and abdominal adiposity assessed by MRI were between 0.47 and 0.58, with the exception of IAT, which was 0.24.
Covariates
Maternal age, ethnicity, educational level, household income, and self-reported prepregnancy weights were ascertained during recruitment. Between 26 and 28 wk of gestation, maternal weight was measured to the nearest 0.1 kg (SECA 803), standing height was measured in duplicate to the nearest 0.1 cm from the top of the head to the heels (SECA 213), maternal plasma folate status was determined by competitive electrochemiluminescence immunoassay on the ADVIA Centaur Immunoassay System (Siemens) (43), and information on physical activity (44), cigarette smoking, and alcohol consumption during pregnancy was ascertained. Prepregnancy BMI was calculated as prepregnancy weight divided by height squared (kg/m2), whereas weight gain until 26–28 wk of gestation was calculated by subtracting prepregnancy weight from weight measured between 26 and 28 wk of gestation.
Statistical analyses
Of the 1247 participants, we excluded women who underwent in vitro fertilization (n = 85) and who were expecting twins (n = 10). Of the remaining 1152 participants, 64 women dropped out during pregnancy due to personal reasons, loss to follow-up, family disapproval, and inconvenience. Women who provided 24-h recalls reported to be reflective of their typical diets and who had information on their offspring's birth anthropometric measurements (n = 1051), total body composition (n = 313), and abdominal adiposity (n = 316) were included in the analysis (Supplemental Figure 1). There were no significant differences in characteristics (birth weight, gestational age, parity, and prepregnancy BMI) between neonates who had undergone MRI and those who did not undergo MRI (42) or Pea Pod measurements (Supplemental Table 1). To evaluate whether possible selection bias may have affected the results, we conducted sensitivity analysis for infant birth anthropometry outcomes by limiting the analysis to a subset of participants with MRI and Pea Pod measurements (n = 166).
HEI-SGP scores derived from 24-h recalls were our primary source of dietary data, because only a subset of participants (n = 259) completed and returned their 3-d food diaries. We conducted sensitivity analyses on participants who provided their 3-d food diaries to assess the consistency and robustness of our study results.
Maternal characteristics and nutrient intakes were summarized according to quintiles of HEI-SGP scores (Table1). P-trends were assessed by modeling the median value of the quintiles in linear regression for continuous variables or by using Cochran-Mantel-Haenszel tests for categorical variables.
TABLE 1.
Characteristics of the study participants according to quintiles of HEI-SGP scores1
| Quintile | ||||||
|---|---|---|---|---|---|---|
| 1 (n = 210) | 2 (n = 210) | 3 (n = 211) | 4 (n = 210) | 5 (n = 210) | P-trend | |
| HEI-SGP score2 | 34.4 (29.8–37.8) | 44.1 (42.0–46.2) | 52.4 (50.3–54.0) | 60 (57.8–61.8) | 70.0 (66.5–74.6) | |
| Age, y | 28.9 ± 5.4 | 30.2 ± 5.0 | 30.4 ± 5.2 | 31.3 ±5.1 | 31.6 ± 4.6 | <0.001 |
| Prepregnancy BMI, kg/m2 | 22.8 ± 4.4 | 22.9 ± 4.8 | 23.3 ± 4.6 | 22.5 ± 4.6 | 22.0 ± 3.5 | 0.04 |
| Weight gain until 26–28 wk of gestation, kg | 9.0 ± 4.4 | 8.3 ± 5.0 | 9.2 ± 5.4 | 8.2 ± 4.3 | 8.6 ± 4.1 | 0.34 |
| Height, cm | 158.1 ± 5.3 | 158.3 ± 5.8 | 157.9 ± 5.7 | 157.9 ± 5.9 | 158.8 ± 5.4 | 0.40 |
| Plasma folate, nmol/L | 33.0 ± 21.6 | 38.9 ± 54.9 | 35.4 ± 23.2 | 44.6 ± 48.1 | 44.5 ± 41.2 | 0.002 |
| Ethnicity, % | 0.13 | |||||
| Chinese | 44 | 55 | 49 | 63 | 65 | |
| Malayan | 43 | 31 | 28 | 17 | 13 | |
| Indian | 12 | 14 | 23 | 20 | 22 | |
| Educational level, % | <0.001 | |||||
| None, primary, or secondary | 40 | 34 | 35 | 28 | 17 | |
| Postsecondary | 39 | 43 | 33 | 35 | 29 | |
| University | 20 | 23 | 33 | 36 | 55 | |
| Household income, % | <0.001 | |||||
| <2000 SGD | 24 | 13 | 14 | 15 | 7 | |
| 2000–6000 SGD | 57 | 66 | 60 | 52 | 50 | |
| >6000 SGD | 18 | 20 | 26 | 33 | 43 | |
| Physical activity, % | 0.92 | |||||
| Inactive (<600 MET-h/wk) | 30 | 42 | 29 | 34 | 31 | |
| Sufficiently active (600–3000 MET-h/wk) | 47 | 43 | 52 | 49 | 50 | |
| Highly active (>3000 MET-h/wk) | 23 | 15 | 19 | 18 | 19 | |
| Primiparous, % | 42 | 41 | 45 | 41 | 45 | 0.63 |
| Current smoker, % | 4.8 | 4.8 | 2.6 | 0.5 | 0.5 | <0.001 |
| Alcohol use during pregnancy, % | 2.4 | 1.6 | 2.0 | 1.5 | 2.4 | 0.98 |
| Nutrient intakes | ||||||
| Total energy intake, kcal/d | 1976 ± 605 | 1832 ± 586 | 1791 ± 590 | 1814 ±485 | 1824 ± 531 | 0.008 |
| Carbohydrate, % of energy | 46.0 ± 8.5 | 49.5 ±8.0 | 53.5 ± 9.2 | 54.4 ± 7.4 | 56.0 ± 6.9 | <0.001 |
| Protein, % of energy | 14.5 ± 3.8 | 15.3 ± 3.9 | 15.8 ± 4.2 | 16.1 ± 3.4 | 16.4 ± 3.6 | <0.001 |
| Total fat, % of energy | 39.5 ± 6.6 | 35.3 ± 6.3 | 30.7 ± 7.3 | 29.5 ± 6.0 | 27.7 ± 5.2 | <0.001 |
| Saturated fat, % of energy | 17.4 ± 4.5 | 14.0 ± 3.2 | 12.0 ± 3.6 | 11.3 ± 3.0 | 10.5 ± 2.8 | <0.001 |
| Dietary fiber, g/1000 kcal | 6.6 ± 2.6 | 7.4 ± 3.2 | 8.9 ± 4.4 | 9.8 ± 4.8 | 11.2 ± 4.5 | <0.001 |
| Dietary calcium, mg/1000 kcal | 252 ± 136 | 306 ± 170 | 328 ± 168 | 372 ± 180 | 473 ± 224 | <0.001 |
| Dietary iron, mg/1000 kcal | 6.6 ± 4.6 | 8.2 ± 7.3 | 9.2 ± 7.2 | 9.9 ± 7.6 | 14.7 ± 11.1 | <0.001 |
Values are means ± SDs unless otherwise indicated; n = 1051. P-trends were assessed by modeling the median value of the quintiles in linear regression for continuous variables or with the use of the Cochran-Mantel-Haenszel tests for categorical variables. There were missing data for prepregnancy BMI (n = 81), weight gain until 26–28 wk of gestation (n = 88), height (n = 9), educational level (n = 14), household income (n = 67), physical activity (n = 9), smoking status (n = 27), alcohol consumption (n = 25), and plasma folate (n = 82). HEI-SGP, Healthy Eating Index for pregnant women in Singapore; MET-h, metabolic equivalent task-hours; SGD, Singapore dollars.
Median (IQR).
The associations of maternal HEI-SGP scores with infant birth outcomes were analyzed by linear regression for continuous outcomes and logistic regression for preterm birth (Table2). These models were adjusted for infant's sex and birth order and mother's age, ethnicity, prepregnancy BMI, weight gain up until 26–28 wk of gestation, height, energy intake, educational level, household income, physical activity, alcohol use, and smoking during pregnancy. Infant body composition and abdominal adiposity analyses were further adjusted for the infant's postnatal age on the Pea Pod and MRI measurement days, respectively.
TABLE 2.
Associations between maternal HEI-SGP scores during 26–28 wk of gestation with infant birth outcomes1
| Unadjusted model, | Multivariable model,2 | ||
|---|---|---|---|
| Value | β (95% CI) | β (95% CI) | |
| Preterm birth,3n (%) | 76 (7.2) | 0.90 (0.75, 1.07)4 | 0.91 (0.75, 1.11)4 |
| Infant anthropometric measures at birth3 | |||
| Birth weight, g | 3090 ± 451 | 4.08 (−16.05, 24.20) | −2.00 (−22.57, 18.57) |
| Birth length, cm | 48.6 ± 2.3 | 0.22 (0.12, 0.32)** | 0.14 (0.03, 0.24)* |
| BMI, kg/m2 | 13.0 ± 1.3 | −0.09 (−0.15, −0.03)** | −0.07 (−0.13, −0.01)* |
| Head circumference, cm | 33.6 ± 1.4 | 0.05 (−0.02, 0.11) | 0.03 (−0.03, 0.10) |
| Abdominal circumference, cm | 28.3 ± 2.4 | −0.11 (−0.22, 0.0003) | −0.06 (−0.18, 0.06) |
| Midupper arm circumference, cm | 10.8 ± 1.0 | −0.01 (−0.05, 0.04) | 0.002 (−0.05, 0.05) |
| Sum of triceps and subscapular skinfold thickness, mm | 10.3 ± 2.2 | −0.18 (−0.29, −0.08)** | −0.15 (−0.26, −0.05)** |
| Infant body composition5 | |||
| Fat-free mass, g | 2780 ± 309 | 11.57 (−15.15, 38.30) | 3.81 (−23.95, 31.58) |
| Fat mass | |||
| g | 315 ± 138 | −14.25 (−26.15, −2.35)* | −17.23 (−29.52, −4.94)** |
| % | 9.95 ± 3.62 | −0.46 (−0.77, −0.15)** | −0.52 (−0.84, −0.20)** |
| Infant abdominal adiposity distribution,6 % | |||
| Superficial subcutaneous abdominal adipose tissue | 9.75 ± 1.77 | −0.13 (−0.27, 0.02) | −0.16 (−0.30, −0.01)* |
| Deep subcutaneous abdominal adipose tissue | 1.66 ± 0.57 | −0.04 (−0.09, 0.003) | −0.06 (−0.10, −0.01)* |
| Internal abdominal adipose tissue | 2.85 ± 0.68 | −0.01 (−0.07, 0.04) | −0.02 (−0.08, 0.04) |
Values are means ± SDs or linear regression coefficients (95% CIs) for continuous variables and logistic regression coefficients (95% CIs) for preterm birth per 10-point increment in HEI-SGP scores unless otherwise indicated. *P < 0.05; **P < 0.01. HEI-SGP, Healthy Eating Index for pregnant women in Singapore.
The multivariable model was adjusted for infants’ sex and birth order and maternal age, ethnicity, prepregnancy BMI, weight gain up until 26–28 wk of gestation, height, energy intake, educational level, household income, physical activity, alcohol use, and smoking during pregnancy. For analysis of infant body composition and abdominal adiposity distribution, the models were additionally adjusted for the infant's exact postnatal age at Pea Pod (Cosmed) and MRI measurements, respectively.
n = 1051.
OR (95% CI).
n = 313.
n = 316.
Missing covariate data [i.e., maternal height (n = 9), physical activity (n = 9), educational level (n = 14), alcohol consumption (n = 25), smoking status (n = 27), household income (n = 67), prepregnancy BMI (n = 81), and pregnancy weight gain up until 26–28 wk of gestation (n = 88)] were estimated by multiple imputation (45). We generated 100 independent imputations, and the results of the pooled analyses are presented. To evaluate whether the imputation of missing data may have affected the results, we performed sensitivity analyses on participants with complete data (n = 886).
We investigated potential effect modification by infant sex and ethnicity by including an interaction term (ethnicity × HEI-SGP score or infant sex × HEI-SGP score) in the multivariable regression models. We also performed sensitivity analyses to examine the robustness of our results by restricting our analyses to term infants (n = 975) and women without medical conditions (i.e., chronic hypertension, pregnancy-induced hypertension, pre-eclampsia, and gestational diabetes) (n = 773). In addition, we alternately excluded one component of the HEI-SGP score to determine whether any individual component was driving the association between HEI-SGP and infant birth outcomes (46).
All of the statistical analyses were performed with the use of SPSS version 19.0 (IBM Corp.). Two-sided P values <0.05 were considered significant.
RESULTS
Characteristics of the study population
In 1051 pregnant women, the mean ± SD HEI-SGP score was 52.1 ± 13.6 (range: 12.7–94.3). Women with higher HEI-SGP scores tended to be older, nonsmokers, and have a higher educational level and household income, higher plasma folate concentrations, and higher dietary intakes of carbohydrates, protein, dietary fiber, calcium, and iron but have lower intakes of fat, particularly saturated fat (Table 1).
Associations with preterm birth and birth anthropometric measurements
In this study, 76 births (7.2%) were preterm, the mean ± SD birth weight was 3090 ± 451 g, and birth length was 48.6 ± 2.3 cm. A higher HEI-SGP score was associated with longer birth length (0.14 cm; 95% CI: 0.03, 0.24 cm; per 10-point increment in HEI-SGP scores), lower BMI (in kg/m2) at birth (−0.07; 95% CI: −0.13, −0.01; per 10-point increment), and lower sum of triceps and subscapular skinfold thicknesses (−0.15 mm; 95% CI: −0.26, −0.05 mm; per 10-point increment) (Table 2). The results remained largely similar when we examined z scores, where each 10-point increment in HEI-SGP score was associated with a 0.06 SD (95% CI: 0.02, 0.11 SD) increase in birth length and a 0.09 SD (95% CI: 0.02, 0.15 SD) decrease in weight for length. No associations were observed for preterm birth, birth weight, and other neonatal body circumference measurements. There were no interactions between infant sex, ethnicity, and HEI-SGP score in relation to preterm birth and infant birth anthropometric measurements (all P-interactions between 0.12 and 0.75).
Associations with infant body composition and abdominal adiposity distribution
In the subset of infants with body-composition data (n = 313), a higher HEI-SGP score was associated with a lower percentage of body fat (−0.52%; 95% CI: −0.84%, −0.20%; per 10-point increment) and lower fat mass (−17.23 g; 95% CI: −29.52, −4.94 g; per 10-point increment) but not with fat-free mass (Table 2).
In 316 infants who underwent MRI, a higher HEI-SGP score was associated with a lower percentage of sSAT (−0.16%; 95% CI: −0.30%, −0.01%; per 10-point increment) and a lower percentage of dSAT (−0.06%; 95% CI: −0.10%, −0.01%; per 10-point increment) but was not associated with percentage of IAT (Table 2). There were no interactions between infant sex, ethnicity, and HEI-SGP score in relation to infant body composition and abdominal adiposity distribution (all P-interactions between 0.07 and 0.86).
Sensitivity analyses
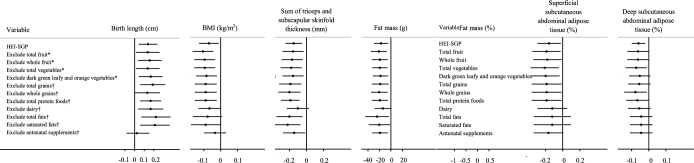
The results remained largely similar when we restricted our analyses to participants with complete data (n = 886), term infants (n = 975), or to women without medical conditions (i.e., chronic hypertension, pregnancy-induced hypertension, pre-eclampsia, or gestational diabetes) (n = 773). In general, alternately excluding one component of the HEI-SGP score did not substantially change our results for all of the outcomes (Figure1), with the exception of birth length. The association between HEI-SGP score with birth length was attenuated (0.02 cm; 95% CI: −0.11, 0.15 cm; per 10-point increment) after the exclusion of antenatal supplements containing iron, folate, and calcium from the HEI-SGP score.
FIGURE 1.
The association between maternal HEI-SGP scores alternately excluding individual components and infant birth outcomes (per 10-point increment). Black dots denote linear regression coefficients and horizontal lines denote 95% CIs. The models were adjusted for infants’ sex and birth order and maternal age, ethnicity, prepregnancy BMI, weight gain up until 26–28 wk of gestation, height, energy intake, educational level, household income, physical activity, alcohol use, smoking during pregnancy, and excluded component. For analysis of infant body composition and abdominal adiposity distribution, the models were additionally adjusted for the infant's exact postnatal age with Pea Pod (Cosmed) and MRI measurements, respectively. *Effect estimates were multiplied by 100/95 to correct for the 100-point scale. †Effect estimates were multiplied by 100/90 to correct for the 100-point scale. HEI-SGP, Healthy Eating Index for pregnant women in Singapore.
In the subset of participants with Pea Pod and MRI data (n = 166), we observed a similar magnitude of association (albeit a wider CI) between a higher HEI-SGP score with a lower BMI at birth (−0.08; 95% CI: −0.23, 0.07; per 10-point increment) and a lower sum of subscapular and skinfold thickness (−0.17 mm; 95% CI: −0.43, 0.10 mm; per 10-point increment) but not with birth length (−0.03 cm; 95% CI: −0.27, 0.02 cm; per 10-point increment).
When we restricted our analyses to a subset of participants with 3-d food diaries (n = 259), we observed a similar direction of association (albeit a wider CI) for birth length (0.10 cm; 95% CI: −0.11, 0.31 cm; per 10-point increment), BMI (−0.13; 95% CI: −0.26, −0.01; per 10-point increment), sum of skinfold thickness (−0.18 mm; 95% CI: −0.38, 0.02 mm; per 10-point increment), percentage body fat (−0.99%; 95% CI: −1.99%, −0.22%; per 10-point increment), and fat mass (−3.60 g; 95% CI: −7.52, 0.32 g; per 10-point increment) but not with sSAT (0.01%; 95% CI: −0.36%, 0.39%; per 10-point increment) and dSAT (0.02%; 95% CI: −0.09%, 0.12%; per 10-point increment), which might be due to the reduced sample size (n <80) and insufficient statistical power for abdominal adiposity measures.
DISCUSSION
In this study, maternal diet quality during pregnancy, as assessed by the HEI-SGP, was not associated with preterm birth or birth weight. Higher maternal diet quality was, however, associated with longer birth length and lower neonatal adiposity, characterized by the reduction in BMI at birth, sum of neonatal skinfold thicknesses, overall body fat, and abdominal subcutaneous adipose tissue in neonates.
Consistent with others, we did not observe an association between maternal diet quality and preterm birth (13, 15, 16, 19), with the exception of 2 studies that showed a lower risk of preterm birth with greater adherence to the DASH diet (21) and a Mediterranean diet (18). The latter study was restricted to participants with low-risk pregnancies (i.e., no pre-existing health conditions, no complications during previous pregnancies, were nonsmokers, etc.), and the results may not be applicable to other populations of pregnant women (18). The intake of sweetened beverages was accounted for in the DASH diet (21) but less emphasized in other diet quality indexes (13, 15, 16, 19, 30). Earlier studies have shown that the intake of sweetened beverages during pregnancy is associated with an increased risk of preterm birth (47–49). Whether the lower intakes of sweetened beverages on the DASH diet contribute to a lower risk of preterm birth warrants further investigation in independent studies.
Previous studies in the Mediterranean area of Spain (10, 14, 17) and Norway (20) have shown that improving diet quality during pregnancy is associated with longer birth length (10, 14), higher birth weight (10, 14, 17), and a lower risk of fetal growth restriction (10, 14, 20), but most cohorts in the United States (9, 11, 12), France (13), Greece (14), and the Atlantic area of Spain (14) did not observe an association with birth size. These studies did not examine the role of individual components of the dietary indexes, and there are insufficient data to determine the reasons for the conflicting results. We showed that the association between HEI-SGP score with birth length was attenuated after excluding the use of antenatal supplements containing iron, folate, and calcium from the HEI-SGP score, which suggests that these micronutrients contributed most to the association between higher HEI-SGP score and longer birth length. This finding is also in line with our previous work (43), which showed trends between higher maternal plasma folate concentrations and longer birth length. Because the requirements for these 3 micronutrients increase during pregnancy, antenatal supplementation may be beneficial if the intake of these micronutrients from foods is inadequate. From our national nutrition survey, it was observed that ∼25% of women aged 18–39 y had insufficient dietary iron and calcium intakes (<70% of the Recommended Dietary Allowance) (50). Similarly, ∼10% of pregnant women in our cohort had low plasma folate concentrations (<13.6 nmol/L). Therefore, it is possible that a better-quality diet, including adequate intakes of these 3 micronutrients, may improve birth outcomes even in well-nourished populations.
In our cohort, a higher HEI-SGP score was associated with lower BMI at birth, a reduced sum of subscapular and triceps skinfold thicknesses at birth, and a lower percentage of body fat, fat mass, and neonatal sSAT and dSAT. Consistent with an earlier finding (9), a higher-quality maternal diet is generally associated with lower neonatal adiposity and this effect is independent of maternal prepregnancy BMI. These results did not appear to be driven by specific components of the HEI-SGP score, because alternately excluding one component of the HEI-SGP score did not materially change our results. We have previously shown that mothers with sufficient vitamin D concentrations (>75.0 nmol/L) (in review), higher maternal betaine status, or higher dietary protein intake were associated with lower neonatal adiposity in our GUSTO cohort (51, 52). This result further attests to our conclusion that neonatal adiposity is dependent on various components of the diet (i.e., the overall diet), rather than a single nutrient or diet component. Abdominal adiposity may be more closely linked to metabolic risk than peripheral adiposity (53). In particular, dSAT and visceral adipose tissue are strongly associated with insulin resistance and the development of obesity-related complications (54, 55). However, further investigation is required to determine whether the distribution of neonatal abdominal adipose tissue persists in adulthood and affects later morbidity.
The clinical significance of maternal diet quality and neonatal adiposity is unclear at present. Given the compelling epidemiologic evidence and clinical emphasis on maternal obesity and infant adiposity (56–58), we sought to compare the association between maternal HEI-SGP score and maternal prepregnancy BMI and infant body-composition outcomes but interpreted the results with caution because they have a different etiology. Among the whole cohort, the mean ± SD HEI-SGP score was 52.1 ± 13.6 and prepregnancy BMI was 22.7 ± 4.3. We noted that the effect estimates for skinfold thickness (0.21 mm per SD decrease in HEI-SGP score compared with 0.35 mm per SD increase in prepregnancy BMI), fat mass (23.3 compared with 19.2 g), percentage body fat (0.71% compared with 0.36%), sSAT (0.21% compared with 0.38%), and dSAT (0.07% compared with 0.08%) were generally comparable. These results highlight the clinical relevance and importance of maternal diet quality during pregnancy as it relates to neonatal adiposity and consequent health outcomes in adulthood.
Strengths of our study include its prospective design, which minimizes recall and interviewer bias (59); the use of first-trimester ultrasound dating, which is more precise in estimating GA (60); the comprehensive assessment of neonatal anthropometric and body-composition outcomes, including a gold-standard, nonionizing method (i.e., MRI) for assessing specific abdominal adipose tissue compartments (61); and the use of the HEI-SGP, which has adequate content validity and suitability for assessing diet quality in pregnant women in Asia because it was developed in a diverse ethnic population (30).
Several limitations of our study need to be noted. First, maternal dietary intake was obtained primarily from 24-h recalls, which may not be representative of an individual's typical dietary intake, which varies from day to day. However, sensitivity analyses in participants who completed a 3-d food diary produced similar estimates, albeit wider CIs (but not with abdominal adiposity measures, which might be due to the reduced sample size). In addition, we also showed that our 24-h recall is moderately valid and has good reproducibility in previously published results (27, 29, 30, 52, 62). Second, maternal diet was only assessed during 26–28 wk of gestation, which may not be representative of a diet followed throughout pregnancy. However, previous studies have reported that dietary intakes and patterns did not change substantially during pregnancy (63–65). Last, although we considered many potential confounding factors, our findings could still be influenced by residual confounding and causality cannot be claimed, as in any observational study.
In conclusion, we observed that maternal diet quality, as assessed by the HEI-SGP, was associated with longer birth length and lower neonatal adiposity in a multiethnic Asian cohort. However, maternal diet quality during pregnancy was not associated with birth weight or preterm birth. These findings warrant further investigation in independent studies.
Supplementary Material
ACKNOWLEDGMENTS
The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit FP Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, Fabian Yap, George Seow Heong Yeo, Helen Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D Holbrook, Joshua J Gooley, Keith M Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, PC Wong, Peter D Gluckman, Pratibha Agarwal, Rob M van Dam, Salome A Rebello, Seang-Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Chin-Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan, and Yung Seng Lee.
The authors’ responsibilities were as follows—A-RC and MF-FC: designed the research and had primary responsibility for the final content; K-HT, FY, LP-CS, Y-SC, KMG, and YSL: designed and led the GUSTO study; M-TT, CYH, L-WC, MC, and IMA: contributed to data collection, cleaning, and analysis; A-RC: performed the statistical analysis and wrote the manuscript; M-TT, L-WC, IMA, and MF-FC: provided statistical input; M-TT, CYH, L-WC, IMA, M-CC, KMG, MVF, YSL, and MF-FC: reviewed the manuscript for important intellectual content; and all authors: read and approved the final manuscript. KMG and Y-SC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG and Y-SC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. The other authors had no financial or personal conflicts of interest to declare.
Supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Program and administered by the Singapore Ministry of Health's National Medical Research Council (NMRC), Singapore (NMRC/TCR/004-NUS/2008 and NMRC/TCR/012-NUHS/2014). Additional funding was provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore, and Nestec. KMG is supported by the National Institute for Health Research (NIHR) through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Program (FP7/2007-2013) and the EarlyNutrition and ODIN projects under grant agreements 289346 and 613977. The study sponsors were not involved in the design of the study, statistical analysis, or interpretation of the results.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used
- DASH
Dietary Approaches to Stop Hypertension
- dSAT
deep subcutaneous adipose tissue
- GA
gestational age
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- HEI
Healthy Eating Index
- HEI-SGP
Healthy Eating Index for pregnant women in Singapore
- IAT
Internal adipose tissue
- sSAT
superficial subcutaneous adipose tissue
REFERENCES
- 1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379(9832):2162–72. [DOI] [PubMed] [Google Scholar]
- 2. Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, Adair L, Baqui AH, Bhutta ZA, Caulfield LE, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 2013;1(1):e26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng S-K, Olog A, Spinks AB, Cameron CM, Searle J, McClure RJ. Risk factors and obstetric complications of large for gestational age births with adjustments for community effects: results from a new cohort study. BMC Public Health 2010;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumfield M. Update on the role of maternal diet in pregnancy and the programming of infant body composition. Nutr Bull 2015;40(4):286–90. [Google Scholar]
- 5. Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009;90(5):1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO. Obesity: preventing and managing the global epidemic. Geneva (Switzerland): WHO; 2000. [PubMed] [Google Scholar]
- 7. Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev 2010;32(1):5–25. [DOI] [PubMed] [Google Scholar]
- 8. Martin-Gronert M, Ozanne S. Maternal nutrition during pregnancy and health of the offspring. Biochem Soc Trans 2006;34(5):779–82. [DOI] [PubMed] [Google Scholar]
- 9. Shapiro AL, Kaar JL, Crume TL, Starling AP, Siega-Riz AM, Ringham BM, Glueck DH, Norris JM, Barbour LA, Friedman JE, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (Lond) 2016;40(7):1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodríguez-Bernal CL, Rebagliato M, Iñiguez C, Vioque J, Navarrete-Muñoz EM, Murcia M, Bolumar F, Marco A, Ballester F. Diet quality in early pregnancy and its effects on fetal growth outcomes: the Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr 2010;91(6):1659–66. [DOI] [PubMed] [Google Scholar]
- 11. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 2009;109(6):1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poon AK, Yeung E, Boghossian N, Albert PS, Zhang C. Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Scientifica 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saunders L, Guldner L, Costet N, Kadhel P, Rouget F, Monfort C, Thomé JP, Multigner L, Cordier S. Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: results from a French Caribbean mother–child cohort study (TIMOUN). Paediatr Perinat Epidemiol 2014;28(3):235–44. [DOI] [PubMed] [Google Scholar]
- 14. Chatzi L, Mendez M, Garcia R, Roumeliotaki T, Ibarluzea J, Tardon A, Amiano P, Lertxundi A, Iniguez C, Vioque J, et al. Mediterranean diet adherence during pregnancy and fetal growth: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr 2012;107(1):135–45. [DOI] [PubMed] [Google Scholar]
- 15. Mikkelsen TB, Østerdal ML, Knudsen VK, Haugen M, Meltzer HM, Bakketeig L, Olsen SF. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand 2008;87(3):325–30. [DOI] [PubMed] [Google Scholar]
- 16. Haugen M, Meltzer HM, Brantsæter AL, Mikkelsen T, Østerdal ML, Alexander J, Olsen SF, Bakketeig L. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand 2008;87(3):319–24. [DOI] [PubMed] [Google Scholar]
- 17. Monteagudo C, Mariscal-Arcas M, Heras-Gonzalez L, Ibañez-Peinado D, Rivas A, Olea-Serrano F. Effects of maternal diet and environmental exposure to organochlorine pesticides on newborn weight in southern Spain. Chemosphere 2016;156:135–42. [DOI] [PubMed] [Google Scholar]
- 18. Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. American journal of obstetrics and gynecology 2005;193(4):1292–301. [DOI] [PubMed] [Google Scholar]
- 19. Hillesund ER, Øverby NC, Engel SM, Klungsøyr K, Harmon QE, Haugen M, Bere E. Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). Eur J Epidemiol 2014;29(10):753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hillesund ER, Bere E, Haugen M, Øverby NC. Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth—a study performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr 2014;17(09):1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin CL, Sotres-Alvarez D, Siega-Riz AM. Maternal dietary patterns during the second trimester are associated with preterm birth. J Nutr 2015;145(8):1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cespedes EM, Hu FB. Dietary patterns: from nutritional epidemiologic analysis to national guidelines. Am J Clin Nutr 2015;101(5):899–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dietary Guidelines Advisory Committee. Scientific report of the 2015 Dietary Guidelines Advisory Committee. Washington (DC): USDA; US Department of Health and Human Services; 2015. [Google Scholar]
- 24. Yajnik C, Fall C, Coyaji K, Hirve S, Rao S, Barker D, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes 2003;27(2):173–80. [DOI] [PubMed] [Google Scholar]
- 25. Modi N, Thomas EL, Uthaya SN, Umranikar S, Bell JD, Yajnik C. Whole body magnetic resonance imaging of healthy newborn infants demonstrates increased central adiposity in Asian Indians. Pediatr Res 2009;65:584–7. [DOI] [PubMed] [Google Scholar]
- 26. Soh S-E, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stünkel W, Holbrook JD, Kwek K, Chong Y-S, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol 2014;43(5):1401–9. [DOI] [PubMed] [Google Scholar]
- 27. Chong MF-F, Chia A-R, Colega M, Tint M-T, Aris IM, Chong Y-S, Gluckman P, Godfrey KM, Kwek K, Saw S-M, et al. Maternal protein intake during pregnancy is not associated with offspring birth weight in a multiethnic Asian population. J Nutr 2015. [DOI] [PubMed] [Google Scholar]
- 28. Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr 2003;77(5):1171–8. [DOI] [PubMed] [Google Scholar]
- 29. Chia A-R, de Seymour JV, Colega M, Chen L-W, Chan Y-H, Aris IM, Tint M-T, Quah PL, Godfrey KM, Yap F, et al. A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study. Am J Clin Nutr 2016. [DOI] [PubMed] [Google Scholar]
- 30. Han CY, Colega M, Quah EPL, Chan YH, Godfrey KM, Kwek K, Saw S-M, Gluckman PD, Chong Y-S, Chong MF-F. A healthy eating index to measure diet quality in pregnant women in Singapore: a cross-sectional study. BMC Nutr 2015;1(1):1. [Google Scholar]
- 31. Li L-J, Ong PG, Colega MT, Han CY, Chen LW, Man Eyn Kidd R, Lamoureux E, Gluckman P, Kwek K, Chong YS, et al. The impact of macronutrients on retinal microvasculature among Singapore pregnant women during the mid-late gestation. PloS One 2016;11(8):e0160704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95(10):1103–8. [DOI] [PubMed] [Google Scholar]
- 33. Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108(11):1896–901. [DOI] [PubMed] [Google Scholar]
- 34. Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013;113(4):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76(6):1261–71. [DOI] [PubMed] [Google Scholar]
- 36. Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108. [DOI] [PubMed] [Google Scholar]
- 37. Aris IM, Gandhi M, Cheung YB, Soh SE, Tint MT, Gluckman PD, Lee YS, Yap FK, Chong YS. A new population-based reference for gestational age-specific size-at-birth of Singapore infants. Ann Acad Med Singapore 2014;43(9):439–47. [PubMed] [Google Scholar]
- 38. Cole T. A new index of child weight-for-height based on weight and height Z scores. Ann Hum Biol 1994;21:96. [Google Scholar]
- 39. Aris IM, Soh SE, Tint MT, Liang S, Chinnadurai A, Saw SM, Kwek K, Godfrey KM, Gluckman PD, Chong YS, et al. Body fat in Singaporean infants: development of body fat prediction equations in Asian newborns. Eur J Clin Nutr 2013;67(9):922–7. [DOI] [PubMed] [Google Scholar]
- 40. Aris IM, Bernard JY, Chen LW, Tint MT, Lim WY, Soh SE, Saw SM, Shek LP, Godfrey KM, Gluckman PD, et al. Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. Int J Obes (Lond) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aris IM, Soh SE, Tint MT, Liang S, Chinnadurai A, Saw SM, Kwek K, Godfrey KM, Gluckman PD, Chong YS, et al. Body fat in Singaporean infants: development of body fat prediction equations in Asian newborns. Eur J Clin Nutr 2013;67(9):922–7. [DOI] [PubMed] [Google Scholar]
- 42. Tint MT, Fortier MV, Godfrey KM, Shuter B, Kapur J, Rajadurai VS, Agarwal P, Chinnadurai A, Niduvaje K, Chan YH, et al. Abdominal adipose tissue compartments vary with ethnicity in Asian neonates: Growing Up in Singapore Toward Healthy Outcomes birth cohort study. Am J Clin Nutr 2016;103(5):1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen L-W, Lim AL, Colega M, Tint M-T, Aris IM, Tan CS, Chong Y-S, Gluckman PD, Godfrey KM, Kwek K, et al. Maternal folate status, but not that of vitamins B-12 or B-6, is associated with gestational age and preterm birth risk in a multiethnic Asian population. J Nutr 2015;145(1):113–20. [DOI] [PubMed] [Google Scholar]
- 44. Padmapriya N, Shen L, Soh SE, Shen Z, Kwek K, Godfrey KM, Gluckman PD, Chong YS, Saw SM, Muller-Riemenschneider F. Physical activity and sedentary behavior patterns before and during pregnancy in a multi-ethnic sample of Asian women in Singapore. Matern Child Health J 2015;19(11):2523–35. [DOI] [PubMed] [Google Scholar]
- 45. Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Method Med Res 2007;16(3):219–42. [DOI] [PubMed] [Google Scholar]
- 46. Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 2009;338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Halldorsson TI, Strøm M, Petersen SB, Olsen SF. Intake of artificially sweetened soft drinks and risk of preterm delivery: a prospective cohort study of 59,334 Danish pregnant women. Am J Clin Nutr 2010. [DOI] [PubMed] [Google Scholar]
- 48. Petherick ES, Goran M, Wright J. Relationship between artificially sweetened and sugar-sweetened cola beverage consumption during pregnancy and preterm delivery in a multi-ethnic cohort: analysis of the Born in Bradford cohort study. Eur J Clin Nutr 2014;68(3):404–7. [DOI] [PubMed] [Google Scholar]
- 49. Englund-Ögge L, Brantsæter AL, Haugen M, Sengpiel V, Khatibi A, Myhre R, Myking S, Meltzer HM, Kacerovsky M, Nilsen RM. Association between intake of artificially sweetened and sugar-sweetened beverages and preterm delivery: a large prospective cohort study. Am J Clin Nutr 2012;96(3):552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Health Promotion Board Report of the National Nutrition Survey 2010 [cited 2017 Feb 20]. Available from: http://www.hpb.gov.sg/HOPPortal/content/conn/HOPUCM/path/Contribution%20Folders/uploadedFiles/HPB_Online/Publications/NNS-2010.pdf. [Google Scholar]
- 51. van Lee L, Tint MT, Aris IM, Quah PL, Fortier MV, Lee YS, Yap FK, Saw SM, Godfrey KM, Gluckman PD, et al. Prospective associations of maternal betaine status with offspring weight and body composition at birth: the GUSTO (Growing Up in Singapore Toward healthy Outcomes) cohort study. Am J Clin Nutr 2016. [DOI] [PubMed] [Google Scholar]
- 52. Chen LW, Tint MT, Fortier MV, Aris IM, Bernard JY, Colega M, Gluckman PD, Saw SM, Chong YS, Yap F, et al. Maternal macronutrient intake during pregnancy is associated with neonatal abdominal adiposity: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study. J Nutr 2016;146(8):1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith SR, Lovejoy JC, Greenway F, Ryan D, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001;50(4):425–35. [DOI] [PubMed] [Google Scholar]
- 54. Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000;278(5):E941–8. [DOI] [PubMed] [Google Scholar]
- 55. Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F, Liuzzi A, Blasio AM. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity 2007;15(8):1933–43. [DOI] [PubMed] [Google Scholar]
- 56. Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006;195(4):1100–3. [DOI] [PubMed] [Google Scholar]
- 57. Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol 2008;198(4):416.e1–6. [DOI] [PubMed] [Google Scholar]
- 58. Modi N, Murgasova D, Ruager-Martin R, Thomas EL, Hyde MJ, Gale C, Santhakumaran S, Dore CJ, Alavi A, Bell JD. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res 2011;70(3):287–91. [DOI] [PubMed] [Google Scholar]
- 59. Mann C. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J 2003;20(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taipale P, Hiilesmaa V. Predicting delivery date by ultrasound and last menstrual period in early gestation. Obstet Gynecol 2001;97(2):189–94. [DOI] [PubMed] [Google Scholar]
- 61. Demerath EW, Fields DA. Body composition assessment in the infant. Am J Hum Biol 2014;26(3):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Seymour J Chia A, Colega M, Jones B, McKenzie E, Shirong C, Godfrey K, Kwek K, Saw S-M, Conlon C, et al. Maternal dietary patterns and gestational diabetes mellitus in a multi-ethnic asian cohort: the GUSTO study. Nutrients 2016;8(9):574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol 2006;20(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women's dietary patterns change little from before to during pregnancy. J Nutr 2009;139(10):1956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cuco G, Fernandez-Ballart J, Sala J, Viladrich C, Iranzo R, Vila J, Arija V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr 2006;60(3):364–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.