ABSTRACT
Background
The substitution of omega (ω)-6 (n–6) polyunsaturated fatty acids (PUFAs) for saturated fatty acids (SFAs) is advocated in cardiovascular disease prevention. The impact of this substitution on lipoprotein metabolism in subjects with dyslipidemia associated with insulin resistance (IR) remains unknown.
Objective
In men with dyslipidemia and IR, we evaluated the impact of substituting ω-6 PUFAs for SFAs on the in vivo kinetics of apolipoprotein (apo) B-containing lipoproteins and on the intestinal expression of key genes involved in lipoprotein metabolism.
Design
Dyslipidemic and IR men (n = 36) were recruited for this double-blind, randomized, crossover, controlled trial. Subjects consumed, in a random order, a fully controlled diet rich in SFAs (SFAs: 13.4% of energy; ω-6 PUFAs: 4.0%) and a fully controlled diet rich in ω-6 PUFAs (SFAs: 6.0%; ω-6 PUFAs: 11.3%) for periods of 4 wk, separated by a 4-wk washout period. At the end of each diet, the in vivo kinetics of apoB-containing lipoproteins were measured and the intestinal expression of key genes involved in lipoprotein metabolism was quantified in duodenal biopsies taken from each participant.
Results
The substitution of ω-6 PUFAs for SFAs had no impact on TRL apoB-48 fractional catabolic rate (Δ = –3.8%, P = 0.7) and production rate (Δ = +1.2%, P = 0.9), although it downregulated the intestinal expression of the microsomal triglyceride transfer protein (Δ = –18.4%, P = 0.006) and apoB (Δ = –16.6%, P = 0.005). The substitution of ω-6 PUFAs for SFAs decreased the LDL apoB-100 pool size (Δ = –7.8%; P = 0.005). This difference was attributed to a reduction in the LDL apoB-100 production rate after the substitution of ω-6 PUFAs for SFAs (Δ = –10.0%; P = 0.003).
Conclusions
This study demonstrates that the substitution of dietary ω-6 PUFAs for SFAs decreases the production and number of LDL particles in men with dyslipidemia and IR. This trial was registered at clinicaltrials.gov as NCT01934543.
Keywords: insulin resistance, lipoprotein metabolism, intestinal mRNA expression, omega-6 polyunsaturated fatty acids, saturated fatty acids
INTRODUCTION
A significant proportion of cardiovascular disease (CVD) is attributable to insulin resistance (IR), which affects >25% of the population in North America (1, 2). The atherogenic dyslipidemia associated with IR is characterized by both decreased HDL cholesterol concentrations and an overaccumulation of triglyceride-rich lipoproteins (TRLs) (3). The increased production rate (PR) of both hepatic apolipoprotein (apo) B-100-containing lipoproteins and intestinally derived apoB-48-containing TRLs, as well as the reduced catabolism of these subfractions, cause the overaccumulation of TRLs associated with IR (4). Recently, our group also showed that subjects with IR present important alterations in the expression of key intestinal genes involved in lipoprotein homeostasis that could play a role in the overall dysregulation of TRL metabolism (5). This is of significant interest because atherogenic dyslipidemia is undisputedly a major CVD risk factor for populations with IR (6, 7).
Dietary guidelines advocate the consumption of omega-6 PUFAs instead of SFAs for the prevention of CVD, as they decrease LDL cholesterol concentrations (8–10). Moreover, acute and chronic dietary studies show that the substitution of ω-6 PUFAs for SFAs decreases postprandial lipemia and apoB-48 secretion in insulin-sensitive subjects (11–14). The cholesterol- and triglyceride-lowering effects of ω-6 PUFA consumption in place of SFAs are of particular interest for dyslipidemic and IR subjects. However, data on the impact of this dietary substitution on lipoprotein metabolism in subjects with dyslipidemia associated with IR are limited and mixed (15–18). Therefore, our ability to define optimal, evidence-based dietary guidelines for this population at high CVD risk remains limited.
The general objective of this study was to characterize the homeostasis of lipoproteins in men with dyslipidemia and IR by examining how the substitution of dietary ω-6 PUFAs for SFAs modifies hepatic and intestinal lipoprotein metabolism. The primary objective was to examine the impact of the substitution of dietary ω-6 PUFAs for SFAs on the in vivo kinetics of apoB-containing lipoproteins. The secondary objective was to examine the impact of the consumption of dietary ω-6 PUFAs instead of SFAs on the expression of key intestinal genes that regulate the absorption, synthesis, assembly, and secretion of lipids and lipoproteins. We hypothesized that, in dyslipidemic and IR men, the substitution of ω-6 PUFAs for SFAs would reduce the PR, stimulate the fractional catabolic rate (FCR) of apoB-containing lipoproteins, and regulate the intestinal expression of key genes involved in lipid and lipoprotein metabolism, as dietary fatty acids are known to affect the transcription of several genes (19).
METHODS
The Laval University Medical Center ethical review committee approved the research protocol. Written consent was obtained from all participants. This trial was registered at clinicaltrials.gov (NCT01934543). The study was conducted between December 2013 and December 2014 at the Institute of Nutrition and Functional Food (INAF) of Laval University in the Quebec City area (Canada).
Study subjects
Thirty-six unrelated, dyslipidemic men with IR were recruited. To be enrolled in the study, participants had to be between 18 and 65 y old, had to have fasting plasma TG concentrations ≥1.3 mmol/L (114 mg/dL) and <7.0 mmol/L (619 mg/dL), and fasting insulin concentrations ≥90 pmol/L. Fasting TG and insulin concentrations were measured during 2 different screening visits. The average concentration of TG and insulin had to meet these criteria. Participants had to have had a stable body weight for ≥3 mo prior to the screening, a BMI (in kg/m2) ≥25 and a waist circumference ≥94 cm. Exclusion criteria included smoking (>1 cigarette/d); alcoholism; illicit drug consumption; extreme dyslipidemias (e.g., familial hypercholesterolemia); type 2 diabetes; a history of CVD or cancer; acute hepatic or renal disease (aspartate aminotransferase/alanine transaminase >1.5× the upper limit of normal; creatinine concentrations >176 μmol/L; creatine phosphokinase >2× the upper limit of normal); HIV; uncontrolled high blood pressure (>160/110 mm Hg); or any other condition that could interfere with participation in the study. Lipid-lowering medication had to be withdrawn 3 wk prior to the first screening visit and abstained from for the duration of the study. The use of antidepressants, antihypertensive drugs, or levothyroxine was allowed only if the dose had been stable for ≥3 mo prior to the study. Any other drug, dietary supplement, or natural health product had to be stopped for the duration of the study. Participants had to notify one of the study coordinators if they had to initiate or modify any medication during the course of the study.
Study design
This study was designed as a double-blind, randomized, controlled, crossover study. Once enrolled, subjects initiated a 2-wk run-in period to familiarize themselves with the instructions of the study (weeks –2 to 0). At week 0, subjects were randomly assigned to either the SFA-PUFA or PUFA-SFA diet sequence. The randomization was performed with 4 blocks of 10 subjects with an allocation ratio of 1:1. Subjects with the SFA-PUFA sequence first consumed a fully controlled diet rich in SFAs and low in ω-6 PUFAs for 4 wk (weeks 0–4). The participants were then subjected to a fully controlled diet rich in ω-6 PUFAs and low in SFAs for 4 wk (weeks 8–12) after a 4-wk washout period separating the 2 experimental diets (weeks 4–8). Given the crossover design, participants with the PUFA-SFA sequence consumed the 2 fully controlled diets in the reverse order. The 2 experimental diets were consumed under carefully controlled, isocaloric conditions to enable participants to maintain a constant weight during the study. The energy requirements of the study participants were estimated using Harris-Benedict's formula (20) and an assessment of their energy intakes from their completed, validated, web-based, self-administered food frequency questionnaires (21). Body weight was monitored on weekdays before lunch. If a participant's weight changed by ±2 kg from the diet-specific baseline value, the participant's caloric intake was adjusted to stop the weight variation. Three meals and 1 snack were provided daily to all participants. A 7-d cyclic menu was used. On weekdays, subjects consumed their lunch meal at the Clinical Investigation Unit at the Institute of Nutrition and Functional Foods under the supervision of a research assistant. Other meals were packed and consumed at home. Participants were instructed to consume all the food, and only the food, that was provided to them. Subjects had free access to caffeine- and calorie-free beverages. Alcohol consumption was forbidden during the experimental diets and limited to ≤2 servings/d and ≤7 servings/wk during the run-in and the washout periods. Participants had to complete a daily checklist to identify foods that they had or had not consumed. Dietary compliance was measured using this checklist. Participants had to maintain a constant physical activity level throughout the study. Fasting blood samples were collected at the 2 screening visits, at the beginning of each diet and on the morning of the kinetic study at the end of each diet (weeks –4, –3, 0, 4, 8, 12). Kinetic studies were conducted on the last day (day 28) of each diet and duodenal biopsies were obtained on the day 26 of each diet. High-intensity physical activity was forbidden 48 h prior to the duodenal biopsies and kinetic studies. Participants and coordinators were blinded until the final statistical analyses were conducted.
Composition of the experimental diets
The nutritional composition of the SFA diet was designed to represent the typical US diet while the nutritional composition of the ω-6 PUFA diet corresponded to the current US nutritional guidelines (8). The 2 experimental diets provided ∼35% of energy as fat, ∼50% as carbohydrates, and ∼16% as protein (Table 1). The SFA diet contained 13.4% of energy as SFAs, 14.4% as MUFAs, and 4.0% as ω-6 PUFAs. Dietary fats and SFAs were mainly provided from lard. The ω-6 PUFA diet provided 6.0% of energy as SFAs, 14.2% as MUFAs, and 11.5% as ω-6 PUFAs. Safflower oil instead of lard was used to provide ω-6 PUFAs. Other foods were fat free or low in fat in both diets.
TABLE 1.
Composition of the experimental diets1
| SFA diet | ω-6 PUFA diet | |
|---|---|---|
| Energy, kcal | 3288 ± 302 | 3296 ± 299 |
| Alcohol, % | 0.0 | 0.0 |
| Protein, % | 16.0 | 16.0 |
| Carbohydrate, % | 49.3 | 49.7 |
| Lipids, % | 34.7 | 34.3 |
| Lipids, g | 127.9 ± 11.7 | 128.2 ± 11.6 |
| SFAs, % | 13.4 | 6.0 |
| SFAs, g | 49.4 ± 4.5 | 22.6 ± 2.1 |
| Butyric acid, (4:0) | 0.5 ± 0.0 | 0.3 ± 0.0 |
| Caproic acid (6:0) | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Caprylic acid (8:0) | 0.3 ± 0.0 | 0.2 ± 0.0 |
| Capric acid (10:0) | 0.5 ± 0.1 | 0.3 ± 0.0 |
| Lauric acid (12:0) | 1.2 ± 0.1 | 0.9 ± 0.1 |
| Myristic acid (14:0) | 3.0 ± 0.3 | 1.2 ± 0.1 |
| Palmitic acid (16:0) | 28.2 ± 2.6 | 14.0 ± 1.3 |
| Margaric acid (17:0) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Stearic acid (18:0) | 14.5 ± 1.3 | 4.8 ± 0.4 |
| Arachidic acid (20:0) | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Behenic acid (22:0) | 0.1 ± 0.0 | 0.1 ± 0.0 |
| MUFAs, % | 14.4 | 14.2 |
| MUFAs, g | 52.9 ± 4.9 | 53.0 ± 4.8 |
| PUFAs, % | 4.5 | 11.8 |
| PUFAs, g | 16.6 ± 1.5 | 44.0 ± 4.0 |
| Linoleic acid (18:2) | 14.4 ± 1.3 | 41.8 ± 3.8 |
| Linolenic acid (18:3) | 1.6 ± 0.1 | 1.6 ± 0.1 |
| Arachidonic acid (20:4) | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Eicosapentanoic acid (20:5) | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Docosapentanoic acid (22:5) | 0.1 ± 0.0 | 0.0 ± 0.0 |
| Docosahexanoic acid (22:6) | 0.2 ± 0.0 | 0.2 ± 0.0 |
| ω-6 PUFAs, % | 4.0 | 11.3 |
| ω-6 PUFAs, g | 14.7± 1.4 | 42.1 ± 3.8 |
| ω-3 PUFAs, % | 0.5 | 0.5 |
| ω-3 PUFAs, g | 1.9 ± 0.2 | 1.9 ± 0.2 |
| PUFA:SFA ratio | 0.3 | 2.0 |
| trans Fatty acid, % | 0.5 | 0.5 |
| Dietary cholesterol, mg | 328 ± 30 | 329 ± 30 |
| Fiber, g | 38.2 ± 3.5 | 38.2 ± 3.5 |
| Sodium, mg | 2934 ± 270 | 2944 ± 267 |
Data are presented as means ± SDs.
In the SFA diet, the use of lard provided an important amount of MUFAs, mainly oleic acid (18:1). To maintain the same MUFA content between the 2 diets, a small quantity of olive oil was substituted for safflower oil in the ω-6 PUFA diet. Thus, the ω-6 PUFA content of the ω-6 PUFA diet was ∼2.0% lower than the SFA content of the SFA diet (11.5% vs. 13.4%). Overall, the main difference between the 2 experimental diets was the substitution of ω-6 PUFAs for SFAs. Other macro- and micronutrients were matched in both diets. The breakfast meal represented ∼30% of the daily energy intake and the lunch and dinner meals each provided 35% of the daily energy intake. The composition of the diets was measured using Nutrition Data System for Research software (University of Minnesota). Dietetic technicians prepared the experimental diets throughout the study. Each food or ingredient was weighed with a precision of ±0.1 g.
Fasting plasma lipoprotein, glucose, and insulin concentration measurements
Twelve-hour fasting blood samples were obtained from an antecubital vein. Serum cholesterol and TG concentrations were determined with a Roche/Hitachi MODULAR analyzer (Roche Diagnostics) using proper reagents. Blood glucose concentrations were measured by colorimetry and insulin concentrations were measured by electrochemiluminescence (Roche Diagnostics). Commercial ELISA kits were used to measure concentrations of apoAV, apoC-II, apoC-III, apoE, angiopoietin-like 3, C-reactive protein, cholesteryl ester transfer protein, hepatic lipase (HL), LDL receptor (LDLR), and lipoprotein lipase (LPL). The complete list of manufacturers is presented in the Supplemental Material.
Kinetic studies
To determine the kinetics of apoB-48- and apoB-100-containing lipoproteins, subjects underwent a primed-constant infusion of l-[5,5,5-d3]leucine while they were maintained in a constant fed state on the last day of each diet. Subjects received 30 small, identical snacks every half hour for 15 h, each containing one-thirtieth of their estimated daily food intake. The composition of the snacks provided during the kinetic studies was identical to that of the diet. The snacks of the kinetic study performed at the end of the ω-6 PUFA diet provided 11.3% of energy as ω-6 PUFAs and 6.0% as SFAs. The snacks of the kinetic study performed at the end of the SFA diet provided 4.0% of energy as ω-6 PUFAs and 13.4% as SFAs. Blood samples were collected at predetermined times during the test. ApoB-48 and apoB-100 concentrations were measured by commercial ELISA kits (Shibayagi Co. Ltd. and Alerchek Inc., respectively). Sample processing, laboratory measurements and lipoprotein kinetic analyses were performed by liquid chromatography-mass spectrometry with multiple reaction monitoring as previously described (22).
Intestinal biopsies and extraction and quantification of total RNA
Biopsy samples were collected from the second portion of the duodenum during gastroduodenoscopy. Three samples (3 × 3 mm) were collected using single-use biopsy forceps and immediately flash frozen in liquid nitrogen and stored at –80°C before RNA extraction. Intestinal tissue samples were homogenized in 1 mL of QIAzol (Qiagen). RNA was extracted using an RNeasy kit (Qiagen). To eliminate any contaminating DNA, biopsies were treated with an RNase-free DNase set. Total RNA extraction and quantitative real-time polymerase chain reaction were performed as previously described (23). Primer sequences and gene descriptions are available in Supplemental Table 1. Quantitative real-time polymerase chain reaction measurements were performed by the CHU de Québec-Université Laval Research Center Gene Expression Platform (Quebec, Canada) and were compliant with MIQE guidelines.
Sample size estimate
A power calculation was conducted using the change in TRL apoB-48 PR as the primary outcome, and this calculation indicated that a sample size of 30 subjects would enable us to detect a clinically significant difference of 37% in TRL apoB-48 PR between the 2 diets with a power of 80% at a 2-sided 5% significance level. The within-patient SD for TRL apoB-48 PR used for this calculation was 50%, based on previous studies from our group (24, 25). Studies conducted in healthy subjects (n < 10) reported significant effects on chylomicron metabolism associated with this dietary substitution (11). A power calculation using the change in LDL apoB-100 FCR as a secondary outcome was also conducted. The within-patient standard deviation was fixed at 35%, based on previous studies from our group (24, 25). A sample size of 30 subjects would allow us to detect a clinically significant difference of 26% in LDL apoB-100 FCR between the SFA and ω-6 PUFA diets with a power of 80% at a 2-sided 5% significance level. This assumption is based on previous studies that reported significant effects on LDL apoB-100 clearance with the substitution of ω-6 PUFAs for SFAs (12, 26). An estimated attrition rate of 20% was taken into account to determine that 36 subjects had to be recruited.
Statistics
Statistical analyses were performed using mixed models with JMP Pro software version 12.2.0. In the mixed models, treatment was treated as a fixed effect and subjects were treated as a random effect. Treatment sequence and diet-specific baseline values were included in the models as fixed effect covariates. Models for biochemical characteristics, kinetics, and mRNA gene expression were also adjusted for anthropometric characteristics that differed between the 2 treatments. Only covariates with significant effects were maintained in the models. The covariance structure was adjusted for each dependent variable to increase the fit of the data to the model. The model normality was assessed by the distribution of the scaled residual values. For additional exploratory analyses, a nonparametric Spearman's rank correlation test was performed to evaluate the associations among key intestinal genes involved in cholesterol metabolism with respect to changes in mRNA expression levels following the PUFA diet versus the SFA diet. Differences were considered to be statistically significant at P < 0.05.
RESULTS
A flow chart of the study is presented in Figure 1. During the intervention, 5 subjects dropped out of the study for personal reasons (protocol judged too restrictive, n = 2; aversion to foods, n = 3). One subject was randomly assigned although he did not meet the eligibility criteria and was excluded from the study during week 0. Baseline demographic and fasting biochemical characteristics of the 30 subjects who completed the study are presented in Table 2. Subjects exhibited features of IR and atherogenic dyslipidemia with elevated HOMA-IR indexes, waist circumferences, and fasting TG concentrations and reduced HDL cholesterol concentrations.
FIGURE 1.
Flow chart of participants.
TABLE 2.
Baseline (week 0) anthropometric and fasting biochemical characteristics of the subjects (n = 30)1
| Characteristics | |
|---|---|
| Age, y | 39.5 ± 12.2 |
| Weight, kg | 100.4 ± 14.3 |
| BMI, kg/m2 | 32.7 ± 3.6 |
| Waist circumference, cm | 110.8 ± 9.6 |
| Systolic blood pressure, mm Hg | 126 ± 10 |
| Diastolic blood pressure, mm Hg | 75 ± 9 |
| TC, mmol/L | 5.39 ± 1.15 |
| TG, mmol/L | 2.44 ± 1.49 |
| HDL-C, mmol/L | 1.09 ± 0.20 |
| LDL-C, mmol/L | 3.12 ± 0.85 |
| Non HDL-C, mmol/L | 4.30 ± 1.10 |
| TC/HDL-C | 5.06 ± 1.18 |
| Glucose, mmol/L | 5.34 ± 0.56 |
| Insulin, pmol/L | 136 ± 52 |
| HOMA-IR index | 4.68 ± 1.91 |
Data are presented as the mean ± SD. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; TC, total cholesterol; TG, triglyceride.
The reported compliance to the experimental diets was very high for both diets (>99%). No difference was observed at the end of the 2 diets in weight (P = 0.5) and BMI (P = 0.9) of the subjects. Waist circumference was slightly lower at the end of the SFA diet in comparison with the end of the ω-6 PUFA diet (Δ = –0.6 cm, P = 0.02).
The substitution of ω-6 PUFAs for SFAs had no impact on TRL apoB-48 PR, FCR, and pool size (PS) (Table 3). Kinetic analyses, however, indicated that the substitution of ω-6 PUFAs for SFAs had a significant impact on LDL apoB-100 metabolism. The LDL apoB-100 PS was significantly lower after the ω-6 PUFA diet compared with the SFA diet. This was attributable to the lower LDL apoB-100 PR, as LDL apoB-100 FCR remained unchanged between the 2 diets. The kinetics of VLDL apoB-100 and of IDL apoB-100 were not modified by the diets. Also, no significant differences between the 2 diets were observed in the VLDL-to-LDL and the IDL-to-LDL conversion rates. Extensive biochemical analyses were conducted to determine if the change in LDL apoB-100 kinetics was associated with a variation in circulating molecules known to impact lipoprotein metabolism (Table 4). No changes were observed in the post-diet fasting concentrations of apoA-V, apoE, apoC-II, apoC-III, LPL, HL, angiopoietin-like 3, cholesteryl ester transfer protein, and LDLR. Finally, the substitution of ω-6 PUFAs for SFAs had no impact on insulin and glucose homeostasis, C-reactive protein concentrations, and cholesterol and fasting TG concentrations. Nonetheless, LDL cholesterol concentrations tended to be lower after the ω-6 PUFA diet compared to the SFA diet (P = 0.06).
TABLE 3.
Kinetic parameters of TRL apoB-48 and VLDL, IDL, and LDL apoB-100 of the subjects (n = 30) at the end of each diet1
| SFAs | ω-6 PUFAs | %Δ | P 2 | |
|---|---|---|---|---|
| TRL apoB-48 | ||||
| PS, mg | 55.0 ± 32.1 | 57.4 ± 28.6 | +4.2 ± 14.2 | 0.7 |
| FCR, pools/d | 8.38 ± 4.47 | 8.07 ± 3.19 | –3.8 ± 11.9 | 0.7 |
| PR, mg · kg−1 · d−1 | 4.05 ± 2.42 | 4.10 ± 1.44 | +1.2 ± 12.6 | 0.9 |
| VLDL apoB-100 | ||||
| PS, mg | 596 ± 378 | 632 ± 423 | +6.0 ± 15.3 | 0.5 |
| FCR, pools/d | 7.79 ± 4.07 | 7.65 ± 2.62 | –1.8 ± 11.3 | 0.9 |
| PR, mg · kg−1 · d−1 | 39.4 ± 13.5 | 42.7 ± 13.0 | +8.4 ± 8.6 | 0.2 |
| Direct catabolic | 18.1 ± 23.4 | 16.8 ± 20.8 | –7.2 ± 31.6 | 0.7 |
| rate, % | ||||
| IDL apoB-100 | ||||
| PS, mg | 124.9 ± 47.3 | 118.8 ± 49.1 | –4.9 ± 9.9 | 0.2 |
| FCR, pools/d | 20.5 ± 21.6 | 19.5 ± 9.4 | –4.9 ± 21.0 | 0.7 |
| PR, mg · kg−1 · d−1 | 23.3 ± 22.2 | 22.1 ± 9.8 | –5.2 ± 18.9 | 0.7 |
| Direct catabolic | 73.4 ± 39.9 | 80.4 ± 35.4 | +9.5 ± 13.3 | 0.3 |
| rate, % | ||||
| LDL apoB-100 | ||||
| PS, mg | 5126 ± 2215 | 4726 ± 2261 | –7.8 ± 11.3 | 0.005 |
| FCR, pools/d | 0.37 ± 0.12 | 0.37 ± 0.14 | +0.0 ± 8.1 | 0.9 |
| PR, mg · kg−1 · d−1 | 17.9 ± 7.3 | 16.1 ± 7.2 | –10.1 ± 10.6 | 0.003 |
| Conversion rate | ||||
| VLDL to IDL, % | 48.5 ± 18.9 | 50.2 ± 18.1 | +3.5 ± 9.8 | 0.6 |
| VLDL to LDL, % | 33.5 ± 16.3 | 33.0 ± 16.6 | –1.5 ± 12.7 | 0.9 |
| IDL to LDL, % | 26.5 ± 39.9 | 19.5 ± 35.4 | –26.4 ± 36.8 | 0.3 |
Diet-specific data are presented as means ± SDs. Differences between diets are presented as means ± SEMs. apo, apolipoprotein; FCR, fractional catabolic rate; IDL, intermediate-density lipoprotein; PR, production rate; PS, pool size; TRL, triglyceride-rich lipoprotein; Δ, change.
P values were calculated with mixed models for repeated measures with subjects as a random effect.
TABLE 4.
Fasting biochemical characteristics of the subjects (n = 30) at the end of each diet1
| SFA diet | ω-6 PUFA diet | %Δ | P 2 | |
|---|---|---|---|---|
| ApoA-V, ng/mL | 58.2 ± 34.9 | 48.6 ± 21.1 | –16.5 ± 12.8 | 0.1 |
| ApoC-II, μg/mL | 149 ± 48 | 155 ± 54 | +4.0 ± 8.8 | 0.5 |
| ApoC-III, μg/mL | 144 ± 69 | 134 ± 54 | –6.9 ± 11.1 | 0.5 |
| ApoE, μg/mL | 37.5 ± 11.5 | 35.6 ± 8.3 | –5.2 ± 6.9 | 0.2 |
| CETP, ng/mL | 2089 ± 406 | 1999 ± 379 | –4.3 ± 4.8 | 0.2 |
| HL, ρg/mL | 7907 ± 1312 | 8178 ± 1354 | +3.4 ± 4.4 | 0.4 |
| LPL, ng/mL | 3899 ± 8943 | 3963 ± 9100 | +1.6 ± 59.7 | 0.6 |
| ANGPTL3, ng/mL | 130 ± 31 | 130 ± 33 | 0.0 ± 6.37 | 0.9 |
| LDLR, ng/mL | 61.2 ± 22.9 | 62.3 ± 17.6 | +1.7 ± 8.6 | 0.3 |
| Glucose, mmol/L | 5.25 ± 0.61 | 5.21 ± 0.69 | –0.8 ± 3.2 | 0.6 |
| Insulin, ρmol/L | 129 ± 41 | 133 ± 36 | +3.1 ± 7.8 | 0.3 |
| HOMA-IR index | 4.37 ± 1.56 | 4.43 ± 1.29 | +1.4 ± 8.5 | 0.5 |
| CRP, mg/L | 7.72 ± 15.92 | 5.87 ± 4.87 | –24.0 ± 39.4 | 0.2 |
| TC, mmol/L | 4.91 ± 0.83 | 4.67 ± 0.86 | –4.9 ± 4.5 | 0.3 |
| TG, mmol/L | 2.02 ± 0.91 | 1.86 ± 0.77 | –7.9 ± 10.9 | 0.9 |
| HDL-C, mmol/L | 1.02 ± 0.21 | 1.00 ± 0.17 | –2.0 ± 4.9 | 0.9 |
| LDL-C, mmol/L | 2.96 ± 0.68 | 2.79 ± 0.73 | –5.7 ± 6.4 | 0.06 |
| Non HDL-C, mmol/L | 3.90 ± 0.82 | 3.67 ± 0.84 | –5.9 ± 5.4 | 0.4 |
| TC/HDL-C | 5.01 ± 1.23 | 4.78 ± 1.15 | –4.4 ± 6.2 | 0.8 |
Diet-specific data are presented as means ± SDs. Differences between diets are presented as means ± SEMs. Apo, apolipoprotein; ANGPTL3, angiopoietin-like 3; CETP, cholesteryl ester transfer protein; CRP, C-reactive protein; HDL-C, HDL cholesterol; HL, hepatic lipase; LDL-C, LDL cholesterol; LDLR, LDL receptor; LPL, lipoprotein lipase; TC, total cholesterol; TG, triglyceride; Δ, change.
P values were calculated with mixed models for repeated measures with subjects as a random effect.
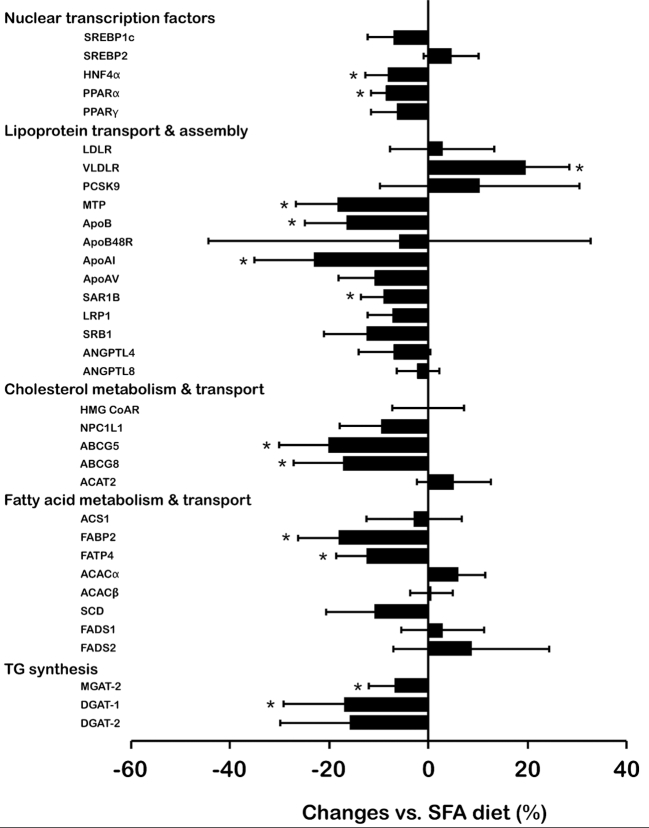
Figure 2 presents the diet-induced changes in the duodenal mRNA expression levels of key genes involved in lipid metabolism. The substitution of dietary ω-6 PUFAs for SFAs significantly downregulated intestinal expression of hepatic nuclear factor-4α (HNF4α: Δ = –8.2 ± 4.6%; P = 0.008) and PPARα (Δ = –8.6 ± 3.1%, P = 0.009). Among genes involved in lipoprotein assembly and transport, substituting ω-6 PUFAs for SFAs significantly upregulated intestinal expression of the VLDL receptor (VLDLR) (Δ = +19.5 ± 9.0%; P = 0.01) and downregulated intestinal expression of microsomal triglyceride transfer protein (MTP) (Δ = –18.4 ± 8.3%; P = 0.006), apoB (Δ = –16.6 ± 8.4%; P = 0.005), apoAI (Δ = –23.1 ± 12.0%; P = 0.002), and SAR1 homolog B (SAR1B) (Δ = –9.0 ± 4.7%; P = 0.04). Of the genes involved in cholesterol metabolism and transport, intestinal expression of ATP-binding cassette (ABC) G5 and ABCG8 were significantly lower after the ω-6 PUFA diet (–20.2 ± 9.9%; P = 0.002 and –17.3 ± 10.0%; P = 0.005, respectively). Similarly, intestinal expression of fatty acid binding protein-2 (FABP2) and fatty acid transport protein-4 (FATP4) were significantly lower after the ω-6 PUFA diet (Δ = –18.2 ± 8.2%; P = 0.006 and Δ = –12.5 ± 6.1%; P = 0.009, respectively). Finally, intestinal mRNA levels of mannosyl (α-1,6-)-glycoprotein β-1,2-N-acetylglucosaminyltransferase (MGAT2) and diacylglycerol O-acyltransferase 1 (DGAT1) were significantly lower following the ω-6 PUFA diet compared to the SFA diet (Δ = –6.8 ± 5.3%; P = 0.04 and Δ = –17.1 ± 12.1%; P = 0.01, respectively).
FIGURE 2.
Percent changes in intestinal mRNA levels following the ω-6 PUFA diet compared with the SFA diet in men with dyslipidemia and insulin resistance (n = 30). Intestinal mRNA levels were normalized according to the expression of the house-keeping gene, TATA box binding protein, prior to statistical analyses. Values are presented as the mean change (%) ± SEM. *Significant change vs. SFA diet (P < 0.05). P values were calculated with mixed models for repeated measures with subjects as a random effect. ABC, ATP-binding casstte; ACAC, acetyl-CoA carboxylase; ACAT2, acetyl-CoA acetyltransferase 2; ACS1, acyl-CoA synthase 1; ANGPTL, angiopoietin-like; Apo, apolipoprotein; DGAT, diglyceride acyltransferase; FABP2, fatty acid-binding protein 2; FADS, fatty acid desaturase; FATP4, fatty acid transport protein 4; HMG CoAR, hydroxymethylglutaryl-CoA reductase; HNF4α, hepatocyte nuclear factor 4 α; LDLR, LDL receptor; LRP1, LDL receptor-related protein 1; MGAT-2, α-1,6-mannosyl-glycoprotein 2-β-N-acetylglucosaminyltransferase; mRNA, messenger RNA; MTP, microsomal triglyceride transfer protein; NPC1L1, Niemann-Pick C1-like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; SAR1B, SAR1 gene homolog B; SCD, stearoyl-CoA desaturase; SRB, scavenger receptor class B t ype 1; SREBP2, sterol regulatory element-binding protein; VLDLR, VLDL receptor.
The validity of the duodenal model used was ascertained by correlation between changes in mRNA levels of different genes known to be regulated together. Among others, the diet-induced changes in HNF4α mRNA levels were significantly correlated with changes in FABP2, FATP4, MTP, apoB, ABCG5, ABCG8, MGAT2, and DGAT1 (Supplemental Table 2).
DISCUSSION
This study evaluated the impact of the substitution of dietary ω-6 PUFAs for SFAs on lipoprotein metabolism in men with dyslipidemia associated with IR. Substituting ω-6 PUFAs for SFAs had no impact on TRL apoB-48 kinetics, but significantly decreased LDL apoB-100 PR and PS by 10.1% and 7.8%, respectively. The substitution of ω-6 PUFAs for SFAs also regulated intestinal expression of several key genes involved in lipoprotein metabolism, including MTP, apoB, and VLDLR. In summary, this study demonstrated that, in dyslipidemic and IR men, substitution of dietary ω-6 PUFAs for SFAs reduces LDL particle number by decreasing production of these lipoproteins.
To our knowledge, this study is the first to evaluate the impact of the substitution of ω-6 PUFAs for SFAs on TRL apoB-48 metabolism using tracer analyses in IR subjects. Postprandial overproduction of intestinal TRLs is a major concern for subjects with dyslipidemia and IR because of the atherogenic properties of apoB-48-containing remnant particles (7, 27). Substitution of ω-6 PUFAs for SFAs reduces postprandial lipemia in non-dyslipidemic and insulin sensitive subjects (11, 12, 14, 28, 29). However, the consumption of various types of dietary fat, including MUFAs, PUFAs, ω-3 PUFAs, SFAs, and medium-chain TGs, has also been reported to have a very limited impact on TRL apoB-48 metabolism and postprandial lipemia in subjects with IR (18, 23, 30, 31). It is well recognized that VLDL apoB-100 PS and insulin sensitivity are the principal determinants of the overproduction of TRL apoB-48 in dyslipidemic and IR subjects (5, 32). In this context, the absence of impact of ω-6 PUFAs on TRL apoB-48 metabolism may result from the neutral effect of ω-6 PUFA on VLDL apoB-100 PS and insulin sensitivity. The present study demonstrated that the substitution of ω-6 PUFAs for SFAs has no beneficial effects on TRL apoB-48 kinetics and postprandial lipemia in subjects with dyslipidemia associated with IR. Accordingly, accumulating evidence now suggests that the impact of dietary fat on postprandial lipoprotein metabolism and CVD risk factors is attenuated by IR (33–35).
In comparison with SFAs, PUFAs are known to repress lipogenesis (19). In this study, the substitution of ω-6 PUFAs for SFAs induced a nearly significant downregulation in the intestinal expression of the lipogenic nuclear transcription factor sterol regulatory element-binding protein 1-C (SREBP1c) (P = 0.1). Several other genes involved in cholesterol and fatty acid transport (ABCG5, ABCG8, FABP2, FATP4), TG synthesis (MGAT2, DGAT1) and chylomicron assembly and secretion (apoB, MTP and SAR1B) were also downregulated following the ω-6 PUFA diet. The concomitant downregulation in the intestinal expression of PPARs was unexpected considering that PUFAs are direct agonists of PPARs (19, 36). Considering that IR is associated with a downregulation in PPAR expression and with important alterations in fat oxidation metabolism (35, 37), it remains likely that the impact of PUFAs on the intestinal expression of PPARs was repressed by IR. Overall, changes in the intestinal mRNA levels of key genes involved in intestinal lipid transport, synthesis, and oxidation, as well as in chylomicron assembly and secretion following the ω-6 PUFA diet, suggest that the substitution of ω-6 PUFAs for SFAs promoted lipid uptake and retention within enterocytes. However, the absence of change in TRL apoB-48 PR between the 2 diets contrasts with these numerous changes in gene expression. Considering that plasma free fatty acids have the ability to directly enter enterocytes via the basolateral membrane (38), exposure to high plasma concentrations of free fatty acids associated with IR probably maintained constant enterocyte lipid content and TRL apoB-48 PR concentrations between the 2 diets (5, 32, 39, 40). Further mechanistic investigations are required to corroborate this hypothesis.
Previous kinetic studies in insulin-sensitive subjects showed that the consumption of ω-6 PUFAs in comparison with SFAs decreased LDL cholesterol concentrations and LDL PS by increasing LDL apoB-100 FCRs (12, 14, 26, 28, 29, 41). Studies conducted in cell and animal models showed that ω-6 PUFAs increase LPL activity (29) and the expression of hepatic lipoprotein-receptor via PPAR-dependent and independent pathways (42–44). Conversely, SFAs increase the apoC-III concentration (45) and reduce HL activity (46) and LDLR concentrations (47), leading to decreased lipoprotein uptake by the liver (48). However, these mechanisms were all described in insulin-sensitive models. In this study, the –7.8% reduction in LDL apoB-100 PS after the ω-6 PUFA diet resulted from the –10.1% decrease in LDL apoB-100 PR. Our results suggest that the reduction in LDL apoB-100 PR could be the outcome of an increased direct clearance of larger TG-rich particles, upstream of LDL production. In that regard, the upregulation of the VLDLR gene induced by the substitution of ω-6 PUFAs for SFAs may have increased the uptake of TG-rich apoB-100–containing particles in the intestine and, potentially, in other peripheral tissues (49–51). This remains, however, only a hypothesis at this stage. Nonetheless, this study demonstrated that substitution of dietary ω-6 PUFAs for SFAs decreases LDL apoB-100 PS by reducing LDL apoB-100 PR in dyslipidemic and IR men.
The recommendation that patients with dyslipidemia associated with IR or type 2 diabetes should reduce SFA consumption to prevent CVD was recently reaffirmed (10). However, these guidelines were primarily based on data from studies conducted in an insulin-sensitive population (52–54) and the cardiovascular benefits of a high consumption of ω-6 PUFAs in place of SFAs was challenged by reappraisal of data from the Sydney Diet Heart Study and the Minnesota Coronary Experiment (55–57). Nonetheless, considering that the LDL apoB-100 particle number is more closely related to coronary risk than the mass of cholesterol within the particles (58), the reduction in LDL apoB-100 PS measured in our fully controlled dietary investigation is of particular interest. Our data suggest that the substitution of ω-6 PUFAs for SFAs could provide cardiovascular benefits in the long term for subjects with dyslipidemia associated with IR. Additional investigation in IR subjects on the impact of this dietary substitution on other cardiometabolic risk factors (e.g., LDL oxidation, inflammation) are required.
The crossover design and fully controlled diets are major strengths of our dietary intervention and enabled evaluation of the impact of the substitution of ω-6 PUFAs for SFAs per se, as recommended in dietary guidelines. Additionally, the kinetic analyses and the quantification of intestinal gene expression provided extensive mechanistic explanations of the reported observations. However, the absence of hepatic and peripheral expression of key genes involved in lipoprotein metabolism may limit the interpretation of changes measured at the duodenal level. It cannot be excluded that the limited length of the dietary interventions attenuated the magnitude of some effects. Finally, the generalizability of the results is limited by the fact that only men were included in the present study.
In conclusion, our study showed that the substitution of dietary ω-6 PUFAs for SFAs in men with dyslipidemia associated with IR had no impact on TRL apoB-48 kinetics but regulated the intestinal expression of several key genes involved in lipoprotein metabolism. More importantly, this dietary substitution reduced LDL particle numbers by decreasing the production of these particles.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to the study subjects for their excellent collaboration and to the dedicated work of Steeve Larouche, Michèle Valotaire and the staff of the metabolic kitchen.
The authors’ responsibilities were as follows—PC and BL: designed the study; J-PD-C, AJT, M-CL, VL: conducted the research; J-PD-C, AJT, BL, and PC: analyzed the data; J-PD-C, AJT, BL, and PC: wrote the manuscript; PC: had the primary responsibility for the final content of the manuscript; and all authors: read and approved the manuscript.
This study was funded by the Canadian Institutes of Health Research (CIHR, MOP-106629). The funder had no role to play in the design of the study and in the interpretation of the results. In the last 5 y, PC has received funding from the CIHR, Agriculture and Agri-Food Canada (Growing Forward program supported by the Dairy Farmers of Canada, Canola Council of Canada, Flax Council of Canada, and Dow Agrosciences), Dairy Research Institute, Dairy Australia, Danone Institute, Merck Frosst, Pfizer, Amgen, Sanofi, Kaneka Corporation, and Atrium Innovations. BL is Chair of Nutrition at Université Laval, which is supported by private endowments from Pfizer, La Banque Royale du Canada and Provigo-Loblaws. BL has received funding in the last 5 years from the CIHR, the Natural Sciences and Engineering Research Council of Canada, Agriculture and Agri-Food Canada (Growing Forward program supported by the Dairy Farmers of Canada, Canola Council of Canada, Flax Council of Canada, Dow Agrosciences), Dairy Research Institute, Dairy Australia, Danone Institute, Merck Frosst, Pfizer and Atrium Innovations. BL serves as the Chair of the peer-review Expert Scientific Advisory Council of Dairy Farmers of Canada. He is also an advisory board member of the Canadian Nutrition Society and the Conseil pour les initiatives de progrès en alimentation and has served as an advisory expert for the Saturated Fat panel of the Heart and Stroke Foundation of Canada. BL has also received speaker honoraria from the International Chair on Cardiometabolic Risk, Dairy Farmers of Canada and the World Dairy Platform. JPDC is the recipient of doctoral scholarships from the Canadian Institute of Health Research and the Fonds de Recherche du Québec—Santé. J-PD-C has received speaker honoria from the Dairy Farmers of Canada. The remaining authors have no conflicts of interest to declare related to this study.
Supported by the Canadian Institutes of Health Research (CIHR, MOP-106629). The funder had no role to play in the design of the study and in the interpretation of the results.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used
- ABC
ATP-binding cassette
- apo
apolipoprotein
- CVD
cardiovascular disease
- DGAT
diglyceride acyltransferase
- FABP2
fatty acid-binding protein 2
- FATP4
fatty acid transport protein 4
- FCR
fractional catabolic rate
- HL
hepatic lipase
- IR
insulin resistance
- LDLR
LDL receptor
- LPL
lipoprotein lipase
- MGAT2
α-1,6-mannosyl-glycoprotein 2-β-N-acetylglucosaminyltransferase
- MTP
microsomal triglyceride transfer protein
- PR
production rate
- PS
pool size
- TG
triglyceride
- TRL
triglyceride-rich lipoprotein
- VLDLR
VLDL receptor
REFERENCES
- 1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9. [DOI] [PubMed] [Google Scholar]
- 2. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004;110:1245–50. [DOI] [PubMed] [Google Scholar]
- 3. Després JP. Dyslipidaemia and obesity. Baillieres Clin Endocrinol Metab 1994;8:629–60. [DOI] [PubMed] [Google Scholar]
- 4. Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagne C, Couture P. Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J Lipid Res 2007;48:1336–42. [DOI] [PubMed] [Google Scholar]
- 5. Couture P, Tremblay AJ, Kelly I, Lemelin V, Droit A, Lamarche B. Key intestinal genes involved in lipoprotein metabolism are downregulated in dyslipidemic men with insulin resistance. J Lipid Res 2014;55:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 7. McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PW, Ordovas JM, Schaefer EJ. Remnant-like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis 2001;154:229–36. [DOI] [PubMed] [Google Scholar]
- 8. Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902–7. [DOI] [PubMed] [Google Scholar]
- 9. Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 10. 8. Cardiovascular Disease and Risk Management. Diabetes Care 2016;39 Suppl 1:S60–71. [DOI] [PubMed] [Google Scholar]
- 11. Weintraub MS, Zechner R, Brown A, Eisenberg S, Breslow JL. Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J Clin Invest 1988;82:1884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demacker PN, Reijnen IG, Katan MB, Stuyt PM, Stalenhoef AF. Increased removal of remnants of triglyceride-rich lipoproteins on a diet rich in polyunsaturated fatty acids. Eur J Clin Invest 1991;21:197–203. [DOI] [PubMed] [Google Scholar]
- 13. Zampelas A, Peel AS, Gould BJ, Wright J, Williams CM. Polyunsaturated fatty acids of the n-6 and n-3 series: effects on postprandial lipid and apolipoprotein levels in healthy men. Eur J Clin Nutr 1994;48:842–8. [PubMed] [Google Scholar]
- 14. Bergeron N, Havel RJ. Influence of diets rich in saturated and omega-6 polyunsaturated fatty acids on the postprandial responses of apolipoproteins B-48, B-100, E, and lipids in triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol 1995;15:2111–21. [DOI] [PubMed] [Google Scholar]
- 15. Summers LK, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML, Moore NR, Frayn KN. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002;45:369–77. [DOI] [PubMed] [Google Scholar]
- 16. Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, et al. . Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 2012;95:1003–12. [DOI] [PubMed] [Google Scholar]
- 17. Heine RJ, Mulder C, Popp-Snijders C, van der Meer J, van der Veen EA. Linoleic-acid-enriched diet: long-term effects on serum lipoprotein and apolipoprotein concentrations and insulin sensitivity in noninsulin-dependent diabetic patients. Am J Clin Nutr 1989;49:448–56. [DOI] [PubMed] [Google Scholar]
- 18. Masson CJ, Mensink RP. Exchanging saturated fatty acids for (n-6) polyunsaturated fatty acids in a mixed meal may decrease postprandial lipemia and markers of inflammation and endothelial activity in overweight men. J Nutr 2011;141:816–21. [DOI] [PubMed] [Google Scholar]
- 19. Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev 2006;86:465–514. [DOI] [PubMed] [Google Scholar]
- 20. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A 1918;4:370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labonte ME, Cyr A, Baril-Gravel L, Royer MM, Lamarche B. Validity and reproducibility of a web-based, self-administered food frequency questionnaire. Eur J Clin Nutr 2012;66:166–73. [DOI] [PubMed] [Google Scholar]
- 22. Tremblay AJ, Lamarche B, Kelly I, Charest A, Lepine MC, Droit A, Couture P. Effect of sitagliptin therapy on triglyceride-rich lipoprotein kinetics in patients with type 2 diabetes. Diabetes Obes Metab 2014;16:1223–9. [DOI] [PubMed] [Google Scholar]
- 23. Tremblay AJ, Lamarche B, Labonte ME, Lepine MC, Lemelin V, Couture P. Dietary medium-chain triglyceride supplementation has no effect on apolipoprotein B-48 and apolipoprotein B-100 kinetics in insulin-resistant men. Am J Clin Nutr 2014;99:54–61. [DOI] [PubMed] [Google Scholar]
- 24. Tremblay AJ, Lamarche B, Cohn JS, Hogue JC, Couture P. Effect of ezetimibe on the in vivo kinetics of apoB-48 and apoB-100 in men with primary hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006;26:1101–6. [DOI] [PubMed] [Google Scholar]
- 25. Tremblay AJ, Lamarche B, Hogue JC, Couture P. Effects of ezetimibe and simvastatin on apolipoprotein B metabolism in males with mixed hyperlipidemia. J Lipid Res 2009;50:1463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shepherd J, Packard CJ, Grundy SM, Yeshurun D, Gotto AM Jr, Taunton OD. Effects of saturated and polyunsaturated fat diets on the chemical composition and metabolism of low density lipoproteins in man. J Lipid Res 1980;21:91–9. [PubMed] [Google Scholar]
- 27. Pal S, Semorine K, Watts GF, Mamo J. Identification of lipoproteins of intestinal origin in human atherosclerotic plaque. Clin Chem Lab Med 2003;41:792–5. [DOI] [PubMed] [Google Scholar]
- 28. Cortese C, Levy Y, Janus ED, Turner PR, Rao SN, Miller NE, Lewis B. Modes of action of lipid-lowering diets in man: studies of apolipoprotein B kinetics in relation to fat consumption and dietary fatty acid composition. Eur J Clin Invest 1983;13:79–85. [DOI] [PubMed] [Google Scholar]
- 29. van Schalkwijk DB, Pasman WJ, Hendriks HF, Verheij ER, Rubingh CM, van Bochove K, Vaes WH, Adiels M, Freidig AP, de Graaf AA. Dietary medium chain fatty acid supplementation leads to reduced VLDL lipolysis and uptake rates in comparison to linoleic acid supplementation. PLoS One 2014;9:e100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tremblay AJ, Lamarche B, Hogue JC, Couture P. n-3 Polyunsaturated fatty acid supplementation has no Effect on postprandial triglyceride-rich lipoprotein kinetics in men with type 2 diabetes. J Diabetes Res 2016;2016:2909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teng KT, Chang CY, Kanthimathi MS, Tan AT, Nesaretnam K. Effects of amount and type of dietary fats on postprandial lipemia and thrombogenic markers in individuals with metabolic syndrome. Atherosclerosis 2015;242:281–7. [DOI] [PubMed] [Google Scholar]
- 32. Lewis GF, O'Meara NM, Soltys PA, Blackman JD, Iverius PH, Pugh WL, Getz GS, Polonsky KS. Fasting hypertriglyceridemia in noninsulin-dependent diabetes mellitus is an important predictor of postprandial lipid and lipoprotein abnormalities. J Clin Endocrinol Metab 1991;72:934–44. [DOI] [PubMed] [Google Scholar]
- 33. Lefevre M, Champagne CM, Tulley RT, Rood JC, Most MM. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr 2005;82:957–63; quiz 1145–6. [DOI] [PubMed] [Google Scholar]
- 34. Chiu S, Williams PT, Dawson T, Bergman RN, Stefanovski D, Watkins SM, Krauss RM. Diets high in protein or saturated fat do not affect insulin sensitivity or plasma concentrations of lipids and lipoproteins in overweight and obese adults. J Nutr 2014;144:1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyle KE, Canham JP, Consitt LA, Zheng D, Koves TR, Gavin TP, Holbert D, Neufer PD, Ilkayeva O, Muoio DM, et al. . A high-fat diet elicits differential responses in genes coordinating oxidative metabolism in skeletal muscle of lean and obese individuals. J Clin Endocrinol Metab 2011;96:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, et al. . Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A 1997;94:4318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li G-S, Liu X-H, Zhu HUA, Huang LAN, Liu Y-L, Ma C-M. Skeletal muscle insulin resistance in hamsters with diabetes developed from obesity is involved in abnormal skeletal muscle LXR, PPAR and SREBP expression. Exp Ther Med 2016;11:2259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Storch J, Zhou YX, Lagakos WS. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J Lipid Res 2008;49:1762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duez H, Lamarche B, Valero R, Pavlic M, Proctor S, Xiao C, Szeto L, Patterson BW, Lewis GF. Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation 2008;117:2369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fielding BA, Callow J, Owen RM, Samra JS, Matthews DR, Frayn KN. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr 1996;63:36–41. [DOI] [PubMed] [Google Scholar]
- 41. Turner JD, Le NA, Brown WV. Effect of changing dietary fat saturation on low-density lipoprotein metabolism in man. Am J Physiol 1981;241:E57–63. [DOI] [PubMed] [Google Scholar]
- 42. Fernandez ML, McNamar DJ. Dietary fat-mediated changes in hepatic apoprotein B/E receptor in the guinea pig: effect of polyunsaturated, monounsaturated, and saturated fat. Metabolism 1989;38:1094–102. [DOI] [PubMed] [Google Scholar]
- 43. Rumsey SC, Galeano NF, Lipschitz B, Deckelbaum RJ. Oleate and other long chain fatty acids stimulate low density lipoprotein receptor activity by enhancing acyl coenzyme A:cholesterol acyltransferase activity and altering intracellular regulatory cholesterol pools in cultured cells. J Biol Chem 1995;270:10008–16. [DOI] [PubMed] [Google Scholar]
- 44. Dallongeville J, Bauge E, Tailleux A, Peters JM, Gonzalez FJ, Fruchart JC, Staels B. Peroxisome proliferator-activated receptor alpha is not rate-limiting for the lipoprotein-lowering action of fish oil. J Biol Chem 2001;276:4634–9. [DOI] [PubMed] [Google Scholar]
- 45. Jackson KG, Wolstencroft EJ, Bateman PA, Yaqoob P, Williams CM. Greater enrichment of triacylglycerol-rich lipoproteins with apolipoproteins E and C-III after meals rich in saturated fatty acids than after meals rich in unsaturated fatty acids. Am J Clin Nutr 2005;81:25–34. [DOI] [PubMed] [Google Scholar]
- 46. Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr 1998;67:828–36. [DOI] [PubMed] [Google Scholar]
- 47. Mustad VA, Etherton TD, Cooper AD, Mastro AM, Pearson TA, Jonnalagadda SS, Kris-Etherton PM. Reducing saturated fat intake is associated with increased levels of LDL receptors on mononuclear cells in healthy men and women. J Lipid Res 1997;38:459–68. [PubMed] [Google Scholar]
- 48. Jackson KG, Maitin V, Leake DS, Yaqoob P, Williams CM. Saturated fat-induced changes in Sf 60-400 particle composition reduces uptake of LDL by HepG2 cells. J Lipid Res 2006;47:393–403. [DOI] [PubMed] [Google Scholar]
- 49. Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J, Miyamori I, Yamamoto TT. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb 2004;11:200–8. [DOI] [PubMed] [Google Scholar]
- 50. Tao H, Aakula S, Abumrad NN, Hajri T. Peroxisome proliferator-activated receptor-gamma regulates the expression and function of very-low-density lipoprotein receptor. Am J Physiol Endocrinol Metab 2010;298:E68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tacken PJ, Hofker MH, Havekes LM, van Dijk KW. Living up to a name: the role of the VLDL receptor in lipid metabolism. Curr Opin Lipidol 2001;12:275–9. [DOI] [PubMed] [Google Scholar]
- 52. Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9. [DOI] [PubMed] [Google Scholar]
- 53. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 54. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013;346:e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ 2016;353:i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. . Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 58. Thanassoulis G, Williams K, Ye K, Brook R, Couture P, Lawler PR, de Graaf J, Furberg CD, Sniderman A. Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: a meta-analysis of randomized trials. J Am Heart Assoc 2014;3:e000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.