Abstract
Traumatic brain injury (TBI) is one of the most devastating injuries experienced by military personnel, as well as the general population, and can result in acute and chronic complications such as cognitive impairments. Since there are currently no effective tools for the treatment of TBI, it is of great importance to determine the mechanisms of neuronal death that characterize this insult. Several studies have indicated that TBI-induced neuronal death arises in part due to excessive activation of poly(ADP-ribose) polymerase-1 (PARP-1), which results in nicotinamide adenine dinucleotide (NAD+) depletion and subsequent energy failure. In this study, we investigated whether intranasal administration of NAD+ could reduce neuronal death after TBI. Rats were subjected to a weight-drop TBI model that induces cortical and hippocampal neuronal death. The intranasal administration of NAD+ (20 mg/kg) immediately after TBI protected neurons in CA1, CA3, and dentate gyrus of the hippocampus, but not in the cortex. In addition, delayed microglial activation normally seen after TBI was reduced by NAD+ treatment at 7 days after insult. Neuronal superoxide production and PARP-1 accumulation after TBI were not inhibited by NAD+ treatment, indicating that reactive oxygen species (ROS) production and PARP-1 activation are events that occur upstream of NAD+ depletion. This study suggests that intranasal delivery of NAD+ represents a novel, inexpensive, and non-toxic intervention for preventing TBI-induced neuronal death.
Key words: : hippocampus, intranasal, microglia, nicotinamide adenine dinucleotide, poly(ADP-ribose) polymerase-1, superoxide, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is one of the leading causes of death and disability among young adults in industrialized countries. In the United States, about two million cases are reported every year, with approximately 500,000 people requiring hospitalization (Weight, 1998). TBI is a heterogeneous disorder, producing contusion, laceration, focal or diffuse intracranial hemorrhages, and diffuse axonal injury. These primary injuries lead to secondary events such as oxidative stress, edema, and alterations of endogenous neurochemical mechanisms. Survival and recovery from TBI have been dramatically improved over the past few decades with modern clinical management practices, including antibiotic treatment and control of intracranial pressure (Singh, 2003). However, many survivors of TBI still display delayed neuronal death and permanent cognitive impairment. Prior studies have identified several mechanisms that contribute to this secondary cell death, including excitotoxicity due to excessive glutamate release into the synaptic cleft, reactive oxygen species (ROS) generation, and intracellular Zn2+ accumulation (Bullock et al., 1992; Faden et al., 1989; Katayama et al., 1990; Lewen and Hillered, 1998; Lewen et al., 2001; Marklund et al., 2001; Mikawa et al., 1996; Suh et al., 2000,2006).
Nicotinamide adenine dinucleotide (NAD+) acts as a major coenzyme in the process of energy metabolism. NAD+ is an essential co-enzyme for mitochondrial lactate-dehydrogenase that converts lactate into pyruvate. Recently, evidence has begun accumulating that NAD+ may mediate certain aspects of cell death and aging. Among NAD+-consuming enzymes, poly(ADP-ribose) polymerases (PARPs) are activated by DNA damage. Excessive activation of PARP by excitotoxicity or oxidative stress depletes NAD+ and ATP, resulting in neuronal death. PARP-1 activation leads to cytosolic NAD+ depletion and mitochondrial release of apoptosis-inducing factor (AIF). Alano and associates demonstrated that NAD+ depletion is a causal event in PARP-1-mediated cell death, and placed NAD+ depletion and glycolytic failure upstream of mitochondrial AIF release (Alano et al., 2010). Zn2+ toxicity (intracellular zinc accumulation) has also been shown to induce neuronal death accompanied by reduced NAD+ and ATP levels, which are attenuated by NAD+ restoration by nicotinamide and pyruvate (Sheline et al., 2000). Additionally, a reduction in NAD+ levels was observed in several animal models of neurological diseases, such as ischemia and TBI (Plaschke et al., 2000). Several studies have shown that cerebral NAD+ content is decreased by PARP activation after TBI in rodents (Clark et al., 2007; Satchell et al., 2003).
Our previous study showed that intranasal administration of NAD+ at 2 and 5 h after ischemic brain injury reduced neuronal death by 60–90% (Ying et al., 2007). Based on that study, and the known effect of PARP-1 on NAD+ depletion in TBI, we hypothesized that intranasal administration of NAD+ should decrease neuronal death in a rat model of TBI. Intranasal delivery provides a practical, non-invasive method of bypassing the blood–brain barrier (BBB) to deliver therapeutic agents to the brain. Intranasal delivery of NAD+ resulted in an increase in brain NAD+ content (Tsuchiyama et al., 2009). To test our hypothesis, rats were subjected to a weight-drop TBI model, and neuronal cell death was evaluated by Fluoro-Jade B staining. We found that intranasal delivery of NAD+ significantly prevented neuronal death and microglial activation in the hippocampus.
Methods
Weight-drop-induced traumatic brain injury in rats
All animal experiments were approved by the animal studies committee of the San Francisco Veterans Affairs Medical Center, and of the Hallym University. Male Sprague-Dawley rats (250–350 g) were deeply anesthetized with ketamine and xylazine. The rats were placed in a stereotaxic frame, the scalp and temporal muscle were reflected, and a 3-mm-diameter hole was drilled through the skull (2 mm lateral to the midline and 4.3 mm rostral to the bregma). TBI was performed using a weight-drop model. For the mechanical trauma, a blunt steel impactor was dropped onto the intact dura (Clark et al., 1994; Suh et al., 2000,2006). With this method, a brief displacement and deformation of the brain was induced by simply dropping a weight with consistent velocity (1.5 m/sec), depth (3.0 mm), and contusion time (120 msec). All rats were maintained at a core temperature of 36.5–37.5°C during and after surgery, until ambulatory. Rats with seizures were excluded from data analysis.
NAD+ or nicotinamide administration
NAD+ or nicotinamide was delivered either to the intranasal space or the intraperitoneal space. For intranasal delivery, NAD+ or nicotinamide (20 mg/kg of body weight) was dissolved in 60 μL saline and 6 μL was intranasally dropped every 30 sec 10 times (i.e., 6 mg in 60 μL for a 300-g rat=20 mg/kg body weight) beginning immediately after TBI. For intraperitoneal delivery, NAD+ or nicotinamide was injected at the same dose (20 mg/kg) immediately after TBI.
Confocal microscopy
Fluorescence signals were detected using a Zeiss LSM 510 confocal imaging system (Zeiss, New York, NY), with a sequential scanning mode for Alexa-Fluor 488 and 594. Stacks of images (1024×1024 pixels) from consecutive slices 0.66–0.7 μm in thickness were obtained by averaging four scans per slice, and were processed with Adobe Photoshop (Adobe Systems, Mountain View, CA).
Assessment of neuronal death
The rats were deeply anesthetized with 3% (vol/vol) isoflurane 24 h after TBI and perfused transcardially with 200 mL of 0.9% saline followed by 4% formaldehyde (FA) for 5 min. The harvested brains were post-fixed with 4% FA for 24 h and immersed in 20% sucrose until sinking to the bottom of the solution. Cryostat sections (25-μm thick) were mounted on frosted slides. For identifying degenerating neurons in the hippocampus, Fluoro-Jade B (FJB) staining was performed as described previously (Schmued and Hopkins, 2000; Suh et al., 2007). Briefly, the sections were immersed in a basic alcohol solution for 5 min and 0.06% KMnO4 for 15 min, and then the sections were incubated in 0.0004% FJB (Histo-Chem, Jefferson, AR) for 20 min. The slides were washed in distilled water and then dried. To quantify neuronal death, sections were collected every third slice from 4.0 mm posterior to the bregma, and five coronal sections were analyzed from each animal. An observer blinded to treatment condition counted the number of FJB-positive neurons in the hippocampal CA1 and subiculum from the ipsilateral hippocampus. Mean numbers of FJB-positive neurons from each region were used for statistical analyses.
NAD+/NADH assay
NAD+ and reduced NAD (NADH) levels in the hippocampus were measured by an NAD+/NADH assay kit (Abcam, San Francisco, CA), according to the manufacturer's instructions. Briefly, the animals were perfused with cold saline, and ∼20 g of the hippocampus (−3.3 to −5.3 mm from the bregma) was dissected and homogenized. Total NAD (NADt) and NADH were extracted in extraction buffer and measured spectrophoretically at 450 nm following the manufacturer's instructions. The NAD+:NADH ratio was calculated as: [NADt – NADH]/NADH. The protein concentrations of lysates were determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA).
Superoxide detection
For evaluation of differences in superoxide production after TBI, rats were intraperitoneally received dihydroethidium (dHEt; Molecular Probes, Invitrogen, Eugene, Oregon) at a dose of 1 mg/kg 30 min before TBI (Murakami et al., 1998; Suh et al., 2008). The rats were euthanized 3 h after TBI and transcardially perfused with 0.9% saline followed by 4% FA fixation. In 40-μm cryostat sections, ethidium (Et) signals were photographed with a confocal fluorescence microscope with an excitation of 510–550 nm and an emission greater than 580 nm. Images were taken from the hippocampal CA3 and dentate gyrus (DG), and the peri-injury area of the parietal cortex. Et signal intensity was expressed as the ratio of the mean fluorescence in the neuronal perikariya to that of the striatum radiatum of the hippocampal CA1 region.
Immunohistochemistry
Forty-micron coronal sections were prepared and immunostained as described previously, with modifications (Suh et al., 2003). Sections were blocked and permeabilized with 10% goat serum/0.1% Triton-X 100 in pH 7.4 phosphate-buffer (PB). Anti-poly ADP ribose (PAR) monoclonal antibody (Trevigen, Gaithersburg, MD) was added at a 1:2000 dilution and incubated at 4°C overnight. After washing, the sections were incubated with Alexa-Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen) at a 1:500 dilution for 1 h at room temperature. The sections were mounted and photographed with a Leica confocal laser-scanning microscope. Negative controls were prepared by omitting the primary antibodies.
Immunostaining for evaluation of microglial activation
Immunostaining was performed on 40-μm brain sections. After a 0.1 M PB rinse, nonspecific protein binding of the brain tissue was blocked by 1 h incubation in blocking buffer (10% goat serum and 0.1% Triton X-100 in 0.1 M PB) at room temperature. The sections were then immunostained with a mouse antibody to rat CD11b (AbD Serotec, Raleigh, NC) at a 1:200 dilution. After washing, the sections were incubated with Alexa-Fluor 488-conjugated goat anti-mouse IgG secondary antibody (Molecular Probes) at a dilution of 1:500 for 2 h at room temperature. Negative controls performed with secondary antibody alone showed no staining. Microglial activation was evaluated by an observer blinded to treatment condition. Three sections from each animal were evaluated for scoring. Microglial activation criteria were based on the number of CD11b-immunoreactive cells and their morphology, as previous published (Kauppinen et al., 2008).
Statistical analysis
Data are shown as means±standard error of the mean (SEM). Statistical significance was assessed by analysis of variance (ANOVA), and post-hoc testing was accomplished using Scheffe's test. A p value <0.05 was considered statistically significant. Microglial activation data were assessed by Kruskal-Wallis non-parametric one-way ANOVA testing, followed by Dunn's test for multiple group comparisons.
Results
TBI-induced cortical neuronal death is not prevented by intranasal administration of NAD+
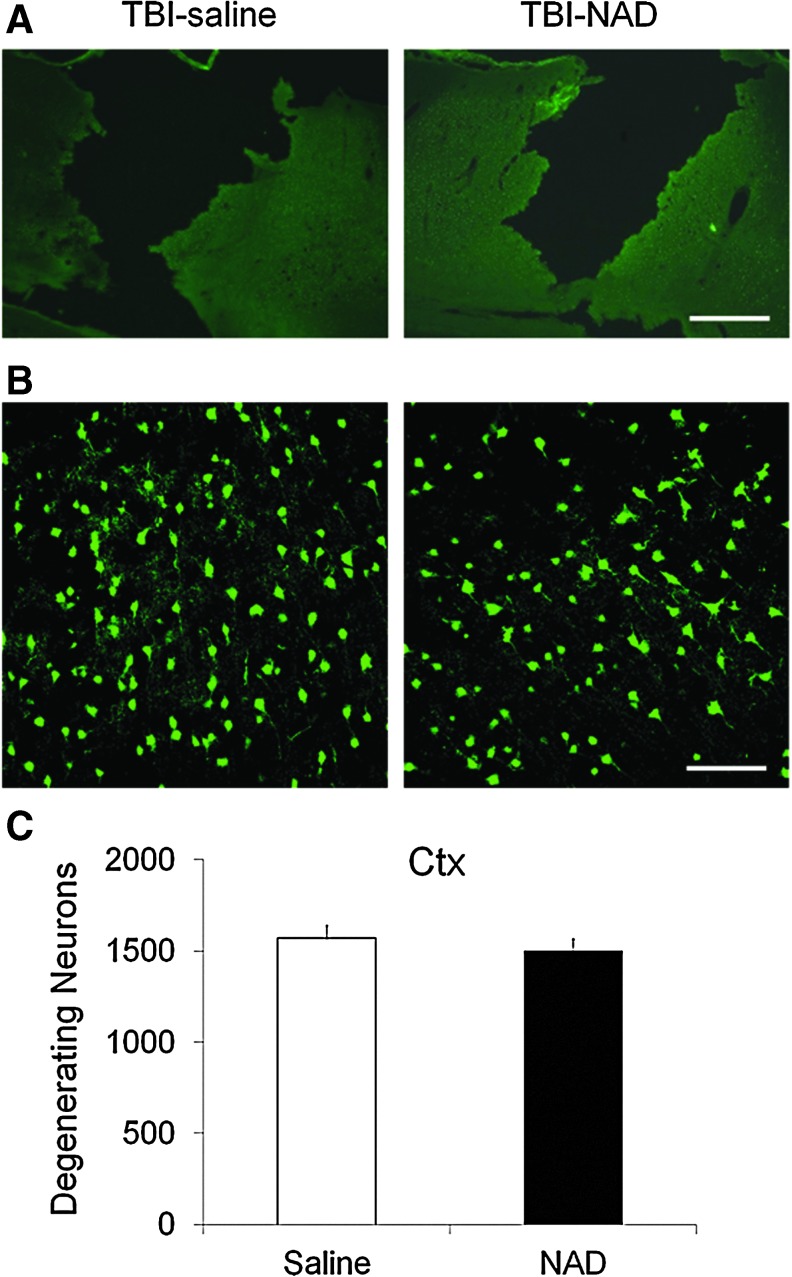
Neuronal injury evaluated by FJB staining at 24 h after TBI showed widespread cortical neuronal injury in vehicle-treated rats. An image taken with low-power magnification shows a large necrotic area of parietal cortex (Fig. 1A). Intranasal treatment with NAD+ showed no neuroprotective effects on the TBI-induced cavity or on peri-lesional neuron death. Compared with saline-treated rats, NAD+-treated rats showed a similar number of FJB-positive neurons in the parietal cortex (Fig. 1B and C). FJB-positive neuron counting was performed 400 μm away from the center of the cortical impact site.
FIG. 1.
Traumatic brain injury (TBI)-induced cortical neuronal death is not prevented by intranasal administration of nicotinamide adenine dinucleotide (NAD+). (A) TBI produced a hemisphere-shaped zone of necrosis. These low-magnification images show a loss of cortical tissue throughout the impact zone. Cortical tissue loss is almost identical in the saline-treated (TBI-saline) and NAD+-treated groups (TBI-NAD; scale bar=500 μm). (B) TBI induced severe neuron death (Fluoro-Jade B [FJB]-positive neurons) in the peri-lesional cortical impact area at 24 h after cortical impact. Confocal fluorescence images show several FJB-positive neurons in the cortex (Ctx) at 24 hours after TBI. Intranasal treatment with NAD+ provided no protective effects against cortical neuronal death (scale bar=100 μm). (C) Bar graph shows the quantified neuronal degeneration in the cortex. The number of FJB-positive neurons was not statistically significantly different in saline-treated and NAD+-treated animals. Color image is available online at www.liebertonline.com/neu
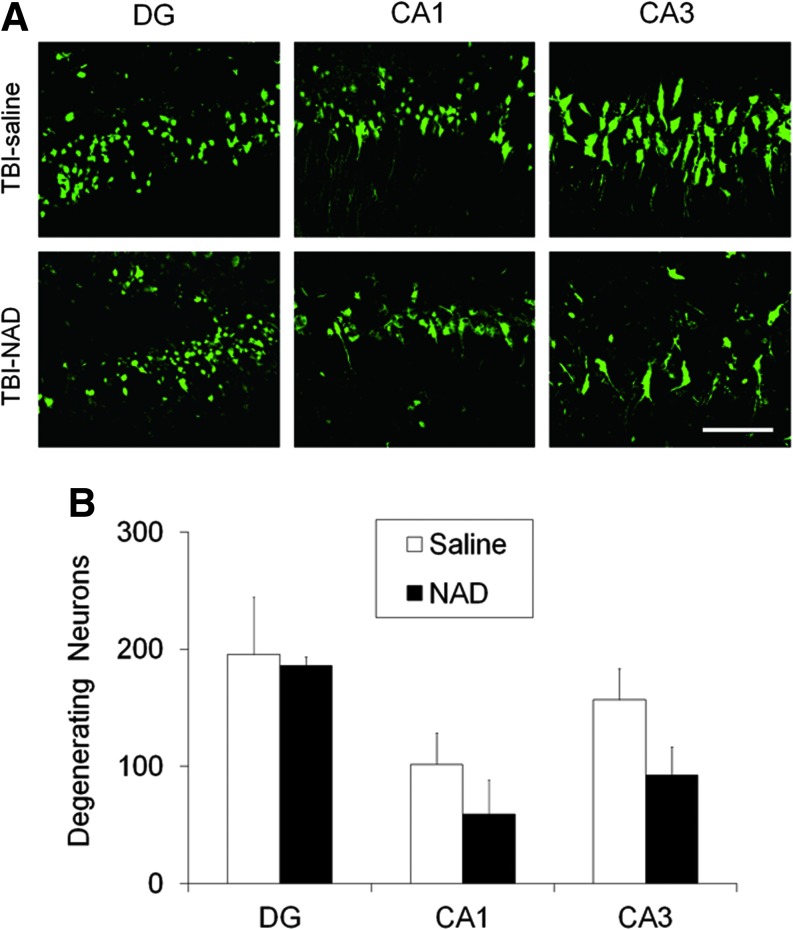
TBI-induced neuronal cell death in the hippocampus is prevented by intranasal administration of NAD+
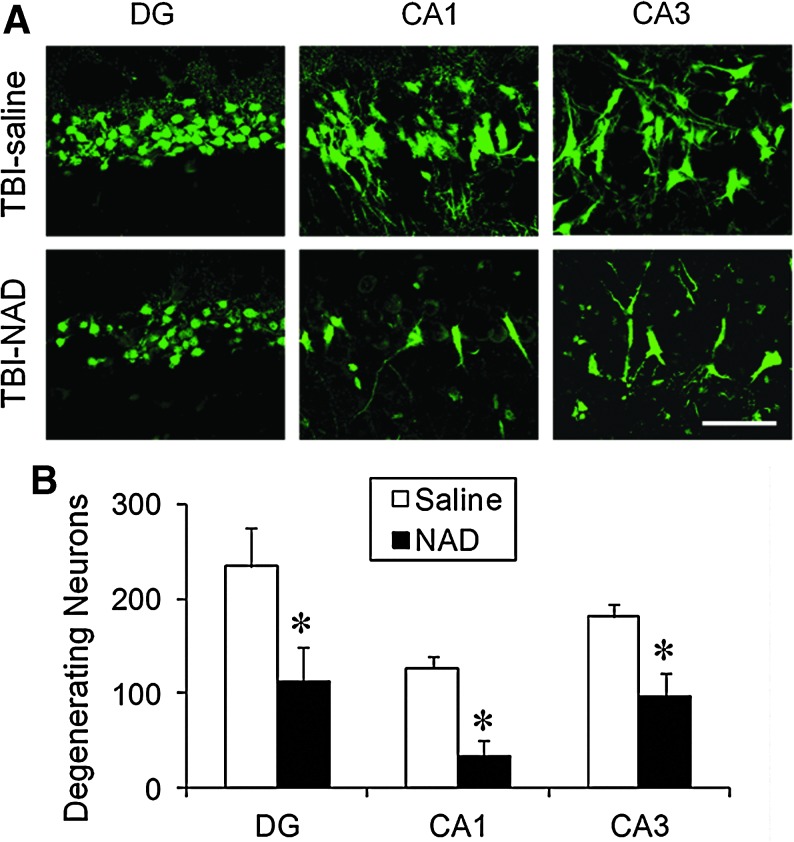
Neuronal injury evaluated by FJB staining at 24 h after TBI showed widespread hippocampal neuronal injury in the hemisphere ipsilateral to the injured side of vehicle-treated rats, which was comparable with that seen in previous studies (Fig. 2; Suh et al., 2000,2006). In sham-operated rats, no FJB-positive neurons were observed. Compared with the vehicle-treated group, the intranasal NAD+-treated group showed significantly fewer FJB-positive neurons (Fig. 2A). Quantification of the number of FJB-positive neurons in the hippocampus showed significant differences between the two groups (Fig. 2B).
FIG. 2.
Traumatic brain injury (TBI)-induced hippocampal neuronal death is prevented by intranasal administration of nicotinamide adenine dinucleotide (NAD+). (A) Confocal fluorescence images show neuronal death in the hippocampal dentate gyrus (DG), CA1, and CA3, at 24 h after TBI. A significant number of FJB-positive neurons (green-colored neurons) were observed in saline-treated (TBI-saline) rats after TBI. The number of Fluoro-Jade B (FJB)-positive neurons was significantly reduced by intranasal administration of NAD+ (TBI-NAD; scale bar=50 μm). (B) Bar graph showing the quantified degree of neuronal degeneration in the ipsilateral hippocampus. Data are mean±standard error of the mean; n=6 each group; *p<0.05 compared with the saline-treated group. Color image is available online at www.liebertonline.com/neu
Intranasal administration of NAD+ restored hippocampal NAD+ levels after TBI
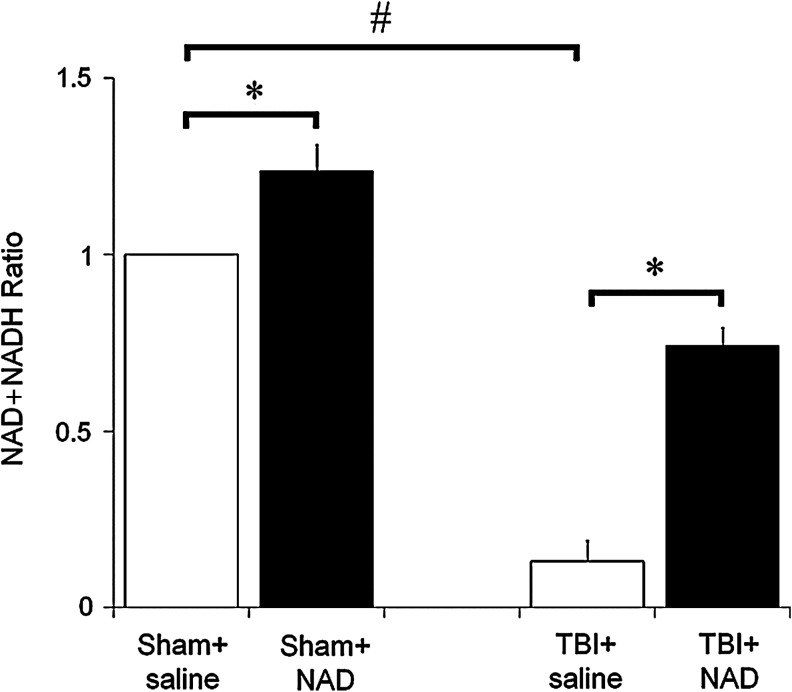
To test whether intranasal administration of NAD+ increases hippocampal NAD+ levels, total hippocampal NAD+ content was measured with or without TBI. When compared to the sham-operated group, TBI resulted in a significant decrease in the hippocampal NAD+:NADH ratio. The NAD+:NADH ratio was increased by intranasal administration of NAD+, either with or without TBI (Fig. 3).
FIG. 3.
Intranasal administration of nicotinamide adenine dinucleotide (NAD+) restored hippocampal NAD+ levels after traumatic brain injury (TBI). To test whether intranasal administration of NAD+ increases hippocampal NAD+ levels, total hippocampal NAD+ content was measured. When compared to the sham-operated group, TBI resulted in a significant decrease in the hippocampal NAD+ levels. NAD+ levels were increased by intranasal administration of NAD+ with or without TBI. Data are mean±standard error of the mean; n=2–5 for each group; #p<0.05 compared with the sham-operated group; *p<0.05 compared with the saline-treated group.
TBI-induced microglial activation in the hippocampus is inhibited by intranasal NAD+ administration
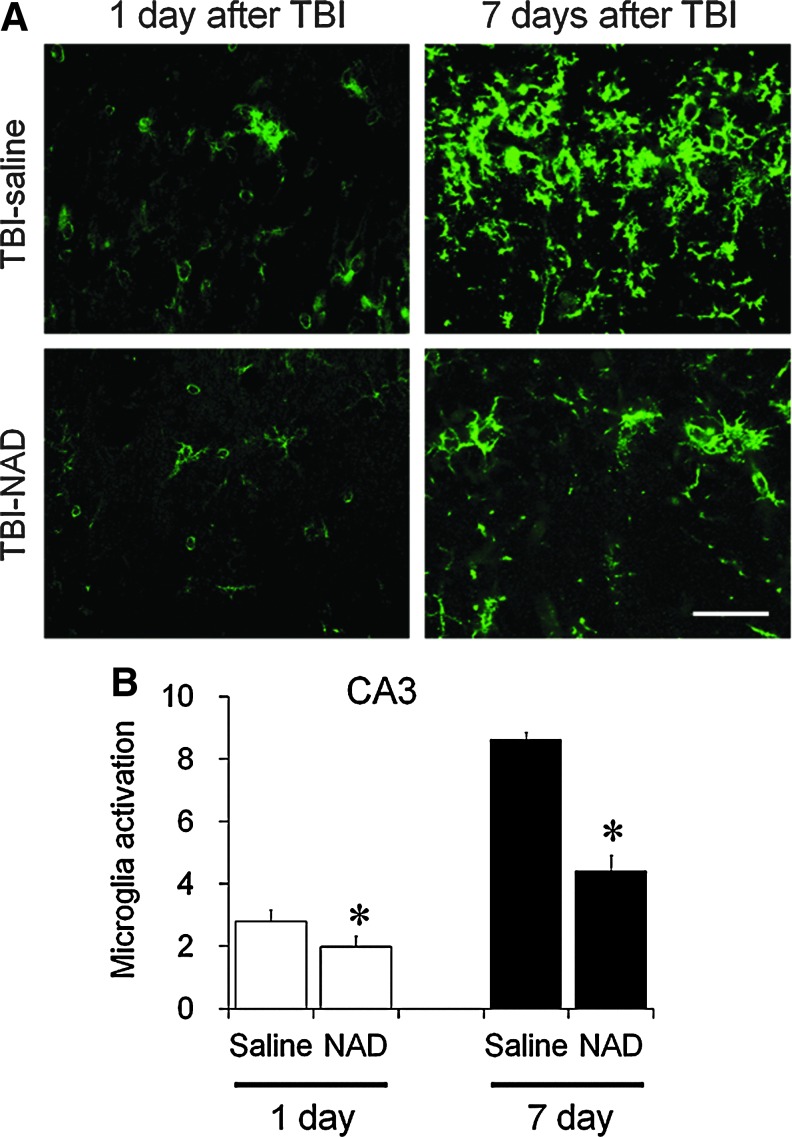
Microglia activation was evaluated in the hippocampal CA1 area at 1 or 7 days after TBI. One day after TBI, moderate microglial activation was detected in the hippocampal CA1 area. Several amoeboid-shaped CD11b-stained microglia were detected. Seven days after TBI, substantial microglial activation was detected in the hippocampal CA1 area. Intense microglial activation was prominent in the pyramidal layer of the CA1 area. To test whether NAD+ had a preventive effect on microglial activation after TBI, intranasal NAD+ was administered immediately after TBI. We found that intranasal administration of NAD+ substantially decreased TBI-induced microglial activation in the hippocampal CA1 pyramidal area at 1 day and 7 days after TBI (Fig. 4). However, intranasal administration of NAD+ caused no reduction of TBI-induced microglial activation in the cerebral cortex (data not shown).
FIG. 4.
Microglial activation after traumatic brain injury (TBI) is inhibited by intranasal administration of nicotinamide adenine dinucleotide (NAD+). (A) The sections harvested at 1 or 7 days after TBI were immunostained with CD11b. Morphological changes and intensity of microglial immunostaining was prominent after TBI. TBI increased microglial activation in the hippocampal CA3 area 1 day after the insult, and substantially increased it 7 days after the insult. Intranasal administration of NAD+ (TBI-NAD) reduced microglial activation in the hippocampal area (scale bar=50 μm). (B) Quantification of microglial activation was performed on the hippocampal CA3 pyramidal area. Microglial activation is quantified based on morphological changes and intensity of CD11b staining. Data are mean±standard error of the mean; n=3–6 for each group; *p<0.05 compared with the saline-treated group. Color image is available online at www.liebertonline.com/neu
TBI-induced superoxide production in the hippocampus and cortex
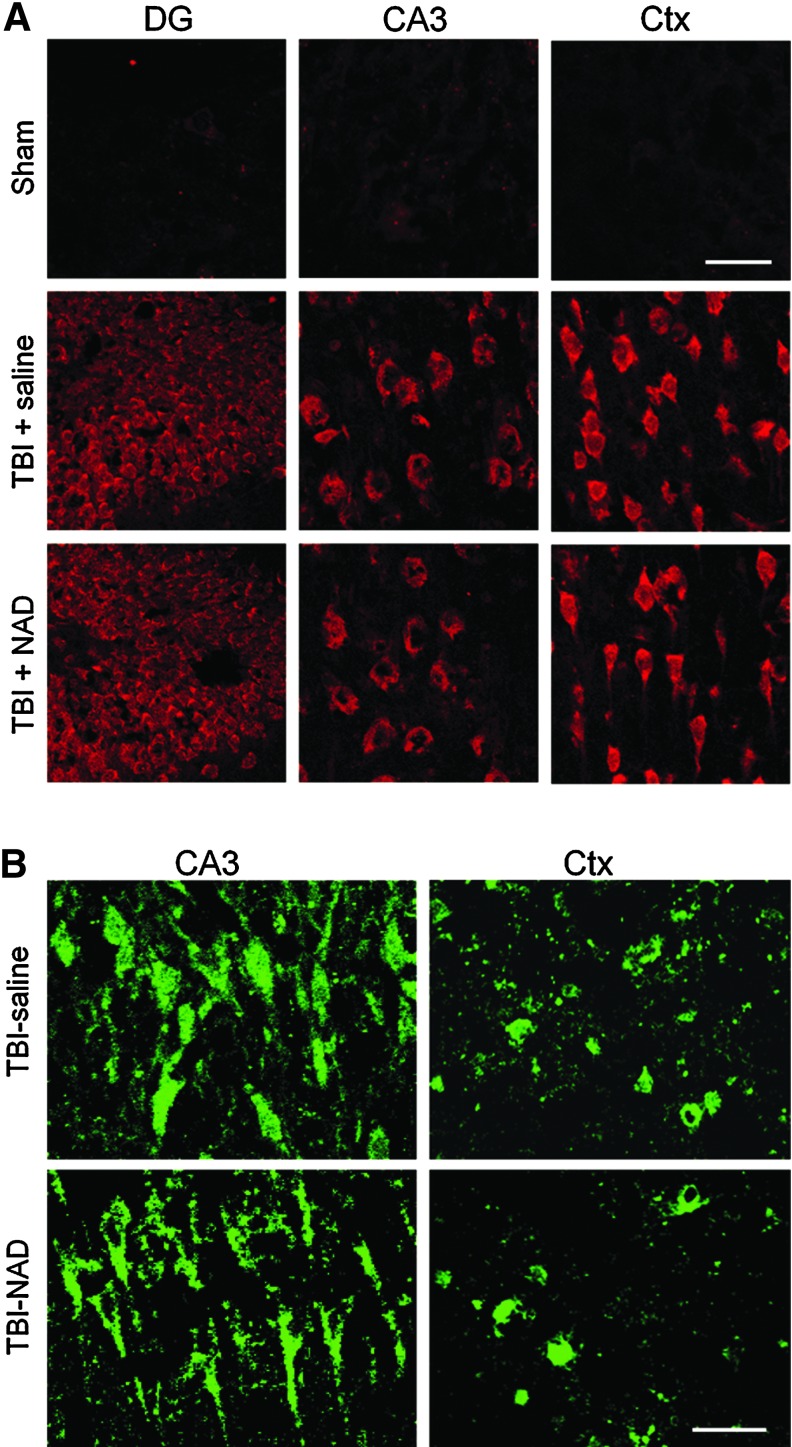
To test whether superoxide production occurs after TBI in hippocampal CA3 and DG neurons, as well as in cortical neurons, rats were injected with dHEt 30 min before TBI, and then brain sections were harvested 1 h after TBI. Superoxide production was estimated in hippocampal CA3 and DG neurons, or in cortical neurons, by the Et intensity ratio compared with the background intensity. Et fluorescence intensity was increased in the hippocampal and cortical neurons after TBI (Fig. 5A). To test whether NAD+ inhibits superoxide production after TBI, rats were treated with intranasal NAD+, and then brain sections were evaluated by quantifying Et fluorescence intensity. Here we found that the superoxide production in the hippocampal CA3, DG, and cortical neurons, were not inhibited by NAD+.
FIG. 5.
Traumatic brain injury (TBI)-induced reactive oxygen species (ROS) production and poly(ADP-ribose) polymerase-1 (PARP-1) activation are not prevented by intranasal nicotinamide adenine dinucleotide (NAD+) administration. (A) TBI-induced ROS production is not prevented by intranasal NAD+ administration. Neuronal superoxide production in the hippocampus and cortex was detected by dihydroethidium (dHEt) fluorescence staining 3 h after TBI. In sham-operated animals (Sham), Et fluorescent signal is negligible. TBI substantially increased ethidium (Et) intensity in the hippocampal CA3 and dentate gyrus neurons, and cortical (Ctx) neurons. Intranasal treatment with NAD+ caused no difference in Et fluorescence intensity after TBI (n=5 for each group; scale bar=30 μm). (B) TBI-induced poly(ADP-ribose) (PAR) accumulation is not prevented by intranasal administration of NAD+ in the hippocampus and cortex. Neuronal poly (ADP-ribose) (PAR) accumulation in the hippocampus and cortex was detected by immunohistochemistry 3 h after TBI. TBI substantially increased PAR fluorescence intensity in hippocampal CA3 and in cortical neurons. However, NAD+ treatment showed no difference in intensity of PAR fluorescence after TBI (n=3 for each group; scale bar=30 μm). Color image is available online at www.liebertonline.com/neu
TBI-induced PARP-1 activation is detected by PAR accumulation
To test whether PARP-1 activation occurs in hippocampal CA3 neurons or cortical neurons after TBI, brain sections were immunostained with an antibody against PAR. PAR accumulation in hippocampal CA3 neurons and in cortical neurons was detected at 3 h after TBI. To test whether intranasal treatment with NAD+ can prevent PAR accumulation in the vulnerable neurons after TBI, NAD+ was administered intranasally immediately after TBI. This study found that PAR accumulation in hippocampal CA3 neurons or cortical neurons was not inhibited by NAD+ (Fig. 5B).
TBI-induced neuronal death is not prevented by intraperitoneal injection of NAD+
Intraperitoneal injection of the same NAD+ concentration that we used for intranasal administration showed no neuroprotective effects on TBI-induced neuronal death. Compared with saline-treated rats, NAD+-treated rats showed a slight reduction in the number of FJB-positive neurons in the hippocampal DG, CA1, and CA3 areas; however, the difference was not significant (Fig. 6).
FIG. 6.
Traumatic brain injury (TBI)-induced hippocampal neuronal death is not prevented by intraperitoneal administration of nicotinamide adenine dinucleotide (NAD+). (A) Confocal fluorescence images show neuronal death in the hippocampal dentate gyrus (DG), CA1, and CA3 areas at 24 h after TBI. A significant number of Fluoro-Jade B (FJB)-positive neurons was observed in saline-treated rats after TBI. The number of FJB-positive neurons was not significantly reduced by intraperitoneal injection of NAD+ (scale bar=50 μm). (B) Bar graph shows the quantified neuronal degeneration in the hippocampus. Data are mean±standard error of the mean (n=5 for each group; *p<0.05 compared with the saline-treated group). Color image is available online at www.liebertonline.com/neu
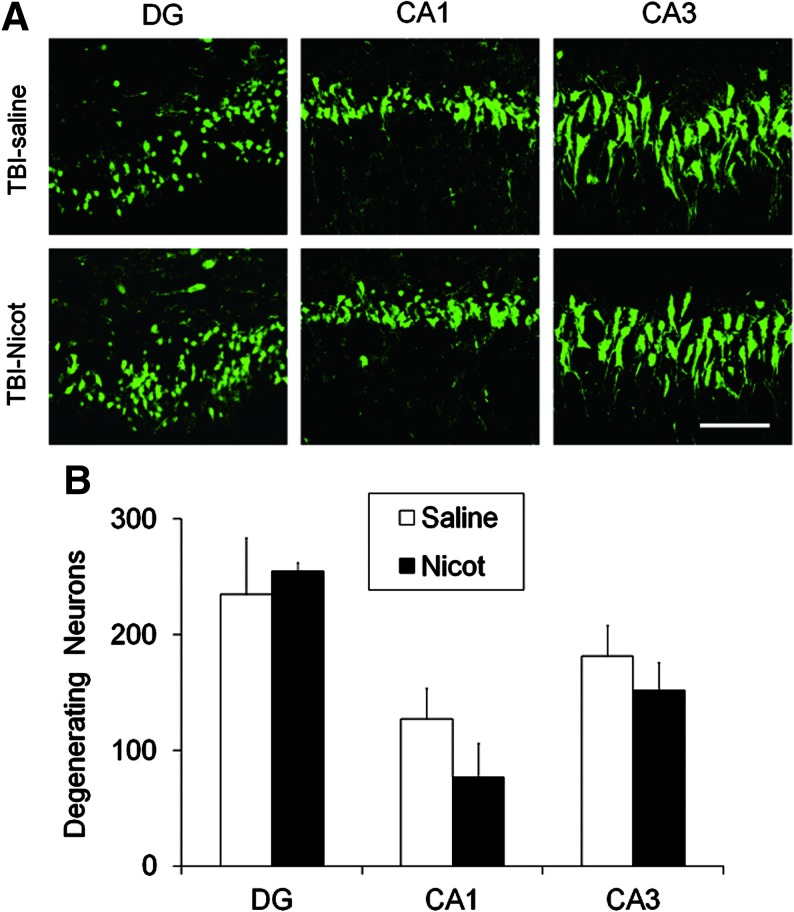
TBI-induced neuronal death is not prevented by intranasal treatment with nicotinamide
Several studies have previously suggested that NAD+ can be metabolized to nicotinamide, and that nicotinamide has an inhibitory effect on PARP-1 activation (Ying et al., 2005). Also, our previous study showed that nicotinamide inhibited N-methyl N′-nitro-N-nitrosoguanidine (MNNG)-induced neuron/astrocyte death through inhibition of PARP-1 (Ying et al., 2003). In the present study, to rule out whether the neuroprotective effects of NAD+ administered after TBI arise from supplementing energy substrates after PARP-1 activation, or via a direct inhibitory effect on PARP-1 activation after TBI, 20 mg/kg nicotinamide was administered intranasally, as was done with NAD+. Compared with NAD+-treated rats, nicotinamide treatment showed no protective effects against TBI-induced neuronal death (Fig. 7).
FIG. 7.
Traumatic brain injury (TBI)-induced hippocampal neuronal death is not prevented by intranasal administration of nicotinamide (Nicot). (A) Confocal fluorescence images show neuron death in the hippocampal dentate gyrus (DG), CA1, and CA3 areas at 24 h after TBI. A significant number of Fluoro-Jade B (FJB)-positive neurons was seen in saline-treated rats after TBI. The number of FJB-positive neurons was not reduced by intranasal administration of nicotinamide (scale bar=50 μm). (B) Bar graph shows the quantified neuronal degeneration in the hippocampus. Data are mean±standard error of the mean (n=6 for each group; *p<0.05 compared with the saline-treated group). Color image is available online at www.liebertonline.com/neu
Discussion
The present study demonstrates that intranasally-administered NAD+ increases hippocampal NAD+ levels and reduces TBI-induced neuronal death in the hippocampus. Here we speculate that TBI-induced PARP-1 activation was a key neuronal death mechanism, and that maintaining adenosine triphosphate (ATP) production by supplementation with intranasal NAD+ can prevent neuronal death after TBI. Microglial activation 7 days after TBI was also significantly attenuated by NAD+ treatment. However, TBI-induced superoxide production and PAR accumulation were not prevented by intranasal administration of NAD+.
The cellular mechanisms underlying TBI-induced neuronal injury are complex and cannot be fully explained by simple mechanical injury. Rather, several contributing factors are involved in downstream events of TBI-induced neuronal death, including sustained activation of glutamate receptors (Carbonell and Grady, 1999), PARP-1 activation (LaPlaca et al., 2001), and zinc translocation (Suh et al., 2000,2006). Although several lines of intervention have been shown to reduce this devastating brain injury in animal models, no clinically-applicable intervention strategies are currently available. Therefore, this study has sought to identify a simple and safe treatment method for reducing TBI-induced neuronal death using intranasal administration of NAD+.
Our lab previously provided the first evidence indicating that treatment with NAD+ can abolish PARP-1-induced death (Alano et al., 2004,2010; Ying et al., 2003,2005; Zhu et al., 2005). Since TBI produces PARP-1 activation, and NAD+ can rescue cells from PARP-1 activation, we tested the possibility that NAD+ may be used in vivo to decrease PARP-1-mediated neuronal injury. Our results confirm that TBI reduces hippocampal NAD+ levels, and intranasal administration restores hippocampal NAD+ levels. Furthermore, the present study provides evidence that intranasal NAD+ administration decreases TBI-induced neuronal death, which is consistent with our previous results in a study of cerebral ischemia (Ying et al., 2007). Intranasal NAD+ is protective even when applied 3–4 h after PARP-1 activation, suggesting that NAD+ administration may have a long window of opportunity in which it can act to decrease tissue injury (Alano et al., 2010).
Several studies have shown that inflammatory processes are involved in the progression of neuronal death after brain ischemia (Inamasu et al., 2000), and brain trauma (Thompson et al., 2005). Activated microglia have been shown to be neurotoxic. Microglia respond rapidly to changes in the central nervous system microenvironment and pathological events, which may play both deleterious and useful roles in neuronal damage. One of the initial stages of microglial activation is morphological transformation from ramified cell shape to amoeboid cell shape. After brain insults such as ischemia, the long, ramified processes of microglia are shortened and the soma is enlarged, finally forming an amoeboid shape (Koshinaga et al., 2000; Mabuchi et al., 2000; Schroeter et al., 1997). Activated microglia release ROS, including superoxide and nitric oxide, glutamate, extracellular protease, and cytokines, which are toxic to neurons (Cai et al., 2006). Inhibition of PARP-1 activation has been shown to attenuate microglial activation after ischemia (Hamby et al., 2007). Therefore, in this study we investigated whether microglial activation is modulated by NAD+ administration after TBI. Here, we found that NAD+ significantly reduced microglial activation after TBI. However, the mechanism by which NAD+ treatment leads to attenuation of microglial activation after TBI is not clear (Han et al., 2002; Inamasu et al., 2000; Yenari and Han, 2006).
Neither oxidative stress nor PAR accumulation were inhibited by NAD+ treatment, indicating that PARP-1 activation occurs upstream of these events. NAD+ can be metabolized to nicotinamide, which has an inhibitory effect on PARP-1 activation (Ying et al., 2005). To exclude the possibility that the neuroprotective effects of NAD+ administered after TBI arise from its metabolism to nicotinamide, equimolar doses of nicotinamide were administered intranasally, as was done with NAD+. Compared with NAD+-treated rats, intranasal nicotinamide treatment showed no protective effects on TBI-induced neuronal death. This, along with the observation that NAD+ supplementation did not reduce PAR formation, indicate that the effects of NAD+ are not attributable to reduced PARP-1 activity. However, since intranasal treatment with nicotinamide showed a trend toward neuroprotection in the hippocampal CA1 area, we cannot exclude the possibility that higher doses of nicotinamide may have therapeutic potential via some other mechanism (Hoane et al., 2003,2006).
Our previous study showed that TBI induced zinc translocation and neuronal death, which could be attenuated by zinc chelators (Suh et al., 2000,2006). Zinc-mediated neuronal death has been shown to reduce NAD+ levels, an effect attenuated by pharmacologically or genetically restoring NAD+ levels (Cai et al., 2006; Sheline et al., 2000,2003). In the present study, the effect of intranasal NAD+ treatment on zinc translocation was not investigated. However, based on several previous studies, we can speculate that TBI likely leads to a deleterious sequence of events that comprises zinc translocation, PARP activation, and subsequent NAD+/ATP depletion. We therefore propose that NAD+ administration exerts its protective effects by directly restoring NAD+ levels, rather than by preventing zinc translocation and PARP activation. Thus the use of a combined regimen consisting of zinc chelators, PARP inhibitors, and NAD+ may have additive effects.
The loss of hippocampal neurons due to TBI has previously been shown to cause deficits in spatial learning and memory (Clark et al., 2007). Preservation of hippocampal neurons with intranasal NAD+ as accomplished here would thus be predicted to reduce this functional impairment. However, a limitation to the present study is that behavioral studies were not performed to evaluate the functional impact of the NAD+ treatment. It may therefore be useful to extend this work to include functional outcome measures, and by evaluating the possibility of additive or synergistic effects of intranasal NAD+ administered with other compounds.
The results of the present study show that PARP activation and subsequent NAD+ depletion contribute to neuronal death after TBI. Intranasal delivery of NAD+ increased hippocampal NAD+ levels and attenuated TBI-induced hippocampal neuronal death. Therefore, administration of NAD+, which is both inexpensive and non-toxic, may warrant clinical evaluation.
Acknowledgments
This work was supported by the U.S. Department of Veterans Affairs and by the U.S. Department of Defense under contract number W81XWH-05-2-0094, and by the Hallym University (HRF-2010-033), and the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A100687).
Author Disclosure Statement
No competing financial interests exist.
References
- Alano C.C. Garnier P. Ying W. Higashi Y. Kauppinen T.M. Swanson R.A. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano C.C. Ying W. Swanson R.A. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J. Biol. Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- Bullock R. Kuroda Y. Teasdale G.M. McCulloch J. Prevention of post-traumatic excitotoxic brain damage with NMDA antagonist drugs: a new strategy for the nineties. Acta Neurochir. Suppl. (Wien.) 1992;55:49–55. doi: 10.1007/978-3-7091-9233-7_15. [DOI] [PubMed] [Google Scholar]
- Cai A.L. Zipfel G.J. Sheline C.T. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur. J. Neurosci. 2006;24:2169–2176. doi: 10.1111/j.1460-9568.2006.05110.x. [DOI] [PubMed] [Google Scholar]
- Carbonell W.S. Grady M.S. Evidence disputing the importance of excitotoxicity in hippocampal neuron death after experimental traumatic brain injury. Ann. NY Acad. Sci. 1999;890:287–298. doi: 10.1111/j.1749-6632.1999.tb08005.x. [DOI] [PubMed] [Google Scholar]
- Clark R.S. Schiding J.K. Kaczorowski S.L. Marion D.W. Kochanek P.M. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J. Neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- Clark R.S. Vagni V.A. Nathaniel P.D. Jenkins L.W. Dixon C.E. Szabo C. Local administration of the poly(ADP-ribose) polymerase inhibitor INO-1001 prevents NAD+ depletion and improves water maze performance after traumatic brain injury in mice. J. Neurotrauma. 2007;24:1399–1405. doi: 10.1089/neu.2007.0305. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Demediuk P. Panter S.S. Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Hamby A.M. Suh S.W. Kauppinen T.M. Swanson R.A. Use of a poly(ADP-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia-reperfusion. Stroke. 2007;38:632–636. doi: 10.1161/01.STR.0000250742.61241.79. [DOI] [PubMed] [Google Scholar]
- Han H.S. Qiao Y. Karabiyikoglu M. Giffard R.G. Yenari M.A. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J. Neurosci. 2002;22:3921–3928. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane M.R. Akstulewicz S.L. Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in rats. J. Neurotrauma. 2003;20:1189–1199. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Tan A.A. Pierce J.L. Anderson G.D. Smith D.C. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J. Neurotrauma. 2006;23:1535–1548. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- Inamasu J. Suga S. Sato S. Horiguchi T. Akaji K. Mayanagi K. Kawase T. Post-ischemic hypothermia delayed neutrophil accumulation and microglial activation following transient focal ischemia in rats. J. Neuroimmunol. 2000;109:66–74. doi: 10.1016/s0165-5728(00)00211-3. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Becker D.P. Tamura T. Hovda D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Kauppinen T.M. Higashi Y. Suh S.W. Escartin C. Nagasawa K. Swanson R.A. Zinc triggers microglial activation. J Neurosci. 2008;28:5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshinaga M. Katayama Y. Fukushima M. Oshima H. Suma T. Takahata T. Rapid and widespread microglial activation induced by traumatic brain injury in rat brain slices. J. Neurotrauma. 2000;17:185–192. doi: 10.1089/neu.2000.17.185. [DOI] [PubMed] [Google Scholar]
- LaPlaca M.C. Zhang J. Raghupathi R. Li J.H. Smith F. Bareyre F.M. Snyder S.H. Graham D.I. McIntosh T.K. Pharmacologic inhibition of poly(ADP-ribose) polymerase is neuroprotective following traumatic brain injury in rats. J. Neurotrauma. 2001;18:369–376. doi: 10.1089/089771501750170912. [DOI] [PubMed] [Google Scholar]
- Lewen A. Fujimura M. Sugawara T. Matz P. Copin J.C. Chan P.H. Oxidative stress-dependent release of mitochondrial cytochrome c after traumatic brain injury. J. Cereb. Blood Flow Metab. 2001;21:914–920. doi: 10.1097/00004647-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Lewen A. Hillered L. Involvement of reactive oxygen species in membrane phospholipid breakdown and energy perturbation after traumatic brain injury in the rat. J. Neurotrauma. 1998;15:521–530. doi: 10.1089/neu.1998.15.521. [DOI] [PubMed] [Google Scholar]
- Mabuchi T. Kitagawa K. Ohtsuki T. Kuwabara K. Yagita Y. Yanagihara T. Hori M. Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Marklund N. Clausen F. McIntosh T.K. Hillered L. Free radical scavenger posttreatment improves functional and morphological outcome after fluid percussion injury in the rat. J. Neurotrauma. 2001;18:821–832. doi: 10.1089/089771501316919184. [DOI] [PubMed] [Google Scholar]
- Mikawa S. Kinouchi H. Kamii H. Gobbel G.T. Chen S.F. Carlson E. Epstein C.J. Chan P.H. Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J. Neurosurg. 1996;85:885–891. doi: 10.3171/jns.1996.85.5.0885. [DOI] [PubMed] [Google Scholar]
- Murakami K. Kondo T. Kawase M. Chan P.H. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998;780:304–310. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- Plaschke K. Kopitz J. Weigand M.A. Martin E. Bardenheuer H.J. The neuroprotective effect of cerebral poly(ADP-ribose)polymerase inhibition in a rat model of global ischemia. Neurosci. Lett. 2000;284:109–112. doi: 10.1016/s0304-3940(00)00988-5. [DOI] [PubMed] [Google Scholar]
- Satchell M.A. Zhang X. Kochanek P.M. Dixon C.E. Jenkins L.W. Melick J. Szabo C. Clark R.S. A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD+ depletion and ribosylation of 14-3-3gamma. J. Neurochem. 2003;85:697–708. doi: 10.1046/j.1471-4159.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- Schmued L.C. Hopkins K.J. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schroeter M. Jander S. Huitinga I. Witte O.W. Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28:382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- Sheline C.T. Behrens M.M. Choi D.W. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J. Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline C.T. Wang H. Cai A.L. Dawson V.L. Choi D.W. Involvement of poly ADP ribosyl polymerase-1 in acute but not chronic zinc toxicity. Eur. J. Neurosci. 2003;18:1402–1409. doi: 10.1046/j.1460-9568.2003.02865.x. [DOI] [PubMed] [Google Scholar]
- Singh P. Missile injuries of the brain: results of less aggressive surgery. Neurol India. 2003;51:215–219. [PubMed] [Google Scholar]
- Suh S.W. Aoyama K. Chen Y. Garnier P. Matsumori Y. Gum E. Liu J. Swanson R.A. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J. Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S.W. Chen J.W. Motamedi M. Bell B. Listiak K. Pons N.F. Danscher G. Frederickson C.J. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- Suh S.W. Frederickson C.J. Danscher G. Neurotoxic zinc translocation into hippocampal neurons is inhibited by hypothermia and is aggravated by hyperthermia after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2006;26:161–169. doi: 10.1038/sj.jcbfm.9600176. [DOI] [PubMed] [Google Scholar]
- Suh S.W. Gum E.T. Hamby A.M. Chan P.H. Swanson R.A. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J. Clin. Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S.W. Hamby A.M. Gum E.T. Shin B.S. Won S.J. Sheline C.T. Chan P.H. Swanson R.A. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J. Cereb. Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- Thompson H.J. Hoover R.C. Tkacs N.C. Saatman K.E. McIntosh T.K. Development of posttraumatic hyperthermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J. Cereb. Blood Flow Metab. 2005;25:163–176. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- Tsuchiyama R. Sozen T. Manaenko A. Zhang J.H. Tang J. The effects of nicotinamide adenine dinucleotide on intracerebral hemorrhage-induced brain injury in mice. Neurol. Res. 2009;31:179–182. doi: 10.1179/174313209X393609. [DOI] [PubMed] [Google Scholar]
- Weight D.G. Minor head trauma. Psychiatr. Clin. North Am. 1998;21:609–624. doi: 10.1016/s0193-953x(05)70026-5. [DOI] [PubMed] [Google Scholar]
- Yenari M.A. Han H.S. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB) Neurochem. Int. 2006;49:164–169. doi: 10.1016/j.neuint.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Ying W. Alano C.C. Garnier P. Swanson R.A. NAD+ as a metabolic link between DNA damage and cell death. J. Neurosci. Res. 2005;79:216–223. doi: 10.1002/jnr.20289. [DOI] [PubMed] [Google Scholar]
- Ying W. Garnier P. Swanson R.A. NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem. Biophys. Res. Commun. 2003;308:809–813. doi: 10.1016/s0006-291x(03)01483-9. [DOI] [PubMed] [Google Scholar]
- Ying W. Wei G. Wang D. Wang Q. Tang X. Shi J. Zhang P. Lu H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front. Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- Zhu K. Swanson R.A. Ying W. NADH can enter into astrocytes and block poly(ADP-ribose) polymerase-1-mediated astrocyte death. Neuroreport. 2005;16:1209–1212. doi: 10.1097/00001756-200508010-00015. [DOI] [PubMed] [Google Scholar]