Abstract
One of the causes of infants’ hospitalizations is bronchiolitis, while different viral agents could be causative agents. As there is little information regarding the common agents of bronchiolitis in Iranian infants, we designed this study to determine which agents were responsible for hospitalization due to bronchiolitis among infants in an Iranian tertiary center. Two hundred and three infants with bronchiolitis who were hospitalized in Bahrami hospital were enrolled. Data regarding age, sex, duration of hospitalization, exposure to smoking, previous antibiotic usage and fever were collected for all enrolled cases. Throat sample by means of soap was collected and rapid test with immunochromatography (IC) test was performed. Rapid test was positive in 59 (29%) cases and three cases had concomitant infection with two viruses. The most common viral agent was RSV (Respiratory Syncytial Virus). Mean age was significantly lower in cases with RSV or RSV+ adenovirus infectious in comparison with other two groups (adenovirus or influenza only), while mean duration of hospitalization was significantly longer in RSV/RSV+ adenovirus group. RSV is the most common viral etiology of bronchiolitis in Iranian infants less than one year old, which is related with younger age and longer duration of hospitalization. Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease. Near 10% of affected children have a relative with SLE. Autoimmune diseases are more common in relatives of children with SLE. As there is no study regarding the prevalence of autoimmune disease in cases with pediatric SLE, we designed this study to evaluate the prevalence of autoimmune disease in children with SLE. In this cross sectional study, 50 children with SLE and 50 healthy children were enrolled. A structured questionnaire was used to collect data regarding the presence of autoimmune diseases in relatives. One thousand eight hundred and thirty two relatives were evaluated in the case group and 1699 in the control group. The number of relatives with autoimmune diseases was significantly higher in the case group (26 vs 10). The most common autoimmune diseases were lupus, followed by thyroid diseases among cases, and thyroid diseases and rheumatoid arthritis in controls.
According to the results of this study, the prevalence of autoimmune disorders is more common in relatives of children with SLE than in those of controls.
Keywords:lupus, SLE, autoimmune disorder, family history, children.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystem autoimmune connective tissue disease, with 20% of all cases having onset in childhood (1). The exact cause of the disease is not clear, while dysregulation of the immune system along with activation of polyclonal B-cells are considered in pediatric SLE (2). It mostly occurs between 12-14 years of age and is characterized by distinctive clinical and histological features (3). Children with SLE have a more aggressive clinical presentation than adult patients (4). In most cases, the presentation is not specific, while in pediatric cases renal involvement is more common and severe (5).
Juvenile SLE is associated with juvenile idiopathic arthritis, dermatomyositis, polymyositis, scleroderma and Crohn’s disease (6), and the reported female to male ratio varies between 2-5 to 1 (7, 8).
Near 10% of all SLE patients have a relative with SLE diagnosis and the risk for autoimmune diseases is higher among first degree relatives than in the general population (7, 8). Genetics along with environmental factors play an important role in the development of pediatric SLE. The prevalence of autoimmune diseases in relatives of children with SLE was reported to range between 42% and 74% (8, 9). As there is no study regarding the prevalence of autoimmune disease in cases with pediatric SLE, we designed this study to evaluate the prevalence of autoimmune disease in children with SLE.
MATERIALS AND METHODS
This cross sectional study conducted in Children Medical Center (hospital affiliated to Tehran University of Medical Sciences) between September 2013 and August 2014.
Systemic lupus erythematosus diagnosis was based on criteria established by the American College of Rheumatology in 2004. Exclusion criteria for patients were: parental separation, children who were in welfare and any chronic diseases except SLE.
We selected controls from children who referred to the emergency department with gastroenteritis and viral pneumonia. Exclusion criteria for controls were: chronic diseases, parental separation, children who were in welfare.
A structured questionnaire was used to collect data regarding the presence of autoimmune diseases in relatives. First degree relatives comprised parents, sisters and brothers, second degree relatives grandparents, uncles and aunts, and third degree relatives, cousins. Sampling was done by a convenient method to reach the sample size of 50 in the case group and 50 in the control group.
All parents filled the informed consent forms before study entrance. Data analysis was done by means of SPSS 22 (IBM, Chicago, IL, USA). Data were presented as mean±SD for continuous variables or frequencies for categorical variables. Chi square test with Fisher’s exact test was used for comparison of categorical variables. Independent sample t test was used to compare continuous variables. P value 0.05 was considered as significant.
RESULTS
One hundred children (50 in each group) were enrolled in the study. One thousand eight hundred and thirty two relatives were evaluated in the case group and 1699 in the control group. Thirty-five patients were females vs. 28 among controls. The mean age of patients was 14.1±4.5.
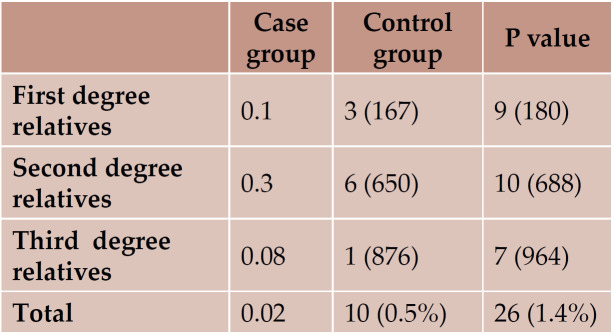
The number of relatives with autoimmune diseases was significantly higher in the case group (Table 1).
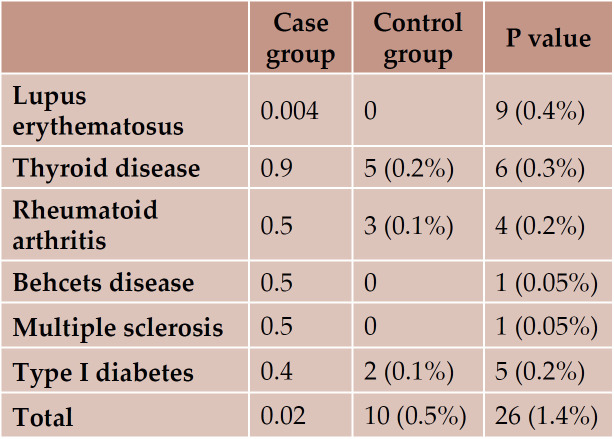
The most common autoimmune diseases were lupus, followed by thyroid diseases in the case group and thyroid diseases and rheumatoid arthritis in controls (Table 2).
DISCUSSION
The results of the current study showed that autoimmune diseases are more common in children with lupus erythematosus, which are frequent in the first and second degree relatives of children with SLE. They also showed that SLE was the most common autoimmune disease in relatives of SLE children, which could reveal the genetic part of the disease. In a previous study, Walters et al. reviewed the medical records of 69 patients with pediatric-onset SLE, and found the SLE and thyroid disease as the most common autoimmune disease in relatives, which is in accordance with our results (8). Wong et al. reported that near 10% of all SLE patients had a family history of SLE, while this rate was 0.4% in the current study (7). Similarly to Wong’s report, in our previous study, family history of autoimmune disease in juvenile SLE was 10% (10). Buckman et al. evaluated 340 SLE patients and found SLE among the relatives of 41 (12%) cases (11). The frequency of having relatives with SLE was higher among men.
In previous studies, thyroid diseases were more prevalent in relatives of children with SLE (9, 12). In this study, the prevalence of thyroid diseases in relatives of SLE cases was 0.3%, which was lower than the value reported in previous studies.
These findings could show the role of genetics in the disease progression as well as a genetic overlap of SLE with other autoimmune diseases (9).
It is known that SLE patients will have relatives with SLE, and the occurrence of the disease will be higher in the first degree relatives followed by second and third degree relatives (13-15).
Familial aggregation of autoimmune diseases in children with SLE may be the result of carrying genes that belong to the major histocompatibility complex (HLA), CTLA-4, and complement genes (16). Healthy relatives of SLE children had a higher rate of antinuclear antibodies (ANAs) positivity, including anti-double-stranded DNA (anti-dsDNA), as compared to controls (17). In a study on juvenile idiopathic arthritis (JIA) with positive history of autoimmune disorders, ANA positivity was more common and the duration of active disease was longer in patients with positive family history of autoimmune disorders in comparison to controls (18). In addition, age onset of JIA in patients with positive family history of autoimmune disorders was significantly smaller than in the control group (18).
Walters et al. considered that family history of autoimmune diseases did not predict the severity of SLE in children, but cases with severe disease had an early onset disease (8). In our previous study, family history of autoimmune disorders in juvenile SLE had no effect on the mortality rate (10).
This study had some limitations, including the fact that the severity of the disease was not measured, which was later evaluated in a tertiary hospital.
CONCLUSION
According to the results of this study, the prevalence of autoimmune disorders is more common in relatives of children with SLE than in those of controls. Also, the prevalence of SLE and thyroid disorders was higher in relatives of children with SLE. Third degree relatives had autoimmune disorders more frequently. Multicentric larger studies are further recom- mended.
Acknowledgments: This study was part of a dissertation of Dr. P. Ashouri and approved by Vice-chancellor for Research of School of Medicine, Tehran University of Medical Sciences.
Conflicts of interest: none declared.
TABLE 1.
Number of relatives with autoimmune diseases in each group
TABLE 2.
Frequency of different autoimmune diseases in two groups
Contributor Information
Parisa ASHOURNIA, Children’s Medical Center, Pediatrics Center of Excellence, Tehran, Iran.
Payman SADEGHI, Department of Pediatrics, Tehran University of Medical Sciences, Tehran, Iran.
Nima REZAEI, Molecular Immunology Research Center, Tehran University of Medical Sciences, Tehran, Iran; Research Center for Immunodeficiencies, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran.
Mohammad-Hassan MORADINEJAD, Children’s Medical Center, Pediatrics Center of Excellence, Tehran, Iran; Department of Pediatrics, Tehran University of Medical Sciences, Tehran, Iran.
Vahid ZIAEE, Department of Pediatrics, Tehran University of Medical Sciences, Tehran, Iran; Pediatric Rheumatology Research Group, Rheumatology Research Center, Tehran University of Medical Sciences, Tehran, Iran.
REFERENCES
- Barsalou J, Levy DM, Silverman ED. An update on childhood-onset systemic lupus erythematosus. Current opinion in rheumatology. 2013;5:616–622. doi: 10.1097/BOR.0b013e328363e868. [DOI] [PubMed] [Google Scholar]
- Moser KL, et al. Recent insights into the genetic basis of systemic lupus erythematosus. Genes and Immunity. 2009;5:373. doi: 10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen T, Madihi Y. Bullous systemic lupus erythematosus and lupus nephritis in a young girl. Oman medical journal. 2016;6:453. doi: 10.5001/omj.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, et al. Recurrent major infections in juvenile-onset systemic lupus rythematosus— a close link with long-term disease damage. Rheumatology. 2007;8:1290–1296. doi: 10.1093/rheumatology/kem102. [DOI] [PubMed] [Google Scholar]
- Youssef DM, et al. Pediatric systemic lupus erythematosus in a single nephrology unit. Saudi Journal of Kidney Diseases and Transplantation. 2015;2:314. doi: 10.4103/1319-2442.152493. [DOI] [PubMed] [Google Scholar]
- Swart JF, Wulffraat NM. Diagnostic workup for mixed connective tissue disease in childhood. The Israel Medical Association Journal. 2008;8-9:650–652. [PubMed] [Google Scholar]
- Wong M, Tsao BP. Current topics in human SLE genetics. Springer seminars in immunopathology. 2006;Springer doi: 10.1007/s00281-006-0031-6. [DOI] [PubMed] [Google Scholar]
- Walters HM, et al. Patterns and influence of familial autoimmunity in pediatric systemic lupus erythematosus. Pediatric Rheumatology. 2012;1:22. doi: 10.1186/1546-0096-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon-Segovia D, et al. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis & Rheumatology. 2005;4:1138–1147. doi: 10.1002/art.20999. [DOI] [PubMed] [Google Scholar]
- Tavangar-Rad F, Ziaee V, Moradinejad MH, Tahghighi F. Morbidity and Mortality in Iranian Children with Juvenile Systemic Lupus erythematosus. Iran J Pediatr. 2014;4:365–370. [PMC free article] [PubMed] [Google Scholar]
- Buckman KJ, et al. Familial systemic lupus erythematosus. Archives of internal medicine. 1978;11:1674–1676. [PubMed] [Google Scholar]
- Huang CM, Yang YH, Chiang BL. Different familial association patterns of autoimmune diseases between juvenileonset systemic lupus erythematosus and juvenile rheumatoid arthritis. Journal of microbiology, immunology, and infection. 2004;2:88–94. [PubMed] [Google Scholar]
- Eroglu G, Kohler P. Familial systemic lupus erythematosus: the role of genetic and environmental factors. Annals of the rheumatic diseases. 2002;1:29–31. doi: 10.1136/ard.61.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, et al. Risk factors for development of systemic lupus erythematosus: allergies, infections, and family history. Journal of clinical epidemiology. 2002;10:982–989. doi: 10.1016/s0895-4356(02)00429-8. [DOI] [PubMed] [Google Scholar]
- Sato E , et al. Systemic lupus erythematosus: a family study of 25 probands. Clinical and experimental rheumatology. 1991;5:455–461. [PubMed] [Google Scholar]
- Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;6939:506. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- Mohan C, et al. Anti-subnucleosome reactivities in systemic lupus erythematosus (SLE) patients and their first-degree relatives. Clinical & Experimental Immunology. 2001;1:119–126. doi: 10.1046/j.1365-2249.2001.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani M, Ziaee V, Moradinejad MH, Parvaneh N. The effect of positive family history of autoimmunity in juvenile idiopathic arthritis characteristics; a case control study. Iran J Pediatr. 2013;5:569–673. [PMC free article] [PubMed] [Google Scholar]