Abstract
Background
Few trials have examined long-term outcomes of advance care planning (ACP) interventions. We examined the efficacy of an ACP intervention on preparation for end-of-life decision making for dialysis patients and surrogates and for surrogates’ bereavement outcomes.
Study Design
A randomized trial compared an ACP intervention (Sharing Patient’s Illness Representations to Increase Trust [SPIRIT]) to usual care alone, with blinded outcome assessments.
Setting & Participants
420 participants (210 dyads of prevalent dialysis patients and their surrogates) from 20 dialysis centers.
Intervention
Every dyad received usual care. Those randomly assigned to SPIRIT had an in-depth ACP discussion at the center and a follow-up session at home 2 weeks later.
Outcomes & Measurements
Primary outcomes: preparation for end-of-life decision making, assessed for 12 months, included dyad congruence on goals of care at end of life, patient decisional conflict, surrogate decision-making confidence, and a composite of congruence and surrogate decision-making confidence. Secondary outcomes: bereavement outcomes, assessed for 6 months, included anxiety, depression, and posttraumatic distress symptoms completed by surrogates after patient death.
Results
Primary outcomes: adjusting for time and baseline values, dyad congruence (OR, 1.89; 95% CI, 1.1–3.3), surrogate decision-making confidence (β = 0.13; 95% CI, 0.01–0.24), and the composite (OR, 1.82; 95% CI, 1.0–3.2) were better in SPIRIT than controls, but patient decisional conflict did not differ between groups (β = −0.01; 95% CI, −0.12 to 0.10). Secondary outcomes: 45 patients died during the study. Surrogates in SPIRIT had less anxiety (β = −1.13; 95% CI, −2.23 to −0.03), depression (β = −2.54; 95% CI, −4.34 to −0.74), and posttraumatic distress (β = −5.75; 95% CI, −10.9 to −0.64) than controls.
Limitations
Study was conducted in a single US region.
Conclusions
SPIRIT was associated with improvements in dyad preparation for end-of-life decision making and surrogate bereavement outcomes.
INDEX WORDS: Advance care planning (ACP), end-of-life decision making, surrogate decision maker, medical decision, patient-surrogate dyad, dyad congruence, treatment options, life-sustaining treatment, bereavement, death, emotional distress, hemodialysis, end-stage renal disease (ESRD), advanced kidney disease, randomized controlled trial (RCT), patient education intervention
Advance care planning (ACP) is a process in which patients and family members or surrogate decision makers anticipate and discuss future health states and treatment options.1,2 It has the potential to improve end-of-life care and reduce costs associated with unwanted or nonbeneficial aggressive treatment near the end of life.3–6 Initial ACP efforts focused on documenting patients’ decisions about end-of-life care.7 However, given evidence that advance directives do not adequately improve end-of-life care, ACP for patients with serious chronic illness has evolved to focus on preparing patients and surrogates for treatment decision making at the end of life.8–12 The importance of surrogates also has been recognized because they are frequently involved in key medical decisions at the end of life.2,13,14 However, rarely have trials examined the long-term impact of ACP, including surrogate outcomes.
For patients with end-stage renal disease (ESRD), with mortality exceeding that for most types of cancer,15,16 dialysis may extend life but it might not improve the quality of survival time. Experts suggest that clinicians initiate timely discussions with patients with ESRD and surrogates to help them express desires about end-of-life care.17 However, these discussions often focus narrowly on advance directives and are delayed until near death.18,19 Further, no trials have examined whether ACP helps both patients with ESRD and their surrogates prepare for end-of-life decision making, the beneficial impact of ACP sustains over time, or ACP improves surrogates’ bereavement outcomes.18
Our ACP intervention, Sharing Patient’s Illness Representations to Increase Trust (SPIRIT), was based on the Representational Approach to Patient Education20,21 reflecting theories of illness cognition and conceptual change. In the representational approach, the interventionist first obtains a clear understanding of the patient’s perspective on their illness, symptoms, or prognosis before providing information to correct misunderstandings. SPIRIT sessions establish comprehension of the cognitive, emotional, and spiritual facets of the patients’ representation (understandings) of their illness, laying the groundwork for the interventionist to provide individualized information such as the effectiveness of mechanical supports at the end of life and to aid patients in examining their own values about such supports.
In a pilot study, SPIRIT had beneficial effects on patient and surrogate preparation for end-of-life decision making.14 The present trial tested the long-term effects of SPIRIT on preparation for end-of-life decision making (preparedness outcomes) for patients with ESRD and their surrogates and bereavement outcomes for surrogates.
METHODS
Design
We conducted a 2-group randomized trial with measures of patient and surrogate preparedness at baseline and 2, 6, and 12 months later and measures of surrogate bereavement outcomes at baseline, 2 weeks, and 3 and 6 months after the patient’s death. Before the first dyad reached the 12-month follow-up, the protocol was modified to ask dyads to extend their participation until study end in order to maximize the number of surrogates with bereavement outcomes. The University of North Carolina at Chapel Hill Institutional Review Board approved the study.
Setting and Participants
Patients were recruited from March 2010 through December 2012 from 20 outpatient dialysis centers in 8 counties in North Carolina. Inclusion criteria were 18 years or older, self-identified African American or white (acceptability of SPIRIT had not been tested with other groups), on dialysis therapy for at least 6 months, Charlson Comorbidity Index22,23 score of 6 or higher or Charlson Comorbidity Index score of 5 and hospitalization in the last 6 months (criteria associated with 1-patient-year mortality of 30%24), English-speaking, no hearing impairment, fewer than 3 errors on the Short Portable Mental Status Questionnaire,25 and an English-speaking surrogate older than 18 years who could participate.
A short battery of questions26 was used to help patients identify and confirm a previously designated surrogate. Patients and surrogates provided written consent and received compensation for completing measures ($15 at baseline, $20 at 2 months, $25 at 6 months, and $30 at 12 months). Each dyad received $15 at baseline for transportation to the dialysis center. Surrogates who completed bereavement measures received $20 at 2 weeks, $25 at 3 months, and $30 at 6 months.
Randomization and Interventions
Group assignments were generated prior to enrollment and concealed in sequentially numbered opaque envelopes opened after participants completed baseline measures. Patient-surrogate dyads were randomly assigned (1:1 ratio) to usual care plus SPIRIT or usual care only (control) using permuted blocks (size of 4) stratified by race (African American vs white), dialysis center type (university affiliated vs nonaffiliated), and dialysis modality (hemodialysis vs peritoneal dialysis).
Usual Care
As required by the Centers for Medicare & Medicaid Services (CMS),27 written information for advance directives was provided to every patient on the first day of dialysis, and a social worker encouraged patients to complete an advance directive and addressed questions about life-sustaining treatments. A nephrologist, physician assistant, or nurse practitioner reviewed resuscitation statements with the patient to determine whether the patient wanted a do-not-resuscitate (DNR) order in the center. If there was no DNR order in the record, a desire for “full code” (receiving cardiopulmonary resuscitation) was presumed.
Intervention
Dyads randomly assigned to intervention received usual care plus SPIRIT, conducted by 1 of 3 nurse interventionists using a structured intervention guide. The interventionists had at least 2 years of clinical experience and completed a 3½-day training program designed for competency in communication skills and knowledge in ESRD and end-of-life care.
SPIRIT is a psychoeducational intervention designed to assist patients to clarify their end-of-life preferences, help surrogates increase their understanding of the patient’s wishes, and prepare surrogates for the role and responsibilities of being a surrogate. The SPIRIT intervention included 2 sessions, and all sessions included both patient and surrogate. During the first session in a private room at the dialysis center, the interventionist assessed cognitive, emotional, and spiritual/religious aspects of the dyad’s representations of the patient’s illness, prognosis, and end-of-life care. This allowed the interventionist to provide individualized information about topics such as the effectiveness of life-sustaining treatment for people with end-organ failure and assisted the patient in examining his or her values about life-sustaining treatment at the end of life. The interventionist aimed to help the surrogate prepare for being a decision maker and for the emotional burden of end-of-life decision making by actively involving the surrogate in the discussion. A goals-of-care document was completed at the end of the session to indicate the patient’s preferences.
In a brief second session delivered 2 weeks later at the patient’s home (to reduce travel burden), the goals-of-care document and resuscitation preferences were reviewed. If the surrogate was someone out of the order of the hierarchical compensatory model28 (eg, a sibling was chosen when the patient had a spouse), the interventionist explored potential family conflicts and encouraged the dyad to talk with other family members and complete a health care power of attorney.
The interventionist then summarized the patient’s end-of-life preferences, listed the surrogate’s name and relationship to the patient, and indicated whether the patient desired a DNR order or assistance in completing an advance directive. The interventionist communicated this information to dialysis staff (the social worker and nurse manager or the medical director), and the document was placed in the medical record.
Sessions were audiorecorded. The first author reviewed every session for 6 months and provided one-on-one feedback to interventionists. After that, 20% of sessions were randomly selected every 6 months for evaluation of adherence using the Treatment Fidelity Assessment Tool.29 Refresher training was offered as needed.
Outcomes and Follow-up
At baseline, a research assistant collected outcome variables and sociodemographics. At follow-up, research assistants blinded to group assignments collected measures by telephone.
Preparedness Outcomes
Preparedness outcomes were dyad congruence, patient decisional conflict, and surrogate decision-making confidence (Table 1). Dyad congruence was assessed using the goals-of-care document,14 which included 2 scenarios describing medical conditions commonly occurring in patients with ESRD. In the first, the patient developed a severe complication and could not speak for him- or herself; the medical team believed recovery was unlikely and continuing life-sustaining treatment, including dialysis, would no longer be beneficial. In the second scenario, the patient developed advanced dementia. Each scenario had 3 response options: “The goals of care should focus on delaying my death, and thus I want to continue life-sustaining treatment,” “The goals of care should focus on my comfort and peace, and thus I do not want life-sustaining treatment, including dialysis,” and “I am not sure.” Patients and surrogates completed this document independently and their responses were then compared to determine dyad congruence: either congruent in both scenarios or incongruent. If both members of the dyad endorsed “I am not sure,” they were considered incongruent.
Table 1.
Summary of Instruments to Assess Outcomes
| Outcome and Variable | Instrument | Completed by | Measurement Time Point | |
|---|---|---|---|---|
|
| ||||
| Patient | Surrogate | |||
| Preparedness | ||||
| Dyad congruence | Goals-of-care document (2 end-of-life scenarios) | ✔ | ✔ | Baseline and 2, 6, and 12 mo |
| Patient decisional conflict | Decisional Conflict Scale (range, 1–5) | ✔ | Baseline and 2, 6, and 12 mo | |
| Surrogate decision-making confidence | Decision Making Confidence scale (range, 0–4) | ✔ | Baseline and 2, 6, and 12 mo | |
| Bereavement | ||||
| Anxiety symptoms | HADS–anxiety subscale (range, 0–21) | ✔ | Baseline and 2 wk postdeath and 3 and 6 mo postdeath | |
| Depression symptoms | HADS–depression subscale (range, 0–21) | ✔ | Baseline and 2 wk postdeath and 3 and 6 mo postdeath | |
| Posttraumatic distress symptoms | PTSS-10 (range, 10–70) | ✔ | Baseline and 2 wk postdeath and 3 and 6 mo postdeath | |
Abbreviations: HADS, Hospital Anxiety and Depression Scale; PTSS-10, Post-Traumatic Symptoms Scale-10.
Patient decisional conflict was measured using the 13-item Decisional Conflict Scale, a validated measure in the context of end-of-life decision making30; higher scores indicate greater difficulty weighing benefits and burdens of life-sustaining treatments and decision making (range, 1–5). Surrogate decision-making confidence was measured using the 5-item Decision Making Confidence scale,14,31 on which higher scores reflect greater comfort in performing as a surrogate (range, 0–4).
We created a composite outcome combining dyad congruence and surrogate Decision Making Confidence scale score because surrogates can feel highly confident even if they misunderstand patients’ wishes.14,31 Thus, to differentiate surrogates who understand the patient’s wishes and feel confident in their role from those who do not (ie, understand the wishes but lack confidence, misunderstand the wishes but feel confident, or neither understand nor feel confident), dyads were grouped as congruent in both scenarios and surrogate Decision Making Confidence scale score of 3 or higher (“confident” to “very confident”), or not.14
Bereavement Outcomes
The 3 most common bereavement outcomes were measured: symptoms of anxiety, depression, and posttraumatic distress.32,33 Anxiety and depression were measured using the Hospital Anxiety and Depression Scale34 (subscale score range, 0–21; higher scores indicate greater symptom severity). The intensity of post-traumatic distress symptoms was assessed using the Post-Traumatic Symptoms Scale 10 (PTSS-10)35 (range, 10–70; higher scores indicate more intense symptoms).
Statistical Analysis
Based on pilot data,14 a priori power analysis of a 2-sample test of proportions at each time point indicated that to detect an odds ratio (OR) of 3.5 for the composite outcome with 2-sided α ≤ 0.017 (= 0.05/3 for multiple tests at 3 points), 80 dyads per group were needed for 90% power, and 100 dyads per group, for 95% power. The sample size allowed for ~30% patient deaths so that surrogate bereavement outcomes could be assessed. Analyses were intention to treat with all available data.
Most missing data on preparedness outcomes were due to deaths of patients (n = 30). Baseline characteristics of those lost to follow-up (n = 8) were similar in the groups, suggesting data missing at random.
Generalized estimating equation (GEE) methods36 with exchangeable working covariance structure were used to examine group differences in preparedness and bereavement outcomes, adjusting for changes over time. The GEE provides unbiased estimates of intervention effects.36 Logistic link function for binary outcomes and identity for continuous outcomes were used. Additional GEE analyses were performed for preparedness outcomes with an intervention group indicator, time, the baseline value, and the interaction between intervention and time. For bereavement outcomes, the intervention-time interaction was not analyzed due to the small sample (n = 45). Analyses were conducted using SAS, version 9.3 (SAS Institute Inc).
RESULTS
Sample Description
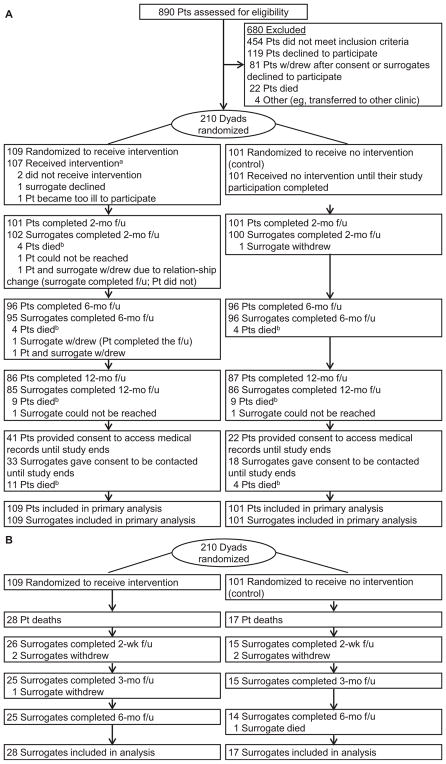
Of 890 patients screened, 436 were eligible; of those, 210 (48%) patient-surrogate dyads were randomly assigned (Fig 1). The groups were slightly imbalanced (n = 109 vs 101) because several dyads consented but did not come for baseline assessment and randomization. After numerous attempts to reschedule appointments, these dyads were considered passive refusals. Their randomization envelopes were neither opened nor reused.
Figure 1.
Flow through the Sharing Patient’s Illness Representations to Increase Trust (SPIRIT) trial of an advance care planning intervention for long-term dialysis patients (pts) and their chosen surrogates. (A) Participant flow. aFive dyads did not receive the second SPIRIT session (a brief follow-up [f/u] discussion): 3 dyads repeatedly canceled, 1 patient died before the scheduled session, and 1 home visit could not be made due to safety concerns for the interventionist. bSee Fig 1B for surrogate participant flow after patient’s death. (B) Surrogate participant flow after patient’s death.
Of the 85 dyads receiving the SPIRIT intervention and the 86 in the control group completing 12-month follow-up, 33 (39%) and 18 (21%), respectively, consented to extend participation until December 2013. Patient survival at 12 months and in December 2013 was similar between groups (P = 0.5 and P = 0.3, respectively). Mean survival, from randomization to patient death, was 11.4 (interquartile range [IQR], 5.3–18.0) months for SPIRIT and 13.1 (IQR, 5.5–15.5) months for control. There were 45 deaths by December 2013. Attrition over 6 months for surrogate bereavement outcomes was 6 (13%). The last 6-month assessment was completed in April 2014.
African Americans constituted 67.4% of participants (141 patients and 142 surrogates). A higher percentage of control patients than SPIRIT patients had no religious preference (19.8% vs 2.8%; P < 0.001); there were no other baseline group differences (Table 2). Baseline characteristics of dyads who agreed to extend study participation were similar between groups.
Table 2.
Baseline Characteristics of Randomly Assigned Participants
| Characteristic | SPIRIT (n = 109) | Control (n = 101 | ||
|---|---|---|---|---|
|
|
|
|||
| Patient | Surrogate | Patient | Surrogate | |
| Sociodemographics | ||||
| Age, y | 61.1 ± 11.4 | 54.1 ± 13.1 | 63.2 ± 11.1 | 54.1 ± 14.2 |
| Female sex | 65 (59.6) | 75 (68.8) | 55 (54.5) | 77 (76.2) |
| African American racea | 72 (66.1) | 74 (67.9) | 69 (68.3) | 68 (67.3) |
| Marital status | ||||
| Married/living with partner | 56 (51.4) | 73 (67.0) | 43 (39.9) | 63 (62.4) |
| Divorced/separated/widowed | 40 (36.7) | 19 (17.4) | 49 (48.5) | 22 (21.8) |
| Never married | 13 (11.9) | 17 (15.6) | 9 (8.9) | 16 (15.8) |
| Formal education completed, y | 12.5 ± 2.8 | 13.5 ± 2.5 | 12.8 ± 2.9 | 13.3 ± 2.0 |
| High school graduate or equivalent | 54 (49.5) | 50 (45.9) | 56 (55.4) | 47 (46.5) |
| Have a religious preference | 106 (97.2) | 99 (90.8) | 81 (80.2) | 91 (90.1) |
| Protestant | 96 (90.6) | 89 (89.9) | 74 (91.4) | 89 (97.8) |
| Extent of following religious customs | ||||
| Never/sometimes | 28 (26.4) | 17 (17.2) | 20 (24.7) | 10 (11.0) |
| Frequently/always | 78 (73.6) | 82 (82.8) | 61 (75.3) | 81 (89.0) |
| Importance of spirituality in life | ||||
| Not at all/somewhat important | 18 (16.5) | 8 (7.3) | 14 (13.9) | 14 (13.9) |
| Very/extremely important | 91 (83.5) | 101 (92.7) | 87 (86.1) | 87 (86.1) |
| Annual income | ||||
| <$20,000 | 53 (48.6) | 28 (25.7) | 53 (52.5) | 28 (27.7) |
| $20,000–$50,000 | 40 (36.7) | 51 (46.8) | 33 (32.7) | 43 (42.6) |
| >$50,000 | 14 (12.8) | 26 (23.9) | 12 (11.9) | 25 (24.8) |
| Refused to answer | 2 (1.8) | 4 (3.7) | 3 (3.0) | 5 (9.0) |
| Have had a close family member/friend die | 108 (99.1) | 103 (94.5) | 98 (97.0) | 98 (97.0) |
| Have been involved in tough medical decisions for family member/friend who died | 36 (33.3) | 39 (36.4) | 25 (25.0) | 35 (34.7) |
| Surrogate’s relationship to patient | ||||
| Spouse/partner | — | 44 (40.4) | — | 37 (36.6) |
| Parent | — | 27 (24.8) | — | 38 (37.6) |
| Sibling | — | 16 (14.7) | — | 11 (10.9) |
| Child | — | 8 (7.3) | — | 4 (4.0) |
| Friend | — | 6 (5.5) | — | 4 (4.0) |
| Other | — | 8 (7.3) | — | 7 (6.9) |
| Patient medical history and records | ||||
| Hemodialysis | 105 (96.3) | — | 96 (95.0) | — |
| Years on dialysis | ||||
| Median [IQR] | 3.8 [4.3] | — | 2.4 [3.8] | — |
| Mean ± SD | 4.5 ± 3.4 | — | 4.2 ± 4.9 | — |
| CCI illness severity20 | 8.2 ± 1.8 | — | 8.1 ± 1.8 | — |
| Has an advance directive | 21 (19.3) | — | 18 (17.8) | — |
| Surrogate listed in the medical record | 2 (1.8) | — | 2 (2.0) | — |
| DNR order at clinic | 5 (4.6) | — | 3 (3.0) | — |
Note: Unless otherwise indicated, values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation.
Abbreviations: CCI, Charlson Comorbidity Index; DNR, do not resuscitate; IQR, interquartile range; SD, standard deviation; SPIRIT, Sharing Patient’s Illness Representations to Increase Trust.
Assessed by self-report using National Institutes of Health reporting categories for federally funded clinical research.
Mean age of the 45 patients who died was 64.6 ± 12.6 (standard deviation) years. Twenty-one (47%) were women, 24 (53%) were African Americans, and 44 (98%) were on hemodialysis therapy. Mean age of bereaved surrogates was 57.1 ± 13.7 years, 34 (76%) were women, and 25 (56%) were African Americans. Infection and cardiovascular complications were causes of death for many patients (n = 18 [40%]). Ten patients (22%) died suddenly, requiring no surrogate decision making, but 35 surrogates (78%) were involved in end-of-life decision making. These characteristics were similar between groups.
Intervention Participation and Fidelity
Of the 109 dyads randomly assigned to SPIRIT, 107 (98%) received the first session; of those, 102 (95.3%) received the second session (Fig 1). The first session averaged 82 minutes and the second session averaged 20 minutes. No session was stopped because of participants’ emotional distress. Interventionist adherence to SPIRIT using the Treatment Fidelity Assessment Tool29 averaged 2.6 of 3.
Outcomes
Preparedness
Dyad congruence in goals of care for both scenarios was higher in SPIRIT than in controls at 2 and 6 months, but that effect was not significant across all time points (Table 3). Patient Decisional Conflict Scale scores decreased over time in SPIRIT while increasing in control, a significant intervention effect across time points (β = −0.12; 95% confidence interval [CI], −0.22 to −0.02; P = 0.01). Surrogate Decision Making Confidence scale scores were high at all time points and did not differ by group. The composite outcome did not differ at any point.
Table 3.
Preparedness and Bereavement Outcomes by Treatment Group
| Outcome | SPIRIT | Control | OR or βa (95% CI) | P |
|---|---|---|---|---|
| Preparedness outcomes | (n = 109) | (n = 101) | ||
| Dyad congruentb | 1.4 (0.9 to 2.1)c | 0.2 | ||
| Baseline | 47 (43.1) | 43 (42.6) | — | |
| 2 mo | 64 (63.4) | 48 (48.0) | 1.9 (1.1 to 3.3) | 0.03 |
| 6 mo | 66 (69.5) | 59 (61.5) | 1.4 (0.8 to 2.6) | 0.2 |
| 12 mo | 51 (60.0) | 52 (60.5) | 1.0 (0.5 to 1.8) | 0.9 |
| Patient DCSd | −0.12 (−0.22 to −0.02)c | 0.01 | ||
| Baseline | 1.6 ± 0.5 | 1.7 ± 0.5 | — | |
| 2 mo | 1.7 ± 0.5 | 1.7 ± 0.5 | −0.03 (−0.15 to 0.09) | 0.6 |
| 6 mo | 1.6 ± 0.5 | 1.8 ± 0.4 | −0.16 (−0.28 to −0.04) | 0.007 |
| 12 mo | 1.6 ± 0.4 | 1.8 ± 0.5 | −0.23 (−0.36 to −0.10) | <0.001 |
| Surrogate DMC scalee | 0.09 (−0.02 to 0.19)c | 0.1 | ||
| Baseline | 3.5 ± 0.5 | 3.6 ± 0.4 | — | |
| 2 mo | 3.7 ± 0.4 | 3.6 ± 0.5 | 0.12 (−0.002 to 0.23) | 0.05 |
| 6 mo | 3.7 ± 0.4 | 3.6 ± 0.5 | 0.10 (−0.02 to 0.23) | 0.1 |
| 12 mo | 3.7 ± 0.4 | 3.7 ± 0.5 | 0.03 (−0.10 to 0.16) | 0.7 |
| Composite outcomef | 1.4 (0.9 to 2.2)c | 0.1 | ||
| Baseline | 46 (42.2) | 43 (42.6) | — | |
| 2 mo | 62 (61.4) | 47 (47.0) | 1.8 (1.0 to 3.1) | 0.04 |
| 6 mo | 64 (67.4) | 55 (57.3) | 1.5 (0.9 to 2.8) | 0.2 |
| 12 mo | 51 (60.0) | 49 (57.0) | 1.1 (0.6 to 2.1) | 0.7 |
| Bereavement outcomes | (n = 28) | (n = 17) | ||
| HADS–anxietyg | −1.2 (−2.8 to 0.3)c | 0.1 | ||
| Baseline | 6.1 ± 4.2 | 6.1 ± 4.0 | — | |
| 2 wk | 6.3 ± 2.6 | 6.6 ± 4.0 | −0.4 (−2.5 to 1.8) | 0.7 |
| 3 mo | 5.1 ± 2.6 | 6.4 ± 2.7 | −1.3 (−3.0 to 0.5) | 0.1 |
| 6 mo | 4.7 ± 3.4 | 6.6 ± 2.7 | −1.9 (−4.0 to 0.3) | 0.09 |
| HADS–depressiong | −2.2 (−4.2 to −0.3)c | 0.02 | ||
| Baseline | 4.1 ± 3.1 | 3.1 ± 3.2 | — | |
| 2 wk | 4.8 ± 3.2 | 6.4 ± 4.4 | −1.6 (−4.1 to 0.9) | 0.2 |
| 3 mo | 3.3 ± 3.1 | 5.9 ± 3.2 | −2.7 (−4.7 to −0.6) | 0.01 |
| 6 mo | 3.4 ± 2.8 | 5.9 ± 3.2 | −2.6 (−4.6 to −0.6) | 0.01 |
| PTSS-10h | −4.0 (−10.2 to 2.2)c | 0.2 | ||
| Baseline | 20.2 ± 8.7 | 17.3 ± 8.1 | — | |
| 2 wk | 23.6 ± 11.8 | 27.0 ± 14.0 | −3.4 (−12.1 to 5.2) | 0.4 |
| 3 mo | 19.3 ± 9.9 | 22.5 ± 8.3 | −3.2 (−9.4 to 3.0) | 0.3 |
| 6 mo | 20.3 ± 11.1 | 25.5 ± 12.4 | −5.2 (−13.0 to 2.6) | 0.2 |
Note: Unless otherwise indicated, values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation.
Abbreviations: CI, confidence interval; DCS, Decisional Conflict Scale; DMC, Decision Making Confidence; HADS, Hospital Anxiety and Depression Scale; OR, odds ratio; PTSS-10, Post-Traumatic Stress Symptoms-10; SPIRIT, Sharing Patient’s Illness Representations to Increase Trust.
Unadjusted treatment effect; using all available data; a negative coefficient indicates the intervention was associated with a lower score; significance based on 2-sided P < 0.017 (Bonferroni correction) for the comparison at each time point.
Dyads congruent in both scenarios of the goals-of-care document.
Overall treatment effect, adjusted for time; significance based on 2-sided P < 0.05.
Patient DCS scores range from 1 to 5, with higher score indicating greater conflict.
Surrogate DMC scale scores range from 1 to 4, with a higher score indicating greater confidence.
Dyads were grouped into either dyads congruent in both scenarios and surrogate DMC scale score ≥ 3 or not (being one of the following: dyads congruent in both scenarios and surrogate DMC scale score < 3, dyads congruent in 1 or none of the scenarios and surrogate DMC scale score ≥ 3, or dyads congruent in 1 or none of the scenarios and surrogate DMC scale score < 3). The numbers (%) indicate dyads congruent in both scenarios and surrogate DMC scale score ≥ 3.
HADS anxiety and depression scores each range from 0 to 21, with higher score indicating greater symptom severity.
PTSS-10 scores range from 10 to 70, with a higher score indicating greater symptom severity.
Adjusting for time, baseline value, and the intervention-time interaction using multivariate GEE models (Table 4), the intervention effects on dyad congruence (OR, 1.89; 95% CI, 1.1–3.3; P = 0.03), surrogate Decision Making Confidence scale score (β = 0.13; 95% CI, 0.01–0.24; P = 0.03), and the composite outcome (OR, 1.82; 95% CI, 1.0–3.2; P = 0.04) were statistically significant. However, the intervention effect on dyad congruence significantly decreased by 12 months (OR, 0.46; 95% CI, 0.2–1.0; P = 0.04), whereas dyad congruence in controls significantly improved from 2 months to 6 (P = 0.02) and 12 (P = 0.02) months.
Table 4.
Multivariate Models for the Preparedness Outcomes
| Dyad Congruence | Patient DCS | Surrogate DMC Scale | Composite Outcome | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| OR (95% CI) | Pa | β (95% CI) | Pa | β (95% CI) | Pa | OR (95% CI) | Pa | |
| Treatment | ||||||||
| SPIRIT | 1.89 (1.1 to 3.3) | 0.03 | −0.01 (−0.12 to 0.10) | 0.9 | 0.13 (0.01 to 0.24) | 0.03 | 1.82 (1.0 to 3.2) | 0.04 |
| Control | 1.00 (reference) | (reference) | (reference) | 1.00 (reference) | ||||
| Time | ||||||||
| 2 mo | 1.00 (reference) | (reference) | (reference) | 1.00 (reference) | ||||
| 6 mo | 1.89 (1.1 to 3.2) | 0.02 | 0.07 (−0.01 to 0.15) | 0.08 | 0.03 (−0.06 to 0.12) | 0.5 | 1.63 (1.0 to 2.7) | 0.06 |
| 12 mo | 1.84 (1.1 to 3.1) | 0.02 | 0.12 (0.02 to 0.22) | 0.02 | 0.08 (−0.03 to 0.19) | 0.1 | 1.66 (1.0 to 2.8) | 0.06 |
| Baseline | 3.43b (2.1 to 5.6) | <0.001 | 0.30 (0.21 to 0.39) | <0.001 | 0.20 (0.07 to 0.33) | 0.002 | 3.42c (2.1 to 5.5) | <0.001 |
| Treatment × time | ||||||||
| Treatment × 6 mo | 0.66 (0.3 to 1.3) | 0.3 | −0.11 (−0.24 to 0.03) | 0.1 | −0.02 (−0.13 to 0.09) | 0.7 | 0.76 (0.4 to 1.5) | 0.4 |
| Treatment × 12 mo | 0.46 (0.2 to 1.0) | 0.04 | −0.19 (−0.33 to −0.04) | 0.01 | −0.08 (−0.21 to 0.05) | 0.2 | 0.57 (0.3 to 1.2) | 0.1 |
Abbreviations: CI, confidence interval; DCS, Decisional Conflict Scale; DMC, Decision Making Confidence; OR, odds ratio; SPIRIT, Sharing Patient’s Illness Representations to Increase Trust.
Significance based on 2-sided P < 0.05.
Reference indicates dyads not congruent in both scenarios.
Reference indicates dyads not congruent and/or surrogate DMC scale score < 3.
The intervention effect in reducing patient Decision Making Confidence scale score was significant at 12 months (β = −0.19; 95% CI, −0.33 to −0.04; P = 0.01). In contrast, Decisional Conflict Scale scores in controls significantly increased by 12 months (β = 0.12; 95% CI, 0.02–0.22; P = 0.02). Baseline values of all preparedness outcomes significantly predicted outcomes at follow-up points (all P < 0.01).
Bereavement
In both groups, surrogates’ anxiety, depression, and PTSS-10 scores increased at 2 weeks’ bereavement (Table 3). In SPIRIT, scores decreased over time, returning to or below baseline scores. Among controls, these scores never returned to baseline. By 3 months, scores stabilized in both groups. Depression scores in SPIRIT were significantly lower at 3 (P = 0.01) and 6 (P = 0.01) months than among controls, resulting in a significant intervention effect across all time points (β = −2.2; 95% CI, −4.2 to −0.3; P = 0.02).
Adjusting for time and baseline scores, GEE models (Table 5) showed significant intervention effects on anxiety (β = −1.13; 95% CI, −2.23 to −0.03; P = 0.04), depression (β = −2.54; 95% CI, −4.34 to −0.74; P = 0.006), and PTSS-10 scores (β = −5.75; 95% CI, −10.9 to −0.64; P = 0.03). Over time, anxiety was significantly reduced by 6 months (P = 0.04), and so was depression by 3 (P = 0.01) and 6 (P = 0.02) months and PTSS-10 scores by 3 months (P = 0.04). All baseline values significantly predicted outcomes at follow-up (either P = 0.01 or P < 0.001).
Table 5.
Multivariate Models for the Surrogate Bereavement Outcomes
| HADS–Anxiety | HADS–Depression | PTSS-10 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| β (95% CI) | Pa | β (95% CI) | Pa | β (95% CI) | Pa | |
| Treatment | ||||||
| SPIRIT | −1.13 (−2.23 to −0.03) | 0.04 | −2.54 (−4.34 to −0.74) | 0.006 | −5.75 (−10.9 to −0.64) | 0.03 |
| Control | (reference) | (reference) | (reference) | |||
| Time | ||||||
| 2 wk | (reference) | (reference) | (reference) | |||
| 3 mo | −0.91 (−1.85 to 0.03) | 0.06 | −1.06 (−1.89 to −0.24) | 0.01 | −4.52 (−7.59 to −1.45) | 0.004 |
| 6 mo | −1.00 (−1.94 to −0.07) | 0.04 | −0.90 (−1.66 to −0.14) | 0.02 | −2.34 (−5.04 to 0.35) | 0.09 |
| Baseline | 0.39 (0.24 to 0.54) | <0.001 | 0.30 (0.07 to 0.52) | 0.01 | 0.63 (0.32 to 0.95) | <0.001 |
Abbreviations: CI, confidence interval; HADS, Hospital Anxiety and Depression Scale; PTSS-10, Post-Traumatic Stress Symptoms-10; SPIRIT, Sharing Patient’s Illness Representations to Increase Trust.
Significance based on 2-sided P < 0.05.
DISCUSSION
SPIRIT was superior to usual care alone in enhancing dyad congruence in terms of goals of care, surrogate decision-making confidence, and the composite outcome combining the 2. These effects decreased by 12 months. SPIRIT significantly reduced patient decisional conflict and was superior to usual care alone in reducing surrogates’ bereavement anxiety, depression, and posttraumatic distress symptoms.
Previous studies14,30,37–39 have demonstrated short-term ACP intervention effects, but have not examined long-term effects and changes over time. In the current trial, SPIRIT’s effect on dyad congruence decreased after 2 months, decreased to two-thirds by 6 months and to half by 12 months. Although the importance of ongoing periodic ACP discussions has been emphasized,2,11 to date, no randomized trials supported that need. Our data suggest that improvement in dyad congruence may not be sustained over time, underscoring the need for repeated discussions. Patients might change their preferences or surrogates might not recall the patient’s wishes expressed during the discussion.
SPIRIT helped surrogates recover from bereavement distress by 3 months. Although another trial found similar results with geriatric patients and their surrogates,38 our trial is the first to show effects on bereavement outcomes of surrogates of patients with ESRD and to demonstrate changes over time. Knowing their loved ones’ wishes may have reduced surrogates’ bereavement distress,3,38 but reduced distress could also have been due to SPIRIT’s attention to preparing surrogates for being a surrogate and the emotional burden they might experience.
To our knowledge, this is the only randomized trial to demonstrate positive long-term effects of an ACP intervention in a sample with a majority of African Americans. For African Americans, ACP has been considered challenging because they are reportedly less amenable to using advance directives.40,41 However, African Americans in our study were clearly interested in discussing end-of-life care because no participant asked that the SPIRIT sessions be halted. We believe this occurred because instead of aiming at completion of an advance directive, SPIRIT focused on assisting patients and surrogates to think and talk about the possibility of end-of-life decision making and to explore how they would feel about care options near the end of life.
The preparedness outcomes, especially dyad congruence, also improved in the control group. As far as we are aware, our trial is the first randomized controlled trial of an ACP intervention with repeat measures of the preparedness outcomes. Simply answering these thought-provoking questions may have served as an intervention, a phenomenon known as assessment effects.42–44
Although this study included 20 dialysis centers representing both community and academic practice settings, caution is needed in generalizing because it was conducted in a single US region. The control group received usual care, not attention control, but usual care reflected CMS requirements for coverage of ESRD facilities; patients were encouraged to participate in their plan of care, including discussing advance directives and end-of-life concerns.27 Finally, the sample for bereavement outcomes was small, although estimates of intervention effects were stable.
In conclusion, SPIRIT was associated with improvements in dyad preparation for end-of-life decision making and surrogate bereavement outcomes. These findings may be useful in addressing the critical need to implement ACP for patients with advanced kidney disease.45 Advanced nurse practitioners or physician assistants might be appropriate to deliver the SPIRIT intervention after training. Future studies should include trials to determine SPIRIT’s effectiveness when implemented in clinical practice and with other racial/ethnic groups.
Acknowledgments
We acknowledge the participation and support of many people without whom this study could never have been completed, including the patients and their chosen surrogates; administrative and clinical staff, including nurses, social workers, and medical directors, of the 20 dialysis centers that participated in the study; the interventionists employed by the grant who delivered the SPIRIT intervention sessions at the center and patients’ homes; and the research assistants employed by the grant for study coordination, recruitment, data collection, retention activities, transcribing of the intervention and interview recordings, and data management.
Support: This study was funded by National Institutes of Health, National Institute of Nursing grant R01NR011464 (Dr Song). The funding source had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Trial registration: www.ClinicalTrials.gov; study number: NCT01259011.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Study concept and design: M-KS, SEW, JPF, LCH, JBH, GAH; data acquisition: M-KS; data analysis/interpretation: M-KS, JPF, F-CL, SEW, LCH, JBH, GAH, JCB; statistical analysis: F-CL, JPF; Funding obtainment: M-KS; administrative, technical, or material support: M-KS, JCB; study supervision: M-KS, JCB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. M-KS takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
References
- 1.Kolarik RC, Arnold RM, Fischer GS, Tulsky JA. Objectives for advance care planning. J Palliat Med. 2002;5(5):697–704. doi: 10.1089/109662102320880516. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academy of Sciences; 2014. [Accessed March 20, 2015]. http://www.iom.edu/~/media/Files/Report%20Files/2014/EOL/Key%20Findings%20and%20Recommendations.pdf. [PubMed] [Google Scholar]
- 3.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 4.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moses H, 3rd, Matheson DH, Dorsey ER, George BP, Sadoff D, Yoshimura S. The anatomy of health care in the United States. JAMA. 2013;310(18):1947–1963. doi: 10.1001/jama.2013.281425. [DOI] [PubMed] [Google Scholar]
- 6.Powell T. Advance care planning [letter] N Engl J Med. 2004;350(14):1470–1471. author reply 1470–1471. [PubMed] [Google Scholar]
- 7.Houben CH, Spruit MA, Groenen MT, Wouters EF, Janssen DJ. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–489. doi: 10.1016/j.jamda.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Fagerlin A, Schneider CE. Enough. The failure of the living will. Hastings Cent Rep. 2004;34(2):30–42. [PubMed] [Google Scholar]
- 9.Gillick MR. Advance care planning. N Engl J Med. 2004;350(1):7–8. doi: 10.1056/NEJMp038202. [DOI] [PubMed] [Google Scholar]
- 10.Perkins HS. Controlling death: the false promise of advance directives. Ann Intern Med. 2007;147(1):51–57. doi: 10.7326/0003-4819-147-1-200707030-00008. [DOI] [PubMed] [Google Scholar]
- 11.Billings JA. The need for safeguards in advance care planning. J Gen Intern Med. 2012;27(5):595–600. doi: 10.1007/s11606-011-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahan RD, Knight SJ, Fried TR, Sudore RL. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage. 2013;46(3):355–365. doi: 10.1016/j.jpainsymman.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–261. doi: 10.1059/0003-4819-153-4-201008170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song MK, Ward SE, Happ MB, et al. Randomized controlled trial of SPIRIT: an effective approach to preparing African American dialysis patients and families for end-of-life. Res Nurs Health. 2009;32:260–273. doi: 10.1002/nur.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 16.US Renal Data System. USRDS 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. [Google Scholar]
- 17.Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal From Dialysis: Clinical Practice Guideline. 2. Rockville, MD: Renal Physicians Association; 2010. [Google Scholar]
- 18.Luckett T, Sellars M, Tieman J, et al. Advance care planning for adults with CKD: a systematic integrative review. Am J Kidney Dis. 2014;63(5):761–770. doi: 10.1053/j.ajkd.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000–1025. doi: 10.1177/0269216314526272. [DOI] [PubMed] [Google Scholar]
- 20.Donovan HS, Ward S. A representational approach to patient education. J Nurs Scholarsh. 2001;33(3):211–216. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 21.Donovan HS, Ward SE, Song MK, Heidrich SM, Gunnarsdottir S, Phillips CM. An update on the representational approach to patient education. J Nurs Scholarsh. 2007;39(3):259–265. doi: 10.1111/j.1547-5069.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 23.Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337–342. doi: 10.1053/ajkd.2001.21300. [DOI] [PubMed] [Google Scholar]
- 24.Cohen LM, Moss AH, Weisbord SD, Germain MJ. Renal palliative care. J Palliat Med. 2006;9(4):977–992. doi: 10.1089/jpm.2006.9.977. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 26.Song MK, Ward SE. Disconnect between emergency contacts and surrogate decision-makers in the absence of advance directives. Palliat Med. 2013;27(8):789–792. doi: 10.1177/0269216312474486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; Conditions for Coverage for End-Stage Renal Disease Facilities; Final Rule. Department of Health and Human Services; 2008. [PubMed] [Google Scholar]
- 28.Carr D, Khodyakov D. Health care proxies: whom do young old adults choose and why? J Health Soc Behav. 2007;48(2):180–194. doi: 10.1177/002214650704800206. [DOI] [PubMed] [Google Scholar]
- 29.Song MK, Happ MB, Sandelowski M. Development of a tool to assess fidelity to a psycho-educational intervention. J Adv Nurs. 2010;66(3):673–682. doi: 10.1111/j.1365-2648.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song MK, Kirchhoff KT, Douglas J, Ward S, Hammes BJ. A randomized, controlled trial to improve advance care planning among patients undergoing cardiac surgery. Med Care. 2005;43(10):1049–1053. doi: 10.1097/01.mlr.0000178192.10283.b4. [DOI] [PubMed] [Google Scholar]
- 31.Song MK, Ward SE, Lin FC. End-of-life decision-making confidence in surrogates of African-American dialysis patients is overly optimistic. J Palliat Med. 2012;15(4):412–417. doi: 10.1089/jpm.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 33.Siegel MD, Hayes E, Vanderwerker LC, Loseth DB, Prigerson HG. Psychiatric illness in the next of kin of patients who die in the intensive care unit. Crit Care Med. 2008;36(6):1722–1728. doi: 10.1097/CCM.0b013e318174da72. [DOI] [PubMed] [Google Scholar]
- 34.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 35.Eid J, Thayer JF, Johnsen BH. Measuring post-traumatic stress: a psychometric evaluation of symptom– and coping questionnaires based on a Norwegian sample. Scand J Psychol. 1999;40(2):101–108. doi: 10.1111/1467-9450.00105. [DOI] [PubMed] [Google Scholar]
- 36.Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23(6):859–874. doi: 10.1002/sim.1650. discussion 875–857, 879–880. [DOI] [PubMed] [Google Scholar]
- 37.Song MK, Donovan HD, Piraino B, et al. Effects of an intervention to improve communication about end-of-life care among African Americans with chronic kidney disease. Appl Nurs Res. 2010;23:65–72. doi: 10.1016/j.apnr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease-specific advance care planning intervention on end-of-life care. J Am Geriatr Soc. 2012;60(5):946–950. doi: 10.1111/j.1532-5415.2012.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwak J, Haley WE. Current research findings on end-of-life decision making among racially or ethnically diverse groups. Gerontologist. 2005;45(5):634–641. doi: 10.1093/geront/45.5.634. [DOI] [PubMed] [Google Scholar]
- 41.Pew Research Center. [Accessed March 20, 2015];Views on end-of-life medical treatments. 2013 http://www.pewforum.org/2013/11/21/views-on-end-of-life-medical-treatments/
- 42.Song MK, Ward SE. Assessment effects in educational and psychosocial intervention trials: an important but often-overlooked problem. Res Nurs Health. 2015;38(3):241–247. doi: 10.1002/nur.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCambridge J, Butor-Bhavsar K, Witton J, Elbourne D. Can research assessments themselves cause bias in behaviour change trials? A systematic review of evidence from solomon 4-group studies. PLoS One. 2011;6(10):e25223. doi: 10.1371/journal.pone.0025223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PLoS One. 2011;6(10):e23748. doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holley JL, Davison SN. Advance Care planning for patients with advanced CKD: a need to move forward. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.00290115. [DOI] [PMC free article] [PubMed] [Google Scholar]